Abstract
The study describes the Cardiff Anomalous Perceptions Scale (CAPS), a new validated measure of perceptual anomalies. The 32-item CAPS measure is a reliable, self-report scale, which uses neutral language, demonstrates high content validity, and includes subscales that measure distress, intrusiveness, and frequency of anomalous experience. The CAPS was completed by a general population sample of 336 participants and 20 psychotic inpatients. Approximately 11% of the general population sample scored above the mean of the psychotic patient sample, although, as a group, psychotic inpatients scored significantly more than the general population on all CAPS subscales. A principal components analysis of the general population data revealed 3 components: “clinical psychosis” (largely Schneiderian first-rank symptoms), “temporal lobe disturbance” (largely related to temporal lobe epilepsy and related seizure-like disturbances) and “chemosensation” (largely olfactory and gustatory experiences), suggesting that there are multiple contributory factors underlying anomalous perceptual experience and the “psychosis continuum.”
Keywords: hallucination, psychometric scale, psychosis continuum, schizophrenia
As a general label for a range of symptoms associated with severe mental illness, “psychosis” is typically characterized as a “loss of contact with reality.” Although lacking a consistent operational definition, one of the most problematic aspects of the term, as traditionally employed, is its assumed categorical nature. In contrast to the traditional categorical approach to psychosis adopted in the Diagnostic and Statistical Manual of Mental Disorders1 (DSM-IV), there is growing interest in a more dimensional view, which argues that psychosis-like beliefs, perceptual distortions, and idiosyncrasies of thought and communication, considered the hallmark diagnostic criteria for psychosis, are distributed (albeit to varying degrees) throughout the general population. Such an approach considers florid psychosis as comprising the most extreme pole of the population spectrum.2–6 The view that psychotic manifestations may exist on a continuum, rather than as a discrete entity, however, is not new. In contrast to the more popular Kraepelinian view, Bleuler, and later others, argued throughout the twentieth century against a clear separation between sanity and madness.7–11
The development of psychometric measures that have attempted to capture the continuum of psychosis and psychosis-like experience has facilitated this noncategorical view. The focus for such scales, however, has varied, with some aiming to measure a general psychosis proneness, while other have focused on particular aspects of the psychosis continuum (such as delusional ideation or hallucination proneness) influenced by the symptom boundaries of clinical psychiatry.
One of the earliest attempts to capture a general concept of psychosis proneness was Eysenck's inclusion of the psychoticism dimension as an aspect of personality.12,13 Adopting a personality-theory standpoint, Eysenck aimed to capture psychosis proneness on a dimensional construct varying from normality (necessarily defined in culturally relative terms) and psychosis. This was subsequently developed into a multidimensional concept of schizotypy,14 based on a factor analysis of various psychosis-proneness scales,15 which has been developed into the “unusual experience,” “cognitive disorganization,” “introvertive anhedonia,” and “impulsive nonconformity” subscales of the Oxford and Liverpool Inventory of Feelings and Experiences (O-LIFE) schizotypy scale.16
In contrast to this approach, most other measures of psychosis proneness are grounded in clinical psychiatry and aimed at measuring attenuated or “soft” psychotic symptoms in the general population. Of particular relevance, understandably, are those measures that attempt to quantify aspects of the “positive symptoms” of psychosis, such as delusions and hallucinations.
The Magical Ideation Scale by Eckblad and Chapman17 covers a range of beliefs and experiences from first-rank symptoms of schizophrenia18 and ideas of reference to popular paranormal and conspiracy theory themes (eg, “The government refuses to tell us the truth about flying saucers”). The Peters et al. Delusions Inventory4,5 (PDI) is a measure of delusional ideation that inquires about beliefs, interpretations, and experiences, using items derived from the Present State Examination,19 an internationally recognized clinical measure, which is often used to detect and assess clinically defined psychotic symptoms. The PDI, however, is unique in that it not only measures the total number of beliefs or experiences endorsed but also the concurrent perceptions of distress, preoccupation, and conviction associated with the endorsed items.
Other measures have focused on perceptual and hallucinatory experiences associated with psychosis. The Perceptual Aberration Scale20 measures the level of body-image aberration, with items based on experiences of somatic distortions and hallucinations, as reported in the clinical literature on schizophrenia and associated diagnoses. Morrison, Wells, and Nothard created and revised the Launay-Slade Hallucinations Scale21,22 (RLSHS) to measure predisposition to hallucinations, in an attempt to capture some clinically recognizable hallucinatory phenomena (such as “hearing voices” and having nonveridical visual experiences), as well as any tendency to have vivid imagery and daydreams.
The Structured Interview for Assessing Perceptual Anomalies23 (SIAPA) is one alternative assessment method that does not rely on self-report. Although it aims to be comprehensive in its coverage of the “5 senses,” it is designed as an interview-based assessment of the frequency of sensory anomalies and, therefore, has the disadvantage of being time-consuming and requiring 1-to-1 assessment. It also is restricted in that, unlike some of the psychosis-inspired scales already mentioned, it does not assess hallucinatory phenomena directly but instead focuses on changes in sensory intensity, attention, and sensory flooding. It is clear that a measure is needed to assess the range of perceptual anomalies not covered by any single existing scale.
Furthermore, many of the psychometric measures of anomalous perceptual experience derive both their content and language from mainstream clinical psychiatry (which depends on frank and often chronic forms of mental illness), and it is apparent that they may lack face validity when trying to assess accurately the full range of perceptual anomalies in the general population.
These biases can make perceptual and cognitive distortions difficult to tease apart adequately. Several of the scales are not “pure” measures of perceptual anomaly (although deliberately so in many cases). For example, the Launay-Slade Hallucinations Scale, despite its name, conflates items concerning both perceptual experience (eg, “I hear the telephone ring and find that I am mistaken”) and delusional ideation (eg, “I fantasize about being someone else”) into a single measure.
There is also an implicit assumption in some scales that respondents are able to distinguish between experiences that stem from perceptions that exist out in the “real world” and those that may arise from distortions with the respondent's own cognitive processes—that is, those that are considered “not to be really there,” as illustrated by this item from the O-LIFE: “When in the dark, do you often see shapes and forms even though there's nothing there?”
Other measures rely on a related concept of strangeness or unusualness (for example, “When I look at things, they appear strange to me,” from the RLSHS) that presupposes a nonveridical perceptual experience will necessarily present as strange or anomalous. Both assumptions are potentially problematic “since virtually all waking perceptual experiences are veridical, a long personal history of validated perception would dictate accepting hallucinations as veridical.”24(p9) Of course, it may be that perceptual anomalies are accompanied by insight into their unusual nature, but it is important that this is not the only criterion by which such anomalies are measured. In fact, there may be several indicators that a perceptual experience is not veridical for an individual, including those that may arise without a clear source, those that do not seem to be shared by other people in the vicinity, and those that that are accompanied by a sense of strangeness.
Another drawback of assessing perceptual anomalies by extrapolating exclusively from the context of clinical psychiatry is the overreliance on hallucinatory phenomena that occur in the visual and auditory modalities. For example, surveys of hallucinatory phenomena in the general population indicate that olfactory and gustatory hallucinations are particularly common,25 yet these modalities are rarely explored in psychometric measures of hallucination or psychosis proneness. Likewise, alterations in sensory intensity, rather than the experience of discrete perceptual phenomena, are not normally covered by existing scales. Another legacy of clinical psychiatry is the lack of coverage of perceptual anomalies associated with temporal lobe disturbance, despite the fact that temporal lobe disturbance has been linked to almost every “stage” on the psychosis continuum, from full-blown psychosis26,27 to paranormal beliefs and experiences,28 as well as to anomalous perceptual phenomena in nonclinical participants.29 Thus, there is a need for a comprehensive scale capable of measuring a range of sensory experience, covering both clinical and nonclinical populations.
Consequently, the purpose in designing the Cardiff Anomalous Perceptions Scale (CAPS) was to construct a valid and reliable psychometric measure of perceptual anomalies. Critically, it is not dependent on the clinical psychiatric context and considers subjective experiences from a range of different perspectives of insight awareness (including knowing that the percept is “not really there,” the percept seeming strange or unusual, or the percept being a nonshared sensory experience). Moreover, the CAPS includes items pertaining to distortions in perceptual intensity, to experiences in all appropriate sensory modalities, and to sensory experiences traditionally associated with temporal lobe disturbances. Following the usefulness of their inclusion in the PDI,4,5 we also included dimensional ratings to measure associated distress, intrusiveness, and frequency for each experience endorsed.
Method
Construction of the CAPS
Item Selection
Measures related to psychosis proneness, hallucination proneness, clinical assessment of psychosis, delusional and magical ideation, and hallucinatory experience, including temporal lobe disturbance, were collected and reviewed (Table 1), and all items relating to sensory experience were considered. To focus particularly on anomalous perceptual experience, rather than on other, more general aspects of schizotypy or psychosis-like experience or proneness, experiences relating to thought broadcast, insertion, blocking, and interference were excluded, unless they had been subsequently experienced via one of the senses (eg, “hearing thoughts out loud”). Similarly, any experiences relating to dissociation, depersonalization, or existential feelings of strangeness or unease (eg, “sometimes everything around me feels strange”) were also excluded, as were those specifically related to hypnopompic, hypnagogic, or other sleep-related states such as dreaming, in order to exclude any experiences that may not have occurred in clear consciousness.
Table 1.
Scales Reviewed in Construction of CAPS Items
| Scale or Assessment | Authors |
| Present State Examination | Wing et al.19 |
| Magical Ideation Scale | Eckblad and Chapman17 |
| Makarec and Persinger Temporal Lobe Scale | Makarec and Persinger30 |
| Scale for the Assessment of Positive Symptoms | Andreasen31 |
| Psychosis Screening Questionnaire | Bebbington and Nayani32 |
| Oxford and Liverpool Inventory of Feelings and Experiences schizotypy scale | Mason et al.16 |
| Structured Interview for Assessing Perceptual Anomalies | Bunney et al.23 |
| Peters et al. Delusions Inventory | Peters et al.5 |
| Revised Launay-Slade Hallucinations Scale | Morrison et al.22 |
Relevant items were generated from candidate experiences, and items that repeated or substantially overlapped with other items were removed, with further items created to cover additional sensory modalities where necessary. The final CAPS items, by category, are listed in Table 2. In the final scale, each item is presented as a question requiring an answer of “yes” or “no,” with the participants required simply to rate the item for distress, intrusiveness, and frequency of occurrence on a 5-point (1–5) Likert scale if they responded with a “yes” to the initial question.
Table 2.
CAPS Items Broken Down by Preselected Category of Anomalous Experience
| Selection Category | CAPS Items |
| Changes in Levels of Sensory Intensity (Relevant Domains: Sight, Sound, Taste, Touch, Smell) | 1. Do you ever notice that sounds are much louder than they normally would be? |
| 18. Do you ever smell everyday odors and think that they are unusually strong? | |
| 20. Do you ever find that your skin is more sensitive to touch, heat, or cold than usual? | |
| 21. Do you ever think that food or drink tastes much stronger than it normally would? | |
| 23. Do you ever have days where lights or colors seem brighter or more intense than usual? | |
| Having a Nonshared Sensory Experience (Relevant Domains: Sight, Sound, Smell) | 13. Do you ever hear voices saying words or sentences when there is no one around that might account for it? |
| 29. Do you ever experience smells or odors that people next to you seem unaware of? | |
| 31. Do you ever see things that other people cannot? | |
| 32. Do you ever hear sounds or music that people near you don't hear? | |
| Inherently Unusual or Distorted Sensory Experience (Relevant Domains: Sight, Sound, Taste, Touch, Smell) | 5. Do you ever experience unusual burning sensations or other strange feelings in or on your body? |
| 16. Do you ever find that sounds are distorted in strange or unusual ways? | |
| 25. Do you ever find that common smells sometimes seem unusually different? | |
| 26. Do you ever think that everyday things look abnormal to you? | |
| 30. Do you ever notice that food or drink seems to have an unusual taste? | |
| Sensory Experience From an Unexplained Source (Relevant Domains: Sight, Sound, Taste, Touch, Smell) | 4. Do you ever see shapes, lights, or colors even though there is nothing really there? |
| 6. Do you ever hear noises or sounds when there is nothing about to explain them? | |
| 8. Do you ever detect smells which don't seem to come from your surroundings? | |
| 12. Do you ever feel that someone is touching you, but when you look nobody is there? | |
| 14. Do you ever experience unexplained tastes in your mouth? | |
| 28. Have you ever heard 2 or more unexplained voices talking with each other? | |
| Distortion of Form (Size, Shape) of Own Body and of External World | 9. Do you ever have the sensation that your body, or a part of it, is changing or has changed shape? |
| 10. Do you ever have the sensation that your limbs might not be your own or might not be properly connected to your body? | |
| 19. Do you ever find the appearance of things or people seems to change in a puzzling way, eg, distorted shapes or sizes or color? | |
| 22. Do you ever look in the mirror and think that your face seems different from usual? | |
| Verbal Hallucinations | 11. Do you ever hear voices commenting on what you are thinking or doing? |
| 13. Do you ever hear voices saying words or sentences when there is no one around that might account for it? | |
| 28. Have you ever heard 2 or more unexplained voices talking with each other? | |
| Sensory Flooding | 15. Do you ever find that sensations happen all at once and flood you with information? |
| 17. Do you ever have difficulty distinguishing one sensation from another? | |
| Thought Echo and Hearing Thoughts Out Loud | 3. Do you ever hear your own thoughts repeated or echoed? |
| 7. Do you ever hear your own thoughts spoken aloud in your head, so that someone near might be able to hear them? | |
| Temporal Lobe | 2. Do you ever sense the presence of another being, despite being unable to see any evidence? |
| 10. Do you ever have the sensation that your limbs might not be your own or might not be properly connected to your body? | |
| 24. Do you ever have the feeling of being uplifted, as if driving or rolling over a road while sitting quietly? | |
| 27. Do you ever find that your experience of time changes dramatically? |
Note: Questions may appear in more than 1 category.
Participants and Procedure
Controls
A total of 358 participants from a nonclinical population participated in the study. Excluded from the analysis were 22 participants who incorrectly completed or missed items on questionnaires, leaving a total of 336 participants in the final analysis (mean age = 21.6; SD = 5.4; range 18–54). Participants were largely drawn from undergraduate students (N = 305), including 111 males and 176 females (not disclosed = 18), with a mean age of 19.9 (SD = 2.6; range 18–44; not disclosed = 13), who took part as part of their induction program or who responded to requests for participants. The remaining 32 participants were drawn from an anonymous postal survey. They responded to advertisements posted on general-purpose Internet discussion groups requesting participants for a study on “beliefs and experiences,” with no reference to the specific aims of the study in the original advertisement or any of the supporting material. This sample consisted of 17 females and 14 males (not disclosed = 1), with a mean age of 32.4 (SD = 10.2; range 18–54; not disclosed = 1).
All participants completed the CAPS and the 21-item Peters et al. Delusions Inventory5,33 (PDI-21). The RLSHS22 was completed by a subset of 288 individuals and the O-LIFE schizotypy scale16 by a subset of 184 participants. The smaller number of participants completing these later questionnaires was due to their not being included in the early phases of the study.
Six months after initially completing the CAPS, undergraduate students were invited to complete the scale again to enable a test-retest reliability assessment, of which 44 responded (males = 7; females = 37).
Clinical
In addition to the nonclinical sample, a sample of 20 psychotic inpatients, consisting of 13 females and 7 males (mean age 40.68; SD = 10.6; range 25–64), completed the CAPS. The group consisted of patients with diagnoses of schizophrenia (9), schizoaffective disorder (1), bipolar disorder (6), psychotic depression (2), delusional disorder (1), and unspecified psychosis (1).
The data from the psychotic patients were collected from 4 acute psychiatric admission wards in the Cardiff area. The patients were selected on the basis of having been diagnosed with a current psychotic episode by the responsible clinician. Patients in this sample were screened with the Psychosis Screening Questionnaire32 (PSQ) to confirm the clinical classification. Out of an original sample of 22 inpatients, 2 were excluded due to screening negative on the PSQ, leaving 20 inpatients from this sample.
Participants in all clinical samples were referred by the responsible clinician as being without a history of brain injury or substance or alcohol abuse, and they were all on a medication regime at the time of testing. This was confirmed by review of the clinical notes. The CAPS was completed with the researcher present to assist with any difficulties in reading or comprehension.
As can be seen from the descriptions of the participant samples, there was a small imbalance in the numbers of male and female participants between the clinical and nonclinical samples, although the distribution was not significantly different from chance when tested with a chi-square test (χ2 = .383, Fisher's exact p = .641). There was, however, a significant difference in age between the samples when tested with an independent samples t-test (t = 13.287, p < .0005), with the nonclinical sample having significantly younger participants than the clinical sample. This was unlikely to have had a confounding effect on the results, however, since age is inversely associated with psychotic symptoms in adults, in both clinical and nonclinical populations.34,35
The study was ethically reviewed and approved by all the participating institutions, and informed consent was obtained from participants (both clinical and nonclinical) before participation.
Results
Psychometric Properties
Four separate scores were obtained from the CAPS: (1) total number of items endorsed; (2) a distress score; (3) an intrusiveness score; and (4) frequency of occurrence. A total score was calculated by summing the number of items endorsed.
For each item endorsed, participants were required to rate the item on 1–5 scales for distress, intrusiveness, and frequency. The total scores for these dimensions were calculated by summing the ratings for all endorsed items, with nonendorsed items considered to have a score of 0 in each of these 3 categories. Therefore, the possible range for the CAPS total was 0 (low) to 32 (high), and for each of the dimensions the possible range was 0 to 160.
Descriptive statistics for the CAPS, PDI-21, RLSHS, and O-LIFE schizotypy scale are given in Table 3. The sum of male and female participants does not add up to the total sample, owing to the fact that participants occasionally did not disclose their gender. As can also be seen from Table 3, there was a significant difference between males and females on all dimensions and total score on the CAPS and on the RLSHS, with males scoring significantly higher. This contrasts with the results from the original study on the 40-item PDI,4 where no significant difference was reported between male and female scores. In our study males scored significantly higher on PDI-21 total score, preoccupation, and conviction dimensions. The sex differences in psychosis proneness reported here, although not consistent with the original PDI study, may be due to the preponderance of younger participants in the nonclinical sample. This finding is consistent with the results reported by Spauwen et al.,36 who found that psychosis-like experiences were more prevalent in males under 21 years than females of the same age. This was thought to reflect the increased vulnerability to psychosis in younger males.37
Table 3.
Descriptive Statistics for CAPS, PDI-21, O-LIFE, and RLSHS in Nonclinical Sample
| Scale (Total N) | CAPS (337) |
PDI-21 (337) |
O-LIFE (184) |
RLSHS (288) |
|||||||||
| Male/Female N | 125/193 |
125/193 |
130/124 |
114/157 | |||||||||
| Subscale | Total | Distress | Intrusiveness | Frequency | Total | Distress | Preoccupation | Conviction | UE | CD | IA | IN | |
| Males, mean (SD) | 9.3 (6.3) | 18.3 (15.3) | 21.9 (17.6) | 18.2 (15.3) | 6.2 (4.0) | 15.0 (12.6) | 15.2 (12.3) | 18.2 (13.9) | 5.1 (6.3) | 12.6 (4.5) | 7.3 (3.1) | 10.7 (3.5) | 41.5 (11.1) |
| Females, mean (SD) | 6.3 (5.3) | 14.0 (14.0) | 16.1 (16.5) | 12.8 (13.4) | 5.0 (3.0) | 12.2 (8.4) | 11.6 (8.4) | 14.7 (10.5) | 5.9 (5.3) | 11.8 (4.2) | 7.2 (3.0) | 9.3 (2.7) | 36.7 (7.5) |
| Total, mean (SD) | 7.3 (5.8) | 15.5 (14.5) | 18.0 (17.0) | 14.6 (14.2) | 5.4 (3.4) | 13.2 (10.2) | 12.9 (10.1) | 15.9 (11.9) | 5.7 (5.4) | 12.1 (4.4) | 7.4 (3.3) | 9.7 (3.0) | 38.6 (9.3) |
| Range | 0–26 | 0–81 | 0–81 | 0–77 | 0–19 | 0–59 | 0–51 | 0–69 | 0–30 | 3–25 | 2–19 | 4–19 | 18–78 |
| Median | 6 | 12 | 13 | 11 | 5 | 11 | 11 | 14 | 4 | 12 | 7 | 10 | 37 |
| Mode | 1 | 0 | 0 | 0 | 4 | 11 | 6 | 11 | 1 | 14 | 6 | 10 | 33 |
| Gender, Z# | −4.31* | −2.73* | −3.31* | −3.68* | −2.41* | −1.08 | −1.97* | −2.05** | −.92 | −.70 | −.27 | −1.91 | −3.46* |
Note: Abbreviations used: CAPS = Cardiff Anomalous Perceptions Scale; CD = Cognitive disorganization; IA = Introvertive anhedonia; IN = Impulsive nonconformity; O-LIFE = Oxford and Liverpool Inventory of Feelings and Experiences; PDI-21 = 21-item Peters et al. Delusions Inventory; RLSHS = Revised Launay-Slade Hallucinations Scale; UE = Unusual experiences.
Mann Whitney U test (2-tailed).
Significant at p < .01;
Significant at p < .05.
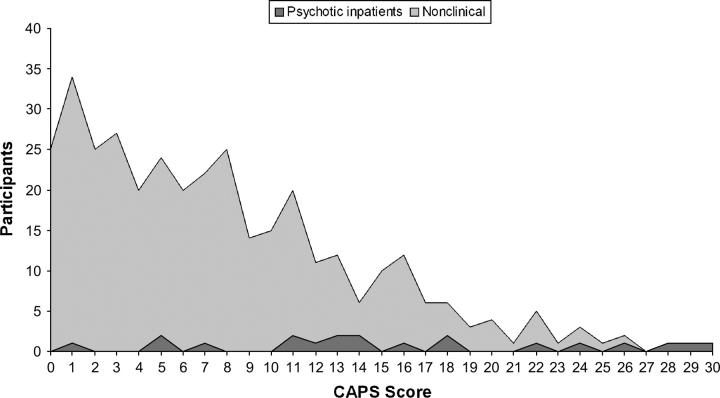
CAPS total score showed a left-skewed frequency distribution in the nonclinical population (Figure 1). Johns and van Os2 previously noted a difference between the normal and left-skewed “half-normal” distribution of measures of psychosis-like experience and argued that a half-normal distribution could result from various causes contributing independently but interacting when expressed. They further suggested that this distribution is most likely a reflection of the “real” distribution of psychosis, suggesting that (on their criteria at least) the CAPS distribution is a statistically accurate reflection of the proposed continuum of psychosis distribution.
Fig. 1.
Frequency distribution of CAPS total score for clinical and nonclinical samples.
Figure 1 also illustrates that the distribution of CAPS total scores from the clinical sample cuts across the entire range of CAPS scores and overlaps considerably with the distribution of scores from the nonclinical population. In terms of CAPS total score, 11.3% of the nonclinical sample score above the mean of psychotic inpatients. Similar patterns have been found with other measures of psychosis proneness and psychosis-like experience, with either participants in the healthy control group endorsing items usually associated with clinical psychosis, or patients with psychosis scoring less than members of the nonclinical population.4,23,38
Reliability
Internal reliability of the CAPS was good, with a Cronbach's alpha coefficient of .87. Test-retest reliability was determined from the group of 44 participants who completed the CAPS a second time, after a 6-month gap, and was also found to be acceptable for all CAPS measures when tested with Pearson correlations: CAPS total score = 0.77 (p < .0005); CAPS distress score = 0.779 (p < .0005); CAPS intrusiveness score = 0.783 (p < .0005); CAPS frequency score = 0.778 (p < .0005). The standard error of measurement for the CAPS total score, therefore, can be calculated as 1.34, showing a low margin of error when measuring the hypothetical true score.39 The Cronbach's alpha coefficient of the test-retest sample was .92, demonstrating that internal reliability remained stable over time.
Validity
Construct validity was assessed by correlating CAPS total score with the RLSHS, O-LIFE subscales, and PDI-21. Pearson's r correlation coefficients between each scale are outlined in Table 4. As a Pearson correlation is not an interval measure (ie, the distance between r = 0.1 to r = 0.2 is not the same as the distance from r = 0.8 to r = 0.9) Fisher's Z transformations are also given, as these reflect an equal-variance scale and are therefore more comparable.40
Table 4.
Correlations Between CAPS Total Score and PDI-21 Total Score, O-LIFE Subscale, and RLSHS in Nonclinical Sample (with Fisher's Z transformations of Pearson's r)
| CAPS Total Score | PDI-21 | O-LIFE UE | O-LIFE CD | O-LIFE IA | O-LIFE IN | RLSHS |
| Pearson's r | .60* | .57* | .36* | .030 | .20** | .65* |
| Fisher's Z | .69 | 0.65 | 0.38 | .03 | 0.20 | 0.78 |
| (N) | (337) | (170) | (170) | (169) | (171) | (288) |
Note: Abbreviations used: CAPS = Cardiff Anomalous Perceptions Scale; CD = Cognitive disorganization; IA = Introvertive anhedonia; IN = Impulsive nonconformity; O-LIFE = Oxford and Liverpool Inventory of Feelings and Experiences; PDI-21 = 21-item Peters et al. Delusions Inventory; RLSHS = Revised Launay-Slade Hallucinations Scale; UE = Unusual experiences.
Significant at p < .01;
Significant at p < .05.
The CAPS total score shows significant positive correlations with the PDI-21, RLSHS, and the O-LIFE Unusual Experiences (UE) subscale, suggesting good convergent validity. In particular, the small or nonsignificant correlations with the other subscales of the O-LIFE schizotypy scale demonstrates good discriminant validity, suggesting that the CAPS is largely selective in tapping perceptual anomalies, rather than measuring schizotypy in general.
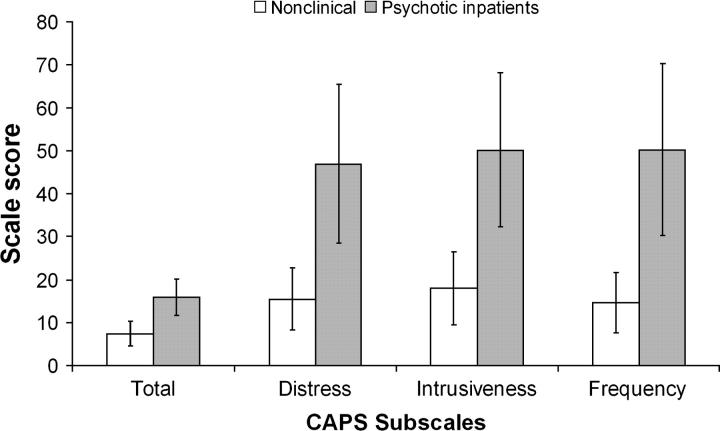
Criterion validity was assessed by comparing the CAPS score of the nonclinical population with the sample of psychotic patients. The mean CAPS scores for all groups are provided in Figure 2. The sample of psychotic inpatients had significantly higher mean scores than the nonclinical sample on CAPS total score and on all CAPS subscale scores, suggesting that the CAPS successfully measures anomalous perceptual experience in a group of patients known to experience high levels of perceptual distortion.
Fig. 2.
CAPS scores for nonclinical participants and psychotic inpatients. When compared using an independent samples 2-tailed t-test, psychotic inpatients differ significantly from the nonclinical sample on all subscales by at least p < .0005.
The CAPS is unique in terms of item selection—it tackles a comprehensive range of perceptual anomalies and does not assume that experiences present in a certain way (eg, as “strange” or “unusual”). In addition, it also includes dimensional scales to measure distress, intrusiveness, and frequency, none of which are present in existing comprehensive measures of anomalous perceptual experience. As such the CAPS does not replicate existing measures and, therefore, has good incremental validity.
Factor Structure
A principal components analysis (PCA) was conducted on data from the nonclinical sample to explore associations between items, without relying on any a priori hypotheses. Items on the CAPS that were endorsed by less than 10% of respondents were removed owing to lack of variance, leading to the removal of 3 items: item 19 (6.8%), item 13 (5.9%) and item 28 (1.2%). The remaining items on the CAPS were entered into the PCA using the oblique rotation (oblimin) procedure. An oblimin rotation was used since it assumes that the underlying factors are not necessarily independent from each other, which may often be the case in perceptual experience (for example, olfactory and gustatory experiences being strongly linked). The Kaiser-Meyer-Oklin value was .9, substantially exceeding the recommended value of .6, and Bartlett's Test of Sphericity was significant (p < .0005), supporting the suitability of PCA with this data set.41
Principal components analysis revealed the presence of 7 factors with eigenvalues exceeding 1, explaining a cumulative total of 50.26% of the variance. An inspection of the scree plot revealed a clear break after the third component, and a further PCA was run with 3 components retained for further investigation. Factor loadings from this analysis are outlined in Table 5. Using a cutoff of .4 for factors loadings, 3 components can be identified: the first (labeled “temporal lobe experience”) consisting of items 26, 4, 32, 10, 12, 24, 2, 1, 16, 27, and 6; the second (labeled “chemosensation”) consisting of items 30, 18, 29, 21, 14, 25, 20, and 8; and the third (labeled as “clinical psychosis”) consisting of items 7, 11, 3, and 31. The 3-factor solution explained a cumulative total of 33.07% of the variance. The component totals correlated moderately (temporal lobe / chemosensation, r = .54, p < .0005; temporal lobe / clinical psychosis, r = .46, p < .0005; chemosensation / clinical psychosis, r = .32; p < .0005), supporting the appropriateness of the direct oblimin rotation for the PCA and suggesting that the components partially overlapped but did not reflect identical sources of variance.
Table 5.
CAPS Items and Factor Loading After Principal Components Analysis (Oblimin Rotation) of Response from Nonclinical Sample
| Component |
|||
| Item | 1 | 2 | 3 |
| 26. Do you ever think that everyday things look abnormal to you? | .666 | ||
| 4. Do you ever see shapes, lights, or colors even though there is nothing really there? | .648 | ||
| 32. Do you ever hear sounds or music that people near you don't hear? | .566 | ||
| 10. Do you ever have the sensation that your limbs might not be your own or might not be properly connected to your body? | .546 | −.311 | |
| 12. Do you ever feel that someone is touching you, but when you look nobody is there? | .534 | ||
| 24. Do you ever have the feeling of being uplifted, as if driving or rolling over a road while sitting quietly ? | .502 | ||
| 2. Do you ever sense the presence of another being, despite being unable to see any evidence? | .453 | ||
| 1. Do you ever notice that sounds are much louder than they normally would be? | .441 | ||
| 16. Do you ever find that sounds are distorted in strange or unusual ways? | .441 | ||
| 27. Do you ever find that your experience of time changes dramatically? | .433 | ||
| 6. Do you ever hear noises or sounds when there is nothing about to explain them? | .400 | ||
| 23. Do you ever have days where lights or colors seem brighter or more intense than usual? | .389 | −.306 | |
| 9. Do you ever have the sensation that your body, or a part of it, is changing or has changed shape? | |||
| 30. Do you ever notice that food or drink seems to have an unusual taste? | −.722 | ||
| 18. Do you ever smell everyday odors and think that they are unusually strong ? | −.711 | ||
| 29. Do you ever notice smells or odors that people next to you seem unaware of? | −.641 | ||
| 21. Do you ever think that food or drink tastes much stronger than it normally would? | −.639 | ||
| 14. Do you ever experience unexplained tastes in your mouth? | −.614 | ||
| 25. Do you ever find that common smells sometimes seem unusually different? | −.493 | ||
| 20. Do you ever find that your skin is more sensitive to touch, heat, or cold than usual ? | −.486 | ||
| 8. Do you ever detect smells which don't seem to come from your surroundings? | −.458 | ||
| 15. Do you ever find that sensations happen all at once and flood you with information? | −.334 | ||
| 22. Do you ever look in the mirror and think that your face seems different from usual? | −.315 | ||
| 17. Do you ever have difficulty distinguishing one sensation from another? | −.308 | ||
| 7. Do you ever hear your own thoughts spoken aloud in your head, so that someone near might be able to hear them? | .674 | ||
| 11. Do you ever hear voices commenting on what you are thinking or doing? | .641 | ||
| 3. Do you ever hear your own thoughts repeated or echoed? | .607 | ||
| 31. Do you ever see things that other people cannot? | .384 | .443 | |
| 5. Do you ever experience unusual burning sensations or other strange feelings in or on your body? | .367 | ||
Note: Factor loadings below 0.3 are not shown.
The first component (temporal lobe experience) encompasses items that describe a number of different perceptual experiences. While many of these items could be associated with psychosis in general, the items present in this component seem a better match for perceptual anomalies reported in the context of temporal lobe disturbance rather than from frank psychosis. Gloor42 reviewed the temporal lobe epilepsy literature and outlined the phenomena associated with temporal lobe seizures as including visual illusions and hallucinations, auditory illusions and hallucinations of music or sounds (usually without clearly defined semantic content, such as coherent verbal utterances, as in the case of auditory hallucinations associated with psychosis), distortions in time perception, the relative lack of gustatory or olfactory experiences, and feelings of familiarity, recognition, or emotion.
The perceptual component of Gloor's description seems to be well represented by the CAPS items loading on this factor. Notably, this component consists of 11 items, whereas the items prechosen to reflect temporal lobe experiences not covered by the other items consist only of 4. The total score of the preselected category, however, correlates highly with the total scores of the temporal lobe component extracted from the PCA (r = .801, p < .0005). Even when the shared items have been removed from the extracted component, the correlation remains strong and significant (r = .552, p < .0005). Indeed, 2 items that load on this component (item 24, “Do you ever have the feeling that of being uplifted, as if driving or rolling over a road while sitting quietly?” and item 2, “Do you ever sense the presence of another being, despite being unable to see any evidence?”) are taken directly from experiences present in the Temporal Lobe Scale of Makarec and Persinger and are known to be present in populations that have temporal lobe disturbances.30,43 Such features are also known to be distributed throughout the general population in attenuated form.44 Similar experiences have been induced by the stimulation of the temporal lobes by magnetic fields,45,46 including anomalous proprioceptive experiences,47 which may account for the loading of item 10 (“Do you ever have the sensation that your limbs might not be your own or might not be properly connected to your body?”) on this component.
The second component consists largely of items related to olfactory and gustatory experiences and has been tentatively labeled “chemosensation.” The presence of item 20 (“Do you ever find that your skin is more sensitive to touch, heat, or cold than usual ?”) initially seems anomalous, although it is perhaps explained by the role of chemoreceptors in mediating perception of hot and cold and the dual role of the trigeminal nerve in conducting olfactory and cutaneous sensitivity (touch, warmth/cold, pain) information.48
Component 3 consists mainly of Schneiderian first-rank symptoms,18 commonly used as clinically unambiguous indicators of schizophrenia, plus an additional item (item 31, “Do you ever see things that other people cannot?”) concerning visual hallucinatory experiences; hence, this component has been tentatively labeled “clinical psychosis.” Notably, item 5 (“Do you ever experience unusual burning sensations or other strange feelings in or on your body?”) also loads on this factor and is also a first-rank symptom, although it has a factor loading of .367 so is below the .4 cutoff for inclusion in this component (albeit only marginally so).
Discussion
The aim behind developing the CAPS was to construct a scale that would be selective for perceptual anomalies, without being conceptually tied to the assumptions and language of previous clinical and psychometric scales. In particular, the object was to create a scale that taps a range of experiences within relevant sensory domains, without relying solely on judgments of “strangeness” or “unusualness” to establish the presence of perceptual anomalies. Dimensional ratings of distress, intrusiveness, and frequency were also included for each item. The results of this study suggest that the Cardiff Anomalous Perceptions Scale is a reliable, valid measure of the presence of perceptual anomalies.
The correlation between the CAPS and RLSHS demonstrates a relationship between the presence of perceptual anomalies and predisposition to hallucinations. In particular, the correlation between the CAPS total score and the Unusual Experiences subscale of the O-LIFE schizotypy scale, though showing weak or nonsignificant correlations with the other subscales, provides good evidence for the selectivity of the CAPS in measuring perceptual anomalies, indicating that the CAPS is not simply a measure of general psychosis proneness or schizotypy. The results also show a relationship between CAPS total scores and PDI-21 total scores, suggesting that the presence of perceptual anomalies and nonclinical delusional ideation may be linked.
Our results also show that there was remarkably little difference between ratings of distress, intrusiveness, and frequency on the CAPS for each sample, suggesting that for the populations sampled, these factors may be highly linked. Previous studies, however, have shown that metacognitive factors and affective reactions to anomalous experiences may be mediated by the framework and beliefs in which they are interpreted.22,49–52 Although it is not possible to distinguish which factors may be important from the data presented here, this is undoubtedly an important avenue for future research to determine, and perhaps therapeutically target, those aspects of anomalous experience that are particularly associated with distress, preoccupation, or other disabling aspects.
Indeed, in line with other studies that have examined the prevalence of psychosis-like experience in the general population,4,23,38 approximately 11% of the nonclinical sample scored above the mean of psychotic inpatients on CAPS total score. This provides further evidence that such experiences are not, in themselves, pathological and that there may be a significant section of the population who manage to integrate considerable perceptual distortion into their lives without necessarily becoming distressed or disabled. To fully understand the interaction between anomalous experience and impairment, and to develop successful therapeutic approaches for those who are being negatively affected, it will be important, however, to distinguish some of the factors that influence the presence of anomalous experience.
The results of the principal components analysis of CAPS responses from the nonclinical sample suggest that there may multiple factors that contribute to the overall level of perceptual distortion. Three main components were revealed and were interpreted as experiences associated with clinical psychosis, experiences associated with chemosensation (largely olfactory and gustatory experiences), and experiences associated with temporal lobe disturbance.
The existence of a coherent multifactor structure suggests that anomalous perceptual experience cannot simply be treated as a unitary dimension, as is often the case in theories of psychosis and psychosis proneness. In fact, the strong grouping of CAPS items that reflect the presence of Schneiderian first-rank symptoms suggests that experiences associated with psychotic mental illness (and particularly schizophrenia) may make a relatively independent contribution to the overall level of anomalous experience, particularly when compared with the “chemosensation' component,” with which there seems little overlap. This latter component largely reflects anomalous experience in the olfactory and gustatory modalities. These experiences have been reported as particularly common, appearing as the most prevalent hallucinatory experiences in a study of over 13,000 members of the general population,25 despite the fact that they are relatively uncommon in psychotic mental illness when compared with hallucinations in other modalities.
Perhaps more equivocal is the interpretation of the first component as being associated with temporal lobe disturbance. It is important to make the distinction here between perceptual anomalies associated with temporal lobe disturbances and clinical psychosis associated with temporal lobe epilepsy. Although seizures associated with temporal lobe epilepsy may produce a number of perceptual anomalies,42 this does not in itself constitute psychosis, although a minority of people with temporal lobe epilepsy may go on to develop psychosis. Indeed, reviews of the literature on psychosis in temporal lobe epilepsy show a mean prevalence of 15.7%, with a typical onset of 11–15 years after the onset of epilepsy.53,54 This suggests that although there may be some commonalities between temporal lobe seizures and psychosis, the core phenomenology is largely distinct and distinguishable.
The work of Makarec and Persinger has suggested that levels of transient temporal lobe disturbance (so-called microseizures) may be distributed throughout the population and contribute to a continuum of unusual experiences and beliefs, although not necessarily of the same character as those associated with psychosis.28,30,43,44 Additionally, our own work has suggested that temporal lobe disturbance induced by transcranial magnetic stimulation can reliably affect the perception of meaning in visual noise.55
Notably, however, the connection between such findings and work on the continuum model of psychosis has rarely been made, despite the obvious parallels. It is possible that perceptual distortions associated with psychosis and those associated with temporal lobe disturbance may share an overlapping neurological basis; for example, each is known to involve the tempero-limbic areas to varying degrees.53,56 This may account for the fact that a number of items in the temporal lobe component, although characteristic of temporal lobe disturbance, could also be present in psychosis. The question of whether this component is best characterized as “temporal lobe” experience, or to what extent verifiable temporal lobe disturbance contributes to the variance of this component, remains an empirical question.
The current study does have several limitations, however, most notably that the nonclinical sample was largely drawn from undergraduate students and may not be truly representative of the wider population. As mentioned earlier, the effect of the younger age of the nonclinical sample is unlikely to have confounded the results, as younger adults tend to show higher levels of anomalous experience than older adults and suggests that the reported effects might be stronger in an age-matched sample. Socioeconomic status was not recorded, although it is likely that the nonclinical sample were typically from a background of a higher mean socioeconomic status than the clinical sample, who were treated in services that deal with a wide range of clients from various areas of large city centers. Socioeconomic status is known to be inversely related to psychosis continuum experiences,35 and, therefore, it is not possible to rule out an effect of this on the results reported here, although it is unlikely that this effect would have invalidated the main findings.
In summary, the CAPS is a clinically useful and comprehensive measure of anomalous perceptual experience independent of psychiatric diagnosis. A principal components analysis of the CAPS data from the nonclinical sample shows 3 components that can be interpreted as “clinical psychosis,” “chemosensation,” and “temporal lobe disturbance.” This suggests that multiple mechanisms underlie anomalous perceptual experience and the “continuum of psychosis” is influenced by several sources of perceptual distortion.
Supplementary Material
The Cardiff Anomalous Perceptions Scale is available as supplementary material online at http://schizophreniabulletin.oxfordjournals.org/.
Acknowledgments
We would like to thank Emmanuelle Peters and Nicola Smedley for helpful comments and critique. We would also like to thank the participants and patients who kindly volunteered to take part in the study.
Funding to pay the Open Access publication charges for this article was provided by …
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Johns LC, van Os J. The continuity of psychotic experiences in the general population. Clin Psychol Rev. 2001;21:1125–1141. doi: 10.1016/s0272-7358(01)00103-9. [DOI] [PubMed] [Google Scholar]
- 3.van Os J. Is there a continuum of psychotic experiences in the general population? Epidemiol Psychiatr Soc. 2003;12:242–252. doi: 10.1017/s1121189x00003067. [DOI] [PubMed] [Google Scholar]
- 4.Peters ER, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophr Bull. 2005;30:1005–1016. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 5.Peters ER, Joseph SA, Garety PA. Measurement of delusional ideation in the normal population: introducing the PDI (Peters et al., Delusions Inventory) Schizophr Bull. 1999;25:553–576. doi: 10.1093/oxfordjournals.schbul.a033401. [DOI] [PubMed] [Google Scholar]
- 6.Verdoux H, van Os J. Psychotic symptoms in non-clinical populations and the continuum of psychosis. Schizophr Res. 2002;54:59–65. doi: 10.1016/s0920-9964(01)00352-8. [DOI] [PubMed] [Google Scholar]
- 7.Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin J, translator. New York: International Universities Press; 1911. [Google Scholar]
- 8.Laing RD. The Divided Self: An Existential Study in Sanity and Madness. London: Penguin Books; 1960. [Google Scholar]
- 9.Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 10.Rado S. Dynamics and classification of disordered behavior. Am J Psychiatry. 1953;110:406–416. doi: 10.1176/ajp.110.6.406. [DOI] [PubMed] [Google Scholar]
- 11.Strauss JS. Hallucinations and delusions as points on continua function: rating scale evidence. Arch Gen Psychiatry. 1969;21:581–586. doi: 10.1001/archpsyc.1969.01740230069010. [DOI] [PubMed] [Google Scholar]
- 12.Eysenck HJ. Schizothymia-cyclothymia as a dimension of personality. Exp J Pers. 1952;20:345–384. doi: 10.1111/j.1467-6494.1952.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 13.Eysenck HJ, Eysenck SBG. Psychoticism as a Dimension of Personality. London: Hodder and Stoughton; 1976. [Google Scholar]
- 14.Claridge G, McCreery C, Mason O, Bentall R, Boyle G, Slade P, Popplewell D. The factor structure of “schizotypal” traits: a large replication study. Br J Clin Psychol. 1996;35:103–15. doi: 10.1111/j.2044-8260.1996.tb01166.x. [DOI] [PubMed] [Google Scholar]
- 15.Bentall RP, Claridge GS, Slade PD. The multidimensional nature of schizotypal traits: a factor analytic study with normal subjects. Br J Clin Psychol. 1989;28:363–75. doi: 10.1111/j.2044-8260.1989.tb00840.x. [DOI] [PubMed] [Google Scholar]
- 16.Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Pers Individ Dif. 1995;18:7–13. [Google Scholar]
- 17.Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J Consult Clin Psychol. 1983;1:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- 18.Schneider K. In: Clinical Psychopathology. Hamilton MW, translator. New York: Grune and Stratton; 1959. [Google Scholar]
- 19.Wing JK, Cooper JE, Sartorius N. Measurement and Classification of Psychiatric Symptoms. Cambridge, UK: Cambridge University Press; 1974. [Google Scholar]
- 20.Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J Abnorm Psychol. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- 21.Morrison AP, Wells A, Nothard S. Cognitive factors in predisposition to auditory and visual hallucinations. Br J Clin Psychol. 2000;39:67–78. doi: 10.1348/014466500163112. [DOI] [PubMed] [Google Scholar]
- 22.Morrison AP, Wells A, Nothard S. Cognitive and emotional predictors of predisposition to hallucinations in non-patients. Br J Clin Psychol. 2002;41:259–270. doi: 10.1348/014466502760379127. [DOI] [PubMed] [Google Scholar]
- 23.Bunney WE, Hetrick WP, Bunney BG, et al. Structured Interview for Assessing Perceptual Anomalies (SIAPA) Schizophr Bull. 1999;25:577–592. doi: 10.1093/oxfordjournals.schbul.a033402. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA. The meaning of insight. In: Amador XF, David AS, editors. Insight and Psychosis. 1st ed. Oxford, UK: Oxford University Press; 1998. pp. 1–12. [Google Scholar]
- 25.Ohayon MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res. 2000;97:153–164. doi: 10.1016/s0165-1781(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 26.Maier M, Mellers J, Toone B, Trimble M, Ron MA. Schizophrenia, temporal lobe epilepsy and psychosis: an in vivo magnetic resonance spectroscopy and imaging study of the hippocampus / amygdala complex. Psychol Med. 2000;30:571–581. doi: 10.1017/s0033291799001993. [DOI] [PubMed] [Google Scholar]
- 27.Toone BK, Garralda ME, Ron MA. The psychoses of epilepsy and the functional psychoses: a clinical and phenomenological comparison. Br J Psychiatry. 1982;141:256–261. doi: 10.1192/bjp.141.3.256. [DOI] [PubMed] [Google Scholar]
- 28.Persinger MA, Makarec K. Temporal lobe epileptic signs and correlative behaviors displayed by normal populations. J Gen Psychol. 1987;114:179–195. doi: 10.1080/00221309.1987.9711068. [DOI] [PubMed] [Google Scholar]
- 29.Persinger MA, Healey F. Experimental facilitation of the sensed presence: possible intercalation between the hemispheres induced by complex magnetic fields. J Nerv Ment Dis. 2002;190:533–541. doi: 10.1097/00005053-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Makarec K, Persinger MA. Temporal lobe signs: electroencephalographic validity and enhanced scores in special populations. Percept Mot Skills. 1985;60:831–842. doi: 10.2466/pms.1985.60.3.831. [DOI] [PubMed] [Google Scholar]
- 31.Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1994. [Google Scholar]
- 32.Bebbington PE, Nayani T. The Psychosis Screening Questionnaire. Int J Methods Psychiatr Res. 1995;5:11–19. [Google Scholar]
- 33.Peters ER, Garety PA. The Peters et al. Delusions Inventory (PDI): new forms for the 21-item version. Schizophr Res. 1996;18:118–119. [Google Scholar]
- 34.Gonzalez-Pinto A, van Os J, Peralta V, et al. The role of age in the development of Schneiderian symptoms in patients with a first psychotic episode. Acta Psychiatr Scand. 2004;109(4):264–268. doi: 10.1046/j.1600-0447.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 35.van Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res. 2000;45(1–2):11–20. doi: 10.1016/s0920-9964(99)00224-8. [DOI] [PubMed] [Google Scholar]
- 36.Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Sex differences in psychosis: normal or pathological? Schizophr Res. 2003;62:45–49. doi: 10.1016/s0920-9964(03)00063-x. [DOI] [PubMed] [Google Scholar]
- 37.Hafner H, Riecher-Rossler A, Maurer K, Fatkenheuer B, Loffler W. First onset and early symptomatology of schizophrenia: a chapter of epidemiological and neurobiological research into age and sex differences. Eur Arch Psychiatry Clin Neurosci. 1992;242:109–118. doi: 10.1007/BF02191557. [DOI] [PubMed] [Google Scholar]
- 38.Bentall RP, Slade PD. Reality testing and auditory hallucinations: a signal detection analysis. Br J Clin Psychol. 1985;24:159–169. doi: 10.1111/j.2044-8260.1985.tb01331.x. [DOI] [PubMed] [Google Scholar]
- 39.Clark-Carter D. Doing Quantitative Psychological Research: From Design to Report. Hove: Psychology Press; 1997. [Google Scholar]
- 40.Hays WL. Statistics for the Social Sciences. London: Holt, Rinehart and Winston; 1973. [Google Scholar]
- 41.Kaiser H. An index of factorial simplicity. Psychometrika. 1974;39:31–36. [Google Scholar]
- 42.Gloor P. Experiential phenomena of temporal lobe epilepsy: facts and hypotheses. Brain. 1990;113:1673–1694. doi: 10.1093/brain/113.6.1673. [DOI] [PubMed] [Google Scholar]
- 43.Makarec K, Persinger MA. Electroencephalographic validation of a temporal lobe signs inventory in a normal population. J Res Pers. 1990;24:323–337. [Google Scholar]
- 44.Persinger MA, Makarec K. Temporal lobe epileptic signs and correlative behaviors displayed by normal populations. J Gen Psychol. 1987;114:179–195. doi: 10.1080/00221309.1987.9711068. [DOI] [PubMed] [Google Scholar]
- 45.Persinger MA, Healey F. Experimental facilitation of the sensed presence: possible intercalation between the hemispheres induced by complex magnetic fields. J Nerv Ment Dis. 2002;190:533–541. doi: 10.1097/00005053-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Cook CM, Persinger MA. Experimental induction of the “sensed presence” in normal subjects and an exceptional subject. Percept Mot Skills. 1997;85:683–693. doi: 10.2466/pms.1997.85.2.683. [DOI] [PubMed] [Google Scholar]
- 47.Hill DR, Persinger MA. Application of transcerebral, weak (1 microT) complex magnetic fields and mystical experiences: are they generated by field-induced dimethyltryptamine release from the pineal organ? Percept Mot Skills. 2003;97:1049–1050. doi: 10.2466/pms.2003.97.3f.1049. [DOI] [PubMed] [Google Scholar]
- 48.Cain WS. Olfaction. In: Atkinson RC, Herrnstein RJ, Lindzey G, Luce RD, editors. Steven's Handbook of Experimental Psychology. 2d ed. New York: John Wiley and Sons; 1988. [Google Scholar]
- 49.Bhugra D. Psychiatry and Religion. London: Routledge; 1996. [Google Scholar]
- 50.Davies MF, Griffin M, Vice S. Affective reactions to auditory hallucinations in psychotic, evangelical and control groups. Br J Clin Psychol. 2001;40:361–370. doi: 10.1348/014466501163850. [DOI] [PubMed] [Google Scholar]
- 51.Gauntlett-Gilbert J, Kuipers E. Visual hallucinations in psychiatric conditions: appraisals and their relationship to distress. Br J Clin Psychol. 2005;44:77–87. doi: 10.1348/014466504x19451. [DOI] [PubMed] [Google Scholar]
- 52.Chadwick P, Birchwood M. The omnipotence of voices: a cognitive approach to auditory hallucinations. Br J Psychiatry. 1994;164:190–201. doi: 10.1192/bjp.164.2.190. [DOI] [PubMed] [Google Scholar]
- 53.Trimble MR. The Psychoses of Epilepsy. New York: Raven Press; 1991. [Google Scholar]
- 54.Trimble MR, Ring HA, Schmitz B. Neuropsychiatric aspects of epilepsy. In: Fogel BS, Schiffer RB, Rao SM, editors. Neuropsychiatry. Baltimore: Lippincott, Williams and Wilkins; 1996. pp. 771–803. [Google Scholar]
- 55.Bell V, Reddy V, Halligan PW, Kirov G, Ellis HD. Relative suppression of magical thinking: a transcranial magnetic stimulation study. Cortex. In press doi: 10.1016/s0010-9452(08)70249-1. [DOI] [PubMed] [Google Scholar]
- 56.Bear DM. Temporal lobe epilepsy—a syndrome of sensory-limbic hyperconnection. Cortex. 1979;15:357–384. doi: 10.1016/s0010-9452(79)80064-7. [DOI] [PubMed] [Google Scholar]