Abstract
Prior empirical studies suggest that cognitive impairment is the strongest predictor of capacity to consent to research among persons with schizophrenia. Yet, despite the frequency and importance of cognitive deficits and impaired decisional capacity in schizophrenia, the scope of neuropsychological testing in most published reports in this area has been relatively narrow. In the present study of 70 people with schizophrenia aged 40 to 70 years we evaluated decisional capacity with the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR). Participants were also evaluated with standardized rating scales of psychopathology and level of insight and with a comprehensive neuropsychological test battery that permitted evaluation of 7 specific cognitive abilities. Results showed that the strongest correlates of capacity (particularly, understanding and appreciation of disclosed information) were cognitive test scores, but there was little evidence of differential relationships between individual cognitive abilities and specific dimensions of capacity. Understanding was also correlated with severity of negative symptoms and of general psychopathology, but not with age, education, severity of positive or depressive symptoms, or level of insight. Understanding improved over successive presentations of consent-relevant information. The results suggest that age and diagnosis should not be viewed as determinants of decisional capacity; investigators should be alert to the presence of cognitive deficits, as well as negative symptoms. Also, an interactive dialogue between patient and investigator with repeated presentation of information is likely to aid understanding of disclosed information among patients with schizophrenia.
Keywords: neurocognition, informed consent, psychoses, bioethics, competence
Introduction
Advances in developing safer and more effective treatments for schizophrenia will require clinical trials involving research participants with this disorder. In addition to the psychopathological symptoms, schizophrenia and other serious neuropsychiatric disorders may adversely affect some aspects of cognitive functioning and insight.1,2 Some bioethicists have expressed concerns about the potential influence of such deficits on capacity to consent to research.3
As noted by Appelbaum and colleagues,4,5 decisional capacity is thought to involve 4 dimensions: (1) understanding of disclosed information, (2) appreciation of the significance of that information for one's own condition or situation, (3) reasoning with the information, and (4) expression of a choice or decision. Although not fully independent dimensions, impairments in one or another of these domains may have different implications for modifying the consent process in a way that facilitates more capable decisions. Thus, there is value in determining how individual factors may differentially affect each of these 4 components of decisional capacity.
Schizophrenia is often,2 although not always,6 associated with mild to moderate cognitive deficits. Recent empirical data suggest that general levels of cognitive impairment are a particularly important determinant of overall decisional capacity in schizophrenia.7–12 There is no single level or pattern of cognitive deficits that is unique to or common to all patients with schizophrenia, but some of the most frequently observed deficits are those in attention/working memory, executive functions, and the learning of new information.2,13
It seems likely that some of the individual types of cognitive functions may have more weight in certain dimensions of decisional capacity than in others. For instance, to understand disclosed information, one presumably must have sufficient attention and verbal skills to comprehend written or aurally presented information, and one must have adequate learning abilities to acquire the information for subsequent processing. Conversely, the processes of appreciation and reasoning may require greater reliance on executive functions such as abstraction, problem solving, and mental flexibility. Indeed, although using somewhat different terminology, Marson et al.14 specified a similar cognitive model to explain the influence of the individual cognitive deficits in different stages of Alzheimer disease on specific aspects of patients' capacity to consent to treatment.
Despite the frequency and importance of cognitive impairment in schizophrenia,2,15 and the documented association of general cognitive functioning with decisional capacity, the scope of neuropsychological testing in most published reports of capacity to consent to research among schizophrenia patients has been relatively narrow. In several studies7,11,16 the primary cognitive measure (supplemented by a few additional tests) was a 20- to 30-minute measure that was originally designed to detect and characterize cognitive impairment among dementia patients—the Repeatable Battery for Assessment of Neuropsychological Status (RBANS).17 In some other key investigations8,18 the cognitive battery was limited to the Vocabulary, Similarities, and Digit Span subtests from the Wechsler Adult Intelligence Scale–Revised.19 Our previous study of capacity to consent to treatment9 included a fairly comprehensive neuropsychological battery administered to a subset of participants, but cognitive testing in prior studies from our own research group on capacity to consent to research10,12 has been limited to brief dementia-related scales such as the Mini-Mental State Examination20 or the Mattis Dementia Rating Scale.21 To our knowledge, there have been no published, large-scale studies of capacity to consent to research that have included a comprehensive neuropsychological test battery. Thus, it remains unclear whether there are individual cognitive abilities that are particularly important for specific dimensions of decisional capacity.
Besides cognition, a few other variables have been reported to be risk factors for impaired decisional capacity in individuals with schizophrenia. Some investigators have found overall severity of psychopathology to be negatively correlated with decisional capacity,7,8,11 but this pattern has been less robust and consistent than that for the effects of cognition on decisional capacity.5,9 In part, inconsistency in findings may relate to the type of specific symptoms evaluated; there is some evidence that negative symptoms may be stronger negative correlates of decisional capacity than positive symptoms.8,9,11 Another possible factor underlying heterogeneity in decisional capacity may be the normal cognitive changes with aging. Specifically, subtle changes in several cognitive domains are commonly observed with normal aging,22 and advanced age has been suggested as a possible risk factor for impaired understanding in some studies of consent conducted with nonschizophrenia patient populations.23 Age has not emerged as a strong correlate of decisional capacity in schizophrenia studies; however, there have been few published empirical comparisons of capacity to consent to research among older versus non-elderly patients with schizophrenia.
In the present study we evaluated the degree to which specific dimensions of decisional capacity were affected by deficits in individual cognitive domains as evaluated with a comprehensive neuropsychological test battery. We hypothesized that the understanding of disclosed material would be strongly correlated with scores on neuropsychological measures of verbal ability and those emphasizing verbal or visual episodic learning/memory, and that appreciation and reasoning would strongly correlate with performance on measures of executive functioning. Given the subtle cognitive changes seen with normal aging, we also expected older age would be associated with greater deficits in decisional capacity (particularly understanding, appreciation, and reasoning). We also evaluated the degree to which each of these capacity dimensions was correlated with specific demographic characteristics and dimensions of psychopathology (severity of positive symptoms, negative symptoms, depression, insight, and general psychopathology). We hypothesized that severity of negative symptoms and level of insight would be the significant predictors of decisional capacity, but not severity of positive symptoms, depression, or general psychopathology. We also evaluated the degree to which repeated disclosure of initially misunderstood information was associated with improved understanding, hypothesizing that understanding would improve over successive presentations.
METHOD
Participants
Participants in this study were 70 middle-aged and older persons with schizophrenia, aged 40 years or above, 28 in the fifth decade, 28 in the sixth decade, and 14 in the seventh decade of their life. Participants were recruited through a variety of sources, including local residential board-and-care homes, day treatment centers, the psychiatric services of the University of California, San Diego (UCSD) and the Veterans Affairs San Diego Healthcare System (VASDHS), as well as through the UCSD Advanced Center for Interventions and Services Research (ACISR). A majority of the participants were outpatients at the time of evaluation, although 6 were evaluated while in an acute inpatient facility (these individuals were only enrolled and evaluated after the clinical treatment staff indicated that their symptoms had stabilized sufficiently for meaningful consent and research participation.)
Inclusion criteria were (1) DSM-IV24 diagnosis of schizophrenia (as determined by the participant's clinical care providers), (2) current age greater than 40 years, (3) fluency in English, (4) absence of a diagnosis of dementia or other medical conditions likely to influence neurocognitive functioning, and (5) currently receiving an FDA-approved “atypical” antipsychotic medication. The rationale for the last requirement was that capacity to consent to research participation was evaluated in reference to an actual study (hereinafter referred to as the “parent study”; see below for details) being conducted through the ACISR on the long-term side effects of second-generation antipsychotic medications among middle-aged and older patients. The decisional capacity measure (described below) was administered prior to formal consent and enrollment in the parent study (for patients who decided to enroll in that study.)
The age range of participants in this study was 40 to 70 years; those age 40 to 59 were selected from a larger dataset (without reference to any of the decisional capacity scores, clinical/psychopathology data, or cognitive test scores) in our ongoing study of decisional capacity among middle-aged and elderly persons with psychoses. In selecting participants age 40 to 59 for inclusion in the present report, we had attended to demographic characteristics (education, gender, and ethnicity) so that the number and demographic characteristics of the 3 age groups (fifth, sixth, and seventh decades) were more comparable (although the number of participants in the oldest age group remained smaller than that in the 2 younger age groups).
The protocol for this capacity study was reviewed and approved by the Human Research Protections Program for UCSD and VASDHS, and all participants provided written informed consent prior to their participation. This was a procedurally simple and minimal-risk study, so the capacity to consent to this study itself was not generally a primary concern; however, 1 patient was excluded from this study due to apparent inability to understand the nature of the research project.
Measures
Decisional Capacity
Each participant's level of capacity to consent to research was evaluated with a modified MacArthur Treatment Competence Assessment Tool for Clinical Research (MacCAT-CR).25 The MacCAT-CR is a 20- to 30-minute semistructured interview that provides scores for 4 commonly recognized dimensions of decisional capacity (understanding [range 0 to 26 points]; appreciation [range 0 to 6 points], reasoning [range 0 to 8 points], and expression of a choice [range 0 to 2 points]).
The content of MacCAT-CR items referred to the parent study on the safety and side effects of “atypical” antipsychotic medications. The parent study is a project comparing various FDA-approved antipsychotic medications in terms of their risk of tardive dyskinesia and other short- and long-term side effects in middle-aged and older patients. Participants in the parent study had to have a psychiatric condition for which antipsychotic medications are appropriate, and they had to be over the age of 40. Procedures in the parent study involved a number of evaluations for tardive dyskinesia and other side effects, as well as clinical interviews and psychopathology ratings, and brief cognitive testing. The parent study was itself minimal risk in that none of the evaluations were dangerous, there was no placebo control, and participants were permitted to stay on their current medication rather than being randomized to a medication. For the current consent study, we eliminated 2 MacCAT-CR items (1 from understanding and 1 from the reasoning subscale) that did not appear applicable in the context of the parent study, and we prorated the subscale scores using the remaining items to preserve the standard range of each MacCAT-CR subscale.
In order to evaluate the degree to which understanding of information could be improved through an iterative process, information was re-disclosed up to 2 times for the information subscale disclosures. Thus, if a participant earned less than full credit (2 points) for a specific MacCAT-CR understanding item, that information was re-explained and subsequently retested at the end of that particular MacCAT-CR subsection. Up to 2 re-presentations were permitted, providing for up to 3 trials of understanding. The standard MacCAT-CR incorporates 1 re-disclosure per item in the understanding subscale. These procedures do not apply to the other subscales, which relate more to participants' beliefs about, reasoning with, or choices about the information.
Semiannual interrater reliability checks were conducted with research assistants administering the MacCAT-CR for this and related studies to ensure adequate interrater reliability (Intraclass correlation coefficient > .80). The research assistants also met regularly with the first author to review and discuss any administration or scoring questions.
Psychopathology Ratings
Severity of positive symptoms, negative symptoms, and general psychopathology was assessed with the corresponding subscale scores of the Positive and Negative Syndrome Scale (PANSS).26 Severity of depressive symptoms was evaluated with the 17-item version of the Hamilton Depression Rating Scale (HAM-D).27 Insight into illness (awareness of symptoms and the need for treatment) was measured with the total score from the Birchwood Insight Questionnaire.28
Neuropsychological Functioning
A majority of the patients were evaluated with a comprehensive neuropsychological test battery. Reading comprehension was assessed with the Reading Comprehension subtest from the Peabody Individual Achievement Test (PIAT),29 and aural-verbal comprehension was measured with the Token test.30 We wanted to examine these 2 abilities separately from the other neuropsychological abilities because reading and aural comprehension are themselves functional tasks involved in the consent process. In order to reduce the number of comparisons, the other neuropsychological tests were grouped into cognitive ability areas. The specific neuropsychological tests, and the 7 ability areas into which they were grouped, consisted of the following:
Verbal: Wechsler Adult Intelligence Scale–Third Edition (WAIS-III)31 Vocabulary, Similarities, and Information subtests;
Perceptual Organization: WAIS-III Picture Completion, Block Design, and Matrix Reasoning subtests;
Attention/Working Memory: WAIS-III Arithmetic, Digit Span, and Letter-Number Sequencing subtests;
Processing Speed: WAIS-III Digit Symbol and Symbol Search subtests, Trail-Making Test Part A,32 Letter and Animal Fluency;33,34
Executive Functioning: Wisconsin Card Sorting Test—64 Card Version (conceptual level responses),35 Trail-Making Test Part B32 (time to complete), Stroop Task36 (Color Word score);
Verbal Learning: Hopkins Verbal Learning Test–Revised37 (total recall trials 1 through 3), Story Memory Test38 (learning score); and
Visual Learning: Brief Visual-Spatial Memory Test–Revised39 (total recall trials 1 through 3), Family Pictures (immediate recall score) from the Wechsler Memory Scale–Third Edition40.
The above groupings of tests were guided by consideration of the construct each test is commonly interpreted to measure,34 as well as results from our group's and from other investigators' factor analytic studies that have included some of the present tests.41,42 To place scores on a common metric, we transformed each raw score to a z-score scale (using the normalized rank function in SPSS version 12.01). For most of these tests, higher raw scores indicate better performance. The 2 exceptions are Trail-Making Part A and B, where higher scores indicate slower response. The scores on those 2 tasks were reflected (subtracted from 1 + the highest score) so that higher z-values indicated better performance on all tests. Then, for each subject, we computed the mean z-score within each of the ability areas, as well as for all the tests within the cognitive ability areas.
Additional Information
In addition to each of the above measures, we collected basic background information through interview and review of available records, including demographic information (age, education, gender, ethnicity), as well as age of onset of illness. We also recorded type of current antipsychotic medication.
Procedures
Trained research assistants administered each of the above measures. The research assistant who administered the MacCAT-CR was kept unaware of the participant's scores and responses on the psychopathology rating scales and neuropsychological tests, and vice versa.
Statistical Analyses
Overall differences among the 3 age groups in terms of education, age of onset of illness, psychopathology ratings (PANSS subscale score, HAM-D total, and Birchwood Insight Scale total) were compared via one-way analyses of variance, with follow-up pairwise comparisons using Tukey's Honestly Significant Difference procedure. (Education was negatively skewed in the 50- to 59-year-old age group, so it was transformed in all groups via a reflected square root function for parametric analyses.) Differences in gender and ethnicity were compared using Pearson chi-square analyses. The distributions of several MacCAT-CR subscale scores were also negatively skewed in each group, but we were unable to identify a transformation that appropriately reduced this skew; as a result, the MacCAT-CR subscale score differences among the 3 age groups were evaluated with Kruskal-Wallis tests. Using the entire sample, improvement in understanding over the 3 MacCAT-CR Understanding trials was assessed with Friedman's analysis of ranks. Spearman's rho was used to compute bivariate correlations between the MacCAT-CR subscale scores and demographic characteristics, age of onset of illness, psychopathology ratings, and mean z-scores within each of the cognitive ability areas, as well as the overall mean neuropsychological z-score. Significance for all analyses was defined as p < .05 (2-tailed).
Results
The demographic characteristics, age of onset of illness, psychopathology ratings, mean overall neuropsychological z-score, and MacCAT-CR subscale scores for each of the 3 age groups are shown in Table 1. The overall test for differences in age of illness onset was significant (F2,60 = 12.50, p < .001), and post hoc comparisons revealed this was due to a later mean age of onset among the 60- to 70-year-old age group relative to the 2 younger age groups. There were no significant differences among the 3 age groups in demographic characteristics, severity of positive symptoms, negative symptoms, general psychopathology, insight, overall neuropsychological performance, or MacCAT-CR subscale scores.
Table 1.
Demographic Characteristics, Psychopathology Ratings, Overall Cognitive Scaled Score, and Decisional Capacity Among Patients With Schizophrenia in the Fifth, Sixth, and Seventh Decades of Life
| Patients Age 40–49 Years (n = 28) | Patients Age 50–59 Years (n = 28) | Patients Age 60–70 Years (n = 14) | F, χ2, or t | p | Significant Pairwise Differences | |
| Age (years) | 45.3 (3.3) | 53.8 (2.8) | 64.6 (4.0) | n/a | ||
| Education (years) | 11.6 (2.8) | 12.2 (2.4) | 12.1 (3.7) | F2,67 = 0.37 | .691a | |
| Gender (% women) | 46.4% | 46.4% | 63.4% | χ2[2] = 1.43 | .490 | |
| Ethnic Background | χ2[8] = 5.80 | .671 | ||||
| Caucasian | 50.0% | 71.4% | 64.3% | |||
| African American | 25.0% | 21.4% | 21.4% | |||
| Latino | 10.7% | 7.1% | 7.1% | |||
| Asian American | 10.7% | 0.0% | 7.1% | |||
| Other | 3.6% | 0.0% | 0.0% | |||
| Age of Onset | 23.0 (8.8) | 26.7 (10.3) | 41.2 (14.4) | F2,60 = 12.50 | < .001 | 60–69 year olds > 40–49 and 50–59 year olds |
| PANSS | ||||||
| Positive subscale total | 15.6 (6.2) | 14.9 (5.7) | 15.4 (5.8) | F2,65 = 0.12 | .890 | |
| Negative subscale total | 14.6 (6.4) | 13.1 (5.4) | 13.5 (5.9) | F2,65 = 0.48 | 624 | |
| General subscale total | 28.4 (8.0) | 26.4 (7.5) | 32.2 (8.7) | F2,65 = 2.51 | .089 | |
| Hamilton Depression Rating Scale | 9.0 (5.8) | 7.4 (4.4) | 11.3 (7.1) | F2,64 = 2.23 | .116 | |
| Birchwood Insight Scale Total | 8.4 (2.3) | 8.1 (3.0) | 8.5 (3.7) | F2,67 = 0.08 | .923 | |
| Mean Neuropsychological z-score | 0.06 (0.79) | 0.12 (0.59) | –0.21 (0.65) | F2,52 = 0.75 | .476 | |
| MacCAT-CR* | ||||||
| Understanding (range 0 to 26) | ||||||
| Trial 1 | 15.9 (5.7) | 18.3 (5.8) | 14.9 (7.2) | χ2[2] = 3.60 | .166 | |
| Trial 2 | 21.8 (4.7) | 22.5 (5.6) | 19.3 (7.4) | χ 2[2] = 2.33 | .312 | |
| Trial 3 | 23.8 (3.4) | 23.8 (5.0) | 20.6 (6.5) | χ 2[2] = 3.43 | .180 | |
| Appreciation (range 0 to 6) | 4.8 (1.7) | 5.0 (1.6) | 3.9 (1.9) | χ 2[2] = 4.17 | .124 | |
| Reasoning (range 0 to 8) | 7.1 (1.7) | 7.3 (1.3) | 6.1 (2.9) | χ 2[2] = 1.47 | .478 | |
| Expression of a Choice (range 0 to 2) | 1.9 (0.4) | 1.9 (0.3) | 1.7 (0.7) | χ 2[2] = 0.88 | .644 |
Note: PANSS = Positive and Negative Syndrome Scale; MacCAT-CR = MacArthur Competence Assessment Tool for Clinical Research; n/a = not available.
The significance values for education, gender, and ethnicity are not fully meaningful as these variables were considered when selecting subjects for inclusion in the younger age groups.
Due to skewed distributions, Kruskal-Wallis test was used to compare groups on MacCAT-CR scores.
The MacCAT-CR understanding scores significantly improved over the 3 trials, from an overall mean (and SD) of 16.7 (6.1) points at trial 1, 21.6 (5.7) points at trial 2, and 23.1 (4.9) points at trial 3; Friedman's analysis of ranks χ2(2, N = 70) = 126.5, p < .001.
The bivariate correlations between each of the MacCAT-CR subscale scores with age, education, age of onset of illness, psychopathology ratings, and each of the neuropsychological ability area mean z-scores are listed in Table 2. There were no significant correlations between age and any of the MacCAT-CR scores. There were also no significant correlations between MacCAT-CR performance and education, age of onset of illness, severity of positive symptoms (PANSS Positive Symptom subscale), severity of depressive symptoms (HAM-D total), or insight (Birchwood Insight Scale total). Modest but significant correlations were seen between severity of negative symptoms and MacCAT-CR understanding scores at trial 1 and trial 2 (absolute value of both rs > .30, both ps < .05). There were also modest but significant negative correlations between general severity of psychopathology (PANSS General Symptom subscale) and all 3 MacCAT-CR understanding trials (absolute value of rs > .26, all ps < .05).
Table 2.
Bivariate Correlations (Spearman's rho) Between Participant Characteristics and MacCAT-CR Scores
| Understanding |
Expression of a Choice | |||||
| Trial 1 | Trial 2 | Trial 3 | Appreciation | Reasoning | ||
| Age (years) | 0.015 | −0.074 | −0.178 | −0.141 | −0.035 | −0.103 |
| Education (years) | 0.162 | 0.155 | 0.152 | 0.141 | 0.086 | 0.136 |
| Age of Onset of Illness (N = 63) | 0.190 | 0.223 | 0.172 | −0.066 | 0.043 | −0.033 |
| PANSS (N = 68) | ||||||
| Positive subscale total | −0.163 | −0.175 | −0.184 | −0.083 | −0.013 | −0.122 |
| Negative subscale total | −0.346* | −0.309* | −0.196 | −0.190 | −0.063 | −0.170 |
| General subscale total | −0.269* | −0.366* | −0.309* | −0.158 | −0.103 | −0.267* |
| HAM-D Total (N = 67) | 0.084 | 0.023 | 0.009 | 0.025 | −0.117 | 0.007 |
| Birchwood Insight Scale Total (N = 68) | 0.118 | 0.183 | 0.145 | 0.084 | 0.190 | 0.200 |
| Reading Comprehension (PIAT) (N = 54) | .489** | .447** | .375** | .562** | .056 | .053 |
| Aural Comprehension (Token test) (N = 51) | .372* | .387* | .352* | .362* | −.016 | .133 |
| Verbal Ability (N = 55) | 0.528** | 0.517** | 0.460** | 0.354* | 0.151 | 0.150 |
| Perceptual Organization (N = 54) | 0.236 | 0.162 | 0.105 | 0.218 | 0.020 | −0.001 |
| Processing Speed (N = 55) | 0.350* | 0.296* | 0.292* | 0.238 | −0.022 | −0.010 |
| Attention/Working Memory (N = 56) | 0.463** | 0.396* | 0.392* | 0.318* | 0.042 | 0.065 |
| Executive Function (N = 52) | 0.370* | 0.300* | 0.268 | 0.350* | −0.006 | 0.117 |
| Auditory Learning (N = 58) | 0.480** | 0.502** | 0.430** | 0.292* | 0.051 | 0.143 |
| Visual Learning (N = 54) | 0.344* | 0.405* | 0.344* | 0.221 | 0.111 | 0.129 |
| Cognitive Composite (N = 55) | 0.475** | 0.445** | 0.385* | 0.304* | −0.001 | 0.065 |
Note: N = 70, unless otherwise indicated. PANSS = Positive and Negative Syndrome Scale; HAM-D = Hamilton Depression Rating Scale (17-item version); PIAT = Reading Comprehension subtest from the Peabody Individual Achievement Test; MacCAT-CR = MacArthur Competence Assessment Tool for Clinical Research.
*p < .05, **p <.001.
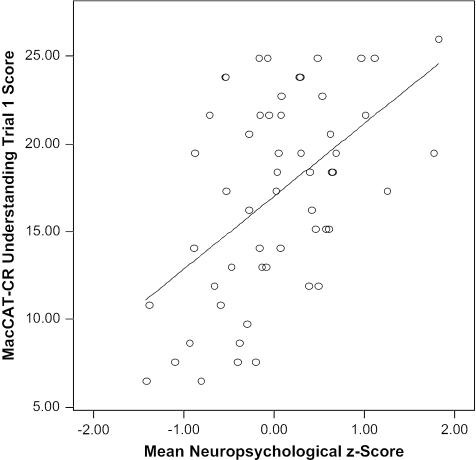
The bivariate correlations between the MacCAT-CR scores and reading comprehension (PIAT), aural comprehension (Token test), and each of the neuropsychological ability areas, as well as the mean z-score across the neuropsychological battery, are also shown in Table 2. Reading comprehension, aural comprehension, and (with the exception of Perceptual Organization) all of the neuropsychological ability areas were correlated with most of the MacCAT-CR understanding scores. Reading comprehension, aural comprehension, and most of the other, specific neuropsychological ability areas (except for Perceptual Organization, Processing Speed, and Visual Learning) were also correlated with the MacCAT-CR appreciation score. There were no significant cognate correlates of the MacCAT-CR reasoning or expression of a choice subscale scores. As an illustration of the strong relationship between cognitive functioning and understanding, the relationship between the mean neuropsychological z-score across the entire battery and the understanding trial 1 score (r = .475, p < .001) is depicted in Figure 1.
Fig. 1.
Relationship of Overall Neuropsychological Performance to Initial Understanding on the MacArthur Competence Assessment Tool for Clinical Research (MacCAT-CR).
Discussion
Consistent with our expectations, neuropsychological test performance was the strongest correlate of capacity to consent to research among middle-aged and older patients with schizophrenia. Other significant correlates of the understanding component of decisional capacity included severity of negative symptoms and severity of general psychopathology, but not severity of positive symptoms or depressive symptoms. Contrary to our hypotheses, the present results did not generally indicate differential relationships between individual cognitive ability areas and specific dimensions of decisional capacity. Also contrary to our expectations, neither age nor level of insight was associated with any of the 4 dimensions of decisional capacity.
The present findings of the primacy of cognitive deficits as predictors of decisional capacity, as well as our findings that negative symptoms (but not positive symptoms) were associated with level of understanding of disclosed information, are consistent with the literature on predictors of functional capacity and independent functioning in schizophrenia.43,44 That is, cognitive deficits, and to a lesser degree negative symptoms, are the strongest determinants of the overall level of independence in daily living skills.
The reason for a lack of significant correlations of psychopathology ratings or cognitive variables with the MacCAT-CR reasoning subscale is unclear, but this pattern is not unique to the present protocol. For instance, Kovnick et al.8 also found the correlations of psychopathology and cognitive functioning to be significant with understanding and appreciation, but not with reasoning. In part, these findings may reflect psychometric limitations of this subscale; the standard MacCAT-CR reasoning subscale has only 4 items compared to the 13 in the standard understanding subscale (a nonapplicable item was deleted from the understanding subscale, and another from the reasoning subscale in the current study). In terms of the lack of cognitive correlates with the MacCAT-CR reasoning subscale, it is also possible that the abilities measured by neuropsychological tests are less relevant to what is being specifically evaluated by this subscale than by the understanding and appreciation subscales. This possibility warrants further empirical attention.
The MacCAT-CR understanding subscale is clearly the strongest of the subscales from a psychometric standpoint (the potential range of scores is at least triple that of the other 3 subscales). Yet, even in the understanding domain, as well as in the appreciation domain, the magnitude of correlations with specific cognitive domains did not suggest a pattern of differential relationships. This lack of specificity may partially reflect the multifactorial nature of common neuropsychological tests.41 It may also reflect a nonspecific pattern of cognitive impairment in schizophrenia and/or sensitivity of the understanding and appreciation domains to deficits in a range of cognitive abilities.
The MacCAT-CR is the most widely used instrument for assessing capacity to consent to research, and it is the one for which there are presently the most supporting data on reliability and validity.45 Yet, for use in studies on capacity to consent to research, there may be value in the further development of comprehensive research capacity instruments. An instrument providing comprehensive assessment and comparison of all 4 dimensions of decision-making capacity (especially appreciation and reasoning) may be less pragmatically useful in many applied settings, but it could be useful for research on decisional capacity when there is a need to make subtle distinctions and comparisons among the various subconstructs. Another consideration for such future instrument development is whether there should also be a “global decisional capacity” score. The MacCAT-CR was intentionally designed without a “total score” because problems in any 1 of the 4 dimensions of decisional capacity could be sufficient to deem a person incapable to provide independent consent, regardless of strengths in the other dimensions.25 However, as an investigator must ultimately decide whether a particular participant has or does not have sufficient capacity to provide research consent, there could be some utility in developing and validating a “global capacity” rating, so that the positive and negative predictive values of various signs of “risk for impaired capacity” could be systematically evaluated (cf. Palmer et al.10 and Kim et al.46).
The overall sample size for patients with MacCAT-CR data, and the slightly smaller proportion for whom we had also had neuropsychological data, is among the largest samples of schizophrenia patients in published studies of decisional capacity (in general, the schizophrenia samples in most studies have included 30 or fewer participants). Another strength of the present study is that decisional capacity was evaluated in reference to an actual study, whereas many studies (including some from our group10,12) relied on hypothetical scenarios. A limitation in using a real protocol is that certain elements of potential interest cannot be evaluated unless those elements in fact characterize the parent protocol (for instance, the parent protocol for the present study did not require randomization or placebo control). Yet, participants may also be less attentive to disclosures when they know they are not actually going to be asked to participate in the study being described, and it may be more difficult to interpret deficits in “appreciating” the significance of a study for which one is not actually being recruited. Both approaches have their limitations, but given the paucity of decisional capacity studies involving real decisions, we believe use of a real protocol in the present study represents a strength.
Limitations of the present study must also be acknowledged. As noted, the various MacCAT-CR subscales are psychometrically not equivalent, and that may complicate detection of differential deficits in the component abilities. There are also limitations related to the study sample. All but 6 participants were evaluated as outpatients. It is likely that greater impairment in decisional capacity might be observed among acute inpatients (cf. Jeste et al.47), and the relationship between insight and appreciation might, in particular, prove stronger among individuals in an acutely psychotic state. However, the primacy of cognitive deficits as a correlate of decisional capacity seems to be a finding that transcends protocol type or inpatient/outpatient status.47 Also, the majority of patients with schizophrenia live in the community,48 so the results may better represent the general abilities of patients with schizophrenia. Also in terms of the sample, no participant was younger than 40 or older than 70 years; it is possible that stronger relationships between age and decisional capacity would be observed in a study spanning the full adult age spectrum. Yet, even the inclusion of individuals in the seventh decade of life with schizophrenia and their direct comparison to those in the fifth and sixth decades make the study unique. We attempted to minimize cohort effects by selecting a subset of those in their fifth and sixths decades of life to be demographically comparable (in terms of education, gender, and ethnicity) to those in the seventh decade. Nonetheless, a possibility that cohort effects obscure aging-associated changes in decisional capacity cannot be fully ruled out without a long-term longitudinal investigation.
These limitations and caveats do not diminish the practical significance of the present findings. As illustrated in Figure 1, there was a wide range of decisional capacity among the patients. Investigators are less likely to overlook the presence of positive psychopathologic symptoms (such as frank delusions or hallucinations) because these tend to be more salient in informal interactions, but the current results suggest that cognitive deficits are particularly important to risk of impaired decisional capacity. The presence of schizophrenia (or other psychiatric diagnosis) alone is an inappropriate basis for determining decisional incapacity. A more effective approach than focusing on diagnosis is to be attentive to cognitive deficits and negative symptoms. Use of brief screening questionnaires10 may also be an efficient means of identifying people who would benefit from more extensive capacity evaluations and/or enhanced consent procedures.
The present results also show that understanding in patients with schizophrenia may be improved by re-disclosure. These findings are consistent with findings from earlier studies,49–51 and indeed, they are to be expected from the common pattern of impaired learning with spared retention seen in schizophrenia,13 yet we suspect this obvious benefit has not yet influenced the way in which the informed consent process is commonly carried out in much clinical research. Specifically, a single disclosure of information (and/or handing and/or reading a formal consent form) to a potential participant may be insufficient except in the context of procedurally simple and minimal-risk studies. In more complex or higher-risk studies, participant understanding of key elements (such as the purpose of the research, general procedures, and key risks) should be specifically queried and re-explained when necessary. An interactive consent process may eliminate many of the common misunderstandings. As exemplified in a study by Roberts and colleagues,52 many patients with schizophrenia are able to express very substantive and discerning thoughts about ethical issues in research participation. Encouraging a genuine dialogue with potential participants about the research and any thoughts or concerns they have about it may be an effective and appropriate means of engaging people in the research endeavor and aid the overall consent process. In this sense, some of the impaired understanding after an initial disclosure should not be viewed as a deficit in consent capacity but rather as a deficit in the commonly employed consent process.
Acknowledgments
This study was supported, in part, by National Institute of Mental Health grants R01 MH64722 and P30 MH66248 and by the Department of Veterans Affairs. The authors express their gratitude to Shahrokh Golshan, Ph.D., for his comments on an earlier draft of this manuscript and to Tia Thrasher, Karen Ueki-Eaton, and Margaret Thompson for their valued assistance with the project, as well as to the research participants for their time and efforts.
References
- 1.Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, Gorman JM. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry. 1994;51:826–836. doi: 10.1001/archpsyc.1994.03950100074007. [DOI] [PubMed] [Google Scholar]
- 2.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 3.Research Involving Persons With Mental Disorders That May Affect Decision-Making Capacity. Rockville, Md: Report and Recommendations of the National Bioethics Advisory Commission; 1998. National Bioethics Advisory Commission. [Google Scholar]
- 4.Appelbaum PS, Roth LH. Competency to consent to research: a psychiatric overview. Arch Gen Psychiatry. 1982;39:951–958. doi: 10.1001/archpsyc.1982.04290080061009. [DOI] [PubMed] [Google Scholar]
- 5.Grisso T, Appelbaum PS. Assessing Competence to Consent to Treatment: A Guide for Physicians and Other Health Professionals. New York: Oxford University Press; 1998. [Google Scholar]
- 6.Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste DV. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter WT, Gold JM, Lahti AC, Queern CA, Conley RR, Bartko JJ, Kovnick J, Appelbaum PS. Decisional capacity for informed consent in schizophrenia research. Arch Gen Psychiatry. 2000;57:533–538. doi: 10.1001/archpsyc.57.6.533. [DOI] [PubMed] [Google Scholar]
- 8.Kovnick JA, Appelbaum PS, Hoge SK, Leadbetter RA. Competence to consent to research among long-stay inpatients with chronic schizophrenia. Psychiatr Serv. 2003;54:1247–1252. doi: 10.1176/appi.ps.54.9.1247. [DOI] [PubMed] [Google Scholar]
- 9.Palmer BW, Dunn LB, Appelbaum PS, Jeste DV. Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Arch Gen Psychiatry. 2004;61:230–236. doi: 10.1001/archpsyc.61.3.230. [DOI] [PubMed] [Google Scholar]
- 10.Palmer BW, Dunn LB, Appelbaum PS, Mudaliar S, Thal L, Henry R, Golshan S, Jeste DV. Assessment of capacity to consent to research among older persons with schizophrenia, Alzheimer disease, or diabetes mellitus: comparison of a three-item questionnaire with a comprehensive standardized capacity instrument. Arch Gen Psychiatry. 2005;62:726–733. doi: 10.1001/archpsyc.62.7.726. [DOI] [PubMed] [Google Scholar]
- 11.Moser D, Schultz S, Arndt S, Benjamin ML, Fleming FW, Brems CS, Paulsen JS, Appelbaum PS, Andreasen NC. Capacity to provide informed consent for participation in schizophrenia and HIV research. Am J Psychiatry. 2002;159:1201–1207. doi: 10.1176/appi.ajp.159.7.1201. [DOI] [PubMed] [Google Scholar]
- 12.Saks ER, Dunn LB, Marshall BJ, Nayak GV, Golshan S, Jeste DV. The California Scale of Appreciation: a new instrument to measure the appreciation component of capacity to consent to research. Am J Geriatr Psychiatry. 2002;10:166–174. [PubMed] [Google Scholar]
- 13.Heaton R, Paulsen J, McAdams LA, Kuck J, Zisook S, Braff D, Harris MJ, Jeste DV. Neuropsychological deficits in schizophrenia: relationship to age, chronicity and dementia. Arch Gen Psychiatry. 1994;51:469–476. doi: 10.1001/archpsyc.1994.03950060033003. [DOI] [PubMed] [Google Scholar]
- 14.Marson DC, Chatterjee A, Ingram KK, Harrell LE. Toward a neurological model of competency: cognitive predictors of capacity to consent in Alzheimer's disease using three different legal standards. Neurology. 1996;46:666–672. doi: 10.1212/wnl.46.3.666. [DOI] [PubMed] [Google Scholar]
- 15.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 16.Moser DJ, Reese RL, Schultz SK, Benjamin ML, Arndt S, Fleming FW, Andreasen NC. Informed consent in medication-free schizophrenia research. Am J Psychiatry. 2005;162:1209–1211. doi: 10.1176/appi.ajp.162.6.1209. [DOI] [PubMed] [Google Scholar]
- 17.Randolph C. RBANS—Manual—Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio: Tex: Psychological Corporation; 1998.
- 18.Grisso T, Appelbaum PS. The MacArthur Treatment Competence Study. III. Abilities of patients to consent to psychiatric and medical treatments. Law Hum Behav. 1995;19:149–174. doi: 10.1007/BF01499323. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. Wechsler Adult Intelligence Scale—Revised, manual. Cleveland, Ohio: Psychological Corporation; 1981. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Mattis S. Dementia Rating Scale. Odessa, Fla: Psychological Assessment Resources, Inc; 1973. [Google Scholar]
- 22.Christensen H, Kumar R. Cognitive changes and the ageing brain. In: Sachdev PS, editor. The Ageing Brain: The Neurobiology and Neuropsychiatry of Ageing. Lisse, Netherlands: Swets and Zeitlinger; 2003. pp. 75–95. [Google Scholar]
- 23.Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA. 2004;292:1593–1601. doi: 10.1001/jama.292.13.1593. [DOI] [PubMed] [Google Scholar]
- 24.Diagnostic Criteria from DSM-IV. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association. [Google Scholar]
- 25.Appelbaum PS, Grisso T. MacCAT-CR: MacArthur Competence Assessment Tool for Clinical Research. Sarasota, Fla: Professional Resource Press; 2001. [Google Scholar]
- 26.Kay S, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 28.Birchwood M, Smith V, Drury V. A self-report insight scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand. 1994;89:62–67. doi: 10.1111/j.1600-0447.1994.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 29.Dunn LM, Markwardt FC. Peabody Individual Achievement Tests. Circle Pines, Minn: American Guidance Service; 1970. [Google Scholar]
- 30.Benton AL, Hamsher KdeS, Sivan AB. Multilingual Aphasia Examination. 3d ed. Iowa City: Iowa: AJA Associates; 1994. [Google Scholar]
- 31.Wechsler D. Wechsler Adult Intelligence Scale—Third Edition (WAIS-III) San Antonio, Tex: Psychological Corporation; 1997. [Google Scholar]
- 32.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. 2d ed. Tucson, Ariz: Neuropsychology Press; 1993. [Google Scholar]
- 33.Gladsjo JA, Schuman C, Evans J, Peavy G, Miller S, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 34.Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fisher JS. Neuropsychological Assessment. 4th ed. New York: Oxford University Press; 2004. [Google Scholar]
- 35.Kongs SK, Thompson LL, Iverson GL, Heaton RK. Wisconsin Card Sorting Test—64 Card Version (WCST-64) Odessa, Fla: Psychological Assessment Resources; 2000. [Google Scholar]
- 36.Golden CJ, Freshwater SM. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Wood Dale, Ill: Stoelting; 2002.
- 37.Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test—Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 38.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, Fla: Psychological Assessment Resources; 2004.
- 39.Benedict RHB. Brief Visual-Spatial Memory Test—Revised. Professional Manual. Odessa, Fla: Psychological Assessment Resources; 1997. [Google Scholar]
- 40.Wechsler D. Wechsler Adult Intelligence Scale (Third Edition): Administration and Scoring Manual. San Antonio, Tex: Psychological Corporation; 1997. [Google Scholar]
- 41.Gladsjo JA, McAdams LA, Palmer BW, Moore D, Jeste DV, Heaton RK. A six-factor model of cognition in schizophrenia and related psychotic disorders: relationships with clinical symptoms and functional capacity. Schizophr Bull. 2004;30:739–754. doi: 10.1093/oxfordjournals.schbul.a007127. [DOI] [PubMed] [Google Scholar]
- 42.Tulsky DS, Ivnik RJ, Price LR, Wilkins C. Assessment of cognitive functioning with the WAIS-III and WMS-III: development of a six-factor model. In: Tulsky D, Saklofske D, Heaton RK, Chelune G, Ivnik R, Bornstein RA, Prifitera A, Ledbetter B, editors. Clinical Interpretation of the WAIS-III and the WMS-III. San Diego, Calif: Academic Press; 2003. pp. 145–177. [Google Scholar]
- 43.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 44.Palmer BW, Heaton RK, Gladsjo JA, Evan JD, Patterson TL, Golshan S, Jeste DV. Heterogeneity in functional status among older outpatients with schizophrenia: employment history, living situation, and driving. Schizophr Res. 2002;55:205–215. doi: 10.1016/s0920-9964(01)00218-3. [DOI] [PubMed] [Google Scholar]
- 45.Dunn LB, Nowrangi MA, Palmer BW, Jeste DV, Saks ER. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2005 doi: 10.1176/ajp.2006.163.8.1323. in press. [DOI] [PubMed] [Google Scholar]
- 46.Kim SYH, Caine ED, Currier GW, Leibovici A, Ryan JM. Assessing the competence of persons with Alzheimer's disease in providing informed consent for participation in research. Am J Psychiatry. 2001;158:712–717. doi: 10.1176/appi.ajp.158.5.712. [DOI] [PubMed] [Google Scholar]
- 47.Jeste DV, Depp CA, Palmer BW. Magnitude of impairment in decisional capacity in people with schizophrenia compared to normal subjects: an overview. Schizophr Bull. 2005 doi: 10.1093/schbul/sbj001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cohen CI, Cohen GD, Blank K, Gaitz C, Katz IR, Leuchter A, Maletta G, Meyers B, Sakauye K, Shamoian C. Schizophrenia and older adults: an overview: directions for research and policy. Am J Geriatr Psychiatry. 2000;8:19–28. doi: 10.1097/00019442-200002000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, Jeste DV. Improving understanding of research consent in middle-aged and elderly patients with psychotic disorders. Am J Geriatr Psychiatry. 2002;10:142–150. [PubMed] [Google Scholar]
- 50.Dunn LB, Jeste DV. Enhancing informed consent for research and treatment. Neuropsychopharmacology. 2001;24:595–607. doi: 10.1016/S0893-133X(00)00218-9. [DOI] [PubMed] [Google Scholar]
- 51.Wirshing DA, Wirshing WC, Marder SR, Liberman RP, Mintz J. Informed consent: assessment of comprehension. Am J Psychiatry. 1998;155:1508–1511. doi: 10.1176/ajp.155.11.1508. [DOI] [PubMed] [Google Scholar]
- 52.Roberts LW, Warner TD, Brody JL. Perspectives of patients with schizophrenia and psychiatrists regarding ethically important aspects of research participation. Am J Psychiatry. 2000;157:67–74. doi: 10.1176/ajp.157.1.67. [DOI] [PubMed] [Google Scholar]