Abstract
Research suggests that first-degree relatives and individuals with schizophrenia spectrum personality disorders (SSPD) may represent nonpenetrant carriers of the genetic diathesis for schizophrenia. This study examined visuospatial working memory (SWM) as a cognitive endophenotype of schizophrenia by expanding the concept of risk for pathophysiological dysfunction beyond overt psychosis. Risk was thus defined by familial status and the presence or absence of SSPD. SWM was assessed in the following groups, in order of decreasing likelihood of genetic vulnerability: 23 patients with schizophrenia, 17 SSPD relatives of patients with schizophrenia, 23 non-SSPD relatives of patients with schizophrenia, 14 SSPD community members with no family history of psychosis, and 36 non-SSPD community members. SWM performance during a computer task was quantified by A-Prime. Relative risk ratios for SWM deficits were compared among the groups. Compared with community non-SSPD volunteers, relative risk (RR) of SWM deficits was significantly elevated in patients with schizophrenia (RR = 3.76, p = .002) and SSPD family members (RR = 2.97, p = .027), but not in the family non-SSPD (RR = 1.88, p = .241) or community SSPD (RR = 1.03, p = .971) groups. The pattern of SWM performance deficits reflected the proposed model of latent genetic liability, upholding SWM as a viable cognitive endophenotype. The results underscore the importance of including both familial liability and the schizophrenia spectrum when considering risk for schizophrenia and schizophrenia-related traits. This is particularly relevant for research efforts to identify pathophysiological components of the disease.
Keywords: relative risk, genetics, schizotypy
Introduction
Schizophrenia is known to be a genetic illness, although the identification of disease susceptibility genes has proven difficult. Findings from family and genetics studies suggest a polygenetic mode of inheritance, whereby multiple genes, each of small effect, combine and contribute to the clinical expression of the disorder.1 Furthermore, the expression of each disease susceptibility gene may depend on specific environmental conditions. As a result of the multifactorial etiology of the illness, possession of a genetic liability for schizophrenia does not conform to the expression of a homogeneous phenotype. However, pathophysiological processes that are more proximal descriptors of simple gene effects may be identified and measured.2 The effort to identify endophenotypic markers of schizophrenia thus aims to elucidate discrete components of the genetic diathesis for the disease.
The characterization of neurobiological traits associated with vulnerability for schizophrenia has extended to the cognitive domain. Spatial working memory (SWM) impairments have been well documented in schizophrenia3 and have been pinpointed as a possible locus of dysfunction in the pathophysiology of the disease.4 Furthermore, Park et al.5 have demonstrated that patients with schizophrenia, as well as a significant number of their unaffected relatives, show impaired performance (i.e., reduced accuracy, numerous uncorrected errors) on a spatial delayed response task when compared with normal controls. These findings implicate a genetically mediated dysfunction in the ability to hold spatial representations “online.” Consistent with evidence linking SWM deficits and genetic risk for schizophrenia, Cannon et al.6 investigated SWM function among monozygotic (MZ) and dizygotic (DZ) twin pairs discordant for schizophrenia and reported a significant effect of genetic predisposition on performance on the spatial span task of the Wechsler Memory Scale Revised. Evidence thus suggests the presence of a measurable cognitive processing disturbance among carriers of the genetic vulnerability for schizophrenia, even in the absence of overt symptomatology or schizophrenia diagnosis. Glahn et al.7 replicated the results of the Cannon et al.6 twin study, reporting poorer SWM performance on a spatial delayed response task with increasing genetic liability for schizophrenia. These results support and extend the findings of Park et al.5 Taken together, these studies suggest that the ability to maintain spatial representations online decreases in a dose-dependent fashion with increasing genetic predisposition toward schizophrenia.
Previous research implicating SWM dysfunction as a cognitive endophenotype has primarily focused on familial transmission of risk. Due to the variability in the genetic liability for schizophrenia, individuals with schizophrenia spectrum personality disorders (SSPD) may be nonpenetrant carriers of the genetic diathesis,8 and SSPD may therefore represent an intermediate clinical phenotype of schizophrenia. Family studies of schizophrenic probands strongly suggest a degree of genetic overlap between risk for SSPD and schizophrenia, indicating elevated prevalence of schizophrenia-related personality disorders in first-degree relatives of schizophrenic patients.9–13 Studies showing increased risk of schizophrenia in families of SSPD probands further support the idea that the 2 disorders share a genetic, or at least a familial, etiology.14 Accordingly, similarities in neurocognitive deficits, including impairments in attention,15–18 information processing,19 and eye tracking performance,20–22 observed among patients with schizophrenia and individuals with SSPD, may be consistent with a shared genetic etiology.
Prior studies of SSPD have also demonstrated cognitive impairment specific to working memory. Psychometric high-risk paradigms provide a method to assess the relationship between schizotypy and working memory deficits. Among normal students selected for schizotypy, high scores on the Perceptual Aberration Scale have been associated with selective impairment on a spatial memory delayed response task,23 while normal performance on measures of verbal recall memory and auditory working memory have been observed.24 In addition, spatial working memory impairment has been demonstrated in studies utilizing social anhedonia to identify psychometric high-risk groups.25,26 Visual memory impairment has also been demonstrated among those with clinically diagnosed schizotypal personality disorder relative to healthy controls.27 Moreover, diagnostic specificity of visuospatial working memory impairment has been confirmed in some studies of schizotypal personality disorder relative to non-schizophrenia-related personality disordered psychiatric groups.28,29 However, visuospatial working memory deficits, relative to healthy normal and other personality disordered control groups, has not been consistently replicated.30 In contrast to the findings of psychometric high-risk paradigms,23,24 the findings of Mitropoulou and colleagues29 suggest that auditory working memory may better account for group differences than other specific working memory functions. These findings can also be interpreted more broadly with respect to those of Barch and colleagues,31 in that schizotypal personality disorder–related working memory impairment may be considered an impairment in the ability to maintain context online. Thus, in both psychometrically and clinically identified populations, working memory deficits generally appear to be associated with the schizophrenia spectrum.
However, the schizophrenia-SSPD etiologic link is not specific. In the absence of a clear family history of schizophrenia, SSPD traits are thought to be heterogeneous in their origins.8,32–34 For example, relatives of probands with several other major psychiatric illnesses, including Fragile X syndrome, show higher rates of SSPD than are observed in the general population. With respect to research design, psychometrically defined groups likely represent an admixture of true schizotypes and individuals reporting experiences that may be associated with other psychopathologic or organic conditions. Likewise, although the genetic link between clinical cases of schizotypal personality disorder and schizophrenia is assumed, studies are inconsistent in reporting on family history of schizophrenia spectrum–related psychopathology within clinical groups. Therefore, the interpretation of cognitive findings in schizophrenia spectrum cases is unclear. Cognitive impairment might reflect either genetic effects relevant for schizophrenia or different genetic or environmental factors that yield overlapping clinical phenotypes.
Thus, in investigating endophenotypic markers of genetic liability for schizophrenia, individuals with SSPD without a family history of schizophrenia may provide an important comparison group. Individuals with SSPD without a clear family history present with similar phenotypes (ie, schizophrenia-like symptoms), although the etiologic link to schizophrenia is not necessarily specific and may be traced to dissimilar genetic or other etiological factors. Therefore, the study of individuals with SSPD in the presence or absence of a family history of schizophrenia provides a unique opportunity to identify mechanisms of pathophysiological dysfunction, particularly in distinguishing those that are associated with genetic liability.
Given prior evidence suggesting a relationship between SWM impairment and genetic predisposition toward schizophrenia, this study proposed to investigate SWM as a cognitive endophenotype more systematically by expanding the definition of risk for schizophrenia. Participants in this study included, in order of decreasing likelihood of genetic carrier status, (1) patients with schizophrenia, (2) first-degree relatives of schizophrenia probands with SSPD traits, (3) first-degree relatives of schizophrenia probands without SSPD traits, (4) individuals with SSPD traits in the absence of family history of psychotic illness, and (5) individuals without SSPD traits in the absence of family history of psychotic illness. We hypothesized that performance on a spatial working memory task would decrease as the probability of genetic liability for schizophrenia increased.
METHOD
Participants
Patients with schizophrenia (N = 23) were recruited from inpatient and outpatient programs at the Maryland Psychiatric Research Center. Diagnoses of MPRC patients were confirmed using the Structured Clinical Interview for DSM-IV (SCID-IV),35 clinical history, and patient and family interviews to support a best estimate diagnosis by a trained clinician. First-degree relatives of patients (family SSPD, N = 17; family normal, N = 23) were family members of individuals with schizophrenia who receive their care at the MPRC. Patients with schizophrenia and their first-degree relatives were not, however, recruited for this study as family units. Although 3 patients with schizophrenia in this sample each had 2 nonpatient family members who also participated, participants in this study were not otherwise related to each other. Relatives were recruited through mail and phone solicitations. Community volunteers from the Baltimore/Washington, DC area were recruited through newspaper advertisements (community normal, N = 36). In order to recruit community volunteers with SSPD symptoms (N = 14), 1 set of advertisements sought individuals who had experienced magical thinking or perceptual distortions (eg, ESP, telepathy, out of body experiences), as well as social isolation or lack of social drive (these methods are described in more detail elsewhere36). All participants gave written informed consent, and data collection procedures were approved by the University of Maryland–Baltimore institutional review board.
Clinical Assessments
The Structured Clinical Interview for DSM-IV (SCID-IV)35 was used to obtain Axis I diagnoses. Community and family participants with a current or lifetime Axis I diagnosis (except those with a single past episode of depression or those with a history of substance abuse ending at least 6 months prior to the study) were excluded. Among the community volunteers, family history of psychotic illness was determined by a modified version of Family History Research Diagnostic Criteria (FH-RDC).37,38 The Structured Interview for DSM-IV Personality Disorder (SIDP)39 was used to examine SSPD symptoms. Questions concerning magical thinking and perceptual distortions from the Structured Interview for Schizotypy40 were added to the SIDP. Negative symptoms were further probed using the Schedule for Deficit Syndrome (SDS).41 The threshold to determine the presence of a particular symptom was lower than is generally used in clinical practice, such that a symptom “definitely present in the absence of functional impairment or distress” was rated as present. Subjects were assigned to SSPD groups according to modified DSM-IV criteria (ie, 3 or more paranoid traits, 3 or more schizoid traits, or 4 or more schizotypal traits). Thresholds for SSPD were lowered to match the design of previous studies.21,42,43 Individuals without SSPD symptoms but with other personality disorders were excluded.
On the basis of these assessments, participants were grouped according to family status (family versus community) and the presence or absence of SSPD. Thus, the community control group (N = 36) was determined on the basis of the lack of family history of psychotic disorder and absence of SSPD. The community spectrum group (N = 14) was defined by the absence of family history and presence of SSPD. The family spectrum group (N = 17) and family normal group (N = 23) were selected on the basis of their first-degree relative status and the presence or absence of SSPD, respectively. Family participants, on average, tended to be older than the community participants. The patient group had received fewer years of education than participants in the other groups. Groups did not differ on race or gender.
Task Stimuli
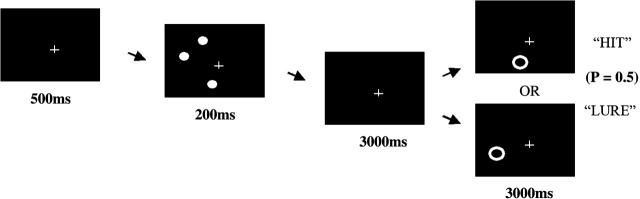
Spatial working memory was assessed using a computerized task based on the work by Jonides et al.44 Three target dots appeared on the circumference of an imaginary circle 14 degrees in diameter centered on the cross. Target dots were spread along the 360 degrees of the circle in varying degrees clockwise (Figure 1). There were 12 possible target dot location “spread” types, which describe the number of degrees separating each of the 3 target dots from each other. Spread types consisted of combinations of 15, 50, 100, and 150 degrees of separation between target dots. For each trial, the ring cue was centered directly over the location of a previous dot (hit) or was located 15 (near), 20 (middle), or 25 (far) degrees away from the location of a previous dot (lure). There were 40 total possible trial types determined by combinations of spread type and hit versus lure conditions. Each trial type was presented once per block for a total of 40 trials per block.
Fig. 1.
Spatial Working Memory Task.
Task Procedure
Subjects were seated comfortably in front of a computer monitor. The task began with the subject fixating on a cross in the center of a black screen for 500 ms. Three white dots were presented for 200 ms, followed by a 3000 ms retention interval during which only the cross was seen. The subject was then probed for spatial location memory by the appearance of a single white ring that either encircled the location of 1 of the previous dots (with a probability of 0.5) or did not. The probe ring was presented for 3000 ms, during which time the subject pressed 1 keyboard button (enter) to answer “yes” that a dot had been present in that location (hit) or pressed another keyboard button (spacebar) to answer “no” that a dot had not been present in that location (lure). Subjects completed a total of 3 blocks with 40 trials per block. The 3 blocks differed in the order of trial presentation, but block and trial order were identical across subjects.
Data Collection
Reaction time was recorded if a response was made during the 3000 ms period in which the probe ring was presented. Correct hits, correct rejections, and nonresponses were recorded for each trial. Because the available response window of 3000 ms extended beyond the range where concerns over slowing of reaction time could impact performance, nonresponses were coded as errors; preliminary analyses revealed that the number of nonresponses was highly correlated with overall accuracy. A correct hit (H) was defined by a “yes” response to a ring cue that encircled the location of a previous dot. A correct rejection was defined by a “no” response to a ring cue that did not encircle the location of a previous dot. False alarms (F) were calculated as a “yes” response to a ring cue that was located at a distance (ie, near, middle, far) from the location of a target dot. The rates of correct hits and false alarms were used to calculate a measure of discriminability, A-prime (A′), that is widely used in signal detection experiments. A′ scores were calculated using the formulae: A′ = 0.5 + (H − F)(1 + H − F) / 4H(1 − F), when H ≥ F, and A′ = 0.5 − (F − H)(1 + F − H) / 4F(1 − H), when F > H (Equation 2).45 Extreme values of H or F were adjusted by replacing zero values with 0.5/n and values of 1.0 with (n − 0.5)/n, where n is the number of trials. A-prime values were obtained for each of the “near,” “middle,” and “far” lure types.
Statistical Analyses
Preliminary analyses determined the effect of ring cue location, as a measure of level of difficulty, across lure trials. Repeated measures analysis of variance (ANOVA) showed no group × location interaction (F = 0.660). Thus the near, middle, and far ring cue location conditions were collapsed, and a mean A-prime value was calculated for each participant. Sociodemographic variables were compared using ANOVA and chi-square tests. Additionally, ANOVA and post-hoc Scheffé tests were used to compare the mean number of clinical traits (paranoid, schizoid, schizotypal) between groups. Primary analyses included group comparisons of A-prime using univariate ANOVA and preplanned contrasts, followed by calculation of effect sizes for A-prime. Calculation of relative risk ratios, which is typically utilized to quantify familial similarity,46 was employed to estimate genetic liability to deficits in spatial working memory. Relative risk ratios for each group were defined by the percentage of participants “affected” by spatial working memory deficits relative to the percentage of affected community controls. An A-prime value of at least 1 standard deviation below the mean of the community normal group defined the cutoff for “affected” status.
Results
Comparison of sociodemographic variables (Table 1) demonstrated a significant difference between groups for age (F = 8.405, p < .001) and years of education (F = 4.956, p = .001) but nonsignificant differences between groups on sex (χ2 = 8.04, p = .09) and race (χ2 = 4.482, p = .811). Due to significant differences in age and education, their influence on task performance was further examined. When age and years of education were added as covariates, no significant effects emerged for age (F = 0.513, p = .476), age × group interaction (F = 2.02, p = .097), years of education (F = 1.685, p = .197), or education × group interaction (F = 0.829, p = .510); adding these variables had little effect on estimates or p-values for the main effect of group. In light of the relatively older age of the family participants, we further tested the association between age and performance. Correlations between age and A-prime were not statistically significant in either the family spectrum group (r = −0.30, p = .24) or family normal group (r = 0.32, p = .88), nor was there a significant correlation in the community spectrum group (r = −0.01, p = .67). The association was only significant in the community normal group (r = −0.43, p = .009). Although the family participants appeared to be older than the other groups, age did not appear to systematically or significantly influence performance in either family group. Subsequent analyses were performed without consideration to the effects of age or years of education on group performance.
Table 1.
Sociodemographic Description of the Study Groups
| Group (N) | Mean Age (SD)a | Mean Years Education (SD)b | Male:Femalec | Caucasian:African American:Otherd |
| Community Normal (36) | 36.67 (13.56) | 14.86 (2.12) | 18:18 | 26:7:3 |
| Schizophrenia Patients (23) | 32.26 (11.25) | 12.00 (2.47) | 16:7 | 14:6:3 |
| Family Spectrum (17) | 45.59 (10.96) | 14.71 (3.69) | 9:8 | 13:3:1 |
| Family Normal (23) | 50.26 (11.53) | 13.82 (2.87) | 7:16 | 17:5:1 |
| Community Spectrum (14) | 38.29 (9.81) | 14.86 (2.07) | 9:5 | 9:2:3 |
ANOVA revealed a significant difference in the ages of the groups (F = 8.405, p < .001). There was no group × age interaction on task performance (F = 2.02, p = .097), and age was not significant as a covariate (F = 0.513, p = .476).
ANOVA revealed a significant difference in years of education (F = 4.956, p = .001). There was no group × years of education interaction on task performance (F = 0.829, p = .510), and years of education was not significant as a covariate (F = 1.685, p = .197).
Chi-square test revealed no significant differences in the proportion of males and females across groups (χ2 = 8.04, p = .09).
Chi-square test revealed no significant differences in the proportion of Caucasian, African American, or “other” participants (χ2 = 4.482, p = .811).
Mean number of paranoid, schizoid, and schizotypal traits expressed (Table 2) differed among the 4 groups (overall ANOVA F = 10.36, p < .001; F = 31.51, p < .001; F = 49.85, p < .001, respectively). Post-hoc Scheffé tests indicated that family and community spectrum groups did not significantly differ on the mean number of clinical traits; neither did family and community normal groups. However, community and family normal groups exhibited significantly fewer symptom traits than both spectrum groups (all comparisons p < .05). The sum total of SSPD traits expressed was examined in a correlational analysis with respect to A-prime for each group. No significant relationships were found between the number of traits of expressed and A-prime for any group; correlations ranged in magnitude from 0.122 to 0.174.
Table 2.
Clinical Ratings: Number of Schizophrenia Spectrum Symptom Traits Expressed
| Group (N) | Paranoid Symptoms Mean (SD) | Schizoid Symptoms Mean (SD) | Schizotypal Symptoms Mean (SD) |
| Community Normal (36) | 0.09 (0.29) | 0.24 (0.50) | 0.24 (0.50) |
| Family Normal (23) | 0.10 (0.45) | 0.40 (0.68) | 0.35 (0.59) |
| Community Spectrum (14) | 1.69 (1.80) | 3.08 (2.1) | 3.23 (1.54) |
| Family Spectrum (17) | 1.38 (1.86) | 3.00 (1.75) | 3.06 (1.57) |
A-prime on the SWM task was significantly different in univariate analysis (F = 3.362, p = .012) for community normals, family normals, community spectrum and family spectrum groups, and patients with schizophrenia. Patients with schizophrenia performed more poorly on the spatial working memory task compared with community controls (p = .001). Mean A-prime values are presented in Table 3.
Table 3.
Comparison of A-Prime Values on a Spatial Working Memory Task with Community Normal Controls
| A-Prime Deviation from Community Normal Mean |
||||||||||||
| Above 1 SD |
Within 1 SD |
Below 1–2 SD |
Below 2 SD |
|||||||||
| Group (N) | Mean (SD) | N | % | N | % | N | % | N | % | RR | χ2 (1 df) | P-Value |
| Community Normal (36) | 0.82 (0.06) | 6 | 17 | 25 | 69 | 3 | 8 | 2 | 6 | 1.00 | ||
| Family Normal (23) | 0.81 (0.09) | 4 | 17 | 13 | 57 | 3 | 13 | 3 | 13 | 1.88 | 1.38 | 0.24 |
| Community Spectrum (14) | 0.78 (0.14) | 1 | 7 | 8 | 79 | 0 | 0 | 2 | 14 | 1.03 | > 0.01 | 0.97 |
| Family Spectrum (17) | 0.76 (0.14) | 2 | 12 | 8 | 47 | 4 | 24 | 3 | 18 | 2.97 | 4.91 | 0.027 |
| Schizophrenia Patients (23) | 0.72 (0.13) | 1 | 4 | 10 | 43 | 4 | 17 | 8 | 35 | 3.76 | 10.03 | 0.002 |
Note: RR = relative risk (compared to community normal) of impaired SWM score (> 1 SD below normal mean). In pairwise comparisons of the percentage with impaired SWM scores among other groups, community spectrum participants had significantly fewer than schizophrenia patients (χ2 = 5.31, p = .021), and family normal participants had marginally fewer than patients with schizophrenia (χ2 = 3.29, p = .070). Comparisons of percentage with impaired SWM between family spectrum versus community spectrum (p = .10), family normal versus community spectrum participants (p = .40), and family normal versus family spectrum participants (p = .31) were not statistically significant.
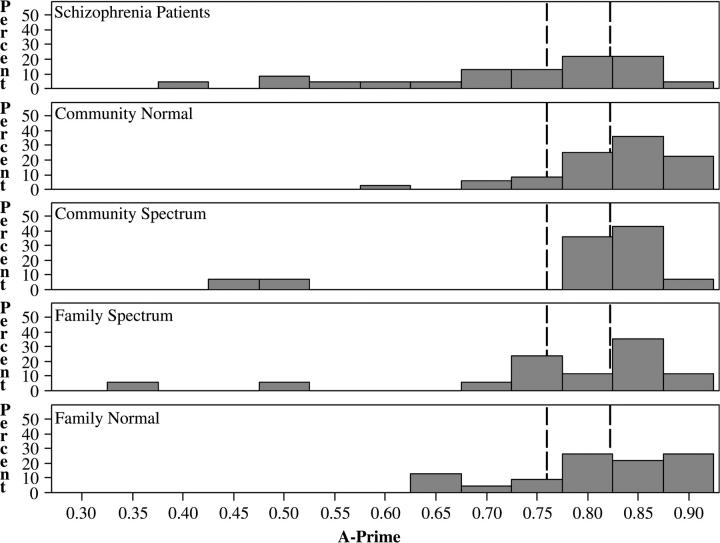
Mean performance of the family normal and community normal groups was virtually identical (A′ = 0.81 versus 0.82, respectively). Family and community SSPD groups were intermediate between the normal groups and schizophrenia patients; their differences from community normals were not statistically significant. Cohen's d was used as a measure of effect size for group differences compared to community normals; effect size estimates were large for patients (0.94), medium for the family SSPD group (0.52) and community SSPD group (0.37), and very small for the family normal group (0.13). Although the community spectrum group performed, on average, more poorly than the family normal group, further examination of the distributions in A-prime values demonstrates that few community spectrum participants actually performed below the mean of the community normal group (Figure 2). In fact, means for both the community and family SSPD groups were heavily influenced by a few outliers with very low A-prime values.
Fig. 2.
Distribution of A-Prime by Group. Dotted lines represent (from right to left) community normal mean, 1 SD below the community normal mean (cutoff for affected status).
The distribution of A-prime in all groups was non-normal, with varying degrees of skew toward small values (Figure 2). Thus, simple comparison of means does not adequately capture the variation in the extent to which groups demonstrated impaired (> 1 SD below the community normal mean) or profoundly impaired (> 2 SD below the community normal mean) performance on the SWM task. Accordingly, comparisons between groups focused on the percentages of participants with impaired SWM performance, as reflected in the relative risk analysis.
The proportion of participants in each group demonstrating impaired performance on the SWM task and the corresponding relative risk ratios are presented in Table 3. Relative risk ratios were calculated based on A-prime values of at least 1 SD below the community normal mean. Magnitude of relative risk for SWM deficits reflected the proposed model of varying genetic liability. In a chi-square analysis, patients with schizophrenia demonstrated moderate to high risk for SWM deficits (3.76; χ2 = 10.03, p = .002), while family SSPD participants demonstrated moderate risk (2.97; χ2 = 4.91, p = .027). Family members without SSPD exhibited higher relative risk (1.88) than the community members diagnosed with SSPD (1.03), although these ratios were not significantly different from the healthy community group (χ2 = 1.38, p = .24 and χ2 = 0.001, p = .97, respectively). Comparisons of percentage with impaired SWM performance between family spectrum, family normal, and community spectrum groups were not statistically significant (all comparisons, p > .05).
Discussion
This study examined spatial working memory impairment as a function of increasing probability of genetic liability for schizophrenia. Genetic liability was defined primarily by familial transmission of risk and secondarily by the presence of schizophrenia spectrum personality symptoms. As expected, the greatest spatial working memory deficits were observed in patients with schizophrenia. The schizophrenia and family spectrum groups both demonstrated large to medium effect sizes for impaired task performance, as well as significantly elevated relative risk ratios for group membership based on poorer spatial working memory performance, compared with community controls. In a study of similar design, Avila and colleagues47 corroborated these findings, demonstrating cognitive deficits in attention and working memory among relatives of schizophrenia patients who exhibited SSPD symptoms. Similarly, other research has shown that SSPD symptomatology is related to deficits in neurocognitive functioning only in the presence of a family history. Johnson et al.48 demonstrated that performance on measures tapping verbal and visual memory, sustained and divided attention, and executive functioning was influenced by symptoms of schizotypy in the co-twins of schizophrenic patients, but that schizotypy symptoms in the absence of a family history were unrelated to neurocognitive impairment. Taken altogether, these results support the assumption that first-degree family members with schizophrenia spectrum traits, as a group, represent the highest genetic loading for disease-related traits (without an Axis I diagnosis); the present study suggests that cognitive impairments, such as in spatial working memory, are among those traits.
We expected that family members, even in the absence of overt symptomatology, would demonstrate poorer cognitive performance due to the heritability of schizophrenia-related cognitive impairments. However, neither this study nor Avila et al.47 found statistically significant impairments among relatives without schizophrenia spectrum traits. It is important to note that the threshold utilized for SSPD diagnoses in this and the study by Avila and colleagues is much lower than required by DSM-IV. Thus, the family normal group likely contains relatives with significantly fewer schizophrenia spectrum traits than those relatives found in other family samples, as even those with mild SSPD symptoms were categorized in the family SSPD group. Despite these nonsignificant results, the relative risk ratios for the family spectrum, family normal, and community spectrum groups were ordered according to our prediction, based on their relative probabilities of carrying a genetic liability. In this study 26% of the family normal group demonstrated impaired performance, compared with 14% of the community spectrum group and 14% of the community normal group. Furthermore, our relative risk ratio of 1.88 for normal family members is similar to the relative risk of 2.10 for deficits in attention reported by Egan et al.,46 using the same 1 SD criterion. Yet, in their analysis, Egan and colleagues utilized a selective group of family members, including only those relatives whose affected siblings were impaired on a distraction version of a Continuous Performance Test. Similarly, Egan et al.49 reported increased risk for a full sample of siblings on Wisconsin Card Sorting Test (WCST) perseverative errors (2.17), categories (2.2), and California Verbal Learning Test (2.5), with the greatest relative risk demonstrated for Trails B (4.0). Thus, despite the selective sample of family members whose data are analyzed here, the risk of impairment in SWM demonstrated among healthy relatives appears to be on par with risk for impairment on other cognitive measures (ie, attention, executive function, verbal memory) that are indicated as viable markers of risk for schizophrenia.50
Although the presence of schizophrenia spectrum symptoms in the absence of a family history appeared to be associated with poorer performance, the relative risk ratios were not significantly elevated compared to the control group. As indicated, only 2 participants out of 14 exhibited deviant performance relative to the community controls. Thus, many individuals expressing schizophrenia spectrum symptomatology in the absence of a family history of psychotic illness are unlikely to exhibit associated neurocognitive deficits; these cases share only the symptomatic aspects of the phenotype. On the other hand, that some community spectrum participants do exhibit specific neurocognitive dysfunction suggests that those individuals may indeed represent those with shared genetic etiology. A significant limitation to the interpretation of these results, however, is that performance may have been a function of the characteristics of the community participants recruited. Individuals with spectrum symptoms were recruited from the community in response to local advertisements, thus introducing the potential for selection bias toward a less functionally impaired group; furthermore, individuals were excluded if they presented with any Axis I or other Axis II diagnosis. In addition, the rate of false-positive schizophrenia spectrum cases among the community members may have been increased by the reduced thresholds for symptom ratings and for diagnostic criteria. Because the mean number of schizophrenia spectrum traits expressed by the community spectrum and family spectrum groups were, however, equivalent while relative differences in spatial working memory performance were observed, the performance of the community spectrum group is more likely associated with the absence of family history than with differences in symptomatology. Nonetheless, sample selection may have contributed to the discrepancy in the level of cognitive impairment found in this versus other community schizophrenia spectrum samples.
Small sample sizes in the current study may have limited the generalizability of the results. That relative risk ratios were broadly consistent with those found in the literature enhances our confidence that our results represent a pattern of impairment in spatial working memory that is significant beyond chance. However, the data indicate that a greater degree of variability exists among our individuals with spectrum characteristics relative to the healthy community controls and nonsymptomatic family members. This suggests that, among those exhibiting spectrum traits, the presence of specific neurocognitive impairment may serve as an additional defining characteristic, differentiating those with greater likelihood of carrying a genetic diathesis for schizophrenia. In this study small sample sizes limited the extent to which patterns of impairment could be investigated within groups. In particular, specific associations between familial relationship (ie, parent, sibling, offspring) or schizophrenia spectrum personality disorder (eg, paranoid, schizoid, schizotypal personality disorder) and neurocognitive function should be further investigated. Thus, due to methodological constraints, the results of the present study are not definitive in determining differential neurocognitive impairment associated with schizophrenia spectrum traits in the presence versus absence of family history. However, the utility of such a distinction is suggested by the data presented herein.
Conclusion
The results of this study indicate a continuum of risk for a schizophrenia-related deficit of spatial working memory, whereby a higher genetic loading for disease-related traits imparts greater cognitive impairment. Our findings support the notion that schizophrenia and schizophrenia-related traits are heterogeneous in their etiology, thus underscoring the importance of expanding the definition of risk to include both familial liability and the schizophrenia spectrum. This research suggests that the power of future family studies will be bolstered by considering the role of symptoms in potentiating physiological impairment, just as studies examining characteristics of SSPD will be enriched by considering assessments of family history. This is a particularly salient consideration for research efforts to identify endophenotypic markers, which serve to inform pathophysiological components of and the genetic diathesis for the disease. We encourage additional research on the relationship between schizophrenia-related personality symptoms and cognitive and neurophysiological functioning. In particular, these findings support future research to consider spatial working memory as a cognitive endophenotypic marker of genetic liability for schizophrenia.
Acknowledgments
This work is supported by National Institutes of Health grants MH49826 and MH40279.
References
- 1.Kendler KS, Diehl SR. Schizophrenia: genetics. In: Kaplan H, Saddock BJ, editors. Comprehensive Textbook of Psychiatry. Baltimore, Md: Williams and Wilkins; 1995. pp. 942–957. Vol 6. [Google Scholar]
- 2.Gottesman, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 3.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 4.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67(2):369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glahn DC, Therman S, Manninen M, et al. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53(7):624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- 8.Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19:261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 9.Baron M, Gruen R, Asnis L, Kane J. Familial relatedness of schizophrenia and schizotypal states. Am J Psychiatry. 1983;140:1437–1442. doi: 10.1176/ajp.140.11.1437. [DOI] [PubMed] [Google Scholar]
- 10.Baron M, Gruen R, Rainer JD, Kane J, Asnis L, Lord S. A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. Am J Psychiatry. 1985;142:447–455. doi: 10.1176/ajp.142.4.447. [DOI] [PubMed] [Google Scholar]
- 11.Kendler KS, Masterson CC, Ungaro R, Davis KL. A family history study of schizophrenia-related personality disorders. Am J Psychiatry. 1984;141:424–427. doi: 10.1176/ajp.141.3.424. [DOI] [PubMed] [Google Scholar]
- 12.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study: III. schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- 13.Kendler KS, Neale MC, Walsh D. Evaluating the spectrum concept of schizophrenia in the Roscommon Family Study. Am J Psychiatry. 1995;152:749–754. doi: 10.1176/ajp.152.5.749. [DOI] [PubMed] [Google Scholar]
- 14.Thaker GK, Adami H, Moran M, Lahti AC, Cassady SL. Psychiatric illnesses in families of subjects with schizophrenia-spectrum personality disorders: high morbidity risks for unspecified functional psychoses and schizophrenia. Am J Psychiatry. 1993;150:66–71. doi: 10.1176/ajp.150.1.66. [DOI] [PubMed] [Google Scholar]
- 15.Roitman SE, Cornblatt BA, Bergman A, et al. Attentional functioning in schizotypal personality disorder. Am J Psychiatry. 1997;154:655–660. doi: 10.1176/ajp.154.5.655. [DOI] [PubMed] [Google Scholar]
- 16.Chen WJ, Liu SK, Chang CJ, Lien YJ, Chang YH, Hwu HG. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry. 1998;155:1214–1220. doi: 10.1176/ajp.155.9.1214. [DOI] [PubMed] [Google Scholar]
- 17.Lenzenweger MF, Cornblatt BA, Putnick M. Schizotypy and sustained attention. J Abnorm Psychol. 1991;100:84–89. doi: 10.1037//0021-843x.100.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Harvey PD, Keefe RSE, Mitropoulou V, et al. Attentional markers of vulnerability to schizophrenia: performance of patients with schizotypal and nonschizotypal personality disorders. Psychiatry Res. 1996;60:49–56. [Google Scholar]
- 19.Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Clementz BA, Reid SA, McDowell JE, Cadenhead KS. Abnormality of smooth pursuit eye movement initiation: specificity to the schizophrenia spectrum? Psychophysiology. 1995;32(2):130–134. doi: 10.1111/j.1469-8986.1995.tb03304.x. [DOI] [PubMed] [Google Scholar]
- 21.Thaker GK, Cassady S, Adami H, Moran M, Ross DE. Eye movements in spectrum personality disorders: comparison of community subjects and relatives of schizophrenic patients. Am J Psychiatry. 1996;153:362–368. doi: 10.1176/ajp.153.3.362. [DOI] [PubMed] [Google Scholar]
- 22.Siever LJ, Friedman L, Moskowitz J, et al. Eye movement impairment and schizotypal psychopathology. Am J Psychiatry. 1994;151:1209–1215. doi: 10.1176/ajp.151.8.1209. [DOI] [PubMed] [Google Scholar]
- 23.Park S, Holzman PS, Lenzenweger MF. Individual differences in spatial working memory in relation to schizotypy. J Abnorm Psychol. 1995;104(2):355–363. doi: 10.1037//0021-843x.104.2.355. [DOI] [PubMed] [Google Scholar]
- 24.Lenzenweger MF, Gold JM. Auditory working memory and verbal recall memory in schizotypy. Schizophr Res. 2000;42:101–110. doi: 10.1016/s0920-9964(99)00121-8. [DOI] [PubMed] [Google Scholar]
- 25.Gooding DC, Tallent KA. Spatial, object, and affective working memory in social anhedonia: an exploratory study. Schizophr Res. 2003;63:247–260. doi: 10.1016/s0920-9964(02)00326-2. [DOI] [PubMed] [Google Scholar]
- 26.Tallent KA, Gooding DC. Working memory and Wisconsin Card Sorting Test performance in schizotypic individuals: a replication and extension. Psychiatry Res. 1999;89(3):161–170. doi: 10.1016/s0165-1781(99)00101-8. [DOI] [PubMed] [Google Scholar]
- 27.Farmer CM, O'Donnell MF, Niznikiewicz MA, Voglmaier MM, McCarley RW, Shenton ME. Visual perception and working memory in schizotypal personality disorder. Am J Psychiatry. 2000;157:781–786. doi: 10.1176/appi.ajp.157.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lees Roitman SE, Mitropoulou V, Keefe RSE, et al. Visuospatial working memory in schizotypal personality disorder patients. Schizophr Res. 2000;41:447–455. doi: 10.1016/s0920-9964(99)00085-7. [DOI] [PubMed] [Google Scholar]
- 29.Mitropoulou V, Harvey PD, Zegarelli G, New AS, Silverman JM, Siever LJ. Neuropsychological performance in schizotypal personality disorder: importance of working memory. Am J Psychiatry. 2005;162:1896–1903. doi: 10.1176/appi.ajp.162.10.1896. [DOI] [PubMed] [Google Scholar]
- 30.Mitropoulou V, Harvey PD, Maldari LA, et al. Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol Psychiatry. 2002;52:1175–1182. doi: 10.1016/s0006-3223(02)01426-9. [DOI] [PubMed] [Google Scholar]
- 31.Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113(4):556–568. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- 32.Stanley MA, Turner SM, Borden JW. Schizotypal features in obsessive-compulsive disorder. Compr Psychiatry. 1990;31(6):511–518. doi: 10.1016/0010-440x(90)90065-z. [DOI] [PubMed] [Google Scholar]
- 33.Squires-Wheeler E, Skodol AE, Friedman D, Erlenmeyer-Kimling L. The specificity of DSM-III schizotypal personality traits. Psychol Med. 1988;18:757–765. doi: 10.1017/s0033291700008461. [DOI] [PubMed] [Google Scholar]
- 34.Lyons MJ, Toomey R, Faraone SV, Tsuang MT. Comparison of schizotypal relatives of schizophrenic versus affective probands. Am J Med Genet. 1994;54:279–285. doi: 10.1002/ajmg.1320540318. [DOI] [PubMed] [Google Scholar]
- 35.First MB, Spitzer RL, Gibbon M, Williams JW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 36.Kunkel R, Adami H, Zetlmeisl M, Ross D, Thaker G. Recruitment of non-patient volunteers with schizophrenia spectrum personality symptoms. Schizophr Res. 1998;34(3):181–186. doi: 10.1016/s0920-9964(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history approach to diagnosis: how useful is it? Arch Gen Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- 38.Adami H, Thaker GK, Moran, et al. Improved diagnosis of schizophrenia spectrum disorders using a modified FH-RDC. Biol Psychiatry. 1990;27:112A. [Google Scholar]
- 39.Pfol B, Blum N, Zimmerman M, Stangl D. Structured Interview for DSM-III-R Personality (SIDP-R) Iowa City: University of Iowa, Department of Psychiatry; 1997. [Google Scholar]
- 40.Kendler KS, Lieberman JA, Walsh D. The Structured Interview for Schizotypy (SIS): a preliminary report. Schizophr Bull. 1989;15:559–571. doi: 10.1093/schbul/15.4.559. [DOI] [PubMed] [Google Scholar]
- 41.Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT., Jr The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 42.Thaker GK, Ross DE, Buchanan RW, et al. Does pursuit abnormality in schizophrenia represent a deficit in the predictive mechanism? Psychiatry Res. 1996;59(3):221–237. doi: 10.1016/0165-1781(95)02759-9. [DOI] [PubMed] [Google Scholar]
- 43.Thaker GK, Ross DE, Cassady SL, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–836. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 44.Jonides J, Smith EE, Koeppe RA, Awh E, Minoshima S, Mintun MA. Spatial working memory in humans as revealed by PET. Nature. 1993;363(6430):623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- 45.Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- 46.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 47.Avila M, Robles O, Hong E, et al. Attention and working memory performance across the schizophrenia spectrum and the mediating effects of familial risk. J Abnorm Psychol. In press doi: 10.1037/0021-843X.115.4.771. [DOI] [PubMed] [Google Scholar]
- 48.Johnson JK, Tuulio-Henriksson A, Pirkola T, et al. Do schizotypal symptoms mediate the relationship between genetic risk for schizophrenia and impaired neuropsychological performance in co-twins of schizophrenic patients? Biol Psychiatry. 2003;54(11):1200–1204. doi: 10.1016/s0006-3223(03)00637-1. [DOI] [PubMed] [Google Scholar]
- 49.Egan MF, Goldberg TE, Gscheidle T, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50(2):98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- 50.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]