Abstract
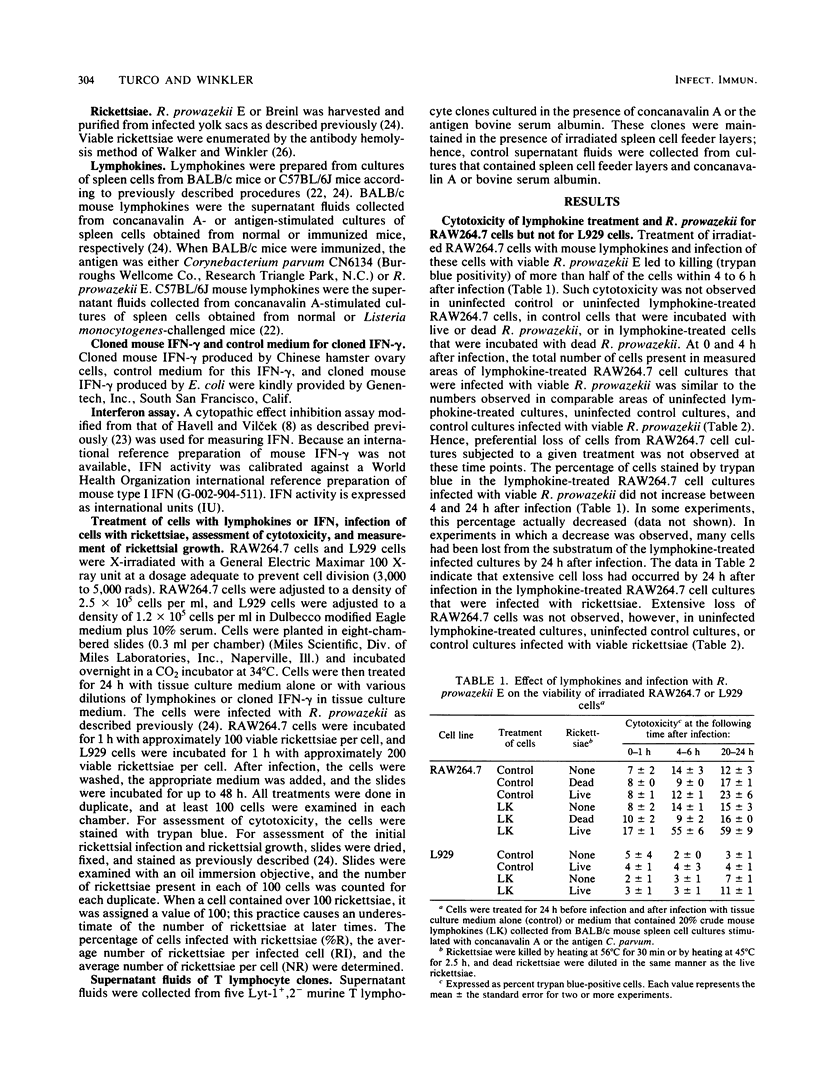
We studied the effects of crude mouse lymphokines and cloned mouse interferon-gamma on the interaction of Rickettsia prowazekii with mouse macrophage-like RAW264.7 cells. Treatment of RAW264.7 cells with lymphokines before infection, after infection, or both before and after infection with R. prowazekii led to killing of a substantial proportion of the RAW264.7 cells. Such cytotoxicity required both lymphokines and viable R. prowazekii and did not occur in mouse fibroblastic L929 cells. Untreated cultures of RAW264.7 cells supported good growth of the Breinl strain of R. prowazekii, but in lymphokine-treated cultures, little or no rickettsial growth occurred in the cells that survived the cytotoxic reaction. In addition, treatment of RAW264.7 cells with lymphokines before rickettsial infection was associated with suppression of the initial infection. The effects of cloned mouse interferon-gamma were similar to the effects of crude mouse lymphokines. Assessment of cytotoxicity, inhibition of the initial infection, and inhibition of rickettsial growth in RAW264.7 cells pretreated with various concentrations of interferon-gamma indicated that the effects of the lymphokines could be explained by the interferon-gamma that was present in these preparations. Treatment of RAW264.7 cells with interferon-gamma makes them unsuitable host cells for R. prowazekii.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaman L., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. VI. Differential opsonizing and neutralizing action of human typhus rickettsia-specific cytophilic antibodies in cultures of human macrophages. Infect Immun. 1976 Oct;14(4):1071–1076. doi: 10.1128/iai.14.4.1071-1076.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeade M. F., Rousset S., Paulin D., Chany C. Reorganization of the cytoskeleton by interferon in MSV-transformed cells. J Interferon Res. 1981 Feb;1(2):323–332. doi: 10.1089/jir.1981.1.323. [DOI] [PubMed] [Google Scholar]
- Degré M., Sonnenfeld G., Rollag H., Mørland B. Effect of gamma interferon preparations on in vitro phagocytosis and degradation of Escherichia coli by mouse peritoneal macrophages. J Interferon Res. 1981;1(4):505–512. doi: 10.1089/jir.1981.1.505. [DOI] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I. On the mechanisms of the antitumor effects of interferon. Tex Rep Biol Med. 1981;41:582–589. [PubMed] [Google Scholar]
- Guyre P. M., Morganelli P. M., Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983 Jul;72(1):393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. P., Jones P. P. Induction of Ia and H-2 antigens on a macrophage cell line by immune interferon. J Immunol. 1983 Jul;131(1):315–318. [PubMed] [Google Scholar]
- McNicholas J. M., King D. P., Jones P. P. Biosynthesis and expression of Ia and H-2 antigens on a macrophage cell line are stimulated by products of activated spleen cells. J Immunol. 1983 Jan;130(1):449–456. [PubMed] [Google Scholar]
- Murray H. W., Rubin B. Y., Rothermel C. D. Killing of intracellular Leishmania donovani by lymphokine-stimulated human mononuclear phagocytes. Evidence that interferon-gamma is the activating lymphokine. J Clin Invest. 1983 Oct;72(4):1506–1510. doi: 10.1172/JCI111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Falk W. Interferon-gamma is required in activation of macrophages for tumor cytotoxicity. Cell Immunol. 1983 Jul 15;79(2):396–402. doi: 10.1016/0008-8749(83)90082-5. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., Meltzer M. S. Macrophages in resistance to rickettsial infection: macrophage activation in vitro for killing of Rickettsia tsutsugamushi. J Immunol. 1979 Dec;123(6):2544–2549. [PubMed] [Google Scholar]
- Nacy C. A., Osterman J. V. Host defenses in experimental scrub typhus: role of normal and activated macrophages. Infect Immun. 1979 Nov;26(2):744–750. doi: 10.1128/iai.26.2.744-750.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Oppenheim J. J. Regulation of murine macrophage Ia-antigen expression by products of activated spleen cells. J Exp Med. 1980 Dec 1;152(6):1734–1744. doi: 10.1084/jem.152.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Cloned mouse interferon-gamma inhibits the growth of Rickettsia prowazekii in cultured mouse fibroblasts. J Exp Med. 1983 Dec 1;158(6):2159–2164. doi: 10.1084/jem.158.6.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Comparison of the properties of antirickettsial activity and interferon in mouse lymphokines. Infect Immun. 1983 Oct;42(1):27–32. doi: 10.1128/iai.42.1.27-32.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Differentiation between virulent and avirulent strains of Rickettsia prowazekii by macrophage-like cell lines. Infect Immun. 1982 Mar;35(3):783–791. doi: 10.1128/iai.35.3.783-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Inhibition of the growth of Rickettsia prowazekii in cultured fibroblasts by lymphokines. J Exp Med. 1983 Mar 1;157(3):974–986. doi: 10.1084/jem.157.3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyring S., Klimpel G. R., Fleischmann W. R., Jr, Baron S. Direct cytolysis by partially-purified preparations of immune interferon. Int J Cancer. 1982 Jul 15;30(1):59–64. doi: 10.1002/ijc.2910300111. [DOI] [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Rickettsial hemolysis: rapid method for enumeration of metabolically active typhus rickettsiae. J Clin Microbiol. 1979 May;9(5):645–647. doi: 10.1128/jcm.9.5.645-647.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker-Dowling P. A., Wilcox D. K., Widnell C. C., Youngner J. S. Interferon-mediated inhibition of virus penetration. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1083–1086. doi: 10.1073/pnas.80.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Miller E. T. Phospholipase A and the interaction of Rickettsia prowazekii and mouse fibroblasts (L-929 cells). Infect Immun. 1982 Oct;38(1):109–113. doi: 10.1128/iai.38.1.109-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisseman C. L., Jr, Waddell A. Interferonlike factors from antigen- and mitogen-stimulated human leukocytes with antirickettsial and cytolytic actions on Rickettsia prowazekii. Infected human endothelial cells, fibroblasts, and macrophages. J Exp Med. 1983 Jun 1;157(6):1780–1793. doi: 10.1084/jem.157.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., McKimm-Breschkin L., Harris A. W., Schrader J. W. Interferon-gamma induces enhanced expression of Ia and H-2 antigens on B lymphoid, macrophage, and myeloid cell lines. J Immunol. 1983 Aug;131(2):788–793. [PubMed] [Google Scholar]


