Abstract
The disrupted in schizophrenia 1 (DISC1) gene has been linked to schizophrenia and other serious mental illnesses in multiple pedigrees. This article will review the neurobiology of DISC1 in normal developing and adult brain and the putative role of the mutant form in major mental illness, particularly schizophrenia. The initial genetic finding of an association between DISC1 and schizophrenia in a Scottish population has now been replicated in Finnish, American, Japanese, and Taiwanese populations. DISC1 is present throughout the brain of a variety of species during development and adulthood, including many of the brain regions known to be abnormal in schizophrenia, such as the prefrontal cortex, hippocampus, and thalamus. The functions of DISC1 in the developing brain include neuronal migration, neurite outgrowth, and neurite extension. In the adult, DISC1 has been identified in multiple populations of neurons and in structures associated with synaptic function, suggesting that one of its adult functions may be synaptic plasticity. DISC1 is associated with numerous cognitive functions that are abnormal in schizophrenia. Converging evidence from cell culture, mice mutants, postmortem brain, and genetics implicates mutant DISC1 in the pathophysiology of schizophrenia and other mental illnesses.
Keywords: development, schizophrenia, genetics, postmortem, plasticity
Introduction
Major mental illnesses such as schizophrenia, schizoaffective disorder, bipolar disorder, and major depression are complex and devastating diseases of the brain. These illnesses share in common certain symptoms, risk factors, and are undoubtedly produced by a combination of genetic and environmental causes. Schizophrenia is a common, chronic disorder characterized by psychosis, cognitive impairments, and deficit symptoms in a subset of patients. Studies of environmental risk factors point to gestation, suggesting that abnormalities in early brain development may play a role in the disorder.1 Genetic factors also play a role in the risk of schizophrenia.2 There are now several genes that are undergoing intensive study because they appear to be susceptibility genes for schizophrenia as well as some of the aforementioned diseases. Disrupted in schizophrenia 1 (DISC1) is one such gene,3–6 and many aspects of its neurobiology are consistent with a role for DISC1 in schizophrenia.7
Genetic Linkage Studies
In addition to increased risk for developing schizophrenia in relatives of those with the disease, numerous studies have found genetic mutations linked to the disease. For example, a (1/11) (q42.1; q14.3) balanced chromosomal translocation was found in a Scottish family.8 In affected subjects, a segment of chromosome 1 was located on chromosome 11 and vice versa. This translocation and subsequent dysregulation are what are associated with schizophrenia. Two genes straddle the breakpoint on chromosome 1, one transcript with an open reading frame, DISC1, is expressed as protein; the other transcript, DISC2, is antisense to DISC1 and appears not to be expressed as a protein product.6 When a psychiatric evaluation of family members was undertaken, this chromosomal abnormality was associated with an increased risk of psychiatric disorders. The strength of the association between the chromosomal translocation and psychiatric illness was greatest for a broad phenotype that included schizophrenia, major depression, and bipolar disorder.6,9–11 This association has now been extended to the general Scottish population and beyond.12,13 For example, a frame shift mutation in DISC1 has been reported in American probands with schizophrenia.14 The most significant finding in a linkage analysis of schizophrenia in a Finnish population was an intragenic marker in the DISC1 gene,15 a finding confirmed with haplotype transmission analysis.16 In a Taiwanese sample, 2 single nucleotide polymorphisms (SNPs) between introns 4 and 5 of the DISC1 gene were identified in single locus and haplotype association analyses.17
Moreover, associations have been found between mutant DISC1 and specific symptoms in schizophrenia and bipolar disorder. An exonic SNP in DISC1 is associated with normal cognitive changes in the aging process.18 Using SNPs, Liu et al17 found an association between abnormal DISC1 and schizophrenic patients who had deficits in sustained attention. The mutant DISC1 genotype also appears to be related to some of the neurocognitive deficits present in schizophrenia, such as memory problems.19,20 Using human lymphoblasts, certain haplotypes of abnormal DISC1 mRNA are associated with manic symptoms in bipolar subjects.9 Although mutant DISC1 has not been identified as a gene of risk in all populations tested thus far, the overwhelming evidence points to a role for DISC1, and/or its associated proteins, in these illnesses.
Location and Function in Schizophrenic Brain
Most of DISC1 studies to date have not examined postmortem brains from schizophrenia subjects. However, evidence from experimental animals and normal human has shown that DISC1 is present in critical brain regions known to be abnormal in schizophrenia,21 including human cerebral cortex22 and hippocampus.23 The subcellular distribution of one of the DISC isoforms is altered in orbitofrontal cortex in schizophrenia.24 A particular allelic variation in DISC1 is associated with altered structure and function of the hippocampus.25 The expression of NUDEL, LIS1, and FEZ1, binding partners of DISC1, are reduced in hippocampus and prefrontal cortex of subjects with schizophrenia; interestingly, DISC1 mRNA was normal in this study.26 Schizophrenic patients with aberrant expression of the DISC1 gene have reduced frontal cortical gray matter volume.20
DISC1 in Animal Models
Animal models of human brain disorders are crucially important in trying to elucidate the pathophysiology of disease. Unfortunately, there are no animal models that mimic hallucinations, delusions, and thought disorder. There are, however, many partial models that provide valuable information on specific symptoms, the role of neurodevelopment, neuropathology in a given brain region, the side effects or therapeutic mechanisms of antipsychotic medications, and the relationships between some of these factors. Importantly, cloned cDNA of DISC1 has very similar sequences in nucleotides and amino acids between human and monkey.27 Although the sequence conservation is poor between humans and rodents, the regional expression profiles are similar.27 Genetically manipulated mice are useful to study the role of schizophrenia susceptibility genes, such as DISC1. These studies can be very informative about the role of DISC1 in normal development and behavior and whether abnormalities in the gene cause similar neuropathology and functional deficits to that present in subjects with schizophrenia.7,28 For instance, impaired working memory is produced in C57BL/6J mice when the mutant DISC1 protein found specifically in the 129S6/SvEv strain of mouse is transferred to the C57BL/6J strain.29 mRNA expression in the brains of rodents treated with antipsychotic drugs indicates that some antipsychotics increase DISC1 expression in prefrontal cortex and hippocampus.30 In cell culture, DISC1 overexpression in COS-7 cells causes mitochondrial reorganization, suggesting a role for DISC1 in mitochondrial fission and/or fusion.31
DISC1 in the Developing Brain
The results of multiple investigations indicate a role for DISC1 in brain development. In the mouse, DISC1 is expressed from embryonic day 10 through adult life.32,33 In mouse brain, neocortex and limbic regions, the bed nucleus of the stria terminalis, and some thalamic nuclei express DISC1 during development. Observations during development suggest that DISC1 is involved in neurite outgrowth34,35 and neuronal migration.36,37 NUDEL, a protein essential for cortical development, neuronal migration, and axon growth, fails to bind to the mutant DISC1. This results in inhibition of neurite outgrowth in vitro and abnormal cortical development in vivo.34,37,38 Disruption of normal development may contribute to the reduced neuropil volume found in postmortem cortex in schizophrenia21 and in reduced frontal cortical gray matter volume in schizophrenic patients with the mutant DISC1 gene.20 The amount of DISC1 peaks in the mouse brain during the time of embryonic neurogenesis and again during puberty,32,33 two critical time points implicated in the pathophysiology of schizophrenia.1 DISC1 appears to change location and function between the developing and the mature brain. Other examples of proteins that do this include reelin, growth-associated protein, neural cell adhesion molecule, and brain-derived neurotrophic factor (Roberts et al39 and references therein).
DISC1 in the Adult Brain
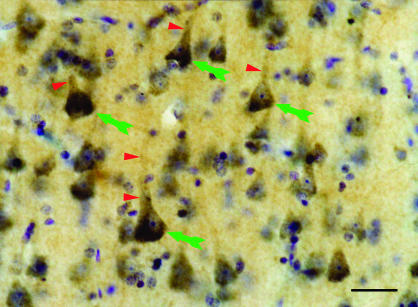
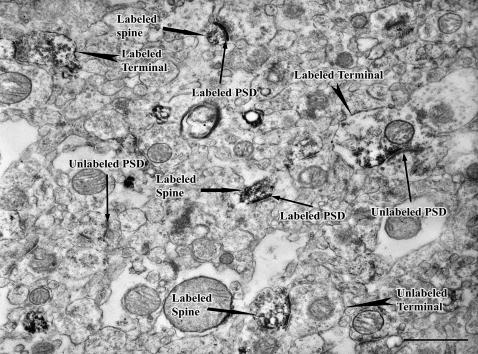
The prevalence of DISC1 in the adult brain is substantial, but maybe somewhat less so than during development, at least for the mouse.3,33 In adult mouse, DISC1 is prominently expressed in the hippocampus, cerebellum, olfactory bulb, and cerebral cortex,32,35 where it is found in both excitatory and inhibitory neurons.33 In adult monkeys, DISC1 is highly localized in many brain regions, is particularly robust in the limbic system, and is more extensively distributed than in the mouse.40 In monkeys, expression was more prominent in the dentate gyrus and lateral septum than in cerebral cortex, amygdala, hypothalamus, cerebellum, and the interpeduncular and subthalamic nuclei. In humans, DISC1 has been located in multiple neuronal populations in both hippocampus23 and neocortex.22 In neocortex, DISC1 staining is widespread and includes both pyramidal and nonpyramidal neurons (figure 1). No obvious differences in labeling pattern were seen across the cortical areas, suggesting a similar cellular function for DISC1 in these regions. At the ultrastructural level in the prefrontal cortex,22 ribosomes, rough endoplasmic reticulum, and synaptic structures were frequently, but not always, labeled (figure 2). In contrast, the machinery of protein excretion and mitochondria were not immunoreactive. The presence of DISC1 on the pre- and/or postsynaptic side of asymmetric synapses suggests the involvement of DISC1 in corticocortical and thalamocortical connections because cortical and thalamic connections both form this type of synapse.41 The presence of DISC1 in symmetric synapses suggests its involvement in inhibitory local circuit connections within the cortex because cortical interneurons form this type of synapse.41 The electron microscopic localization of DISC1 in the nucleus is consistent with molecular evidence showing that the DISC1 gene contains the nuclear localization signal and leucine-zipper motifs that are frequently found in nuclear proteins.22 Moreover, both Sawamura et al24 and James et al23 found DISC1 immunoreactivity in nuclear fractions using subcellular fractionation and cell culture, respectively.
Fig. 1.
Light micrograph of DISC1. Human prefrontal cortex immunolabeled for DISC1 (brown) and counterstained with cresyl violet (blue). Note cell body labeling, especially in pyramidal cells (green arrows), and rich neuropil staining. Labeling in proximal dendrites is common (red arrowheads). Scale bar = 50 μm.
Fig. 2.
Electron micrograph of DISC1. Human prefrontal cortex labeling of axon terminals, spines, and postsynaptic densities (PSD). Note that only a subset of spines, PSDs, and axon terminals are immunoreactive. Scale bar = 1 μm.
Results of molecular and ultrastructural studies suggest that DISC1 interacts with a number of proteins, including centrosome and cytoskeletal proteins, proteins that localize receptors to membranes, and signal transduction proteins.22,34,42,43 DISC1 labeling of some microtubules22 is consistent with other reports showing DISC1 interactions with cytoskeletal elements.44 The relationship of mutant DISC1 with microtubules causes abnormal neurite extension in development and could cause problems with proper cellular movement of mitochondria in both development and adult. In adult humans, DISC1 is prevalent throughout the cortical layers in multiple populations of neurons, axon terminals, and postsynaptic targets.22 The location of DISC1 at many synapses suggests that it may play a role in synaptic function in the adult brain.22 In conclusion, many features of the neurobiology of DISC1 make it a highly likely candidate for a role in some of the many neuropsychiatric problems that afflict patients with schizophrenia and, perhaps, other major mental illnesses.6,45
References
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 2.Shirts BH, Nimgaonkar V. The genes for schizophrenia: finally a breakthrough? Curr Psychiatry Rep. 2004;6:303–312. doi: 10.1007/s11920-004-0081-1. [DOI] [PubMed] [Google Scholar]
- 3.Devon RS, Anderson S, Teague PW, et al. Identification of polymorphisms within disrupted in schizophrenia 1 and disrupted in schizophrenia 2, and an investigation of their association with schizophrenia and bipolar affective disorder. Psychiatr Genet. 2001;11:71–78. doi: 10.1097/00041444-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 5.Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75:862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Millar JK, James R, Brandon NJ, Thompson PA. DISC1 and DISC2: discovering and dissecting molecular mechanisms underlying psychiatric illness. Ann Med. 2004;36:367–378. doi: 10.1080/07853890410033603. [DOI] [PubMed] [Google Scholar]
- 7.Ishizuka K, Paek M, Kamiya A, Sawa A. A review of disrupted-in-schizophrenia-1 (DISC1): neurodevelopment, cognition, and mental conditions. Biol Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- 8.St Clair D, Blackwood D, Muir W, et al. Association within a family of a balanced autosomal translocation with major mental-illness. Lancet. 1990;336:13–16. doi: 10.1016/0140-6736(90)91520-k. [DOI] [PubMed] [Google Scholar]
- 9.Maeda K, Nwulia E, Chang J, et al. Biol Psychiatry. Differential expression of disrupted-in-schizophrenia (DISC1) in bipolar disorder. 2006;60:929–935. [DOI] [PubMed] [Google Scholar]
- 10.Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 11.Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders–cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomson PA, Wray NR, Millar JK, et al. Association between the TRAX/DISC locus and both bipolar disorder and schizophrenia in the Scottish population. Mol Psychiatry. 2005;10:657–668. doi: 10.1038/sj.mp.4001669. [DOI] [PubMed] [Google Scholar]
- 13.Zhang F, Sarginson J, Crombie C, Walker N, St Clair D, Shaw D. Genetic association between schizophrenia and the DISC1 gene in the Scottish population. Am J Med Genet Part B Neuropsychiatr Genet. 2006;141:155–159. doi: 10.1002/ajmg.b.30274. [DOI] [PubMed] [Google Scholar]
- 14.Sachs NA, Sawa A, Holmes SE, Ross CA, Delisi LE, Margolis RL. A frameshift mutation in disrupted in schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol Psychiatry. 2005;10:758–764. doi: 10.1038/sj.mp.4001667. [DOI] [PubMed] [Google Scholar]
- 15.Ekelund J, Hovatta I, Parker A, et al. Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet. 2001;10:1611–1617. doi: 10.1093/hmg/10.15.1611. [DOI] [PubMed] [Google Scholar]
- 16.Hennah W, Varilo T, Kestila M, et al. Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12:3151–3159. doi: 10.1093/hmg/ddg341. [DOI] [PubMed] [Google Scholar]
- 17.Liu YL, Fann CS, Liu CM, et al. A single nucleotide polymorphism fine mapping study of chromosome 1q42.1 reveals the vulnerability genes for schizophrenia, GNPAT and DISC1: association with impairment of sustained attention. Biol Psychiatry. 2006;60:554–562. doi: 10.1016/j.biopsych.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Thomson PA, Harris SE, Starr JM, Whalley LJ, Porteous DJ, Deary IJ. Association between genotype at an exonic SNP in DISC1 and normal cognitive aging. Neurosci Lett. 2005;389:41–45. doi: 10.1016/j.neulet.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Burdick KE, Hodgkinson CA, Szeszko PR, et al. DISC1 and neurocognitive function in schizophrenia. Neuroreport. 2005;16:1399–1402. doi: 10.1097/01.wnr.0000175248.25535.f6. [DOI] [PubMed] [Google Scholar]
- 20.Cannon TD, Hennah W, van Erp TG, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;11:205–213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 21.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick B, Xu L, Cascella N, Ozeki Y, Sawa A, Roberts RC. DISC1 immunoreactivity at the light and ultrastructural level in the human neocortex. J Comp Neurol. 2006;497:436–450. doi: 10.1002/cne.21007. [DOI] [PubMed] [Google Scholar]
- 23.James R, Adams RR, Christie S, Buchanan SR, Porteous DJ, Millar JK. Disrupted in schizophrenia 1 (DISC1) is a multicompartmentalized protein that predominantly localizes to mitochondria. Mol Cell Neurosci. 2004;26:112–122. doi: 10.1016/j.mcn.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Sawamura N, Sawamura-Yamamoto T, Ozeki Y, Ross CA, Sawa A. A form of disrupted-in-schizophrenia-1 (DISC1) enriched in the nucleus has altered subcellular distribution in schizophrenia brains. Proc Natl Acad Sci U S A. 2005;102:1187–1192. doi: 10.1073/pnas.0406543102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callicott JH, Straub RE, Pezawas L, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipska BK, Peters T, Hyde TM, et al. Expression of DISC1 binding partners is reduced in schizophrenia and associated with DISC1 SNPs. Hum Mol Genet. 2006;15:1245–1258. doi: 10.1093/hmg/ddl040. [DOI] [PubMed] [Google Scholar]
- 27.Bord L, Wheeler J, Paek M, et al. Primate disrupted-in-schizophrenia-1 (DISC1): high divergence of a gene for major mental illnesses in recent evolutionary history. Neurosci Res. 2006 doi: 10.1016/j.neures.2006.07.010. 56:286–293. [DOI] [PubMed] [Google Scholar]
- 28.O'tuathaigh CM, Babovic D, O'meara G, Clifford JJ, Croke DT, Waddington JL. Susceptibility genes for schizophrenia: characterisation of mutant mouse models at the level of phenotypic behaviour. Neurosci Biobehav Rev. 2006 doi: 10.1016/j.neubiorev.2006.04.002. In press. [DOI] [PubMed] [Google Scholar]
- 29.Koike H, Arguello PA, Kvajo M, Karayiorgou M, Gogos JA. Disc1 is mutated in the 129S6/SvEv strain and modulates working memory in mice. Proc Natl Acad Sci U S A. 2006;103:3693–3697. doi: 10.1073/pnas.0511189103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiba S, Hashimoto R, Hattori S, et al. Effect of antipsychotic drugs on DISC1 and dysbindin expression in mouse frontal cortex and hippocampus. J Neural Transm. 2006;113:1337–1346. doi: 10.1007/s00702-005-0414-1. [DOI] [PubMed] [Google Scholar]
- 31.Millar JK, Pickard BS, Mackie S, et al. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- 32.Austin CP, Ky B, Ma L, Morris JA, Shughrue PJ. Expression of disrupted-in-schizophrenia-1, a schizophrenia-associated gene, is prominent in the mouse hippocampus throughout brain development. Neuroscience. 2004;24:3–10. doi: 10.1016/j.neuroscience.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Schurov IL, Handford EJ, Brandon NJ, Whiting PJ. Expression of disrupted in schizophrenia 1 (DISC1) protein in the adult and developing mouse brain indicates its role in neurodevelopment. Mol Psychiatry. 2004;9:1100–1110. doi: 10.1038/sj.mp.4001574. [DOI] [PubMed] [Google Scholar]
- 34.Ozeki Y, Tomoda T, Kleiderlein J, et al. Disrupted-in-schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyoshi K, Honda A, Baba K, et al. Disrupted-in-schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- 36.Brandon NJ, Handford EJ, Schurov I, et al. Disrupted in schizophrenia 1 and NUDEL form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. doi: 10.1016/j.mcn.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi MA, Portaro FC, Bastos MF, et al. Inhibition of NUDEL (nuclear distribution element-like)-oligopeptidase activity by disrupted-in-schizophrenia 1. Proc Natl Acad Sci U S A. 2005;102:3828–3833. doi: 10.1073/pnas.0500330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamiya A, Kubo K, Tomoda T, et al. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- 39.Roberts RC, Xu L, Roche JK, Kirkpatrick B. The ultrastructural localization of reelin in the cortex in postmortem human brain. J Comp Neurol. 2005;482:294–308. doi: 10.1002/cne.20408. [DOI] [PubMed] [Google Scholar]
- 40.Austin CP, Ma L, Ky B, Morris JA, Shughrue PJ. DISC1 (disrupted in schizophrenia-1) is expressed in limbic regions of the primate brain. Neuroreport. 2003;14:951–954. doi: 10.1097/01.wnr.0000074342.81633.63. [DOI] [PubMed] [Google Scholar]
- 41.Peters A. Examining neocortical circuits: some background and facts. J Neurocytol. 2002;31:183–193. doi: 10.1023/a:1024157522651. [DOI] [PubMed] [Google Scholar]
- 42.Morris JA, Kandpal G, Ma L, Austin CP. DISC1 (disrupted-in-schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum Mol Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- 43.Miyoshi K, Asanuma M, Miyazaki I, et al. DISC1 localizes to the centrosome by binding to kendrin. Biochem Biophys Res Commun. 2004;317:1195–1199. doi: 10.1016/j.bbrc.2004.03.163. [DOI] [PubMed] [Google Scholar]
- 44.Brandon NJ, Schurov I, Camargo LM, et al. Subcellular targeting of DISC1 is dependent on a domain independent from the Nudel binding site. Mol Cell Neurosci. 2005;28:613–624. doi: 10.1016/j.mcn.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Sawa A, Snyder SH. Two genes link two distinct psychoses. Science. 2005;310:1128–1129. doi: 10.1126/science.1121114. [DOI] [PubMed] [Google Scholar]