Abstract
Schizotypal traits and cognitive disturbances are known to be present in first-degree relatives of people with schizophrenia. However, there is little understanding of how these endophenotypes are related to each other. We explored the nature of this relationship in individuals with schizophrenia, their full siblings, community controls, and their siblings. All participants were assessed in the domains of working memory, attention, episodic memory, and executive function, as well as in their level of positive, negative, and disorganization symptoms. Schizophrenia probands were significantly impaired on all cognitive domains, as compared with the other 3 groups, and displayed the highest levels of positive, negative, and disorganization symptoms. Proband siblings performed significantly worse than controls on tasks of working memory, episodic memory, and executive function, and they displayed significantly more positive and negative symptoms as compared with controls. Poorer task performance across all 4 cognitive domains was most strongly correlated with increased negative symptoms. Mediation analyses revealed that working memory, episodic memory, and executive function deficits partially mediated increases in negative symptoms among proband siblings. Negative symptoms fully mediated deficits in working memory and episodic memory but only partially mediated deficits in executive function. Results suggest that there is a complex relationship between cognitive and clinical factors in this high-risk population.
Keywords: schizophrenia, vulnerability, cognition
Introduction
One of the primary goals of research on schizophrenia is the identification of vulnerability factors related to increased risk for developing this disorder. In the search for such intermediate endophenotypic markers, relatives of individuals with schizophrenia are often a population of interest, as they carry a genetic liability toward developing schizophrenia. In fact, the risk of developing schizophrenia is directly associated with the degree of relatedness to an affected individual.1 Further, subtle disturbances in cognition and subthreshold clinical symptoms, such as schizotypal traits, are present in the relatives of patients with schizophrenia. However, there is relatively little understanding of how these 2 types of potential endophenotypes may be related to each other. The extent to which we can clarify the relationship between deficits in cognition and subthreshold psychopathology may help to reveal the genetic susceptibility to schizophrenia.2 For example, cognitive deficits and subclinical psychopathology may be independent phenomena with separable genetic substrates. Alternatively, they may be correlated, perhaps because they share a common genetic liability. The goal of the current study is to assess these differing possibilities by exploring the relationship between cognitive function and schizotypal symptoms in the first-degree relatives of individuals with schizophrenia.
Prior studies have demonstrated a variety of cognitive deficits among first-degree relatives of individuals with schizophrenia. Kremen et al.3 provides a review of early studies of neuropsychological abnormalities among relatives of schizophrenia probands. More recently, results from the Edinburgh High Risk Study have indicated that high-risk relatives (those with at least 2 family members diagnosed with schizophrenia) performed significantly worse than age- and sex-matched controls on tests of executive function, learning, and memory.4 Staal and colleagues5 also reported deficits in executive functioning and sensory-motor functioning among nonpsychotic siblings of individuals with schizophrenia. A number of different studies have reported deficits in sustained attention,6,7 as well as episodic memory8–14 among first-degree relatives of individuals with schizophrenia. Further, Wolf et al.15 established that these impairments are specific to relatives of individuals with schizophrenia, as compared with relatives of individuals with other psychiatric disorders. Taken together, these studies suggest that relatives of individuals with schizophrenia show impairment in multiple cognitive domains. These impairments are qualitatively similar to those found in individuals with schizophrenia (for a review, see Heinrichs and Zakzanis16), although the severity of impairment is attenuated.17 Furthermore, research suggests that various neurocognitive domains may be intercorrelated in relatives of schizophrenia probands.18
Data from longitudinal studies of relatives of individuals with schizophrenia suggest that the severity of cognitive deficits can predict later development of psychosis. For example, Niendam and colleagues19 found that children who later developed schizophrenia had more severe deficits on selected subtests of the Wechsler Intelligence Scale for Children than their siblings who did not go on to develop a psychotic disorder. Similarly, the severity of deficits on an attentional task (Continuous Performance Test-Identical Pairs [CPT-IP]) predicted the development of adult schizophrenia spectrum disorders in the offspring of individuals with schizophrenia.20 Thus, the presence and severity of cognitive deficits in child relatives of schizophrenia probands may predict the eventual onset of the disorder.
In another line of research, relatives of schizophrenia probands have been shown to have schizotypal traits.21 These traits are often regarded as subclinical forms of symptoms characteristic of schizophrenia. Cadenhead22 provides a brief review of the early literature on how the concept of schizophrenia spectrum disorders originally stemmed from observations of family members of schizophrenia patients, who often displayed odd or eccentric behaviors without having overt psychotic symptoms. Kety et al.23 provided systematic evidence for the genetic basis of this phenomenon by showing that the biological relatives of adoptees with chronic schizophrenia, but not their adoptive family members, had an increased frequency of schizophrenia spectrum disorders. Although schizophrenia spectrum disorders can be present in individuals without a known family history of schizophrenia, patient relatives are more likely to have spectrum disorders (eg, schizotypal personality disorder) than relatives of healthy individuals.24
Longitudinal studies to assess the predictive power of schizotypal traits among first-degree relatives of individuals with schizophrenia are scarce, but there is some evidence to suggest that the presence of such traits predicts the later development of psychosis. For example, in the Edinburgh High Risk Study, Miller et al.25 found that individuals who later developed psychosis scored significantly higher on all 4 factors of the Structured Interview for Schizotypy at their initial assessment than those who remained well. Among schizotypal traits, social withdrawal appeared to have the greatest predictive power. In another report from the same study, Miller et al.26 found that maternal ratings on the Child Behavior Checklist for withdrawn behavior and delinquent-aggressive behavior in adolescents aged 13–16 were also predictive of the later development of schizophrenia.
Despite evidence that both schizotypal traits and cognitive deficits predict the later onset of psychosis in the first-degree relatives of individuals with schizophrenia, there have been few studies on the relationship between these 2 phenomena. In psychometrically ascertained schizotypal populations, high levels of negative symptoms have been related to poor performance on tests of executive function,27–30 and high levels of disorganization symptoms have been related to poor performance on tests of attention.31 However, in studies of first-degree relatives of individuals with schizophrenia, results have been less consistent. For example, Conklin et al.32 found that performance on a task of episodic memory was inversely correlated with scores on the Schizotypal Personality Questionnaire. However, Laurent et al.33 found virtually no significant correlations between self-report measures of schizotypal traits and various measures of neurocognitive functioning in first-degree relatives of schizophrenia probands. Similarly, Byrne et al.34 also found that neuropsychological deficits in high-risk individuals were not associated with psychopathology, as measured by the Present State Examination. Finally, Johnson et al.35 reported an inverse correlation between schizotypal traits and poor performance on tasks of attention, memory, and executive function among nonpsychotic co-twins of schizophrenia probands. However, they also reported poor working memory performance among proband co-twins as compared with controls, regardless of the presence or absence of schizotypal symptoms.
These findings suggest that deficits in some, but not all, cognitive domains might be related to the presence and severity of schizotypal symptoms among first-degree relatives of individuals with schizophrenia. This type of relationship could occur for several reasons. First, specific types of cognitive deficits and schizotypal traits may be caused by a common underlying neurobiological factor. For example, an abnormality in prefrontal dopaminergic function could (1) impair working memory and executive function and (2) interfere with subcortical reward systems and alter motivation and hedonic function. In the case of such a relationship, specific cognitive deficits and schizotypal traits would be correlated in relatives, but there would be no direct cause-and-effect relationship between the two. Second, an underlying neurobiological abnormality could cause a deficit in cognitive function, which in turn gives rise to schizotypal pathology. For example, dopaminergic abnormalities in the prefrontal cortex could produce deficits in the ability to use goal representations to guide discourse planning and motivated behaviors, which directly lead to disorganized speech36–38 and negative symptoms.39 Third, an underlying neurobiological factor could produce psychopathology, which in turn interferes with performance on tests of specific elements of cognition. Although this last explanation seems less intuitive, an increase in negative symptoms (eg, motivation abnormalities) could lead to apparent cognitive dysfunction.
Although previous research suggests that there are at least some relationships between cognitive deficits and schizotypal traits in relatives of individuals with schizophrenia, the findings are inconsistent across studies. One reason for this may be variations in the way in which schizotypal traits have been assessed. In some studies a variety of schizotypal traits are combined into a single estimate of schizotypy.34,35 However, separate dimensions of schizotypy may exist,40–42 and specific elements of schizotypy may be differentially related to specific cognitive deficits. For example, Dinn et al.28 found that only negative symptoms of schizotypy were associated with cognitive deficits. Notably, similar relationships between negative symptoms and cognitive deficits have been found in studies of schizophrenia patients.43–45
The goals of the present study were to examine the relationship between various types of schizotypal traits and performance on tests of several different domains of cognition and to determine whether cognitive impairment mediates schizotypal traits or whether schizotypal traits mediate impaired performance on cognitive tasks in siblings of individuals with schizophrenia. Participants included individuals with schizophrenia, their full siblings who had not yet passed the age of risk for development of schizophrenia (ie, mean age < 25), and community controls. We examined 3 components of schizotypal symptoms—positive symptoms, negative symptoms, and disorganization symptoms—and 4 broad domains of cognitive functioning—working memory, episodic memory, attention, and executive function. Based on previous studies in individuals with schizophrenia, as well as psychometrically identified schizotypes, we hypothesized that cognitive test performance across all domains would be inversely related to negative symptoms and disorganization symptoms but not with positive symptoms.
METHOD
Participants
Participants were recruited through the Conte Center for the Neuroscience of Mental Disorders (CCNMD) at Washington University in St. Louis and included (1) 27 individuals with DSM-IV schizophrenia (25 male, 2 female) (SCZ); (2) 31 siblings of individuals with schizophrenia (16 males, 15 female) (SCZ-SIB); (3) 39 healthy control participants (21 male, 18 female) (CON); and (4) 42 siblings of healthy controls (30 male, 12 female) (CON-SIB). SCZ participants were recruited from local inpatient and outpatient treatment facilities. CON participants were recruited using local advertisements from the same community. Exclusion criteria for CON participants included a lifetime history of any Axis I psychiatric disorder and having a first-degree relative with a psychotic disorder. Both SCZ-SIB and CON-SIB were excluded for a lifetime history of Axis I psychotic disorders (including bipolar disorder) and current major depression, but not other Axis I disorders. Participants from any of the 4 groups were excluded if they (1) met DSM-IV criteria for substance abuse or dependence within the past 6 months; (2) had a clinically unstable or severe medical disorder, or a medical disorder that would confound the assessment of psychiatric diagnosis or render research participation dangerous; (3) had head injury (past or present) with documented neurological sequelae or resulting in loss of consciousness; and (4) met DSM-IV criteria for mental retardation (mild or greater in severity). The SCZ group had significantly more male participants than the other groups [χ2(3) = 27.01, p < .01], but the SCZ-SIB group did not differ from CON. CON and CON-SIB groups had significantly more Caucasians [χ2(3) = 8.1, p < .05] than did SCZ and SCZ-SIB groups. CON and CON-SIB participants had more years of education than SCZ participants, but not more education than SCZ-SIB participants (F3,137 = 4.6, p < .01). The groups did not differ significantly on age (F3,137 = 0.9, p = .45) or parental socioeconomic status (F3,137 = 0.8, p = .48). See Table 1 for demographic information. Given the similarity of the groups on parental socioeconomic status, we did not attempt to control for education in the analyses presented herein, as cognitive disturbances associated with risk for schizophrenia may impair educational achievement.46,47 All analyses remained significant when gender and race were entered as covariates. However, for clarity and ease of presentation, only the primary analyses are presented.
Table 1.
Demographic Information
| Controls | Control Siblings | Schizophrenia Patients | Schizophrenia Patient Siblings | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 21.3 (3.3) | 20.8 (3.4) | 22.1 (3.2) | 20.9 (3.6) |
| Sex (% male)a | 53.8% | 28.6% | 92.6% | 51.6% |
| Race (% Caucasian)b | 92.3% | 90.5% | 77.4% | 70.4% |
| Socioeconomic status rating | 3.0 (0.9) | 3.1 (0.8) | 3.4 (1.2) | 3.1 (1.2) |
| Education (years)a | 13.6 (2.7) | 13.1 (2.4) | 11.3 (2.0) | 12.3 (2.8) |
| WAIS—Vocabulary scaledc | 12.6 (2.7) | 11.8 (2.6) | 8.4 (2.9) | 10.6 (3.1) |
Schizophrenia < Control/Control Siblings/Patient Siblings (p < .01).
Control/Control Siblings > Schizophrenia/Patient Siblings (p < .05).
Control/Control Siblings > Patient Siblings > Schizophrenia (p < .05); WAIS = Wechsler Adult Intelligence Scale.
Diagnoses for all participant groups were determined using the Structured Clinical Interview for DSM-IV (SCID-IV).48 These interviews were conducted by a master's-level research assistant, who had completed SCID-IV training and participated in regular diagnostic training sessions as part of the CCNMD. The SCID-IV interviewer had access to all data from present and past hospital records and corroborative family sources. In addition, an expert clinician (in most cases, author Csernansky) conducted a semistructured interview, also using DSM-IV criteria and all available records. A consensus meeting between the SCID-IV interviewer and the expert clinician determined the participant's final diagnosis. SCZ participants were stabilized on antipsychotic medication for at least 2 weeks before participating in the study.
Clinical Rating Scales
Psychopathology was assessed in all participants using the Scale for the Assessment of Negative Symptoms (SANS),49 the Scale for the Assessment of Positive Symptoms (SAPS),50 and the Structured Interview for Prodromal Syndromes (SIPS).51 These ratings were completed by a master's-level research assistant who regularly participated in training and reliability sessions. All participants also completed the Chapman Psychosis Proneness Scales,52 which included the Perceptual Aberration Scale, the Magical Ideation Scale, the Physical Anhedonia Scale, and the Social Anhedonia Scale. For analysis, 3 clusters of symptoms were quantified—positive symptoms, negative symptoms, and disorganization symptoms. All rating scale scores were z-scored using the mean and standard deviation of the current sample and averaged within symptom clusters. Internal consistency analyses were performed by computing Cronbach's alpha. The positive symptom cluster (α = .89) was composed using the item scores for hallucinations and delusions on the SAPS, unusual thought content, suspiciousness, grandiosity, and perceptual abnormalities on the SIPS, the Perceptual Aberration Scale, and the Magical Ideation Scale. The negative symptom cluster (α = .93) was composed using the item scores for affective flattening, alogia, anhedonia, avolition, and attention on the SANS, social isolation, avolition, decreased expression of emotion, decreased experience of self, decreased ideational richness, and deterioration in role functioning on the SIPS, the Physical Anhedonia Scale, and the Social Anhedonia Scale. The disorganization symptom cluster (α = .72) was composed using the item scores for formal thought disorder and bizarre behavior on the SAPS, and odd behavior, bizarre thinking, trouble with attention, disorganized communication, and personal hygiene on the SIPS.
Neuropsychological Scales
All participants were administered a neuropsychological battery, which consisted of several measures across 4 broad cognitive domains—working memory, episodic memory, executive function, and attention. Prior research has demonstrated that individuals with schizophrenia show performance deficits in all of these domains. The score for each cognitive domain was formed using groupings of individual cognitive tasks. The task groupings were developed using previous reports of the factor structure of cognitive impairment associated with schizophrenia.53 Further, these individual cognitive tasks have been found to have moderate effect sizes, ranging from .20 to .68, in first-degree relatives of schizophrenia probands.54 Raw scores were converted to z-scores using the mean and standard deviation of the current sample and averaged within domains. The working memory domain (α = .59) consisted of scaled scores on letter-number sequencing and digit span, subtests from the Wechsler Memory Scale—Third Edition,55 as well as percentage correct on the 2-back version of the N-back task.56 The episodic memory domain (α = .75) consisted of scaled scores on immediate recall on family pictures and logical memory (also subtests of Wechsler Memory Scale—Third Edition), and the free recall score for trials 1–5 on the California Verbal Learning Test.57 The executive function domain (α = .70) included time to completion on Trails B,58 number of novel words generated on the category and verbal fluency tasks,59 and scaled score on the matrix reasoning subtest from the Wechsler Adult Intelligence Scale—Third Edition (WAIS-III).60 The attention domain (α = .60) consisted of 2 different versions of the Continuous Performance Task (CPT)—AX and Degraded. In the AX-CPT task, participants were instructed to respond to a target (X) when it was followed by a cue (A) and to withhold their response to the target in the absence of the cue. In the degraded condition the stimulus was degraded by randomly removing 85% of the pixels that make up the letters. Details regarding the administration and characteristics of these tasks are described in greater detail elsewhere.61
Data Analysis
Group differences in the cognitive and clinical symptom domains were examined using mixed-model methods that took into account correlations due to familial relationships (ie, sibling relationship). The family identifier was used as a grouping factor for the Level 2 analysis, while ID was used for the Level 1 identifier. Planned a priori contrasts examined differences comparing SCZ vs CON and CON-SIB, SCZ vs SCZ-SIB, and SCZ-SIB vs CON and CON-SIB. To examine the relationship between the 4 cognitive domains and the 3 clinical factors, partial correlations were computed between the various pairs of indices, controlling for group status. To assess group status, 3 dichotomous contrast codes were used (SCZ vs all others, SCZ-SIB vs all others, and CON-SIB vs all others). To protect against false positives, only correlations with a p-value less than .01 were considered significant.
We next examined whether the different symptom domains accounted for common or unique variance in the cognitive domains, and whether there were group differences in the strength of the relationship between the clinical factors and cognitive domains. To do so, we used mixed-model hierarchical regression methods that accounted for familial relationships. In this method, variables are entered in a predetermined order, and significance is assessed by examining the test of the factor in the context of those already in the model. The order of entry was (1) contrast code for SCZ vs others; (2) contrast code for SCZ-SIB vs others; (3) contrast code for CON-SIB vs others; (4) disorganization symptom score and negative symptom score; and (5) group × symptom interaction terms. Lastly, a 2-step procedure was used to determine whether the differences in cognitive domain function between SCZ-SIB and CON mediated the severity of symptoms in SCZ-SIB. First, for each cognitive domain found to be impaired in SCZ-SIB (executive function, working memory, and episodic memory), a Sobel test62 was conducted to determine whether the cognitive domain mediated any of the group-related differences in negative symptoms. If the Sobel test was significant, a hierarchical regression procedure was used to determine if this mediation were full or partial.
Results
Group Differences
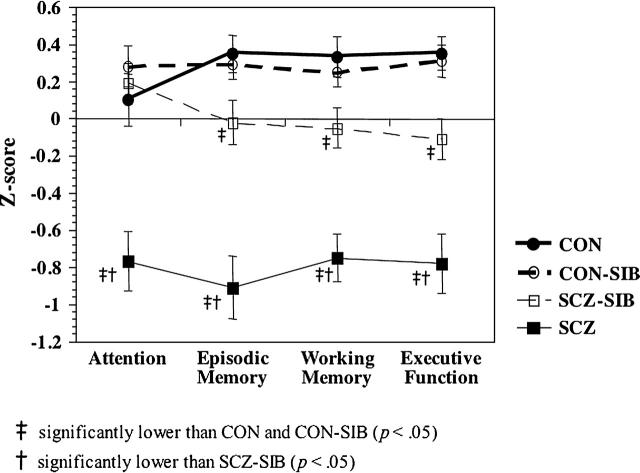
As shown in Figure 1, we found significant main effects of group for the working memory domain (F3,83 = 18.11, p < .01), the episodic memory domain (F3,83 = 22.03, p < .01), the attention domain (F3,83 = 10.99, p < .01), and the executive function domain (F3,83 = 17.60, p < .01). SCZ performed significantly worse than CON and CON-SIB across all 4 cognitive domains (all p < .01) and performed worse than their own siblings across all 4 cognitive domains (all p < .01). SCZ-SIB performed worse than CON and CON-SIB on the executive function, working memory, and episodic memory domains (all p < .05) but did not differ significantly from CON on the attention domain.
Fig. 1.
Graph Plotting the Average Z-score for Each Cognitive Domain, Separately for Each of the 4 Groups.
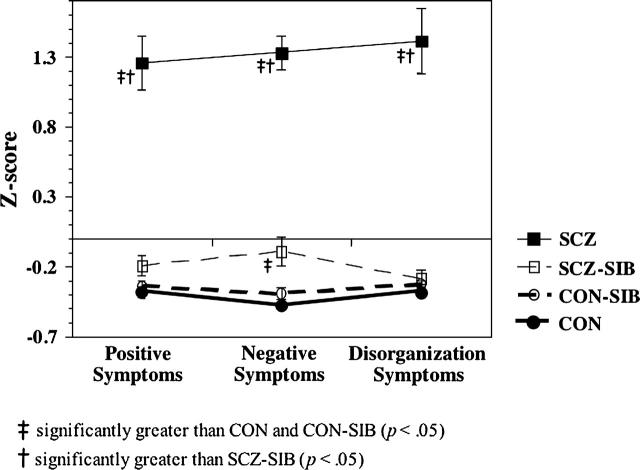
As shown in Figure 2, there were main effects of group for positive (F3,83 = 65.72, p < .01), negative (F3,83 = 125.50, p < .01), and disorganization symptoms (F3,83 = 70.13, p < .01). As expected, SCZ had higher scores on all 3 clinical factors as compared with CON, CON-SIB, and SCZ-SIB (all p < .01). SCZ-SIB also reported significantly more negative symptoms than CON and CON-SIB (all p < .05), but they did not differ significantly from CON or CON-SIB on disorganization (p = .36) or positive symptoms (p = .11).
Fig. 2.
Graph Plotting the Average Z-score for Each Clinical Domain, Separately for Each of the 4 Groups.
Relationship Between Cognitive Function and Clinical Symptoms
As predicted, the severity of negative symptoms was significantly associated with poorer performance in all 4 cognitive domains (Table 2). In addition, the severity of disorganization symptoms was significantly associated with poorer performance in working memory and episodic memory. In contrast, positive symptoms were not significantly correlated with performance on any of the cognitive domains. As shown in Table 2, negative symptoms and disorganization symptoms were strongly positively associated, but positive symptoms were not significantly correlated with either negative or disorganization symptoms. The cognitive domains were, for the most part, strongly intercorrelated. However, there was no significant relationship between the attention domain and either executive function or episodic memory.
Table 2.
Partial Correlation Coefficients for Cognitive and Clinical Variables, Controlling for Group Status
| Working Memory | Episodic Memory | Executive Function | Attention | Positive Symptoms | Negative Symptoms | Disorganization Symptoms | |
| Working Memory | — | ||||||
| Episodic Memory | .57* | — | |||||
| Executive Function | .49* | .38* | — | ||||
| Attention | .41* | .18 | .19 | — | |||
| Positive Symptoms | −.12 | −.03 | −.01 | .09 | — | ||
| Negative Symptoms | −.40* | −.32* | −.26* | −.30* | .14 | — | |
| Disorganization Symptoms | −.23* | −.32* | −.14 | −.18 | .04 | .54* | — |
p < .01.
We next examined whether the symptom domains accounted for common or unique variance in the cognitive domains, and whether there were group differences in the strength of the relationship between clinical symptoms and cognitive function. We only examined negative and disorganization symptoms since these were the only 2 symptom domains related to cognition in the correlational analyses presented above. As shown in Table 3, for the attention domain, the contrast coding for “SCZ vs others” (but neither of the other 2 contrast codes) accounted for significant variance. Additionally, negative symptom severity, but not disorganization symptom severity, accounted for a significant increase in variance. The interaction terms did not account for a significant increment in explained variance. For the episodic memory domain, the contrast codes for “SCZ vs others” and for “SCZ-SIB vs others” (but not “CON-SIB vs others”) added significantly to the explained variance. There was also a significant main effect of negative symptoms and a trend level effect of disorganization symptoms (p < .10). However, the interaction terms were not significant. For the working memory and executive function domains, the contrast codes for “SCZ vs others” and for “SCZ-SIB vs others” (but not “CON-SIB vs others”) were significant. Negative symptoms, but not disorganization symptoms, were also significant in both of these domains. The interaction terms were not significant.
Table 3.
Regression Analyses
| Model Step | Predictor Variables | Beta | F Change | R2 Change | Significance |
| Dependent Variable: Attention | |||||
| 1 | c1 (schizophrenia) | −0.96** | 34.54 | 0.202 | p < .01 |
| 2 | c2 (schizophrenia siblings) | 0.00 | 0.00 | 0.000 | NS |
| 3 | c3 (control siblings) | −0.23 | 2.04 | 0.007 | NS |
| 4 | Negative Symptoms | −0.55** | 4.25 | 0.074 | p < .01 |
| Disorganization Symptoms | −0.02 | ||||
| 5 | Group × Symptom | 0.47 | 0.024 | NS | |
| Dependent Variable: Episodic Memory | |||||
| 1 | c1 (schizophrenia) | −1.04** | 66.45 | 0.316 | p < .01 |
| 2 | c2 (schizophrenia siblings) | −0.34* | 6.09 | 0.030 | p < .05 |
| 3 | c3 (control siblings) | 0.04 | 0.09 | 0.001 | NS |
| 4 | Negative Symptoms | −0.32* | 6.25 | 0.086 | p < .05 |
| Disorganization Symptoms | −0.25 | ||||
| 5 | Group × Symptom | 0.32 | 0.013 | NS | |
| Dependent Variable: Executive Function | |||||
| 1 | c1 (schizophrenia) | −0.81** | 42.10 | 0.257 | p < .01 |
| 2 | c2 (schizophrenia siblings) | −0.42** | 9.47 | 0.054 | p < .01 |
| 3 | c3 (control siblings) | 0.05 | 0.19 | 0.000 | NS |
| 4 | Negative Symptoms | −0.42** | 2.80 | 0.044 | p < .01 |
| Disorganization Symptoms | 0.05 | ||||
| 5 | Group × Symptom | 0.17 | 0.008 | NS | |
| Dependent Variable: Working Memory | |||||
| 1 | c1 (schizophrenia) | −0.82** | 47.98 | 0.263 | p < .01 |
| 2 | c2 (schizophrenia siblings) | −0.36** | 7.55 | 0.036 | p < .01 |
| 3 | c3 (control siblings) | 0.07 | 0.40 | 0.002 | NS |
| 4 | Negative Symptoms | −0.56** | 8.17 | 0.115 | p < .01 |
| Disorganization Symptoms | 0.00 | ||||
| 5 | Group × Symptom | 0.74 | 0.030 | NS | |
Note: df = 83, *p < .05, **p < .01.
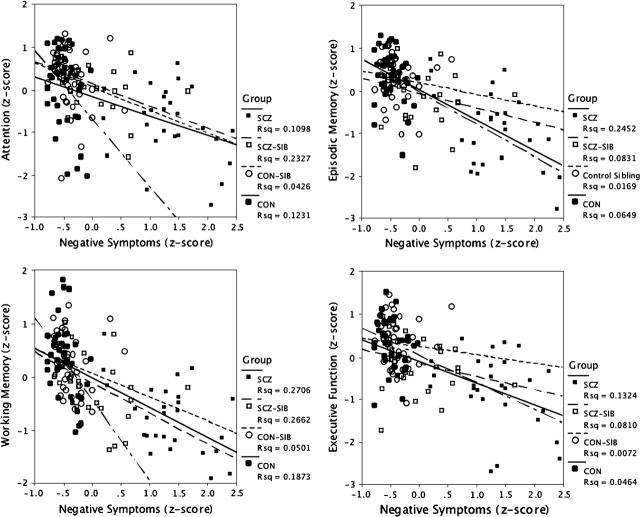
Figure 3 illustrates the relationships between negative symptoms and cognitive domains across groups. Although the magnitude of the relationship between the cognitive domains and negative symptoms (the symptom factor most strongly related to cognitive function) is somewhat smaller in CON and CON-SIB, all 4 groups fall on a similar regression line.
Fig. 3.
Graphs Plotting the Relationships Between Negative Symptoms and Each of the 4 Cognitive Domains. Separate regression lines are plotted for each group, and the R2 values from the regression for each group are shown on the graph.
Mediation Analyses
We focused these mediation analyses on negative symptoms, as this was the domain most strongly associated with cognitive deficits (eg, positive symptoms were not correlated with cognitive function, and disorganization symptoms only demonstrated a unique association with episodic memory). The Sobel tests indicated that the working memory (z = 2.3, p < .05), executive function (z = 2.2, p < .05) and episodic memory (z = 2.0, p < .05) significantly mediated the SCZ-SIB contrast code increase in negative symptoms. However, the attention domain did not significantly mediate the SCZ-SIB increases in negative symptoms (z = 0.1, p > .5). Follow-up hierarchical regressions for the working memory, executive function, and episodic memory domains revealed that group status still accounted for significant variance in negative symptoms in Step 2 (all p < .01), indicating that these cognitive deficits partially, but not fully, mediated the increase in negative symptoms.
To evaluate the reverse relationship between cognition and negative symptom severity, we determined whether increase in negative symptoms mediated performance deficits in any of the 3 cognitive domains found to be impaired in these relatives (working memory, episodic memory, and executive function). The Sobel tests indicated that negative symptoms significantly mediated SCZ-SIB deficits in working memory (z = 3.3, p < .01), episodic memory (z = 2.5, p < .05), and executive function (z = 2.6, p < .05). The follow-up hierarchical regressions for working memory and episodic memory revealed that the effect of group was no longer significant in Step 2 (p > .10), indicating that negative symptoms fully mediated the deficits in working memory and episodic memory found in SCZ-SIB. Finally, the hierarchical regression for executive function revealed that group status still accounted for significant variance in Step 2 (F1,79 = 6.1, p < .05), indicating that negative symptoms partially mediated the deficits in executive function among SCZ-SIB.
Vocabulary and IQ
The groups differed on WAIS-III vocabulary scores, which are often used as a proxy for crystallized IQ. One hypothesis is that reduced IQ in schizophrenia somehow leads to changes in cognitive processes such as working memory and executive function. However, it may also be the case that the cognitive processes involved in working memory, executive function, and episodic memory are the building blocks of crystallized IQ such as vocabulary. If so, controlling for vocabulary would eliminate variance in the cognitive process of interest. To examine the relationship between deficits in verbal IQ and deficits in other cognitive domains among the individuals with schizophrenia and their siblings, we conducted 2 sets of analyses. First, we computed a hierarchical regression for each of the cognitive domains in which vocabulary scores were entered in Step 1, and then the contrast codes for SCZ vs others and SCZ-SIB vs others were entered in Steps 2 and 3. For all 4 cognitive domains, the deficits among SCZ remained significant even after accounting for group differences in vocabulary scores (all p < .0001). The effect for SCZ-SIB showed trend level significance for episodic memory (p = .10), executive function (p = .07), and working memory (p = .07) after accounting for group differences in vocabulary scores. For the attention domain, the effect of SCZ-SIB was not significant, but this effect was not significant in the original analysis. We then computed a hierarchical regression in which vocabulary scores were the dependent measure. We entered the 4 cognitive domain scores in Step 1, and the contrast codes for SCZ vs others in Step 2 and the contrast code for SCZ-SIB vs others in Step 3. Neither the SCZ nor SCZ-SIB contrast codes were significant at even a trend level (p > .30) for vocabulary scores when group differences in the cognitive variables were taken into account.
Discussion
The goal of the current study was to clarify the relationships between deficits in specific cognitive domains and schizotypal traits in first-degree relatives of individuals with schizophrenia. We found group differences in both cognitive function and symptom severity, with SCZ demonstrating the worst performance and SCZ-SIB showing impairments intermediate between SCZ and CON/CON-SIB. Consistent with our hypotheses, we found that the negative symptoms and disorganization symptoms, but not positive symptoms, were correlated with deficits in several cognitive domains. Hierarchical regression analyses revealed that the 4 cognitive domains had different relationships with the negative and disorganization symptom clusters.
Group Differences: Clinical Symptoms
As expected, SCZ had significantly more positive symptoms, negative symptoms, and disorganization symptoms than SCZ-SIB, CON, and CON-SIB. Also, SCZ-SIB had higher levels of negative symptoms, but not disorganization symptoms, as compared with CON and CON-SIB. SCZ-SIB had numerically higher scores on positive symptoms as compared with CON and CON-SIB, but this effect was only a mild trend (p = .11). These results are partially consistent with prior work that first identified the concept of schizotypy in first-degree relatives of individuals with schizophrenia.1,63 In addition, these results are consistent with more recent work separately examining the positive, negative, and disorganization factors of schizotypal traits. There have been consistent reports of elevations in negative symptoms,33,40,64,65 as well as some evidence for enhanced positive symptoms,33,65,66 among relatives of schizophrenia probands.
Group Differences: Neuropsychological Functioning
As expected, SCZ were significantly impaired in all 4 cognitive domains as compared with SCZ-SIB, CON, and CON-SIB. This finding is consistent with previous reports of cognitive dysfunction in SCZ.16 SCZ-SIB generally performed in an intermediate range between their ill siblings and CON and CON-SIB and were significantly impaired on tasks of executive function, working memory, and episodic memory. Several previous studies have documented impairment in executive function,4,5,54,67 working memory,35,54,68 and episodic memory8–14 among first-degree relatives of individuals with schizophrenia.
Notably, the SCZ-SIB group did not differ significantly from CON or CON-SIB on measures of attention. A number of previous studies have documented an attention deficit in relatives of schizophrenia probands, especially as indexed by the CPT-IP.7,54 The discrepancy between our results and these prior reports could be due to several factors. First, in the current study we examined variants of the degraded CPT and the AX-CPT as measures of the attention domain. Although both of these tasks have been found to elicit impairments in first-degree relatives in prior studies,69 it is possible that these tasks may not have been sufficiently challenging to elicit deficits in our relatively young group of SCZ-SIB. We did not examine performance on the CPT-IP, perhaps the most classic task used to study attentional function in individuals at risk for schizophrenia. Although some would consider the CPT to be a measure of working memory as well as attention, we felt it would be more sensitive to attention deficits in our participants. Notably, other studies of first-degree relatives have found variable results using similar versions of the CPT. For example, in a group of 193 siblings of individuals with schizophrenia, Egan et al.70 reported no differences on the CPT, 1–9 version as compared with normal comparison subjects. Similarly, Cosway et al.71 found no differences on the CPT-IP between relatives of schizophrenia individuals and matched controls, examining an age range similar to our study (ages 16–25). Further, Avila et al.72 found that CPT-IP deficits were observed only in those relatives who met criteria for a schizophrenia spectrum disorder. Finally, in a prospective, longitudinal study of the children of parents with schizophrenia, Cornblatt et al.20 reported attentional deficits in a sample of high-risk children on the CPT-IP. However, these deficits were only present in a subgroup of individuals who later met criteria for a schizophrenia spectrum disorder. The entire group of high-risk children was not significantly different on CPT-IP performance as compared with normal controls. In our data, only 5% of SCZ-SIB scored 1 standard deviation below the mean of the CON/CON-SIB group. Taking our findings together with the results of these previous studies, it appears that significant attentional deficits may be present only in a subgroup of those relatives of schizophrenia probands with perhaps a particularly high genetic liability.
Our groups differed on education and vocabulary scores, though they did not differ in parental socioeconomic status. We did not control for educational differences, as the cognitive deficits associated with risk for schizophrenia may themselves impair educational achievement.46,47 A common assumption is that vocabulary scores are a proxy for IQ, and reduced IQ among individuals at risk for schizophrenia somehow leads to changes in cognitive processes such as working memory and executive function. However, it is also possible that the cognitive processes involved in working memory, executive function, and episodic memory are the building blocks of IQ, particularly of measures of crystallized IQ such as vocabulary. If so, controlling for vocabulary would also eliminate variance in the cognitive process of interest. Our results are more consistent with the idea that deficits in working memory, episodic memory, and executive function lead to reduced IQ in individuals with schizophrenia and their siblings, rather than the other way around. If we use vocabulary scores as covariates, all of the differences between SCZ and CON remain significant, and the differences between SCZ-SIB and CON either remain significant or are marginal (p < .10). In contrast, there are no remaining group differences in vocabulary when we enter the 4 cognitive domains as covariates. Such results do not rule out the possibility that a general factor is leading to deficits in a range of cognitive domains in those at risk for schizophrenia (eg, a generalized deficit). However, such results are not consistent with the hypothesis that deficits in working memory, episodic memory, and executive function among those at risk for schizophrenia are simply an artifact of lower IQ.
Relationship Between Neuropsychological Functioning and Clinical Symptoms
Consistent with our hypothesis, deficits in all 4 cognitive domains were inversely related to negative symptoms and disorganization symptoms but were not significantly correlated with positive symptoms. This pattern is consistent with previous reports suggesting that cognitive impairment in SCZ is related to both negative and disorganization symptoms.43,73–75 Moreover, the strength of the relationship to negative versus disorganization symptoms differed across the 4 cognitive domains. Regression analyses revealed that when negative symptoms were in the model, disorganization symptoms did not account for additional variance in the working memory, executive function, and attention domains. For the episodic memory domain, regression analysis showed that in addition to negative symptoms accounting for unique variance, variance accounted for by disorganization symptoms was significant at a trend level (p < .10). Our results are partly consistent with the results of prior studies of SCZ, which themselves have been mixed. O'Leary et al.74 found that negative symptoms were correlated with a wide range of cognitive deficits after controlling for disorganization symptoms, but that disorganization symptoms remained associated with low verbal IQ and poor concept attainment only after controlling for negative symptoms. In contrast, Cuesta and Peralta75 found that after accounting for the effect of negative symptoms, disorganization symptoms were still significantly correlated with verbal memory. Daban et al.76 found that disorganization symptoms were correlated with working memory and executive function after controlling for negative symptoms. Further, other studies have found that disorganization symptoms, but not negative symptoms, are related to working memory or executive function deficits.77,78 The fact that disorganization symptoms were not as strongly related to cognitive function as negative symptoms in the current study may be related to our examination of SCZ-SIB as well as SCZ individuals. Though the general pattern of the relationship between disorganization symptoms and cognitive function was similar across groups (see below), reduced variance in disorganization symptoms among SCZ-SIB may have truncated the strength of the relationship of disorganization symptoms to cognitive function. In fact, if we examine just the individuals with schizophrenia, disorganization symptoms (–.28 > r > –.55) are just as strongly correlated with cognition function as negative symptoms (–.36 > r > –.52).
We also examined the question of whether there were differential relationships between cognitive deficits and psychopathology across groups. Our data suggests that all 4 groups showed similar relationships between cognitive domains and clinical factors, although the magnitudes of these relationships were less in CON and CON-SIB. This reduced magnitude of correlations may be related to the reduced variance in clinical symptomatology in CON and CON-SIB. Our results differ from those reported by Johnson et al.,35 who reported a significant correlation between schizotypal symptoms and cognitive deficits among individuals with a genetic liability toward schizophrenia but not in healthy subjects. However, these authors used 1 composite score to measure schizotypy, while we measured 3 schizotypal symptom clusters independently. Further, our findings are consistent with a large body of research on individuals with schizotypy drawn from the general population (ie, individuals who display schizotypal symptoms but do not have a known family history of schizophrenia).27–29,31,79
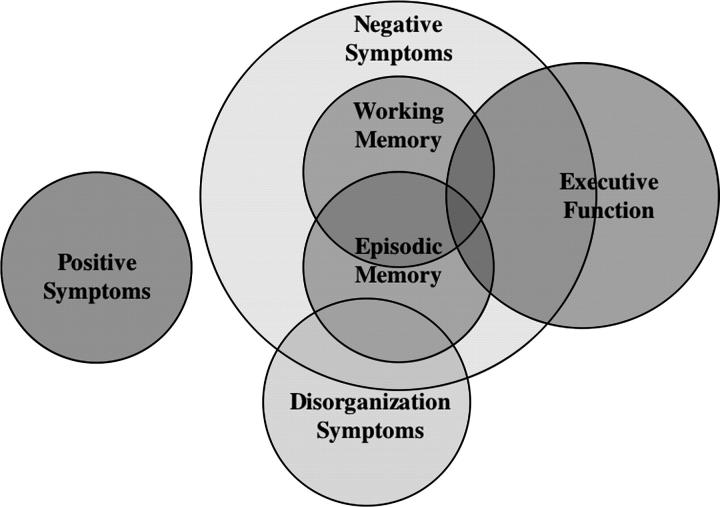
The last goal of our study was to examine whether the clinical symptoms shown by SCZ-SIB were either partially or fully mediated by cognitive deficits (or vice versa). These analyses were correlational; therefore, a cause-and-effect relationship cannot be established. However, they are informative about the degree of shared variance among these constructs. Our data show that positive symptoms are unrelated to cognitive function. However, in the case of negative symptoms, the answer is less clear. Mediation analyses suggest that cognitive function may partially mediate increased negative symptoms among SCZ-SIB, as the increase in negative symptoms among SCZ-SIB remains significant after accounting for variance associated with cognitive function. In contrast, mediation analyses show that negative symptoms fully mediate deficits in working memory and episodic memory and partially mediate deficits in executive function in SCZ-SIB. Taken together, these results suggest that there is a complex relationship between cognitive deficits and schizotypal traits in individuals who are at increased genetic risk for developing schizophrenia (see Figure 4). First, there appear to be vulnerability factors that contribute to increases in positive symptoms that do not share variance with vulnerability factors for cognitive deficits. Second, there are common vulnerability factors that contribute to increases in both negative and disorganization symptoms, as well as deficits in executive function, working memory, and episodic memory. However, negative symptoms may be a more sensitive indicator of the common genetic vulnerability factor, or an additional factor may contribute to increases in negative symptoms over and above those that contribute to cognitive deficits. Third, although executive function shares much variance with working memory, episodic memory, and negative symptoms, there is some variance in executive function deficits that is not shared by these other factors. Again, this may indicate that executive function is a more sensitive indicator of a common genetic vulnerability factor or that an additional factor contributes to deficits in executive function over and above those that contribute to negative symptoms or deficits in other cognitive domains. It may also be the case that the processes assessed by the executive function factor are more diverse and therefore able to capture more variance in cognitive function among those at risk for schizophrenia.
Fig. 4.
Figure Illustrating the Relationships Among Clinical and Cognitive Variables.
The specific vulnerability factors that are common or unique to memory and executive function deficits and to negative symptoms are not yet known. However, a likely candidate is a deficit in dorsolateral prefrontal cortex function. Many lines of research indicate that the dorsolateral prefrontal cortex is involved in all of the cognitive domains shown to be impaired in relatives of individuals with schizophrenia.80–82 Also, a number of theorists have suggested that negative symptoms in SCZ may reflect deficits in prefrontal cortex function.39 The emergence of psychotic symptoms following puberty has also been related to neurodevelopmental processes occurring in the prefrontal cortex during late adolescence.83,84 At a different level of analyses, disturbances in dopaminergic85 and GABAergic function86 within the prefrontal cortex have been linked to both negative symptoms and cognitive deficits.87–89 In addition, dopamine's role in reward processing and motivation90,91 provides a plausible mechanism by which deficits in dopamine function could cause negative symptoms, such as motivation and hedonic disturbances.39
In conclusion, we found that schizotypal symptoms were related to cognitive function in SCZ, SCZ-SIB, and CON/CON-SIB. Further, SCZ-SIB displayed significant increases in negative symptoms, as well as significant deficits in working memory, episodic memory, and executive function. We also found that increases in negative symptoms among SCZ-SIB were not fully mediated by cognitive deficits and that deficits in executive function were not fully mediated by negative symptoms. These results suggest that both negative symptoms and deficits in executive function are sensitive indicators of the genetic liability to develop schizophrenia. Future research in which markers of underlying genetic and neurobiological factors are assessed in combination with cognition and psychopathology can shed further light on the mechanisms by which cognitive deficits and schizotypal traits are related in various populations.
Acknowledgments
We wish to thank the individuals with schizophrenia and their families who participated in this work. In addition, we thank the staff of the Administration and Assessment Core at the Washington University Conte Center for the Neuroscience of Mental Disorders. We also thank Dr. Martha Storandt for her statistical consultation. This work was supported National Institute of Mental Health grants MH60887 and MH56584, as well as the Conte Center for the Neuroscience of Mental Disorders (MH071616).
References
- 1.Gottesman II. Schizophrenia Genesis: The Origins of Madness. New York, NY: Freeman; 1991. [Google Scholar]
- 2.Nuechterlein KH, Asarnow RF, Subotnik KL, et al. The structure of schizotypy: relationships between neurocognitive and personality disorder features in relatives of schizophrenic patients in the UCLA family study. Schizophr Res. 2002;54:121–130. doi: 10.1016/s0920-9964(01)00359-0. [DOI] [PubMed] [Google Scholar]
- 3.Kremen WS, Seidman LJ, Pepple JR, Lyons MJ, Tsuang MT, Faraone SV. Neuropsychological risk indicators of schizophrenia: a review of family studies. Schizophr Bull. 1994;20:103–119. doi: 10.1093/schbul/20.1.103. [DOI] [PubMed] [Google Scholar]
- 4.Byrne M, Hodges A, Grant E, Owens C, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study. Psychol Med. 1999;29:1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- 5.Staal WG, Hijman R, Hulshoff Pol HE, Kahn RS. Neuropsychological dysfunctions in siblings discordant for schizophrenia. Psychiatry Res. 2000;95(3):227–235. doi: 10.1016/s0165-1781(00)00172-4. [DOI] [PubMed] [Google Scholar]
- 6.Michie PT, Kent A, Stienstra R, et al. Phenotypic markers as risk factors in schizophrenia: neurocognitive functions. Aust N Z J Psychiatry. 2000;34(suppl):S74–85. doi: 10.1080/000486700226. [DOI] [PubMed] [Google Scholar]
- 7.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–62. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Sponheim SR, Steele VR, McGuire KA. Verbal memory processes in schizophrenia patients and biological relatives of schizophrenia patients: intact implicit memory, impaired explicit recollection. Schizophr Res. 2004;71:339–348. doi: 10.1016/j.schres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Toulopoulou T, Rabe-Hesketh S, King H, Murray RM, Morris RG. Episodic memory in schizophrenic patients and their relatives. Schizophr Res. 2003;63:261–271. doi: 10.1016/s0920-9964(02)00324-9. [DOI] [PubMed] [Google Scholar]
- 10.Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: a 4-year follow-up study. J Abnorm Psychol. 1999;108:176–181. doi: 10.1037//0021-843x.108.1.176. [DOI] [PubMed] [Google Scholar]
- 11.Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: the effect of genetic loading. Biol Psychiatry. 2000;48:120–126. doi: 10.1016/s0006-3223(99)00263-2. [DOI] [PubMed] [Google Scholar]
- 12.Wittorf A, Klingberg S, Wiedemann G. Secondary verbal memory: a potential endophenotype of schizophrenia. J Psychiatr Res. 2004;38(6):601–612. doi: 10.1016/j.jpsychires.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Schubert EW, McNeil TF. Neuropsychological impairment and its neurological correlates in adult offspring with heightened risk for schizophrenia and affective psychosis. Am J Psychiatry. 2005;162:758–766. doi: 10.1176/appi.ajp.162.4.758. [DOI] [PubMed] [Google Scholar]
- 14.Sitskoorn MM, Aleman A, Ebisch SJ, Appels MC, Kahn RS. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004;71:285–295. doi: 10.1016/j.schres.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Wolf LE, Cornblatt BA, Roberts SA, Shapiro BM, Erlenmeyer-Kimling L. Wisconsin Card Sorting deficits in the offspring of schizophrenics in the New York High-Risk Project. Schizophr Res. 2002;57:173. doi: 10.1016/s0920-9964(01)00301-2. [DOI] [PubMed] [Google Scholar]
- 16.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 17.Cannon TD, Zorrilla LE, Shtasel D, et al. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry. 1994;51:651–661. doi: 10.1001/archpsyc.1994.03950080063009. [DOI] [PubMed] [Google Scholar]
- 18.Toomey R, Faraone SV, Seidman LJ, Kremen WS, Pepple JR, Tsuang MT. Association of neuropsychological vulnerability markers in relatives of schizophrenia patients. Schizophr Res. 1998;31:89–98. doi: 10.1016/s0920-9964(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 19.Niendam TA, Bearden CE, Rosso IM, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 20.Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L. Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol. 1999;11:487–508. doi: 10.1017/s0954579499002175. [DOI] [PubMed] [Google Scholar]
- 21.Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study: III. schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- 22.Cadenhead KS. Vulnerability markers in the schizophrenia spectrum: implications for phenomenology, genetics, and the identification of the schizophrenia prodrome. Psychiatr Clin North Am. 2002;25:837–853. doi: 10.1016/s0193-953x(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 23.Kety SS, Wender PH, Jacobsen B, et al. Mental illness in the biological and adoptive relatives of schizophrenic adoptees: replication of the Copenhagen Study in the rest of Denmark. Arch Gen Psychiatry. 1994;51:442–455. doi: 10.1001/archpsyc.1994.03950060006001. [DOI] [PubMed] [Google Scholar]
- 24.Baron M, Gruen R, Rainer J, Kane J, Asnis L, Lord S. A family study of schizophrenic and normal control probands: implications for the spectrum concept of schizophrenia. Am J Psychiatry. 1985;142:447–455. doi: 10.1176/ajp.142.4.447. [DOI] [PubMed] [Google Scholar]
- 25.Miller P, Byrne M, Hodges A, Lawrie SM, Owens DG, Johnstone EC. Schizotypal components in people at high risk of developing schizophrenia: early findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2002;180:179–184. doi: 10.1192/bjp.180.2.179. [DOI] [PubMed] [Google Scholar]
- 26.Miller PM, Byrne M, Hodges A, Lawrie SM, Johnstone EC. Childhood behaviour, psychotic symptoms and psychosis onset in young people at high risk of schizophrenia: early findings from the Edinburgh High Risk Study. Psychol Med. 2002;32:173–179. doi: 10.1017/s0033291701004779. [DOI] [PubMed] [Google Scholar]
- 27.Barrantes-Vidal N, Fananas L, Rosa A, Caparros B, Dolors Riba M, Obiols JE. Neurocognitive, behavioural and neurodevelopmental correlates of schizotypy clusters in adolescents from the general population. Schizophr Res. 2003;61:293–302. doi: 10.1016/s0920-9964(02)00321-3. [DOI] [PubMed] [Google Scholar]
- 28.Dinn WM, Harris CL, Aycicegi A, Greene P, Andover MS. Positive and negative schizotypy in a student sample: neurocognitive and clinical correlates. Schizophr Res. 2002;56:171–185. doi: 10.1016/s0920-9964(01)00230-4. [DOI] [PubMed] [Google Scholar]
- 29.Diforio D, Walker E, Kestler LP. Executive function in adolescents with schizotypal personality disorder. Schizophr Res. 2000;42:125–134. doi: 10.1016/s0920-9964(99)00119-x. [DOI] [PubMed] [Google Scholar]
- 30.Suhr JA, Spitznagel MB. Factor versus cluster models of schizotypal traits: II. relation to neuropsychological impairment. Schizophr Res. 2001;52:241–250. doi: 10.1016/s0920-9964(00)00185-7. [DOI] [PubMed] [Google Scholar]
- 31.Chen WJ, Hsiao CK, Lin CC. Schizotypy in community samples: the three-factor structure and correlation with sustained attention. J Abnorm Psychology. 1997;106:649–654. doi: 10.1037//0021-843x.106.4.649. [DOI] [PubMed] [Google Scholar]
- 32.Conklin HM, Calkins ME, Anderson CW, Dinzeo TJ, Iacono WG. Recognition memory for faces in schizophrenia patients and their first-degree relatives. Neuropsychologia. 2002;40:2314–2324. doi: 10.1016/s0028-3932(02)00091-x. [DOI] [PubMed] [Google Scholar]
- 33.Laurent A, Biloa-Tang M, Bougerol T, et al. Executive/attentional performance and measures of schizotypy in patients with schizophrenia and in their nonpsychotic first-degree relatives. Schizophr Res. 2000;46:269–283. doi: 10.1016/s0920-9964(99)00232-7. [DOI] [PubMed] [Google Scholar]
- 34.Byrne M, Clafferty BA, Cosway R, et al. Neuropsychology, genetic liability, and psychotic symptoms in those at high risk of schizophrenia. J Abnorm Psychology. 2003;112:38–48. [PubMed] [Google Scholar]
- 35.Johnson JK, Tuulio-Henriksson A, Pirkola T, et al. Do schizotypal symptoms mediate the relationship between genetic risk for schizophrenia and impaired neuropsychological performance in co-twins of schizophrenic patients? Biol Psychiatry. 2003;54:1200–1204. doi: 10.1016/s0006-3223(03)00637-1. [DOI] [PubMed] [Google Scholar]
- 36.Barch DM. The role of working memory in language production and its disturbance in schizophrenia. Schizophr Res. 1999;36:160. [Google Scholar]
- 37.Barch DM, Berenbaum H. The effect of language production manipulations on negative thought disorder and discourse coherence disturbances in schizophrenia. Psychiatry Res. 1997;71:115–127. doi: 10.1016/s0165-1781(97)00045-0. [DOI] [PubMed] [Google Scholar]
- 38.Kerns JB, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J Abnorm Psychology. 2002;111:211–224. [PubMed] [Google Scholar]
- 39.Barch DM. The relationships among cognition, motivation, and emotion in schizophrenia: how much and how little we know. Schizophr Bull. 2005;31:875–881. doi: 10.1093/schbul/sbi040. [DOI] [PubMed] [Google Scholar]
- 40.Calkins ME, Curtis CE, Grove WM, Iacono WG. Multiple dimensions of schizotypy in first-degree biological relatives of schizophrenia patients. Schizophr Bull. 2004;30:317–325. doi: 10.1093/oxfordjournals.schbul.a007081. [DOI] [PubMed] [Google Scholar]
- 41.Stefanis NC, Smyrnis N, Avramopoulos D, Evdokimidis I, Ntzoufras I, Stefanis CN. Factorial composition of self-rated schizotypal traits among young males undergoing military training. Schizophr Bull. 2004;30:335–350. doi: 10.1093/oxfordjournals.schbul.a007083. [DOI] [PubMed] [Google Scholar]
- 42.Vollema MG, van den Bosch RJ. The multidimensionality of schizotypy. Schizophr Bull. 1995;21:19–31. doi: 10.1093/schbul/21.1.19. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwenstein MR, Aleman A, de Haan EH. Relationship between symptom dimensions and neurocognitive functioning in schizophrenia: a meta-analysis of WCST and CPT studies. J Psychiatr Res. 2001;35:119–125. doi: 10.1016/s0022-3956(01)00014-0. [DOI] [PubMed] [Google Scholar]
- 44.Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Neuropsychological correlates of negative, disorganized and psychotic symptoms in schizophrenia. Schizophr Res. 1998;31:99–111. doi: 10.1016/s0920-9964(98)00023-1. [DOI] [PubMed] [Google Scholar]
- 45.Voruganti LN, Heslegrave RJ, Awad AG. Neurocognitive correlates of positive and negative syndromes in schizophrenia. Can J Psychiatry. 1997;42:1066–1071. doi: 10.1177/070674379704201008. [DOI] [PubMed] [Google Scholar]
- 46.Meehl P. Nuisance variables and the ex post facto design. In: Radner M, Winokur S, editors. Minnesota Studies in the Philosophy of Science, IV. Analyses of Theories and Methods of Physics and Psychology. Minneapolis, Minn: University of Minnesota Press; 1970. pp. 373–402. [Google Scholar]
- 47.Resnick SM. Matching for education in studies of schizophrenia. Arch Gen Psychiatry. 1992;49:246. doi: 10.1001/archpsyc.1992.01820030078011. [DOI] [PubMed] [Google Scholar]
- 48.First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 49.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 50.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983. [Google Scholar]
- 51.McGlashan TH, Miller TJ, Woods SW, Rosen JL, Hoffman RE, Davidson L. Structured Interview for Prodromal Syndromes (SIPS) New Haven, Conn: Yale School of Medicine; 2000. [Google Scholar]
- 52.Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Raine T, Lencz T, Mednick S, editors. Schizotypal Personality. New York. NY: Cambridge University Press; 1995. pp. 79–106. [Google Scholar]
- 53.Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Snitz BE, Macdonald AW, 3rd, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32:179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wechsler D. Wechsler Memory Scale—Third Edition. San Antonio, Tex: Psychological Corporation; 1997. [Google Scholar]
- 56.Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- 57.Delis D, Kramer J, Kaplan E, Ober B. California Verbal Learning Test, Research Edition. Cleveland, OH: Psychological Corporation; 1983. [Google Scholar]
- 58.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tuscon, Ariz: Neuropsychology Press; 1985. [Google Scholar]
- 59.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. Iowa City, Iowa: AJA Associates; 1976. [Google Scholar]
- 60.Wechsler D. Wechsler Adult Intelligence Scale—Third Edition. San Antonio, Tex: Psychological Corporation; 1997. [Google Scholar]
- 61.Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ. Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol. 2004;113:556–568. doi: 10.1037/0021-843X.113.4.556. [DOI] [PubMed] [Google Scholar]
- 62.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 63.Kety S. Schizophrenic illness in the families of schizophrenic adoptees: findings from the Danish national sample. Schizophr Bull. 1988;14:217–222. doi: 10.1093/schbul/14.2.217. [DOI] [PubMed] [Google Scholar]
- 64.Kendler KS, Thacker L, Walsh D. Self-report measures of schizotypy as indices of familial vulnerability to schizophrenia. Schizophr Bull. 1996;22:511–520. doi: 10.1093/schbul/22.3.511. [DOI] [PubMed] [Google Scholar]
- 65.Appels MC, Sitskoorn MM, Vollema MG, Kahn RS. Elevated levels of schizotypal features in parents of patients with a family history of schizophrenia spectrum disorders. Schizophr Bull. 2004;30:781–790. doi: 10.1093/oxfordjournals.schbul.a007131. [DOI] [PubMed] [Google Scholar]
- 66.Vollema MG, Sitskoorn MM, Appels MC, Kahn RS. Does the Schizotypal Personality Questionnaire reflect the biological-genetic vulnerability to schizophrenia? Schizophr Res. 2002;54:39–45. doi: 10.1016/s0920-9964(01)00350-4. [DOI] [PubMed] [Google Scholar]
- 67.Szoke A, Schurhoff F, Mathieu F, Meary A, Ionescu S, Leboyer M. Tests of executive functions in first-degree relatives of schizophrenic patients: a meta-analysis. Psychol Med. 2005;35:771–782. doi: 10.1017/s0033291704003460. [DOI] [PubMed] [Google Scholar]
- 68.Conklin HM, Curtis CE, Calkins ME, Iacono WG. Working memory functioning in schizophrenia patients and their first-degree relatives: cognitive functioning shedding light on etiology. Neuropsychologia. 2005;43:930–942. doi: 10.1016/j.neuropsychologia.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 69.MacDonald AW, Pogue-Geile MF, Johnson MK, Carter CS. A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry. 2003;60:57–65. doi: 10.1001/archpsyc.60.1.57. [DOI] [PubMed] [Google Scholar]
- 70.Egan MF, Goldberg TE, Gscheidle T, Weirich M, Bigelow LB, Weinberger DR. Relative risk of attention deficits in siblings of patients with schizophrenia. Am J Psychiatry. 2000;157:1309–1316. doi: 10.1176/appi.ajp.157.8.1309. [DOI] [PubMed] [Google Scholar]
- 71.Cosway R, Byrne M, Clafferty R, et al. Sustained attention in young people at high risk for schizophrenia. Psychol Med. 2002;32:277–286. doi: 10.1017/s0033291701005050. [DOI] [PubMed] [Google Scholar]
- 72.Avila MT, Robles O, Hong E, et al. Deficits on the Continuous Performance Test (CPT) within the schizophrenia spectrum and the mediating effects of family history of schizophrenia. J Abnorm Psychol. In press doi: 10.1037/0021-843X.115.4.771. [DOI] [PubMed] [Google Scholar]
- 73.Barch DM, Carter CS, Cohen JD. Context processing deficit in schizophrenia: diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol. 2003;112:132–143. [PubMed] [Google Scholar]
- 74.O'Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J Neuropsychiatry. 2000;12:4–15. doi: 10.1176/jnp.12.1.4. [DOI] [PubMed] [Google Scholar]
- 75.Cuesta MJ, Peralta V. Cognitive disorders in the positive, negative, and disorganization syndromes of schizophrenia. Psychiatry Res. 1995;58:227–235. doi: 10.1016/0165-1781(95)02712-6. [DOI] [PubMed] [Google Scholar]
- 76.Daban C, Amado I, Bayle F, et al. Disorganization syndrome is correlated to working memory deficits in unmedicated schizophrenic patients with recent onset schizophrenia. Schizophr Res. 2003;61:323–324. doi: 10.1016/s0920-9964(02)00232-3. [DOI] [PubMed] [Google Scholar]
- 77.Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 78.Pantelis C, Harvey CA, Plant G, et al. Relationship of behavioural and symptomatic syndromes in schizophrenia to spatial working memory and attentional set-shifting ability. Psychol Med. 2004;34:693–703. doi: 10.1017/S0033291703001569. [DOI] [PubMed] [Google Scholar]
- 79.Suhr JA, Spitznagel MB. Factor versus cluster models of schizotypal traits: II. relation to neuropsychological impairment. Schizophr Res. 2001;52:241–250. doi: 10.1016/s0920-9964(00)00185-7. [DOI] [PubMed] [Google Scholar]
- 80.Barch DM, Csernansky J, Conturo T, Snyder AZ, Ollinger J. Working and long-term memory deficits in schizophrenia: is there a common underlying prefrontal mechanism? J Abnorm Psychol. 2002;111:478–494. doi: 10.1037//0021-843x.111.3.478. [DOI] [PubMed] [Google Scholar]
- 81.Barch DM, Carter CS, Braver TS, et al. Selective deficits in prefrontal cortex regions in medication naive schizophrenia patients. Arch Gen Psychiatry. 2001;50:280–288. doi: 10.1001/archpsyc.58.3.280. [DOI] [PubMed] [Google Scholar]
- 82.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 83.Walker EF, Gale S. Neurodevelopmental processes in schizophrenia and schizotypal personality disorder. In: Raine A, Lencz T, Mednick S, editors. Schizotypal Personality. New York, NY: Cambridge University Press; 1995. pp. 56–75. [Google Scholar]
- 84.Walker E, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 85.Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coyle JT. The GABA-glutamate connection in schizophrenia: which is the proximate cause? Biochem Pharmacol. 2004;68:1507–1514. doi: 10.1016/j.bcp.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 87.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Constantinidis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nature Neurosci. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- 89.Rao SG, Williams GV, Goldman-Rakic PS. Destruction and creation of spatial tuning by disinhibition: GABA(A) blockade of prefrontal cortical neurons engaged by working memory. J Neurosci. 2000;20:485–494. doi: 10.1523/JNEUROSCI.20-01-00485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 91.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]