Abstract
Smaller medial temporal lobe volume is a frequent finding in studies of patients with schizophrenia, but the relative contributions of the hippocampus and three surrounding cortical regions (entorhinal cortex, perirhinal cortex, and parahippocampal cortex) are poorly understood. We tested the hypothesis that the volumes of medial temporal lobe regions are selectively changed in schizophrenia. We studied 19 male patients with schizophrenia and 19 age-matched male control subjects. Hippocampal and cortical volumes were estimated using a three-dimensional morphometric protocol for the analysis of high-resolution structural magnetic resonance images, and repeated measures ANOVA was used to test for region-specific differences. Patients had smaller overall medial temporal lobe volumes compared to controls. The volume difference was not specific for either region or hemisphere. The finding of smaller medial temporal lobe volumes in the absence of regional specificity has important implications for studying the functional role of the hippocampus and surrounding cortical regions in schizophrenia.
Keywords: medial temporal lobe, entorhinal, perirhinal, parahippocampal, region, functional
Introduction
Medial temporal lobe pathology is one of the most consistent findings in post-mortem and neuroimaging studies of patients with schizophrenia.1–4 In particular, structural neuroimaging studies have reported smaller hippocampal volume in schizophrenia.5,6 A meta-analysis of 18 quantitative magnetic resonance imaging (MRI) studies5 found a subtle (4%), yet significant, bilateral hippocampal volume reduction in patients with schizophrenia. A more recent meta-analysis of 58 MRI studies concluded that regional volume reductions in excess of global differences (i.e., smaller cerebral volume and larger ventricular volume in schizophrenia) were particularly marked in bilateral medial temporal lobe regions (i.e., amygdala, hippocampus, and parahippocampal gyrus).6
The details of medial temporal lobe volume reduction in schizophrenia, however, remain poorly understood. For example, are hippocampus and parahippocampal gyrus equally affected in schizophrenia? Are the three cortical regions of the parahippocampal gyrus (i.e., the entorhinal and perirhinal cortices anteriorly, and the parahippocampal cortex posteriorly) differentially affected in schizophrenia? Since the existing evidence for parahippocampal gyrus volume change is inconclusive, with some studies reporting reduced volume in schizophrenia7–11 and others reporting no significant changes,12–16 we decided to study the volume of the three parahippocampal gyrus regions in greater detail.
There are at least three reasons to explore a differential pathology of the medial temporal lobe in schizophrenia: connectivity, function, and differential susceptibility to injury. First, the three cortical regions of the parahippocampal gyrus establish distinct connectivity profiles with the neighboring hippocampus and the cerebral cortex.17 All unimodal association areas send information to largely nonoverlapping regions of the perirhinal and parahippocampal cortices. Most of this sensory information is then relayed via the entorhinal cortex to the hippocampus, while a small, yet significant, contingent reaches the hippocampus directly. This architecture ensures a significant role of the perirhinal and parahippocampal cortices in hippocampal information processing.18 The entorhinal cortex receives, in addition to the strong projections from the neighboring perirhinal and parahippocampal cortices, polysensory input from multimodal association areas in the medial and lateral frontal lobes, cingulate cortex, and retrosplenial cortex. This makes the entorhinal cortex the major gateway of unimodal as well as multimodal information into the hippocampus. In addition, most of the hippocampal projections back to the cortex are relayed via the entorhinal cortex as well.19
Second, the hippocampus and surrounding cortical regions subserve related yet distinct functions during the encoding of stimuli into memory and during subsequent retrieval.18,20,21 For example, the parahippocampal gyrus and hippocampus make different contributions to single-item versus associative memory and to the detection of novelty.22–26 Selective impairments of either the hippocampus or surrounding cortical regions could result in mental representations and memory deficits similar to those seen in schizophrenia.27–30
Third, medial temporal lobe regions are differentially susceptible to pathological changes. Prominent examples include the selective vulnerability of hippocampal sectors to hypoxia31 and glucocorticoid damage,32 and the prominent role of entorhinal cortex lesions in the early stages of Alzheimer's, Parkinson's, and Huntington's disease.33,34
Considering these anatomical, functional, and etiological implications of selective medial temporal pathology, we tested the hypothesis of differential volume changes in hippocampus, entorhinal cortex, perirhinal cortex, and parahippocampal cortex in a cohort of male patients with schizophrenia.
Methods
Subjects
We studied 19 male patients with a DSM-IV diagnosis of schizophrenia and 19 healthy male controls. The patients were consecutively recruited from an outpatient schizophrenia clinic in Boston. Confirmation of the diagnosis was made for all patients by clinic psychiatrists using the Structured Clinical Interview for DSM-IV Disorders (SCID).35 All patients had chronic illness (mean duration of illness ± SD = 21.1 ± 6.7 years), were maintained on a stable dose of antipsychotic medications (16 on second-generation antipsychotics, three on first-generation antipsychotics), and did not have their medication withdrawn for the purpose of the study. There was no history of any significant neurological illness such as seizure disorder, head trauma, or cerebrovascular accident, and no subject met DSM-IV criteria for alcohol or other substance abuse within the past three months.
Nineteen age-matched healthy male controls were recruited from the community by advertisements. Control subjects were free of any Axis I psychiatric disorder as determined by SCID and had no history of any major neurological illness, medical illness, substance abuse, or psychotropic medication use. All the subjects were right-handed.
The study protocol was approved by the Institutional Review Boards of both Massachusetts General Hospital and the Commonwealth of Massachusetts Department of Mental Health. Written, informed consent was acquired from all the participants after a detailed explanation of the study procedures, and a brief questionnaire was also administered to ensure understanding of the major risks and benefits of participation. Demographic and clinical characteristics of the sample are shown in Table 1. We tested for group differences with the student t-test for normally distributed and the non-parametric Mann-Whitney U test for non-normally distributed continuous data. Controls had a significantly higher level of education (z = −2.72, p = 0.007) and verbal IQ (t = 4.00, df = 35, p < 0.001) compared to the patients with schizophrenia. The two samples did not differ in age (t = −0.95, df = 36, p = 0.48) or mean parental education (z = −0.47, p = 0.64).
Table 1.
Demographic and Clinical Characteristics of the Study Subjects
| Controls (N = 19) |
Schizophrenia (N = 19) |
|||
| Characteristic | Mean | SD | Mean | SD |
| Age (years) | 44.0 | 9.2 | 45.8 | 6.7 |
| Education (years) | 15.1 | 3.1 | 12.6 | 2.2 |
| Parental education (years) | 12.5 | 1.7 | 12.8 | 2.1 |
| Verbal IQa | 113.1 | 10.2 | 98.8 | 11.5 |
| Duration of illness (years) | 21.1 | 6.7 | ||
| PANSS total | 59.8 | 13.1 | ||
| SANS total | 35.4 | 12.4 | ||
| Chlorpromazine equivalentsb | 409.3 | 228.6 | ||
MRI Acquisition
Magnetic resonance images were acquired on a 1.5T whole-body MRI scanner (Siemens, Munich, Germany). Stability of a high signal-to-noise ratio was ensured through a daily automated quality-control procedure. After automated scout and shimming procedures to optimize field homogeneity, total brain volume was measured with a high-resolution, 3D MPRAGE sequence that obtained 128 contiguous sagittal slices of 1.3 mm thickness (TR = 2.5 s, TE = 3 ms, FOV2 = 256 mm, NEX = 1, flip angle = 7°). The in-plane resolution was 1.3 × 1.0 mm.
MRI Preparation
Image analyses were performed on a Linux workstation. To maximize volume measurement accuracy,36 the images were resampled into 1 mm isotropic voxels and standardized Talairach coordinates.37 Transformation parameters were determined by a fully automated technique.38 Morphometric analysis was performed using an interactive software package (DISPLAY) developed at the Brain Imaging Centre of the Montreal Neurological Institute. This program allows simultaneous viewing and segmentation of volumes with reference to the coronal, sagittal, and horizontal orientations. Image rendering within the analysis program was constrained to a grayscale spectrum that yielded optimal contrast between the gray matter, white matter, and cerebrospinal fluid across the cases. This morphometric protocol was adapted from recently published methods of parcellation of the hippocampus and parahippocampal gyrus that were designed to maximize reproducibility.37,39
Hippocampal and Parahippocampal Segmentation
Details of the hippocampal segmentation are described elsewhere.40 The parahippocampal gyrus includes the entorhinal, perirhinal and parahippocampal cortices (see Figure 1). The boundaries of these cortices were delineated with relation to the collateral sulcus, calcarine sulcus, hippocampus, amygdale, and frontotemporal junction.
Fig. 1.
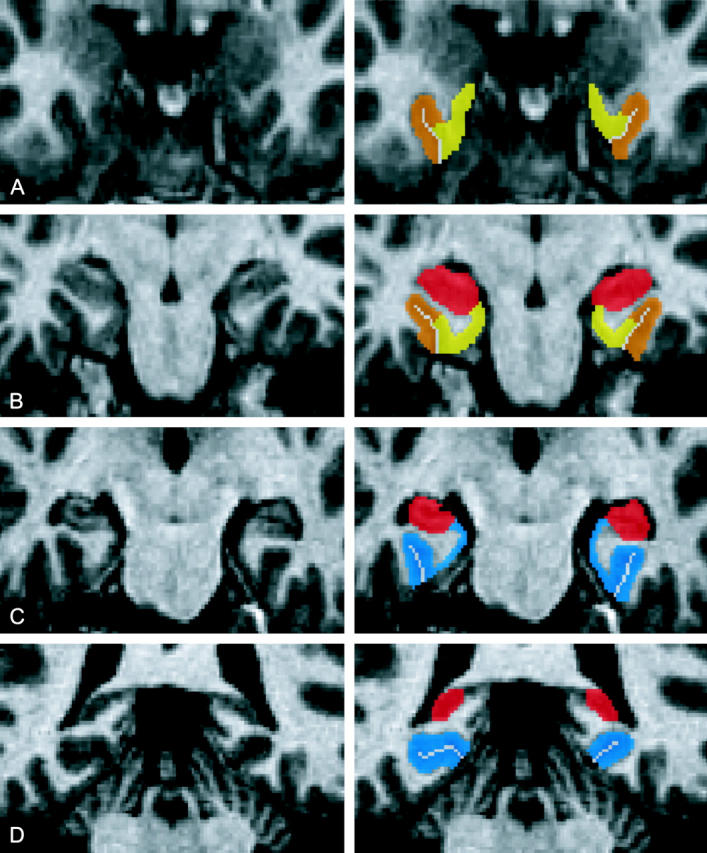
Four coronal slices through the medial temporal lobe demonstrating the parahippocampal subregions at the level of the (A) amygdala, (B) uncus, (C) body, and (D) tail of the hippocampus. (Entorhinal cortex in yellow, perirhinal cortex in orange, hippocampus in red and parahippocampal cortex in blue).
The posterior extent of the parahippocampal cortex was defined by the presence of the hippocampus in the coronal plane. Its anterior extent was defined as the coronal section 4 mm posterior to the most posterior coronal section containing the hippocampal uncus. Its infero-lateral extent was the lateral edge of the collateral sulcus. If two collateral sulci were present, then the parahippocampal cortex extended to the fundus of the more lateral sulcus. If the calcarine sulcus was present, then the supero-medial border of the parahippocampal cortex was defined as the inferior edge of the calcarine sulcus. Anterior to the calcarine sulcus, the supero-medial border of the parahippocampal cortex was taken to be the hippocampus.
The perirhinal and entorhinal cortices were located anterior to the parahippocampal cortex. Their anterior extent was defined by the presence of the collateral sulcus and the frontotemporal junction. The lateral extent of the perirhinal cortex was defined in a manner consistent with that of the parahippocampal cortex. The medial extent of the perirhinal cortex was defined as half the depth of the medial bank of the collateral sulcus. The entorhinal cortex was located immediately medial to the perirhinal cortex. Its medial extent was defined by the presence of the hippocampus or amygdala in the anterior sections.
All regional brain volumes were adjusted for intracranial cavity (ICC) volumes in Talairach space, by computing the ratio (regional brain volume in mm3/ICC volume in mm3). The ICC volumes were measured using an automated, in-house technique (Freesurfer).41 There was no significant difference in ICC volume between the patients and controls (1.616 ± 0.118 × 103 cm3 versus 1.619 ± 0.129 × 103 cm3: t = 0.09, df = 36, p = 0.94). The results were identical for ICC volumes in native space.
Reliability Assessment
The test-retest (intra-rater) reliability of the measurement technique for the hippocampus and parahippocampal gyrus was assessed by repeated measurement of two sets four randomly selected subjects (two from controls and two from patients) over a minimum interval of two weeks. Using a two-factor random-effect model for intraclass correlation coefficient calculation,42 alpha values were greater than 0.96 for the left and right regional brain volumes. Interrater reliability evaluation performed on a separate subset of four subjects (two from controls and two from patients) revealed alpha values of greater than 0.90 for all left and right regional brain volumes.
Statistical Analysis
Data were analyzed using the Statistical Package for Social Sciences-PC version 11.0 (SPSS Inc, Chicago). For the purpose of statistical analyses, all volumes were transformed (in scale but not in orientation) from Talairach to native space using the x,y,z scaling factors of the linear transformation. The native volumes of the hippocampi and parahippocampal subregions (corrected for ICC volumes) were then subjected to repeated measures analysis of variance, using diagnosis (control versus schizophrenia) as between-group factor and both hemisphere (left versus right) and subregions (hippocampus, parahippocampal, perirhinal, and entorhinal cortices) as within-group factors. Significant main effects and interactions were then explored with post hoc t-tests.
We explored the relationship of medial temporal lobe volumes with continuous clinical measures (duration of illness, severity of psychopathology, and medication dosages). Correlations for normally distributed data were made with linear regression (Pearson's r), and non-normally distributed data were correlated with a rank-method (Spearman's rs). Statistical significance was set a priori at alpha of 0.05 (two-tailed).
Results
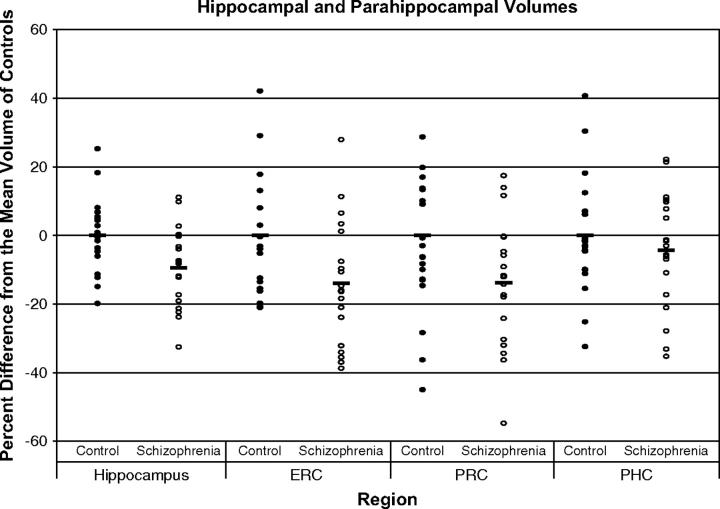
Two main effects explained significant variance components of the medial temporal lobe volumes: diagnosis (i.e., healthy controls had greater volumes than patients) (F(1, 36) = 7.32, p = 0.01) and region (F(3,34) = 99.35, p < 0.001). The significant volume difference between the two groups was not specific for any of the regions (diagnosis by region interaction: F(3,34) = 0.67, p = 0.58) or for hemisphere (diagnosis by hemisphere interaction: F(1,36) = 0.69, p = 0.41 and diagnosis by hemisphere by region interaction: F(3,34) = 2.65, p = 0.06). Post hoc t-tests revealed that left (t = 2.26, df = 36, p = 0.03) and right (t = 2.85, df = 36, p = 0.007) hippocampal volumes were significantly larger in healthy controls, whereas volume differences of the left and right entorhinal cortex (t = 1.72, df = 36, p = 0.09; t = 1.82, df = 36, p = 0.08), perirhinal cortex (t = 1.25, df = 36, p = 0.22; t = 1.58, df = 36, p = 0.12), and parahippocampal cortex (t = 1.50, df = 36, p = 0.14; t = −0.37, df = 36, p = 0.71) did not reach statistical significance (see Table 2 and Figure 2).
Table 2.
Hippocampal and Parahippocampal Volumes and Between-Group Differences Shown by subregion*
| Control volume in mm3 (SD) |
Schizophrenia volume in mm3 (SD) |
% Volume difference (95% CI) |
||||
| Left | Right | Left | Right | Left | Right | |
| Hippocampus | 3131.3 (351.7) | 3282.9 (372.7) | 2848.2 (417.0) | 2947.1 (354.1) | 9.0 (1.2 to 16.8) | 10.2 (2.9 to 16.9) |
| Entorhinal cortex | 1896.7 (446.8) | 1829.9 (385.2) | 1645.5 (351.2) | 1603.9 (381.2) | 12.0 (−1.7 to 25.7) | 12.4 (−0.9 to 25.7) |
| Perirhinal cortex | 1931.1 (495.9) | 1792.3 (376.9) | 1741.5 (436.8) | 1594.7 (395.2) | 9.8 (−5.6 to 25.2) | 11.0 (−2.7 to 24.7) |
| Parahippo-campal cortex | 2786.7 (442.1) | 2465.9 (432.8) | 2554.2 (512.9) | 2514.5 (364.2) | 8.3 (−2.6 to 19.2) | −1.9 (−12.2 to 8.4) |
Boldface indicates that the values are statistically greater than zero at p < 0.05.
Fig. 2.
Mean and percentage volume differences between the two groups in the hippocampal and parahippocampal subregions (Abbreviations: ERC, entorhinal cortex; PHC, parahippocampal cortex; PRC, perirhinal cortex).
We found a region by hemisphere interaction (F(3,34) = 9.25, p < 0.001) with post hoc t-tests demonstrating that, within the entire cohort of subjects, right hippocampus volume was larger than the left (t = −3.70, df = 37, p = 0.001), and the left parahippocampal cortex volume was larger than the right (t = −3.01, df = 37, p = 0.005). Alternate analyses using the Talairach volumes yielded results that were identical to the results based on native volumes. The main findings also did not differ when age was included as a covariate in the repeated measures analysis of variance.
Within the patient group, the severity of psychopathology (total PANSS scores) correlated positively with right hippocampal volume (rs = 0.48, p = 0.038). There was no significant correlation between the duration of illness or medication dosages (daily chlorpromazine equivalents) with any hippocampal or parahippocampal subregional volume.
Discussion
Our study replicates previous findings of smaller medial temporal lobe volume in patients with schizophrenia, but refutes the hypothesis that this volume change is regionally specific to either left or right hippocampus, entorhinal cortex, perirhinal cortex, or parahippocampal cortex. To the best of our knowledge, this is the first study examining the differential involvement of these four medial temporal lobe regions within a cohort of patients with schizophrenia.
The lack of regional specificity (as revealed by the repeated measures ANOVA) suggests that all four regions contribute to the well-known finding of smaller medial temporal lobe volume in schizophrenia. This is not entirely surprising, in view of the close and integrated anatomical and functional relationship of the parahippocampal subregions with the hippocampus.19,43,44 However, previous studies have typically limited their search to either the hippocampus or the parahippocampal gyrus, leading to models of primarily hippocampal27,28,30,45,46 or parahippocampal pathology29, 47–49 in schizophrenia. The more generalized volume change detected in our study is more consistent with a pathological process that affects the hippocampus as well as the parahippocampal gyrus within the medial temporal lobe. This has relevance for neural models explaining psychosis and cognitive deficits of schizophrenia with regionally specific deficits of the hippocampus or parahippocampal gyrus.29
Although we did not find any evidence for regionally specific volume loss in schizophrenia, a comparison of the relative effect sizes revealed that they are greatest for the hippocampus, followed by the entorhinal, perirhinal, and parahippocampal cortical regions (see Figure 2 and the confidence intervals in Table 2). Within the parahippocampal gyrus, the mean differences were largest for the entorhinal and perirhinal cortex, but these differences did not reach statistical significance in post-hoc t-tests. We may be underpowered to detect between-group differences, especially in the extrahippocampal subregions, in view of the greater amount of variance within these subregions. For example, based on the mean volumes, standard deviation, number of subjects in each group, and alpha error value of 5%, we found the power to find a significant difference to be 57% for the right entorhinal cortex and 89% for the right hippocampus. It is possible that a larger study sample would reveal significant group differences not only of hippocampal, but also of parahippocampal, perirhinal, and entorhinal cortex volumes. This would only strengthen our main finding: i.e., that the volumes of hippocampal and extrahippocampal regions within the medial temporal lobe are similarly affected in schizophrenia. The large variance in parahippocampal gyrus regional volumes may also be due to the variable pattern of the collateral sulcus.50 However, a previous study did not find a significant difference in collateral sulcus variability between control and schizophrenia subjects.51 The changes of entorhinal cortex volume reported in this study are consistent with previous reports of decreased parahippocampal gyrus52,53 and entorhinal cortex54 volumes, as well as abnormalities of cytoarchitecture, spatial distribution, neuronal size, and neuron number of the entorhinal cortex.47,48,55,56
We did not find significant diagnosis by hemisphere or diagnosis by hemisphere by region interactions, indicating that medial temporal lobe volume reduction in patients with schizophrenia is not specific to one hemisphere. This is contrary to the hypothesis of Crow et al.,57,58 but consistent with other studies.59–62 In particular, two earlier reports studying solely male patients with schizophrenia59,62 also did not find a diagnosis by hemisphere interaction. We did find a right greater than left hippocampal volume asymmetry in both control and schizophrenia subjects, which is consistent with previous studies.4,38,39
The medial temporal lobe is vulnerable to both genetic and environmental insults.63,64 This is supported by earlier findings that the hippocampal formation is susceptible to injury during pregnancy and perinatal events such as birth complications,65 viral infection,66 and stress,67 with likely involvement of the surrounding parahippocampal regions. On the other hand, insults to the parahippocampal gyrus could secondarily affect the hippocampus. For example, a disturbed architecture of entorhinal cortex neurons could lead to abnormal connections with the hippocampus,48 as in Alzheimer's disease68,69 and some forms of epilepsy,70,71 where the point of initial pathology is located in the parahippocampal subregions. The interaction between genetic and environmental factors may act synergistically and predispose the medial temporal lobe regions toward volumetric changes in schizophrenia,64 and conversely, the volumetric changes in the medial temporal lobe regions may further interact with genetic and environmental factors, leading to the greater vulnerability for the development of subsequent psychopathology.72
It is not clear whether the positive correlation of an overall psychopathology score with right hippocampal volume is related to previously described neuroplastic and compensatory effects within the hippocampus.73 However, the lack of correlation between the entorhinal cortical volumes and the severity of psychopathology is consistent with the findings of Joyal et al.,74 but not that of Prasad et al.,10 although both studies focused on patients with first-onset schizophrenia. The lack of correlation between duration of illness and medial temporal lobe structures was also reported in other studies60,75,76 and could be evidence for a non-progressive pattern of volume change in schizophrenia.75,77
There are several limitations to this study. We studied only chronic, treated, male patients with schizophrenia. The relatively homogeneous study sample might have limited our ability to find the pattern of medial temporal lobe pathology present across the full range of patients with schizophrenia and could have limited our ability to detect significant relationships between regional brain volumes and clinical variables. Studies using a similar parcellation of hippocampus and parahippocampal gyrus in female patients and in first-episode patients are necessary to assess the effect of gender, duration of illness, and possibly medication. However, previous studies have already demonstrated smaller hippocampal78 and entorhinal10,74 cortex volumes in first-episode patients of either gender, making it less likely that our findings are explained by gender, medication, or the stress of a long psychotic illness. We also did not study the cognitive correlates of hippocampal versus parahippocampal gyrus deficits, which could provide further evidence for patterns of medial temporal lobe pathology in schizophrenia.
In conclusion, using a reliable parcellation protocol, we replicated the well-established finding of smaller medial temporal lobe volumes in schizophrenia, but we did not find evidence for regional or hemispheric specificity. This has anatomical and functional implications, which must be considered when studying the etiology and mechanisms of medial temporal lobe pathology in schizophrenia.
Acknowledgments
This study was supported by NIH grant R01 MH070560 (S.H.).
References
- 1.Arnold SE. The medial temporal lobe in schizophrenia. J Neuropsychiatry Clin Neurosci. 1997;9:460–470. doi: 10.1176/jnp.9.3.460. [DOI] [PubMed] [Google Scholar]
- 2.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 3.Heckers S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus. 2001;11:520–528. doi: 10.1002/hipo.1068. [DOI] [PubMed] [Google Scholar]
- 4.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 6.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 7.Andia AM, Zisook S, Heaton RK, et al. Gender differences in schizophrenia. J Nerv Ment Dis. 1995;183:522–528. doi: 10.1097/00005053-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bogerts B, Ashtari M, Degreef G, et al. Reduced temporal limbic structure volumes on magnetic resonance images in first episode schizophrenia. Psychiatry Res. 1990;35:1–13. doi: 10.1016/0925-4927(90)90004-p. [DOI] [PubMed] [Google Scholar]
- 9.Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- 10.Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 11.Razi K, Greene KP, Sakuma M, Ge S, Kushner M, DeLisi LE. Reduction of the parahippocampal gyrus and the hippocampus in patients with chronic schizophrenia. Br J Psychiatry. 1999;174:512–519. doi: 10.1192/bjp.174.6.512. [DOI] [PubMed] [Google Scholar]
- 12.Corey-Bloom J, Jernigan T, Archibald S, et al. Quantitative magnetic resonance imaging of the brain in late-life schizophrenia. Am J Psychiatry. 1995;152:447–449. doi: 10.1176/ajp.152.3.447. [DOI] [PubMed] [Google Scholar]
- 13.DeLisi LE, Hoff AL, Schwartz JE, et al. Brain morphology in first-episode schizophrenic-like psychotic patients: a quantitative magnetic resonance imaging study. Biol Psychiatry. 1991;29:159–175. doi: 10.1016/0006-3223(91)90044-m. [DOI] [PubMed] [Google Scholar]
- 14.Havermans R, Honig A, Vuurman EF, et al. A controlled study of temporal lobe structure volumes and P300 responses in schizophrenic patients with persistent auditory hallucinations. Schizophr Res. 1999;38:151–158. doi: 10.1016/s0920-9964(99)00006-7. [DOI] [PubMed] [Google Scholar]
- 15.Sanfilipo M, Lafargue T, Rusinek H, et al. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000;57:471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- 16.Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- 17.Burwell RD. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 18.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 19.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. In: Scharfman HE, Witter MP, Schwarcz R, editors. The Parahippocampal Region: Implications for Neurological and Psychiatric Diseases. Vol. 911. New York: Annals of the New York Academy of Sciences; 2000. pp. 1–24. [PubMed] [Google Scholar]
- 20.Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. Oxford: Oxford University Press; 2001. [Google Scholar]
- 21.Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann N Y Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- 22.Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duzel E, Habib R, Rotte M, Guderian S, Tulving E, Heinze HJ. Human hippocampal and parahippocampal activity during visual associative recognition memory for spatial and nonspatial stimulus configurations. J Neurosci. 2003;23:9439–9444. doi: 10.1523/JNEUROSCI.23-28-09439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mishkin M, Vargha-Kadem F, Gadian DG. Amnesia and the organization of the hippocampal system. Hippocampus. 1998;8:212–216. doi: 10.1002/(SICI)1098-1063(1998)8:3<212::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 26.Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 27.Hemsley DR. A simple (or simplistic?) cognitive model for schizophrenia. Behav Res Ther. 1993;31:633–645. doi: 10.1016/0005-7967(93)90116-c. [DOI] [PubMed] [Google Scholar]
- 28.Roberts DR. Schizophrenia and the brain. Journal of Neuropsychiatry. 1963;5:71–79. [PubMed] [Google Scholar]
- 29.Talamini LM, Meeter M, Elvevag B, Murre JM, Goldberg TE. Reduced parahippocampal connectivity produces schizophrenia-like memory deficits in simulated neural circuits. Arch Gen Psychiatry. 2005;62:485–493. doi: 10.1001/archpsyc.62.5.485. [DOI] [PubMed] [Google Scholar]
- 30.Venables PH. Hippocampal function and schizophrenia. Experimental psychological evidence. Ann N Y Acad Sci. 1992;658:111–127. doi: 10.1111/j.1749-6632.1992.tb22841.x. [DOI] [PubMed] [Google Scholar]
- 31.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 32.Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Del Tredici K, Bohl J, Bratzke H, Braak E. Pathological changes in the parahippocampal region in select non-Alzheimer's dementias. Ann N Y Acad Sci. 2000;911:221–239. doi: 10.1111/j.1749-6632.2000.tb06729.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer's disease: clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000;911:254–274. doi: 10.1111/j.1749-6632.2000.tb06731.x. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute, Biometrics Research; 1995. [Google Scholar]
- 36.Bartzokis G, Altshuler LL, Greider T, et al. Reliability of medial temporal lobe volume measurements using reformatted 3D images. Psychiatry Res. 1998;82:11–24. doi: 10.1016/s0925-4927(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 37.Pruessner JC, Li LM, Serles W, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 38.Collins DL, Neelin P, Peters TM, et al. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 39.Pruessner JC, Köhler S, Crane J, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- 40.Weiss AP, Dewitt I, Goff D, Ditman T, Heckers S. Anterior and posterior hippocampal volumes in schizophrenia. Schizophr Res. 2005;73:103–112. doi: 10.1016/j.schres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 41.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 42.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 44.Van Hoesen GW. The parahippocampal gyrus: new observations regarding its cortical connections in the monkey. Trends Neurosci. 1982;5:345–350. [Google Scholar]
- 45.Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol Psychiatry. 1999;46:589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 46.Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- 47.Arnold SE. Cellular and molecular neuropathology of the parahippocampal region in schizophrenia. Ann N Y Acad Sci. 2000;911:275–292. doi: 10.1111/j.1749-6632.2000.tb06732.x. [DOI] [PubMed] [Google Scholar]
- 48.Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- 49.Walker MA, Highley JR, Esiri MM, et al. Estimated neuronal populations and volumes of the hippocampus and its subfields in schizophrenia. Am J Psychiatry. 2002;159:821–828. doi: 10.1176/appi.ajp.159.5.821. [DOI] [PubMed] [Google Scholar]
- 50.Hanke J. Sulcal pattern of the anterior parahippocampal gyrus in the human adult. Anat Anz. 1997;179:335–339. doi: 10.1016/S0940-9602(97)80071-4. [DOI] [PubMed] [Google Scholar]
- 51.Heckers S, Heinsen H, Heinsen Y, Beckmann H. Morphometry of the parahippocampal gyrus in schizophrenics and controls: some anatomical considerations. J Neural Transm. 1990;80:151–155. doi: 10.1007/BF01257080. [DOI] [PubMed] [Google Scholar]
- 52.Bogerts B, Meertz E, Schonfeldt-Bausch R. Basal ganglia and limbic system pathology in schizophrenia: a morphometric study of brain volume and shrinkage. Arch Gen Psychiatry. 1985;42:784–791. doi: 10.1001/archpsyc.1985.01790310046006. [DOI] [PubMed] [Google Scholar]
- 53.Brown R, Colter N, Corsellis JA, et al. Postmortem evidence of structural brain changes in schizophrenia: differences in brain weight, temporal horn area, and parahippocampal gyrus compared with affective disorder. Arch Gen Psychiatry. 1986;43:36–42. doi: 10.1001/archpsyc.1986.01800010038005. [DOI] [PubMed] [Google Scholar]
- 54.Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region—a morphometric study. Biol Psychiatry. 1988;24:515–521. doi: 10.1016/0006-3223(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 55.Arnold SE, Franz BR, Gur RC, et al. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- 56.Falkai P, Schneider-Axmann T, Honer WG. Entorhinal cortex pre-alpha cell clusters in schizophrenia: quantitative evidence of a developmental abnormality. Biol Psychiatry. 2000;47:937–943. doi: 10.1016/s0006-3223(99)00250-4. [DOI] [PubMed] [Google Scholar]
- 57.Crow TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull. 1990;16:433–443. doi: 10.1093/schbul/16.3.433. [DOI] [PubMed] [Google Scholar]
- 58.Crow TJ, Ball J, Bloom SR, et al. Schizophrenia as an anomaly of development of cerebral asymmetry: a postmortem study and a proposal concerning the genetic basis of the disease. Arch Gen Psychiatry. 1989;46:1145–1150. doi: 10.1001/archpsyc.1989.01810120087013. [DOI] [PubMed] [Google Scholar]
- 59.Becker T, Elmer K, Schneider F, et al. Confirmation of reduced temporal limbic structure volume on magnetic resonance imaging in male patients with schizophrenia. Psychiatry Res. 1996;67:135–143. doi: 10.1016/0925-4927(96)03002-8. [DOI] [PubMed] [Google Scholar]
- 60.Dauphinais ID, DeLisi LE, Crow TJ, et al. Reduction in temporal lobe size in siblings with schizophrenia: a magnetic resonance imaging study. Psychiatry Res. 1990;35:137–147. doi: 10.1016/0165-1781(90)90156-y. [DOI] [PubMed] [Google Scholar]
- 61.Flaum M, Swayze VW, 2nd, O'Leary DS, et al. Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry. 1995;152:704–714. doi: 10.1176/ajp.152.5.704. [DOI] [PubMed] [Google Scholar]
- 62.Pegues MP, Rogers LJ, Amend D, Vinogradov S, Deicken RF. Anterior hippocampal volume reduction in male patients with schizophrenia. Schizophr Res. 2003;60:105–115. doi: 10.1016/s0920-9964(02)00288-8. [DOI] [PubMed] [Google Scholar]
- 63.Mody M, Cao Y, Cui Z, et al. Genome-wide gene expression profiles of the developing mouse hippocampus. Proc Natl Acad Sci U S A. 2001;98:8862–8867. doi: 10.1073/pnas.141244998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Erp TGM, Saleh PA, Rosso IM, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia of schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- 65.Conrad AJ, Scheibel AB. Schizophrenia and the hippocampus: the embryological hypothesis extended. Schizophr Bull. 1987;13:577–587. doi: 10.1093/schbul/13.4.577. [DOI] [PubMed] [Google Scholar]
- 66.Fatemi SH, Emamian ES, Kist D, et al. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- 67.Vaid RR, Yee BK, Shalev U, et al. Neonatal nonhandling and in utero prenatal stress reduce the density of NADPH-diaphorase-reactive neurons in the fascia dentata and Ammon's horn of rats. J Neurosci. 1997;17:5599–5609. doi: 10.1523/JNEUROSCI.17-14-05599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juottonen K, Laakso MP, Insausti R, et al. Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiol Aging. 1998;19:15–22. doi: 10.1016/s0197-4580(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 69.Van Hoesen GW, Hyman BT, Damasio AR. Entorhinal cortex pathology in Alzheimer's disease. Hippocampus. 1991;1:1–8. doi: 10.1002/hipo.450010102. [DOI] [PubMed] [Google Scholar]
- 70.Du F, Whetsell WO, Abou-Khalil B, Blumenkopf B, Lothman EW, Schwarcz R. Preferential neuronal loss in layer III of the entorhinal cortex in patients with temporal lobe epilepsy. Epilepsy Res. 1993;16:223–233. doi: 10.1016/0920-1211(93)90083-j. [DOI] [PubMed] [Google Scholar]
- 71.Nakasato N, Levesque MF, Babb TL. Seizure outcome following standard temporal lobectomy: correlation with hippocampal neuron loss and extrahippocampal pathology. J Neurosurg. 1992;77:194–200. doi: 10.3171/jns.1992.77.2.0194. [DOI] [PubMed] [Google Scholar]
- 72.Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathological vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frost DO, Tamminga CA, Medoff DR, Caviness V, Innocenti G, Carpenter WT. Neuroplasticity and schizophrenia. Biol Psychiatry. 2004;56:540–543. doi: 10.1016/j.biopsych.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 74.Joyal CC, Laakso MP, Tiihonen J, et al. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–1007. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- 75.Marsh L, Suddath RL, Higgins N, Weinberger DR. Medial temporal lobe structures in schizophrenia: relationship of size to duration of illness. Schizophr Res. 1994;11:225–238. doi: 10.1016/0920-9964(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 76.Young AH, Blackwood DHR, Roxborough H, McQueen JK, Martin MJ, Kean D. A magnetic resonance imaging study of schizophrenia: brain structure and clinical symptoms. Br J Psychiatry. 1991;158:158–164. doi: 10.1192/bjp.158.2.158. [DOI] [PubMed] [Google Scholar]
- 77.Wood SJ, Velakoulis D, Smith DJ, et al. A longitudinal study of hippocampal volume in first episode psychosis and chronic schizophrenia. Schizophr Res. 2001;52:37–46. doi: 10.1016/s0920-9964(01)00175-x. [DOI] [PubMed] [Google Scholar]
- 78.Velakoulis D, Pantelis C, McGorry PD, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- 79.Blair J, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 80.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2001;64:663–667. doi: 10.4088/jcp.v64n0607. (2003) [DOI] [PubMed] [Google Scholar]
- 81.American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Schizophrenia. Washington, D.C.: American Psychiatric Press; 1997. [Google Scholar]