Abstract
There is good evidence that clozapine is more efficacious than first-generation antipsychotic drugs in resistant schizophrenia. It is less clear if clozapine is more effective than the other second-generation antipsychotic (SGA) drugs. A noncommercially funded, pragmatic, open, multisite, randomized controlled trial was conducted in the United Kingdom National Health Service (NHS). Participants were 136 people aged 18–65 with DSM-IV schizophrenia and related disorders whose medication was being changed because of poor clinical response to 2 or more previous antipsychotic drugs. Participants were randomly allocated to clozapine or to one of the class of other SGA drugs (risperidone, olanzapine, quetiapine, amisulpride) as selected by the managing clinician. Outcomes were assessed blind to treatment allocation. One-year assessments were carried out in 87% of the sample. The intent to treat comparison showed no statistically significant advantage for commencing clozapine in Quality of Life score (3.63 points; CI: 0.46–7.71; p = .08) but did show an advantage in Positive and Negative Syndrome Scale (PANSS) total score that was statistically significant (–4.93 points; CI: −8.82 to −1.05; p = .013) during follow-up. Clozapine showed a trend toward having fewer total extrapyramidal side effects. At 12 weeks participants who were receiving clozapine reported that their mental health was significantly better compared with those receiving other SGA drugs. In conclusion, in people with schizophrenia with poor treatment response to 2 or more antipsychotic drugs, there is an advantage to commencing clozapine rather than other SGA drugs in terms of symptom improvement over 1 year.
Keywords: schizophrenia, clozapine, quality of life, clinical trial
Introduction
A substantial minority of people with schizophrenia (20–33%) derive little benefit from antipsychotic drug therapy.1 There is evidence that clozapine, the first so-called atypical or second-generation antipsychotic (SGA) drug, is efficacious in treatment-resistant schizophrenia when compared with older drugs (first-generation antipsychotics, or FGAs).2–7 However, clozapine carries a risk of agranulocytosis, a problem that seems to be largely absent with other SGA formulations. Systematic reviews have provided uncertain evidence to support clinicians' choosing between clozapine and other SGA drugs for people with treatment-resistant schizophrenia.8–10 A recent Cochrane review concluded that “trials of sufficient power, with longer duration, measuring clinically important outcomes, are needed to assess the true comparative clinical effectiveness, tolerability and cost effectiveness of newer drugs in relation to clozapine.”9
We report a pragmatic, open, multicenter, randomized controlled trial (the CUtLASS 2 trial) of clozapine versus other SGA drugs for schizophrenia, with a blind rating of outcome over 1 year. The trial was funded independently of the pharmaceutical industry. The trial concerned the relative clinical effectiveness of the non-clozapine class of SGA drugs rather than efficacy of individual drugs, since they are usually grouped together in clinical guidelines. Our primary hypothesis was that use of clozapine would be associated with improvement in quality of life over 1 year and that this improvement would be associated with fewer symptoms, improved patient satisfaction, and possibly lower total health care costs.
METHOD
Protocol and Rationale of Trial Design
The central issue for policymakers was to clarify the role of SGA drugs and clozapine in people with schizophrenia unresponsive to current treatment. A pragmatic, multicenter, rater-blind, randomized controlled trial was designed to test effectiveness in routine National Health Service (NHS) practice: (1) trial entry was defined by the clinician who was deciding to change drug management; (2) inclusion criteria were broad to best reflect normal clinical practice; (3) there was nonintensive follow-up with 1 primary outcome. The trial included an economic component.
Clozapine is the benchmark SGA drug, and clinical guidelines group together the other SGA drugs. Therefore, patients were randomized to either clozapine or treatment with one of the other SGA drugs. The choice of drug within the SGA class was made in advance by the clinician, supported by best available evidence.
Five UK centers were recruited, covering 14 NHS Trusts in the Northwest of England, Nottingham, West London, South East London, and Cambridge. Ethical approval was granted by the North West Multi-Centre Research Ethics Committee (MREC). Local research ethics approval was obtained for all participating districts. Inclusion criteria were:
DSM-IV schizophrenia, schizoaffective disorder, or delusional disorder11;
Age 18–65;
Responsible clinician electing to change current FGA or SGA drug treatment because of poor clinical response, and considering clozapine; and
Trials of at least 2 previous drugs, with poor clinical response.
Exclusion criteria were substance misuse or medical disorder being the major causative factor for psychotic symptoms and history of neuroleptic malignant syndrome.
Randomization and Assignment
The following drugs were classed as non-clozapine second-generation medications: risperidone, olanzapine, amisulpride, zotepine, and quetiapine. Ziprasadone is not licensed in the United Kingdom. Randomization was undertaken via a remote telephone service. After stratifying by treatment center, the method of allocation was randomized, permuted blocks within strata.
Efforts were made to initiate the first dose of randomized treatment as soon as possible. Participants allocated to clozapine required inpatient admission and baseline hematology. The actual mean and median times to first dose were 19 and 7 days, respectively. Efforts were made to keep the patient within the treatment arm that they were randomized to for a minimum of 12 weeks and preferably for the full year of the study. If the clinical decision was made to change the antipsychotic medication and the participant was in the SGA arm, the clinician was asked to initiate an alternative SGA where possible, so that the new medication should fall within the same treatment arm. Clinicians had access to a desk reference, best-prescribing handbook written for the trial. This allowed adjunctive medication but discouraged prescription of 2 or more antipsychotic drugs in parallel.
Outcome Measures
The primary outcome was Quality of Life Scale score (QLS).12 Quality of life was selected as the primary outcome variable in the trial since it is the construct that best fits the clinician's long-term treatment aim. The QLS is the most widely used scale in the evaluation of psychopharmacological treatments for established schizophrenia.13
Secondary outcome measures were as follows:
Symptoms on the Positive and Negative Syndrome Scale (PANSS)14
The Calgary depression scale15
Participant attitudes and adherence to medication ratings using the Drug Attitudes Inventory (DAI)16 and a 7-point drug adherence scale17
Global Assessment of Functioning Scale (GAF)11
- Side effects scales:
- The Simpson and Angus Scale18 for pseudo-Parkinsonian symptoms and signs (SAS)
- The Barnes Akathisia Scale (BARS)19
- The Abnormal Involuntary Movements Scale for tardive dyskinesia (AIMS)20
- A total neurological side effect score was obtained by summing scores on these scales
- The Antipsychotic Non-Neurological Side-Effects Rating Scale (ANNSERS), a new scale developed to assess the side effects of both FGA and SGA drugs
A quantitative rating of participant satisfaction was carried out at both the 12- and 52-week follow-up assessments, in the areas of satisfaction with new treatment, mental health, and side effects since taking the new medication.
Interrater reliability was assessed using 10 videotaped interviews for both the QLS and the PANSS. Intraclass correlations (n = 9 raters) were 0.99 for the total QLS and 0.84 for the total PANSS.
Masking to Allocation
The trial was rater-blind in nature. Measures taken to prevent breaking the blind included the physical location of assessors in relation to the rest of the team, a system of passwords for access to electronically held data, restrictions on discussions about individual patients within the team, and secured hard copies of case report forms. Study participants were frequently reminded to avoid open discussions of treatment assignment, and randomization lists were sent by encrypted e-mail from the randomization center.
The masked clinical assessor carried out follow-up assessments 12, 26, and 52 weeks following randomization in a range of settings, usually in the community. Telephone interviews were performed on a small number of occasions. Participants were deemed “lost to follow-up” only after a minimum of 4 failed visits at each time point.
Costs
We collected information about use of services for all participants entered in the trial. This included hospital inpatient and outpatient services, primary and community care services, and prescribed medications. The mandatory hematological monitoring for clozapine was performed in all cases by the manufacturer (Novartis) and is included in the acquisition costs of the drug. Direct costs were measured as resource use multiplied by unit cost.
Data Analysis
Routine data manipulation and data exploration was carried out using SPSS for Windows 10.21 Further analysis of the outcomes was carried out using Stata Version 7.22 The aim of the majority of the statistical analyses was to estimate the intention-to-treat effect.
An analysis based on all available outcome data was used to estimate the difference between the treatment arms in the quantitative outcomes for each of the 3 times (12, 26, and 52 weeks) using a longitudinal analysis of covariance, allowing for location and appropriate baseline score as covariates. This analysis was carried out using xtgee, the generalized estimating equations (GEE) command in Stata, specifying the identity link and normally distributed errors. A GEE regression model (using the unstructured assumption for the correlation between repeated measures) was used to estimate the treatment arm effect common to the 3 follow-up times, allowing for covariates as above (with allowance for the effects of the baseline covariates to vary over time). The analysis was repeated using the exchangeable assumption for the correlation between repeated outcome measurements (equivalent to a random effects or mixed model). In all cases robust standard errors and confidence intervals were requested. In no case were the results of the 2 approaches in any disagreement, and the results for the GEE models are the ones presented here. In addition, for all outcomes we tested for a treatment group by follow-up time interaction. In no case was this nonconstant treatment effect found to be statistically significant, and therefore the results of fitting the models with these interactions will not be presented.
Restricting the analysis to QLS and PANSS scores at 12 weeks, the effect of treatment arm crossovers before the 12-week follow-up was examined informally by estimation of “As Treated,” “Per Protocol,” and “Adjusted Treatment Received” (ATR) effects. The ATR estimates adjust for the confounding selection effect, which is a potential source of bias in naive “As Treated” and “Per Protocol” estimates. They are examples of the instrumental variable methods that have been recently advocated for use in psychiatric trial data by Dunn et al.,24, 25 Levy et al.,26 and O'Malley and Normand.27 The ATR estimates were produced using the Stata ivreg command. In all cases treatment received at 12 weeks was the endogenous variable; random allocation was the instrumental variable. Simple analysis of covariance models was used for the estimation of “As Treated” and “Per Protocol” effects. In all models both location and baseline score were used as covariates.
Treatment arm differences for nonlongitudinal secondary binary outcome measures were evaluated using Pearson's chi-square. Treatment arm differences in ordinal outcomes (participant satisfaction, for example) were evaluated using the Mann-Whitney U-test.
Sample Size and Power
The principal outcome used to determine the sample size needed was the QLS total score. Sixty participants in each group were required to give 85% power to show a statistically significant difference in the change scores of 10 points (ie, mean 12-month scores of 35 versus 45). Estimating a 15% dropout rate at 12 months, the trial needed to recruit 138 patients in total.
Results
A total of 168 patients were referred by 60 clinicians to the trial; 32 were not randomized due to ineligibility (n = 7; 4%) or refusal of consent (n = 25; 15%).
Of the 136 patients, 67 (49%) were randomly assigned to receive clozapine and 69 (51%) randomly assigned to receive an SGA drug. Baseline demographic characteristics of the randomized sample were comparable on most variables (Table 1). By chance, the mean QLS was higher (better) in the clozapine-allocated group. Table 2 shows the drugs prescribed within each treatment arm, plus mean end doses. Prior to randomization to the SGA arm, 44 patients (64%) were being treated with a first-generation drug; 30 of these via a depot (some had received more than 1 antipsychotic drug concurrently). The remaining patients were receiving a (non-clozapine) SGA. No patients were treated with clozapine prior to randomization. There were no differences in pattern of chosen drug within the SGA arm according to category of prior antipsychotic drug. All participants in both arms underwent a change in medication as a result of randomization.
Table 1.
Baseline Characteristics of Participants by Treatment Arm
| Clozapine Arm (n = 67) | SGA Arm (n = 69) | |
| Age (years) | ||
| Mean (SD) | 37.2 (12.2) | 37.9 (10.3) |
| Median/Range | 36.2/18–63 | 36.9/20–65 |
| Length of Illness (years) | ||
| Mean (SD) | 13.0 (10.5) | 13.6 (10.2) |
| Median/Range | 11.2/0–39 | 10.9/0–46 |
| Number of Previous Admissions | ||
| Mean (SD) | 4.2 (5.0) | 5.6 (5.0) |
| Median/Range | 3.0/0–30 | 5.0/0–33 |
| Gender (n, %) | ||
| Men | 49 (73%) | 44 (64%) |
| Women | 18 (27%) | 25 (36%) |
| Ethnicity (n, %) | ||
| White | 45 (67%) | 53 (77%) |
| Black | 14 (21%) | 12 (17%) |
| Asian | 6 (9%) | 2 (3%) |
| Other | 2 (3%) | 2 (3%) |
| Diagnosis (n, %) | ||
| Schizophrenia | 60 (90%) | 58 (84%) |
| Other | 7 (10%) | 11 (16%) |
| Patient Status at Baseline (n, %) | ||
| Inpatient | 41 (61%) | 35 (51%) |
| Day patient | 2 (3%) | 2 (3%) |
| Outpatient | 24 (36%) | 32 (46%) |
| First Episode? (n, %) | ||
| Yes | 8 (12%) | 4 (6%) |
| Current Drug Misuse (n, %) | ||
| None | 49 (73%) | 56 (81%) |
| Current Alcohol Misuse (n, %) | ||
| None | 40 (60%) | 38 (55%) |
Table 2.
Drugs Prescribed by Treatment Arm and Details of End Doses
| End Dose (mg) |
End Dose (mg) |
||||||||
| Clozapine Arm | n = 67 | Mean | Median | Range | SGA Arm | n = 69 | Mean | Median | Range |
| Clozapine | 67 | 333 | 300 | 100–600 | Amisulpride | 10 | 683 | 650 | 600–800 |
| Olanzapine | 31 | 19 | 20 | 10–30 | |||||
| Quetiapine | 21 | 520 | 525 | 300–750 | |||||
| Risperidone | 7 | 6 | 6 | 3–8 | |||||
Participant Flow and Follow-Up
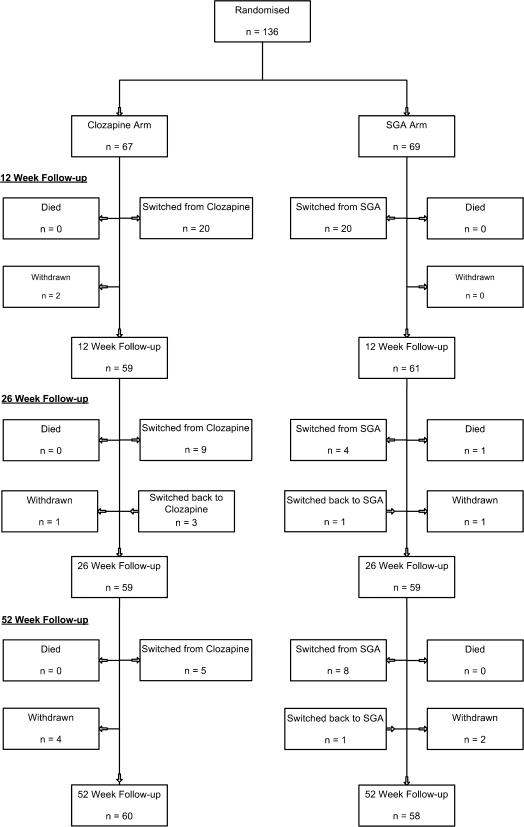
Figure 1 shows the flow of participants through the trial. The follow-up interview assessment rate was 87% at 1 year, compared with the projected rate of 85%. One death occurred in the SGA arm. Overall, 7% withdrew from the study, and 4% were lost to follow-up at 1 year. There were 4 cases of unblinding (3%), where the assessor became aware of the class of drug that the patient was currently receiving. Of those randomized to clozapine, 36 (54%) remained on clozapine at the end of 1 year, at a mean dose of 333 mg (median 300 mg) daily. Of those randomized to the SGA arm, 39 (57%) remained on an SGA at the end of 1 year, although this was a different SGA drug in 4 cases. A total of 15 patients assigned to the SGA arm went on clozapine before the end of the trial. Overall rates of antipsychotic polypharmacy fell to 8% at week 52 (6 SGA patients/5 clozapine patients).
Fig. 1.
Participant Flow.
Outcomes
Tables 3 and 4 give the QLS data at each time point, together with patterns of nonmissing data and mean changes in QLS scores from baseline. The missing data patterns for PANSS scores were practically identical. Table 5 also gives mean changes in the PANSS scores from baseline. Table 6 presents the results of the analyses using longitudinal GEE models. The intention to treat (ITT) treatment arm difference for the primary outcome (QLS) is not statistically significant, and only 1 of the differences for the secondary outcomes is statistically significant (that for the PANSS). Weight gain occurred similarly in both arms over 1 year: in the clozapine arm, a mean of 7.72 kg (SD 13.65, median 6.30, range −34.5 to +61.7) and in the non-clozapine SGA arm, a mean of 7.05 kg (SD 9.60, median 5.30, range −14.4 to +31.7).
Table 3.
Post-Randomization Missing Value Patterns for Quality of Life Scale (QLS) Totals, by Treatment Arm
| Count |
||
| Pattern | Clozapine | SGA |
| X X X | 50 | 49 |
| – X X | 5 | 4 |
| X – X | 3 | 3 |
| X X – | 4 | 4 |
| – – X | 1 | 0 |
| – X – | 0 | 1 |
| X – – | 1 | 4 |
| – – – | 3 | 4 |
Note: X is nonmissing; – is missing.
Table 4.
Primary Outcome: Quality of Life Scale (QLS)
| Clozapine Arm |
SGA Arm |
|||||
| n | Mean | SD | n | Mean | SD | |
| QLS Total Scores | ||||||
| Baseline | 67 | 41.0 | 19.9 | 69 | 34.7 | 17.1 |
| 12 weeks | 57 | 46.4 | 18.9 | 61 | 40.9 | 17.7 |
| 26 weeks | 58 | 50.8 | 19.8 | 59 | 41.5 | 18.6 |
| 52 weeks | 59 | 53.3 | 19.2 | 56 | 45.0 | 18.7 |
| QLS Change Scores | ||||||
| 12 weeks | 57 | 6.1 | 12.6 | 61 | 4.8 | 13.6 |
| 26 weeks | 58 | 10.0 | 14.0 | 59 | 6.9 | 17.3 |
| 52 weeks | 59 | 12.7 | 16.8 | 56 | 10.2 | 18.3 |
Note: QLS Change Scores = follow-up minus baseline.
Table 5.
Main Secondary Outcome: Positive and Negative Syndrome Scale (PANSS) Total and Subscale Scores
| Clozapine Arm |
SGA Arm |
|||||
| n | Mean | SD | n | Mean | SD | |
| PANSS Total | ||||||
| Baseline | 67 | 80.07 | 17.72 | 69 | 84.09 | 22.65 |
| 12 weeks | 57 | 68.67 | 14.80 | 61 | 75.61 | 19.47 |
| 26 weeks | 59 | 66.69 | 17.67 | 59 | 70.83 | 18.52 |
| 52 weeks | 59 | 63.47 | 17.72 | 57 | 68.00 | 18.81 |
| PANSS Positive Subscale | ||||||
| Baseline | 67 | 20.21 | 6.35 | 69 | 19.09 | 7.30 |
| 12 weeks | 57 | 15.42 | 4.96 | 61 | 17.28 | 5.95 |
| 26 weeks | 59 | 15.51 | 5.61 | 59 | 15.29 | 5.56 |
| 52 weeks | 59 | 14.51 | 5.72 | 59 | 15.10 | 6.12 |
| PANSS Negative Subscale | ||||||
| Baseline | 67 | 21.19 | 7.17 | 67 | 23.52 | 8.40 |
| 12 weeks | 57 | 18.18 | 5.82 | 61 | 20.16 | 7.51 |
| 26 weeks | 59 | 17.58 | 5.72 | 59 | 19.83 | 6.18 |
| 52 weeks | 59 | 16.81 | 5.61 | 59 | 19.27 | 6.91 |
| PANSS General Subscale | ||||||
| Baseline | 67 | 38.67 | 8.70 | 67 | 41.48 | 11.42 |
| 12 weeks | 57 | 35.07 | 7.47 | 61 | 38.16 | 10.02 |
| 26 weeks | 59 | 33.61 | 9.13 | 59 | 35.71 | 9.94 |
| 52 weeks | 59 | 32.15 | 8.85 | 57 | 34.00 | 9.81 |
| PANSS Total Change Scores | ||||||
| 12 weeks | 57 | −12.34 | 14.82 | 61 | −7.61 | 15.37 |
| 26 weeks | 59 | −13.45 | 18.29 | 59 | −12.36 | 17.3 |
| 52 weeks | 59 | −16.44 | 19.11 | 57 | −15.96 | 18.3 |
Note: Values for occasional missing scale items were imputed using the median of observed responses within the same subscale for that subject; PANSS Total Change Scores = follow-up minus baseline.
Table 6.
Estimates of Treatment Effect for Primary and Secondary Outcomes (difference between means, clozapine minus SGA, common to all 3 follow-up times)
| Domain and Measure | Effect | SE | 95% Confidence Interval | P-value |
| Quality of Life | ||||
| QLS totala | 3.63 | 2.08 | −0.46 to 7.71 | p = .082 |
| Psychopathology | ||||
| PANNS Totalb | −4.93 | 1.98 | −8.82 to −1.05 | p = .013 |
| General Functioning | ||||
| GAF Totala | 1.68 | 1.44 | −1.13 to 4.50 | p = .242 |
| Depression | ||||
| Calgaryb | −0.48 | 0.45 | −1.36 to 0.40 | p = .284 |
| Parkinsonism | ||||
| SASb | −0.10 | 0.38 | −0.85 to 0.64 | p = .785 |
| Akathisia | ||||
| BARSb | −0.55 | 0.35 | −1.22 to 0.13 | p = .116 |
| Tardive dyskinesia | ||||
| AIMSb | −0.63 | 0.46 | −1.53 to 0.28 | p = .176 |
| Total Neurological Side Effects | ||||
| Total of preceding 3b | −1.45 | 0.90 | −3.21 to 0.30 | p = .105 |
| Compliance and Attitude Toward Antipsychotic Drug | ||||
| DAIb | 1.43 | 1.51 | −1.52 to 4.38 | p = .343 |
| Non-Neurological Side Effects | ||||
| ANNSERSb | −0.63 | 0.98 | −2.54 to 1.28 | p = .520 |
A high score on this item means a better outcome. A positive parameter estimate means that participants in the clozapine arm are doing better
A high score on this scale means a worse outcome. A negative parameter estimate means that participants in the clozapine arm are doing better.
The participant satisfaction questionnaire was completed by 79% of patients in both treatment arms at both the 12-week and the 52-week follow-up assessments. A greater improvement in mental health at 12 weeks was reported by those who had been randomized to receive clozapine compared with those randomized to receive an SGA drug (p = .048, Mann-Whitney U-test).
Overall, there were higher costs at 1 year for people allocated to initiation of clozapine therapy (mean cost $62 523; £33 796, SD £34 296) rather than SGA therapy (mean cost $52 555; £28 408, SD £32 828). These differences were not statistically significant. Of these costs, 4.0% and 3.3% respectively were due to antipsychotic drug costs, and 81% and 84% respectively were due to psychiatric inpatient costs. At the time of trial recruitment and until 2003 the UK license for clozapine stipulated inpatient commencement. To correct for this, the data were adjusted to exclude the costs of inpatient care for the purpose of commencing clozapine, giving an adjusted 1-year figure of £33 157 ($61 340), SD£34 740.
Finally, we examined the possible effects of treatment arm switches within the first 12 weeks of post-randomization follow-up. Summary statistics for the change in QLS and PANSS scores from baseline to week 12 are shown in Table 7. The ITT effects, conditional on location and relevant baseline score, and assuming that data are missing completely at random, for the 12-week QLS and PANSS scores are 2.08 (SE 2.28) and −5.98 (SE 2.24), respectively. They are consistent with the results presented in Table 6—the effect on PANSS being statistically significant, that on QLS not. The “As Treated” estimates are 0.38 (SE 2.33) and −1.16 (SE 2.37) for 12-week QLS and PANSS scores, respectively. Comparing outcomes in only those patients who stayed in their allocated arms, the “Per Protocol” estimates of the treatment effects on QLS and PANSS scores are 2.18 (SE 2.88) and −5.19 (SE 2.76), respectively. None of the “As Treated” or “Per Protocol” effects are statistically significant. Finally, the ATR (instrumental variable) estimates of the effect of receiving clozapine as opposed to the other SGAs are 4.83 (SE 5.39) and −13.81 (SE 5.99) for QLS and PANSS, respectively. In comparison with the last 2 estimates, the corresponding ITT estimates are attenuated by the treatment switches. The ATR estimates adjust for this attenuation, but the statistical significance of the effects is more or less the same as the corresponding ITT results.
Table 7.
The Effects of Treatment Arm Crossover Before Week 12
| Randomized | Treatment (12 week) | n | 12-Week Mean | Mean Change | SD of Change |
| Change in QLS Scores (week 12 minus baseline) | |||||
| Clozapine | Clozapine | 40 | 48.00 | 7.83 | 12.70 |
| Clozapine | SGA | 17 | 42.65 | 2.18 | 11.68 |
| SGA | SGA | 44 | 44.25 | 6.43 | 14.31 |
| SGA | Clozapine | 17 | 32.06 | 0.65 | 10.86 |
| Change in PANSS Total Scores (week 12 minus baseline) | |||||
| Clozapine | Clozapine | 40 | 67.90 | −12.86 | 14.25 |
| Clozapine | SGA | 17 | 70.47 | −11.12 | 16.48 |
| SGA | SGA | 44 | 73.81 | −7.59 | 17.12 |
| SGA | Clozapine | 17 | 80.24 | −7.65 | 9.9 |
Discussion
The intent to treat comparison of clozapine with other SGA drugs in people judged clinically unresponsive to 2 or more antipsychotic drugs failed to show a significant advantage to commencing clozapine in quality of life (QLS score). There was a statistically significant advantage to clozapine on PANSS total score, equating to an approximately 5-point advantage, but not on other secondary outcome measures. There were no significant differences in rates of side effects, including weight, although the clozapine arm showed an advantage at the trend level for total neurological side effects. Participants' satisfaction with their mental health was significantly better at 12 weeks in those assigned to clozapine compared with SGA drugs. There were higher costs in the clozapine group, giving a net additional cost of £5388 ($9968) per person per year of initiating therapy with clozapine. This cost difference was not statistically significant, although the power of the study to show this was limited.
In general, the results from this comparison are similar to those from the large, randomized, 1-year double-blind comparative study of clozapine versus haloperidol at 15 Veterans Affairs (VA) Medical Centers,5 generally considered to be the definitive efficacy trial thus far. The subjects in that study were all inpatients and had a diagnosis of refractory schizophrenia. A total of 57% of patients in the clozapine group continued their assigned treatment for the entire year compared with 28% of the patients in the haloperidol group. As in the current trial, the difference on QLS at 1 year in the VA trial was not significant in the intent to treat analysis. As judged according to the PANSS total score, patients in the clozapine group had 5.4% lower symptom levels than those in the haloperidol group at all follow-up evaluations (p = .02). This was equated to a mean PANSS total score difference of 4.5 points.27 The VA study concluded that, for patients with refractory schizophrenia and high levels of hospital use, clozapine was somewhat more effective than haloperidol and had fewer side effects and similar overall costs.
The trial reported here has limitations. It is relatively small but had sufficient power to detect an expected 10-point difference in the primary outcome measure (QLS score). The results of the randomization were known to clinician and participant. Overall, the use of clinical assessors who did not know the treatment allocation of participants and the high rate of success in concealing allocation suggest that the recording of clinical assessments was not biased by knowledge of treatment allocation. The groups were not well balanced at baseline in terms of QLS scores, by chance. The design of the study, comparing classes of drugs, will serve to “hide” the effects of individual drugs that have particular efficacy or tolerability advantages. Although the present trial was not designed to test the effects of the individual SGAs, future trials could be designed with this in mind, and the design proposed by Lavori et al.28 might prove useful for this purpose. Finally, the mean dose of clozapine was fairly low, at 333 mg. The recommended target range for clozapine dosage is 300 to 450 mg a day,29 but titration of the drug to achieve optimum therapeutic response has generally been considered in terms of plasma clozapine level.30 The consensus is that a level of 350 ng/mL is a reasonable target threshold to ensure an adequate trial,29,31 and so it is arguable that the dosages prescribed in this study would not have ensured that optimum plasma clozapine levels were reached for all patients.32
In conclusion, in this noncommercially funded, randomized controlled trial in people with schizophrenia with clinician-defined poor response to 2 or more prior antipsychotic drugs, commencing clozapine led to significantly more improvement in symptoms but not quality of life over 1 year compared with commencing one of the other SGA drugs. This finding can be judged in the context of a trial run in parallel using a similar design (CUTLASS 1) which showed that in patients with schizophrenia requiring a change of treatment, the class of non-clozapine SGA drugs showed no advantage to first generation drugs over one year.33
Acknowledgments
Financial support was provided by a project grant from the Secretary of State for Health under the UK NHS Technology Assessment Program. The views and opinions expressed in this paper are those of the authors and do not necessarily reflect the views and opinions of the UK NHS Executive.
We are grateful to the statistical referees and to Professor Andrew Pickles for their suggestions for improvements to the analysis strategy. We thank the health professionals and participants for their invaluable help in the running of the project and collection of data.
We thank the independent members of the Steering and Data Monitoring and Ethics Committees: Glyn Lewis, John Geddes, and Peter Elton. Maria Clark, Tanya Hawthorn, Fiona Hynes, Fionnbar Lenihan, Jenny Massie, Ahmed Mahmoud, Paul Monks, Alex Barrow, Tracy Fay, Maurice Gervin, and Susie Morrow were essential in the management and execution of the study. Rob Kerwin and David Taylor contributed to the design of the trial.
Patient assessments were carried out by Candice Blackwell, Nerys Gooding, Rhona Howitt, Natasha Newbery, Eleanor Page, Joanne Shepherd, and Emma Sowden. Secretarial support came from Patricia Smith and Helen Woodiwiss. Data entry and IT support came from Simon Foster, Xinming Jin, Zhenhua Zhu, John Cooley, Paul Schofield, and Lisa Riley.
References
- 1.Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophr Bull. 1997;23:663–674. doi: 10.1093/schbul/23.4.663. [DOI] [PubMed] [Google Scholar]
- 2.Kane JM, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 3.Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151:20–26. doi: 10.1176/ajp.151.1.20. [DOI] [PubMed] [Google Scholar]
- 4.Essock SM, Hargreaves WA, Covell NH, Goethe J. Clozapine's effectiveness for patients in state hospitals: results from a randomized trial. Psychopharmacol Bull. 1996;32:683–697. [PubMed] [Google Scholar]
- 5.Rosenheck R, Cramer J, Xu W, et al. Comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. N Engl J Med. 1997;337:809–815. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- 6.Chakos M, Lieberman JA, Hoffman E, Bradford D, Sheitman B. Effectiveness of second-generation antipsychotics in patients with treatment-resistant schizophrenia: a review and meta-analysis of randomized trials. Am J Psychiatry. 2001;158:518–526. doi: 10.1176/appi.ajp.158.4.518. [DOI] [PubMed] [Google Scholar]
- 7.Wahlbeck K, Cheine M, Tuisku K, Ahokas K, Joffe G, Rimon R. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2000;24:911–922. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 8.Gilbody SM, Bagnall AM, Duggan L, Tuunainen A. Risperidone versus other atypical antipsychotic medication for schizophrenia [abstract] The Cochrane Database of Systematic Reviews. 2000;3 doi: 10.1002/14651858.CD002306. doi: 10.1002/14651858.CD002306. [DOI] [PubMed] [Google Scholar]
- 9.Tuunainen A, Wahlbeck K, Gilbody SM. Newer atypical antipsychotic medication versus clozapine for schizophrenia [abstract] The Cochrane Database of Systematic Reviews. 2000;2 doi: 10.1002/14651858.CD000966. doi: 10.1002/14651858.CD000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagnall AM, Jones L, Ginnelly L, et al. A systematic review of atypical antipsychotic drugs in schizophrenia. Health Technol Assess. 2003;7(13):1–204. doi: 10.3310/hta7130. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. (DSM-IV). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 12.Heinrichs DW, Hanlon TE, Carpenter WT. The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull. 1984;10:388–398. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- 13.Lehman AF. Measures of quality of life among persons with severe and persistent mental disorders. Soc Psychiatry Psychiatr Epidemiol. 1996;31:78–88. doi: 10.1007/BF00801903. [DOI] [PubMed] [Google Scholar]
- 14.Kay SR, Fisbein A, Opler LA. The Positive and Negative Syndrome Scale for schizophrenia. Schizophr Bull. 1989;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1996;3:247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- 16.Hogan TP, Awad AG, Eastwood R. A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med. 1983;13:177–183. doi: 10.1017/s0033291700050182. [DOI] [PubMed] [Google Scholar]
- 17.Hayward P, Chan N, Kemp R, Youle S, David A. Medication self-management: a preliminary report on an intervention to improve medication compliance. J Ment Health. 1995;4:513–519. [Google Scholar]
- 18.Simpson G, Angus JSW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;212(suppl):9–11. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 19.Lingjaerde O, Ahlfors U, Bech P, Dencker SJ, Elgen K. The UKU side effects rating scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side-effects in neuroleptic-treated patients. Acta Psychiatr Scand. 1987;76:1–10. doi: 10.1111/j.1600-0447.1987.tb10566.x. [DOI] [PubMed] [Google Scholar]
- 20.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Government Printing Office; 1976. [Google Scholar]
- 21.SPSS Inc. SPSS for Windows Release 10.1.0. Chicago, Ill: SPSS Inc; 2001. [Google Scholar]
- 22.StataCorp. Stata Statistical Software: Release 7.0. College Station, Tex: Stata Corporation; 2001. [Google Scholar]
- 23.Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear mixed models using adaptive quadrature. Stata J. 2002;2:1–21. [Google Scholar]
- 24.Dunn G, Maracy M, Dowrick C, et al. Estimating psychological treatment effects from an RCT with both non-compliance and loss to follow-up. Br J Psychiatry. 2003;183:323–331. doi: 10.1192/bjp.183.4.323. [DOI] [PubMed] [Google Scholar]
- 25.Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with non-compliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res. 2005;14:369–395. doi: 10.1191/0962280205sm403oa. [DOI] [PubMed] [Google Scholar]
- 26.Levy DE, O'Malley AJ, Normand SL. Covariate adjustment in clinical trials with non-ignorable missing data and non-compliance. Stat Med. 2004;23:2319–2339. doi: 10.1002/sim.1848. [DOI] [PubMed] [Google Scholar]
- 27.O'Malley AJ, Normand SL. Likelihood methods for treatment noncompliance and subsequent nonresponse in randomized trials. Biometrics. 2005;61:325–334. doi: 10.1111/j.1541-0420.2005.040313.x. [DOI] [PubMed] [Google Scholar]
- 28.Lavori PW, Rush JA, Wisniewski SR, et al. Biol Psychiatry. 2001;50:792–801. doi: 10.1016/s0006-3223(01)01223-9. [DOI] [PubMed] [Google Scholar]
- 29.Citrome L, Volavka J. Optimal dosing of atypical antipsychotics in adults: a review of the current evidence. Harv Rev Psychiatry. 2002;10(5):280–291. doi: 10.1080/10673220216279. [DOI] [PubMed] [Google Scholar]
- 30.Van der Zwagg C, McGee M, McEvoy JP, Freudenreich O, Wilson WH, Cooper TB. Response of patients with treatment refractory schizophrenia to clozapine with three serum level ranges. Am J Psychiatry. 1996;153:1579–1584. doi: 10.1176/ajp.153.12.1579. [DOI] [PubMed] [Google Scholar]
- 31.Kronig MH, Munne RA, Szymanski S, et al. Plasma clozapine levels and clinical response for treatment-refractory schizophrenic patients. Am J Psychiatry. 1995;152:179–182. doi: 10.1176/ajp.152.2.179. [DOI] [PubMed] [Google Scholar]
- 32.Rostami-Hodjegan A, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol. 2004;24:70–78. doi: 10.1097/01.jcp.0000106221.36344.4d. [DOI] [PubMed] [Google Scholar]
- 33.Jonas PB, Davies L, Barres TR, et al. Randomized controlled trial of effect on quality of life of second generation versus first generation antipsychotic drugs in schizophrenia. Arch Gen Psychiatry. doi: 10.1001/archpsyc.63.10.1079. in press. [DOI] [PubMed] [Google Scholar]