Abstract
This review synthesizes our current knowledge on the neurobiology of psychosis from an array of in vivo brain-imaging studies. The evidence base consists of hundreds of studies of patients with schizophrenia and fewer on bipolar disorder but rarely providing direct comparisons between the disorders or integration across methods. Replicated findings in schizophrenia include reduced whole-brain and hippocampal volume as potential vulnerability markers, with further progression at onset; reduced N-acetyl aspartate concentrations in hippocampus and prefrontal cortex; striatal dopamine D2 receptors upregulation; and alteration in the relation between frontal and temporal activation. These findings are not attributable to medication effects but are of unclear specificity and may apply across the psychosis spectrum. There are consistently replicated associations of psychotic symptoms and cognitive impairment in both structural and functional imaging in schizophrenia but not, as yet, in bipolar disorder. Therefore, it would be premature to dispense with current diagnostic categories because direct comparisons among them are rare, insufficient studies have examined longitudinal changes, and long-term imaging outcome studies in first-episode psychosis have not yet been done. To address these issues and make neuroimaging “clinically relevant,” investigators will need to standardize their approaches to data acquisition and analysis, and construct the necessary range of “human brain maps,” to implement studies that are sufficiently powered to provide reliable data pertinent to deconstructing psychosis.
Keywords: neuroimaging, schizophrenia, psychosis, brain structure, brain function
Introduction
Advances in neuroimaging technologies have created both opportunities and challenges in the study of psychosis. Eager to obtain a “window to the mind,” neuroimaging has been embraced by investigators applying diverse methods to examine brain structure and function in psychiatric disorders. With progress in quantitative computational anatomy methodologies, we are at the threshold of an exciting era in psychiatric research that can capitalize on the ability to study the living brain with refined approaches both for hypothesis testing and for exploration. In vivo measurement is afforded by magnetic resonance imaging (MRI) examining neuroanatomy through structural MRI (sMRI), connectivity through diffusion tensor imaging (DTI), and neurochemistry through magnetic resonance spectroscopy (MRS). Magnetic resonance also enables examination of brain physiology using functional MRI (fMRI) methods. Other functional neuroimaging methods include positron emission tomography (PET), which enables measurement of local cerebral glucose metabolism, blood flow, and receptor function. Single-photon computed emission tomography (SPECT) can also be used to measure cerebral perfusion and receptor function.
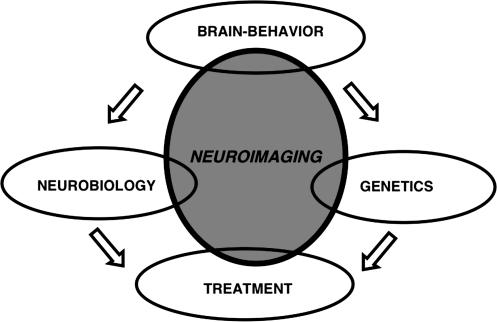
The diversity and complementarity of neuroimaging methods can place them in a crucial position for integrative translational research. Neuroimaging can intersect basic and clinical efforts in elucidating the underlying processes of complex psychotic disorders. By supplying data obtained on patients, neuroimaging has a firm hold on the clinical phenotype, and by informing on brain systems, it can link to molecular substrates. Furthermore, combining neuroimaging with genetic strategies can yield a powerful methodology with unprecedented potential for novel treatments (figure 1). The challenge we face is making this happen by mobilizing the increasing array of procedures and measures relevant to clinically important questions such as diagnosis, course of illness, and outcome.
Fig. 1.
A Schematic Representation of the Central Role of Neuroimaging Intersecting Between Basic Science and Clinical Applications.
After 3 decades of neuroimaging research, is the technology informative to efforts to deconstruct psychosis? Based on brain-imaging studies can we examine a patient with first-episode psychosis and determine with some confidence whether schizophrenia or bipolar disorder are on the horizon? Might we even be able to use imaging as an early diagnostic aid in those at genetic or symptomatic high risk?
The research agenda in neuroimaging and psychosis has not been geared from the outset to be clinically relevant in differential diagnosis. Rather, most studies in psychosis have focused on 1 disorder with the explicit primary goal of understanding its specific pathophysiology. An implicit secondary goal has been to improve diagnosis and clinical management. When imaging, commonly structural, has been applied clinically as part of the workup of a psychotic patient, the purpose has been to rule out a space occupying lesion or developmental malformation that may potentially cause the psychosis. Although incidental findings have been reported in MRI studies of even healthy people1 and patients who present with psychosis,2 such findings are infrequent and commonly asymptomatic. This is not to say that obtaining a scan is of no value where an organic psychosis is suspected; a recent analysis of 253 adult psychiatric patients who underwent a clinical MRI, 38 (15%) had some form of treatment modification as a result of the neuroimaging findings, and in 6 patients a medical condition was identified as a result of the MRI.3 However, in the absence of quantitative analysis, routine brain imaging cannot aid in the differential diagnosis of psychosis without considering the clinical presentation.4 Thus far, studies using imaging techniques to determine prognosis or treatment response have not generated sufficiently replicated findings. There are, however, encouraging results from several studies evaluating these technologies as possible predictors of diagnosis.
Most neuroimaging studies have been conducted in schizophrenia. A PubMed search in October 2006 shows 490 citations for “schizophrenia and neuroimaging” and only 134 for “bipolar and neuroimaging.” Only 31 studies are cited for the conjunctive “schizophrenia and bipolar and neuroimaging” query. Few prospective studies contain the information that would permit comparison between patients with schizophrenia and those with bipolar illness. Inconsistent findings within disorders have often led to controversy and have been attributed to disease heterogeneity. Over the past decade, advances in quantitative techniques have established some firm findings about schizophrenia and related disorders. As importantly, these techniques have also highlighted areas where further study is required and where methodological practices need to be improved.
This article will briefly highlight the knowledge we have gained about psychosis using brain-imaging methods by emphasizing the results from consistently replicated studies, systematic reviews, and meta-analyses of the relevant literature. We shall consider structural imaging (sMRI, DTI), neurochemical imaging (MRS, receptor studies), and functional imaging techniques in patients with schizophrenia and the affective psychoses, including studies of at-risk populations. The latter enable integration of genetic and neuroimaging paradigms in our efforts to elucidate neurobiological mechanisms that underlie these disorders that may guide treatments.
Structural Magnetic Resonance Imaging and Diffusion Tensor Imaging
sMRI Studies of Patients
An extensive literature, presented in reviews4–6 and meta-analyses,7–11 documents consistent morphometric differences between patients with schizophrenia and healthy people. There is whole-brain volume reduction of about 3% in patients, particularly in gray matter,7,8 and a concomitant increase in cerebrospinal fluid (CSF). Volume reductions have been most notable in frontotemporal regions. Medial temporal lobe (MTL) structures and, particularly, the hippocampus and amygdala are reduced by a greater amount than the whole brain.6,10 This is also probably true of the prefrontal cortex (PFC) and other parts of the temporal lobe, particularly the superior temporal gyrus (STG).5,8 There is evidence that the thalamus is likewise reduced in volume to a greater extent than the whole brain.11 The size of the corpus callosum, a white matter fiber bundle, is reduced to a roughly similar extent as the whole brain.9
The region of interest (ROI) analytic approach initially applied has been replaced by automated methods for regional parcellation and voxel-based morphometry that can efficiently yield information on the entire brain, permitting validation of reported findings and new discovery of other affected regions. Based on morphological parameters, it is possible to apply high-dimensional nonlinear pattern classification techniques to quantify the degree of separation of patients with schizophrenia and healthy controls. Such procedures enable testing the potential of sMRI as an aid to diagnosis. In a recent study of patients with schizophrenia and healthy controls, such a procedure demonstrated average classification accuracy of 82% for women and 85% for men.12 While such automated methods are promising, further investigation is needed and we cannot yet rely solely upon such approaches.
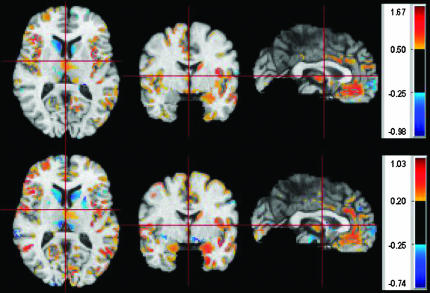
Whole-brain size reductions observed in schizophrenia have been demonstrated to have “concurrent validity” by quantitative review of postmortem studies.13 A review of computational voxel-based morphometry studies highlighted that they consistently find gray matter density reductions in MTLs and the STG.14 Furthermore, there are replicated associations between STG volumes and positive symptoms and between MTL reductions and memory impairment.6,15,16 Figure 2 illustrates application of deformation-based morphometry to compare a sample of patients with schizophrenia to healthy controls.12
Fig. 2.
Effect sizes of control/patient group difference, calculated separately for neuroleptic-naive (top) and treated patients with Schizophrenia (bottom). The spatial patterns are similar, except that treated patients display generally stronger effect sizes. Blue means that the respective structures were relatively larger in patients than in healthy controls. Thus, treated patients showed a pronounced increase in basal ganglia volumes. (from Davatzikos et al12)
These abnormalities are unlikely to be confounded by factors such as antipsychotic medication or substance abuse. Most MRI studies that examined the specific ROIs, highlighted above, also evaluated possible relationships with antipsychotic medication status or doses and very rarely find any—with the exception of some parts of the basal ganglia. In particular, increases of up to 20% in the volume of the globus pallidus are regularly related to first-generation (typical) antipsychotic medication dose.8 A review examining the effect of typical antipsychotics on brain structure revealed basal ganglia volume increases and cortical gray matter decreases, detectable even after a 12-week treatment period.17 However, most studies have involved indirect and nonrandomized comparisons in analyses that seemingly seek to establish that the second-generation (atypical) antipsychotics have beneficial effects on neuroanatomy. There are no consistently replicated accounts of particular drugs having beneficial effects in specific brain regions. Because patients with psychosis may have comorbid substance abuse, the possible effects of such substances should also be considered. The effects of alcohol abuse on the brain are usually generalized, or show a PFC rather than temporal lobe bias, and the abnormalities in schizophrenia noted above are present in patients with no history of alcohol abuse. It is unclear if cannabis has any effects on brain structure, and other substances are used too infrequently to be likely confounders.
More pertinent limitations of this literature are highlighted in a recent review of sMRI in first-episode schizophrenia studies that confirms only a reduction in the volumes of the whole brain and of the hippocampus.18 This raises the clear need for further studies of recent onset patients to determine if other abnormalities are evident at that time or if they are progressive19; although it will clearly be very difficult, if not impossible, to distinguish the effects of illness duration from the effects of ongoing antipsychotic treatment.
Notably, similar findings, at least concerning the whole brain and hippocampus, are evident in dementia. The cognitive deficits in schizophrenia and its early characterization as dementia praecox buttress that similar brain systems may be affected, with an underlying different neuropathology and decades apart. Of more direct clinical concern, as the structural neuroimaging literature in bipolar disorder and depression accrues, it seems that the neuroanatomy of affective disorder is qualitatively similar to that in schizophrenia but merely less marked in quantitative terms. At present, the only disease-specific finding is that patients with bipolar disorder may not have whole-brain volume reduction that is evident in schizophrenia,20–23 may not show volume reductions in amygdala and may even show volume increases in amygdala at particular stages of the illness.23 It is now clear that the hippocampus is reduced in volume even in depression.24,25 These hippocampal reductions may be related to the number of depressive episodes and may even be more marked in patients with severe depression than in the general population of patients with schizophrenia. Finally, there are consistent reports and meta-analyses of an increased frequency of signal hyperintensities in affective disorder22–27 that may be specific but of uncertain pathologenesis.
There have been too few direct comparisons of patients with schizophrenia and bipolar disorder, let alone other psychoses, to evaluate neuroanatomical differences among the disorders. These studies have relatively small samples and few have addressed changes over time—the basis on which the disorders were originally separated. A useful strategy that can address the issue of diagnostic specificity is the study of patients with first-episode psychosis who are followed longitudinally. Once the diagnosis is established, intake sMRI measures are examined for possible differences among groups.28–32 The available reports are inconsistent. For example, left prefrontal gray matter volume reduction was noted in first-episode schizophrenia and not in affective psychosis.28 However, in male adolescents, increased CSF and reduced gray matter volumes in the frontal lobes did not distinguish those who developed schizophrenia from those who did not.31 Such studies are important because they enable testing the hypothesis that there is more progression of abnormalities in those with first-episode psychosis who go on to develop schizophrenia as compared with affective disorder, but this key question would be much more practicably and quickly addressed in multicenter than single-center studies.
There is a relative lack of studies in the affective disorders examining the associations of sMRI findings to clinical and neurobehavioral features. In schizophrenia, there are demonstrated associations between memory difficulties and positive psychotic symptoms and the size of the hippocampus, the STG, and the temporal lobe in general and between executive function, negative symptoms, and PFC measures,6,15,16 but these relationships have not been documented in bipolar disorder.
Diffusion Tensor Imaging
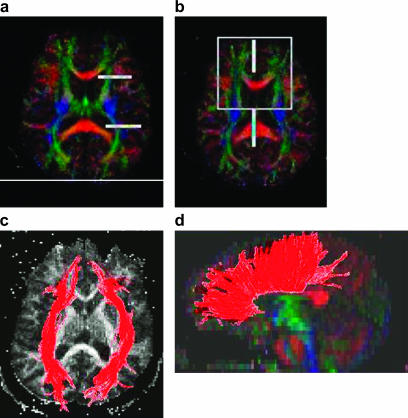
DTI examines white matter integrity and is a more recent addition to structural measures (figure 3). As might be expected with such a rapidly developing technology, there are some replicated findings in the schizophrenia literature, but it has been particularly hampered by the wide array of different approaches both to acquire the data and to analyze it.33 With the development of tractography techniques, a common approach by the imaging community could facilitate progress. Although gray matter volume deficits are more marked than white matter abnormalities in schizophrenia, reduced anisotropy (a measure of directionality of flow of water molecules in axons, thereby an index of white matter integrity) is observed with DTI in many brain regions. This finding suggests that white matter structure may be disorganized in schizophrenia rather than reduced in size.33 One major appeal of DTI is that it can directly test the prevailing view of schizophrenia (and psychosis in general) as a disconnection disorder.
Fig. 3.
Illustration of DTI measures showing fractional anisotropy (a, b) and with delineation of specific ROIs corresponding white matter tracts can be visualized showing front-back (c) and left-right callosal connectivity (d) (courtesy of R. Verma, University of Pennsylvania).
Studies of Relatives and Others “at Risk”
sMRI studies of the MTL have been the focus of most attention in people at risk. Early ROI studies tended to examine the amygdala and hippocampus together and consistently found reductions in relatives compared with controls, but most relatives did not have volume reductions to pathological levels.15,34 The balance of the evidence was for hippocampal differences in particular, although there were some notable and quite large negative studies. A comprehensive review concluded that reduced hippocampi were likely to be a vulnerability marker for schizophrenia.15 This view has recently been supported by a systematic review and meta-analysis of studies of relatives that finds hippocampal reductions in relatives, with an effect size of about 0.3, and additional differences between relatives and patients.35
Despite the small number of studies, there are already replicated computational voxel-based morphometry studies in the relatives of patients with schizophrenia vs bipolar disorder. Both Job et al36 and Diwadkar et al37 found reduced gray matter in PFC in relatives at high risk for schizophrenia. Similarly, both McIntosh et al38 and McDonald et al39 have reported reductions in gray matter density in prefrontal regions and thalamus in schizophrenia as distinct from no reductions in gray matter in these regions in bipolar disorder. Reductions in the thalamus have been reported as a measure of genetic liability to psychosis in general.39,40
The implication of such findings is that there are dissociable state and trait-imaging markers of psychosis. Therefore, vulnerability markers may predict schizophrenia before clinical presentation, expecting further volume reduction near the onset of psychosis. The 2 main studies to have addressed these issues to date are the Edinburgh High-Risk Study (EHRS) and the study conducted in the Personal Assessment and Crisis Evaluation clinic in Melbourne, Australia. These pioneering studies have examined large populations of people at risk, for genetic or clinical reasons, over almost 10 years. A total of 5 articles have been published by these 2 research groups concerning the possible predictive utility of a reduced hippocampal volume, and even different reports from the same study are conflicting. Thus, it seems that any predictive effect is inconsistent and at most weak.41–45 More encouragingly, both groups have also evaluated changes in brain structure over time and reported complementary results. Pantelis et al42 demonstrated reductions in gray matter in the left parahippocampal and fusiform gyri, as well as other regions in frontal and left cerebellar cortex, over approximately a year in 11 people as they developed a diagnosis of psychosis, usually schizophrenia. Job et al43 revealed reductions in gray matter density in left (para) hippocampal uncus, fusiform gyrus, and right cerebellar cortex in 8 individuals at high risk, for familial reasons, who developed schizophrenia on average 2.5 years after the first of 2 scans, obtained approximately 18 months apart. This replication suggests reductions in temporal lobe structure around the time of transition to diagnosis of psychosis and, to some extent, predating the conversion. The EHRS in particular makes it clear that such changes may occur years prior to diagnosis and cannot be attributable to medication as none of the participants were medicated until after their second scan and establishing their diagnosis.
The Edinburgh group has gone a step further and evaluated the diagnostic properties of these reductions in gray matter density as a possible “early diagnostic test,” by comparing the 8 subjects who had 2 scans and developed schizophrenia with either 10 patients, with similar psychotic symptoms at the time of scanning, who did not go on to have schizophrenia, and 57 high-risk subjects who had 2 scans regardless of whether or not they had symptoms. In both cases, temporal lobe volume reductions show very promising diagnostic properties, with positive predictive values (PPVs) of around 70% for these regional reductions individually, and about 80% in combination.46 These PPVs can be contrasted with much lower values for psychotic symptoms and behavioral measures. In the high-risk study, approximately 12.5% of those “at risk” developed schizophrenia as did (only) 25% of those with psychotic symptoms as well and about 30% of those scoring poorly on the Rey Auditory Verbal Learning Test and approximately 50% of those scoring above the cutoff on schizotypy measures.44 Structural imaging clearly adds clinical value here, but there are important questions about the practicality of using such an approach in clinical practice and early diagnosis would only be justified if an intervention was available for such patients.
A major limitation is the very small sample sizes in these studies. Replication with larger samples is needed and can best be achieved in multicenter collaborations. The standardization of imaging techniques and approaches to analysis is essential for deriving a ‘human brain map’ with detailed information about relevant changes in brain structure during the normal range of neurodevelopment. Such extensive information might be required before significant progress can be made in applying structural imaging techniques to clinical issues in psychosis.
Neurochemical Imaging
Magnetic Resonance Spectroscopy
MRS provides a noninvasive tool to investigate metabolites in the living human brain. Being safe, this technique allows investigation of the effects of the illness course as well as the medications on these metabolites. Much MRS work has focused on investigating phosphorus (31P-MRS) and proton-containing metabolites (1H-MRS).47,48
Proton MRS metabolites include N-acetyl aspartate (NAA), creatine, choline, myoinositol, glutamine, glutamate, glutathione, and Gamma-aminobutyric acid (GABA). NAA is mainly synthesized in neurons and is therefore regarded as a putative marker for neuronal loss or dysfunction.49,50 However, NAA levels may also reflect the integrity of glial cells.51 NAA is also important for membrane phospholipid and mitochondrial metabolism.52,53
A reduction in NAA peaks is found in most studies of patients with chronic schizophrenia. Such deficits encompass several brain regions, notably hippocampus and frontal cortex. A recent systematic review and meta-analysis of 64 published studies involving 1209 schizophrenia patients and 1256 controls suggested consistent evidence of NAA reductions in the frontal lobes and the hippocampus.54 NAA reductions appear to be associated with cortical atrophy, cognitive impairment, and negative symptoms.48 Furthermore, NAA reductions have been correlated with increased illness duration55 supporting the possibility of a progressive impairment of neuronal integrity as the illness unfolds.
NAA reductions are established and clinically used in studies of several neurological disorders including stroke and multiple sclerosis. Among psychiatric disorders, euthymic bipolar patients have decreases in NAA in frontal lobe structures and hippocampus, reported in a review of 22 studies involving 328 adult bipolar and 349 control subjects.56 On the other hand, a systematic review and meta-analysis by the same authors of Major Depressive Disorder (MDD) indicated increased choline-containing metabolites in the basal ganglia but no alteration of NAA.57 The diagnostic specificity of NAA reduction remains to be further clarified.
NAA reductions are present in first-degree relatives, who are at Genetic High Risk (GHR) for Schizophrenia, though the results are more variable than in patients. Nonpsychotic relatives of schizophrenia patients showed NAA/choline ratio reductions in the anterior cingulate.34,58 By contrast, Tibbo et al59 observed elevated glutamatergic metabolites but no other metabolite alterations in high-risk offsprings of schizophrenia patients in a 3T MRS study. Jessen et al60 used proton MRS to examine neurochemical characteristics of the brain in people deemed clinically at high risk (CHR) for schizophrenia (the prodromal state, defined by the presence of subthreshold psychotic-like symptoms). They observed that reduced NAA/choline ratios in the anterior cingulate predicted psychosis during longitudinal follow-up. Wood et al61 reported increased NAA/creatine ratios in the dorslolateral prefrontal cortex in CHR subjects; this findings did not predict those who “converted” to schizophrenia during follow-up. Collectively, these observations suggest that alterations of NAA in prefrontal structures may represent a vulnerability indicator for schizophrenia in GHR subjects and even less consistently in CHR subjects. More data are needed to replicate these observations if they have to be of any value as clinically useful predictive markers for schizophrenia.
31P-MRS investigations in drug-naive first-episode psychosis patients suggest increased membrane breakdown at the onset of psychosis,62–65 and in most studies, there appears to be reduced membrane generation in early and chronic schizophrenia. Cell membrane changes occur prominently during cell generation, and synaptogenesis, but also during cell degenerative processes such as apoptotic elimination of dendrites and axons (pruning) and cell death. Cell membrane alterations of patients with schizophrenia are also well documented in peripheral and postmortem brain tissue at different stages of the disorder (for review see Berger et al66). Such findings may reflect a reduction in neurons, glia, or synapses in schizophrenia. Studies of adolescent offspring at increased genetic risk for schizophrenia show membrane alterations similar to those observed in patients with early schizophrenia67; these changes are more pronounced in the at-risk adolescents who have already begun to manifest psychopathology.68
Interestingly, patients with manic psychosis appear to have an increase in membrane precursors,69 that may reflect a compensatory increase in cell generation or synaptogenesis during manic exacerbation of psychotic disorders. This suggests that there might be some measure of diagnostic specificity for 31P-MRS changes, but the number of studies in bipolar patients and other psychiatric disorders is too small to have confident application of these findings as clinical markers for diagnosis.
Neuroreceptor Studies
PET and SPECT provide an important avenue to examine in vivo neurochemistry. The investigation of receptor function with PET followed progress with in vitro binding measurements and autoradiography. Earlier ligand studies in schizophrenia have examined primarily dopamine (DA) receptor properties and particularly D2. The application of D2 receptor PET studies to neuroleptic-naive patients yielded initially somewhat inconsistent results; data from Johns Hopkins investigators showed increased occupancy with11 C-N-methylspiperone,70 but Karolinska investigators using11 C-Raclopride did not.71 These discrepancies in the literature might be related to several factors, such as differences in patient population, ligands used, and modeling methods.72 The emphasis in studying neuroleptic-naive patients in a limited number of settings that can apply the technology resulted in relatively small samples with commonly less than 20 patients per study. However, an early systematic review of 17 postmortem and PET studies found a large effect size of almost 1.5,73 accompanied by increases in both D2 receptor density and affinity. Several comprehensive reviews have come to the same conclusion.74,75
A consistent literature has emerged indicating increased presynaptic dopaminergic turnover in schizophrenia. Such studies measured striatal fluorodopa uptake as an index of increased dopa decarboxylase activity and greater presynaptic DA turnover in the striatum. Increased activity of DA neurons in the striatum appears to be associated with clinical status and is more evident during acute exacerbations and presence of positive symptoms.75 Notably, such effects are consistent with studies of neuropharmacological stimulants, such as amphetamine, and cannot be attributed to antipsychotic medication because, approximately, half the studies have been conducted in medication-free, including neuroleptic-naive, patients.
Increased striatal DA, most evident in patients with active psychotic symptoms, has been related to the positive symptoms of schizophrenia. More recently, neuroreceptor studies have related DA function to cognitive processes in schizophrenia. Cortical DA transmission via D1 receptors may play a role in impaired working memory and negative symptoms,76 whereas striatal DA activity via D2 receptors may modulate response inhibition, temporal organization, and motor performance.77
Most neuroreceptor studies have been conducted in patients with schizophrenia, and it is unclear if the relation between striatal DA function and psychosis is unique to schizophrenia or is evident in other disorders with psychotic features. Recent PET studies in bipolar disorder have examined different systems implicated in the pathophysiology of the disorder including serotonin transporter binding78 and the muscarinic receptor.79 Thus, there is insufficient knowledge to determine whether receptor neuroimaging can be helpful is differentiating among psychotic disorders.
Receptor imaging by PET and SPECT allows investigation of in vivo targets for antipsychotic drug action.80 It is now known that extrapyramidal (parkinsonian) side effects of first-generation antipsychotic drugs result from high striatal DA D2 receptor blockade (∼75%), while second-generation antipsychotic drugs produce therapeutic benefit in relation to modest and transient striatal D2 receptor occupancy levels (∼65%). These neuroimaging observations point to a rationale for the use of relatively low doses of first-generation antipsychotics and equivalent doses of second generation antipsychotics,81 though use of neuroimaging to determine dose ranges in a given patient is far from practical. Neuroreceptor PET/SPECT studies are valuable research tools that can help examine compounds that may regulate or stabilize DA, as well as nondopaminergic pathways, such as serotonin, glutamate, and GABA that may offer promising targets for drug development.
Functional Imaging
Studies of Patients
The functional imaging literature in schizophrenia has evolved from PET studies measuring glucose metabolism and blood flow to fMRI studies with activation paradigms. Diverse neurobehavioral probes have been applied in activation paradigms, designed to elucidate the underlying brain circuitry. Tasks applied have evaluated executive function such as attention, abstraction, and working memory, as well as declarative and procedural memory, language, spatial, sensorimotor, and emotion processing. The breadth of approaches has precluded the establishment of a functional imaging phenotype of schizophrenia. Nonetheless, there is an emerging consistency of findings.82
The early emphasis on “hypofrontality” in schizophrenia has been refined. A review of the PET literature found 21 resting studies with an overall effect size of 0.64 and 9 activation studies with an overall effect size of 1.13.83 A more recent review of PET and SPECT studies examined 47 reports with relative resting measures of cerebral activity, 29 with absolute resting baseline measures, and 14 activation paradigms. Studies with neurobehavioral probes included similar numbers of those using the Wisconsin Card Sort Test, the Continuous Performance Task, and a variety of other probes. While some similarity in the pattern of brain activity was observed across experiments, there was substantial heterogeneity.84 A potential strength in activation studies is the ability to relate the extent of activation to performance obtained “on line.” However, relative underactivation in patients who have difficulties performing a task may reflect a deficit in underlying processes related to that task or lack of engagement.84,85 Notably, PET and fMRI studies that attempted to correct for patients' impairment, by balancing performance of patients and healthy controls, often found no hypofrontality or even hyperfrontality.14 In 2 recent systematic reviews, however, 12 N-back (working memory) fMRI studies and 18 episodic memory studies with PET or fMRI found “hypofrontality” in dorsolateral and inferolateral prefrontal cortex, respectively.86,87 Glahn et al87 also reported hyperfrontality in medial areas including (dorsal) anterior cingulate. Antipsychotic medication is likely to normalize performance on these tasks and hypofrontality.88 While the majority of studies were conducted in patients with schizophrenia, reduced or increased frontal lobe activity is also evident in bipolar disorder,21,22 but direct comparisons of the groups are rare.89
Regarding the temporal lobe, an early review found fairly consistent evidence of increased temporal lobe activity in 13 SPECT studies and 6 PET studies.90 These increases were cortical, but Achim and Lepage86 recently reported bilateral reductions in perfusion in the MTLs. Perhaps, a hypothesis that will incorporate these findings will evaluate the interaction between laterality and frontality. For example, lateral cortex hypofrontality and hypertemporality may interact with a mirror image in medial hyperfrontality-hypotemporality.
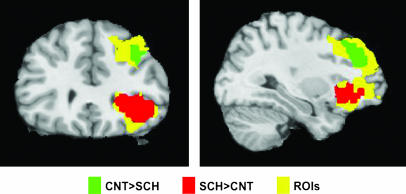
Such a synthesis of the available literature for lateral cortical regions is certainly in keeping with early and contemporary accounts of the disconnectivity hypothesis of schizophrenia and replicated findings of reduced frontotemporal and frontoparietal functional connectivity.91,92 PET, SPECT, and fMRI studies (figure 4) of disconnectivity are also supported by accounts of reduced coherence and gamma asynchrony with electroencephalograpy and magnetoencephalography in schizophrenia.93,94 Where medial regions have been invoked in such systems, it has usually been in terms of medial frontal regions modulating lateral frontotemporal interactions. There is, as yet, no systematic review of this literature and no generally adopted approach to acquiring and processing data for functional and effective connectivity analyses. Establishing such a framework must be another priority for the imaging community.
Fig. 4.
An fMRI word-encoding study showing connectivity differences between patients with schizophrenia and healthy controls in left superior temporal gyrus (STG) to dorsolateral prefrontal cortex (DLPFC) and to ventrolateral prefrontal cortex (VLPFC). A region of DLPFC shows greater connectivity with STG in controls, while a region in VLPFC shows greater connectivity with STG in patients (from Wolf et al99).
Studies in Relatives and Clinical Utilities
Functional imaging studies in family member of patients with schizophrenia are limited. There are less than 10 perfusion studies and fewer still reports of disconnectivity. There are approximately equal numbers of accounts of hypofrontality, no significant perfusion deficits, and hyperfrontality under different conditions.95 More obviously consistent are replicated accounts of PFC disconnectivity.96–99
Comparative studies of diagnostic specificity are again few. The possibilities of using functional imaging, and particularly ligand binding, to predict treatment response and prognosis have also been understudied, with few replicated results. In a preliminary “proof of concept” study from the EHRS, fMRI could indeed predict the later development of schizophrenia—but this was in a post hoc analysis of only 4 patients.98
Functional Imaging in Relation to Treatment
Several studies have examined changes in abnormal brain function in relation to antipsychotic medications. Davis et al88 reviewed 21 functional imaging (fMRI and PET) studies involving a pretreatment baseline study and at least one posttreatment follow-up study. Overall, the studies suggested normalization of brain function (ie, patients were more similar to controls following treatment, especially with second-generation antipsychotic drugs), though a wide variability of findings was evident due to methodological limitations such as lack of reliability of data, varying subject populations, research designs, and statistical approaches. Functional imaging can also contribute in pharmacologic provocative studies as well as in nonpharmacological behavioral interventions.100,101
Conclusions and Recommendations
The field of neuroimaging in psychotic disorders has made progress especially in schizophrenia, where methods have been initially applied. While there is increased consistency within disorders across methods, there is paucity of work comparing diagnostic specificity of findings. These are exactly the studies required to deconstruct psychosis.
Steps are underway that begin to provide important information: there is a growing literature of structural imaging studies that prospectively examine patients with schizophrenia, bipolar disorder, and healthy people; first-episode patients with psychosis followed longitudinally and family studies of individuals at risk. We have the tools to move the field ahead and need to apply experimental designs that will address fundamental questions. Studies have to include sufficiently large samples to permit clinical correlations and be longitudinal.
Several steps are essential for progress toward the eventual clinical utility of brain imaging in psychiatry. First, collaborating research groups need to standardize their approach to data acquisition, processing, and analyses. This will permit the construction of “atlases” of normal and abnormal brain development. Such “4-dimensional” (3D brains over time) imaging studies must incorporate neurobehavioral paradigms necessary for elucidating brain-behavior relationships most pertinent to these disorders. This approach necessitates multicenter studies in order to obtain sufficiently large samples. Second, we need to incorporate pharmacologic probes into fMRI studies because this may provide valuable information linking with molecular substrates and with direct therapeutic implications. Third, cohort studies need to be set up around the time of onset of psychosis to establish the extent to which such abnormalities could be used to define schizophrenia at an early stage, with a view to early intervention and possibly even prevention. These studies could incorporate longitudinal follow-up examinations. Finally, more data are needed to examine the extent to which distinct neuroimaging alterations exist across and within traditional diagnostic boundaries. Such work could inform on whether the observed abnormalities map onto clinical features of symptomatoloy, course, and treatment response dimensions (the phenome) and to specific genetic polymorphisms (the genome). The availability of such data will permit an evaluation of the usefulness of neuroimaging in the distinction between schizophrenia and affective psychosis and to address a crucial question on how neural activity changes in association with different levels and different types of psychosis.
Acknowledgments
This report was supported by the following grants: MH64045 and MH60722 (Gur), MH64023 and MH45156 (Keshavan), Dr Mortimer and Theresa Sackler Foundation (Lawrie).
References
- 1.Illes J, et al. Working group on incidental findings in brain imaging research. Ethics. Incidental findings in brain imaging research. Science. 2006;311:783–784. doi: 10.1126/science.1124665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lisanby SH. Psychosis secondary to brain tumor. Semin Clin Neuropsychiatry. 1998;3:1–12. [PubMed] [Google Scholar]
- 3.Erhart SM. Clinical utility of magnetic resonance imaging radiographs for suspected organic syndromes in adult psychiatry. J Clin Psychiatry. 2005;66:968–973. doi: 10.4088/jcp.v66n0802. [DOI] [PubMed] [Google Scholar]
- 4.Lawrie SM. Qualitative cerebral morphology in schizophrenia: a magnetic resonance imaging study and systematic literature review. Schizophr Res. 1997;25:155–166. doi: 10.1016/S0920-9964(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 5.Shenton ME. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawrie SM. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 7.Ward KE. Meta-analysis of brain and cranial size in schizophrenia. Schizophr Res. 1996;22:197–213. doi: 10.1016/s0920-9964(96)00076-x. [DOI] [PubMed] [Google Scholar]
- 8.Wright IC. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 9.Woodruff PWR. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson MD. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 11.Konick LC. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- 12.Davatzikos C, et al. Whole brain morphometric study of schizophrenia reveals a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 13.Harrison PJ. Meta-analysis of brain weight in schizophrenia. Schizophr Res. 2003;64:25–34. doi: 10.1016/s0920-9964(02)00502-9. [DOI] [PubMed] [Google Scholar]
- 14.Honea R. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 15.Lawrie SM. Schizophrenia: From Neuroimaging to Neuroscience. Oxford, UK: Oxford University Press; 2004. [Google Scholar]
- 16.Antonova E. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Scherck H. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19:145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- 18.Vita A. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Ho BC. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 20.Hoge EA. Meta-analysis of brain size in bipolar disorder. Schizophr Res. 1999;37:177–181. doi: 10.1016/s0920-9964(98)00149-2. [DOI] [PubMed] [Google Scholar]
- 21.Strakowski SM. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 22.Bearden CE. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- 23.McDonald C, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Campbell S. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 25.Videbech P. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 26.Altshuler LL. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiatry. 1995;152:1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- 27.Videbech P. MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatr Scand. 1997;96:157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirayasu Y, et al. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11:374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- 29.Dickey CC, et al. Follow-up MRI study of prefrontal volumes in first-episode psychotic patients. Schizophr Res. 2004;71:349–351. doi: 10.1016/j.schres.2004.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad KM. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry. 2004;161:1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 31.Molina V. Dorsolateral prefrontal and superior temporal volume deficits in first-episode psychoses that evolve into schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2006;256:106–111. doi: 10.1007/s00406-005-0615-5. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand LC, et al. An in vivo MRI study of prefrontal cortical complexity in first-episode psychosis. Am J Psychiatry. 2005;162:65–70. doi: 10.1176/appi.ajp.162.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanaan RA. Diffusion tensor imaging in schizophrenia. Biol Psychiatry. 2005;58:921–929. doi: 10.1016/j.biopsych.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Keshavan MS, et al. Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:1285–1295. doi: 10.1016/s0278-5846(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 35.Boos HBM. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2006;81:41. [Google Scholar]
- 36.Job DE. Voxel based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64:1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 37.Diwadkar VA. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 38.McIntosh AM. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58:254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 39.McDonald C, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 40.McIntosh AM, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141:76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 41.Phillips LJ, et al. Non-reduction in hippocampal volume is associated with higher risk of psychosis. Schizophr Res. 2002;58:145–158. doi: 10.1016/s0920-9964(01)00392-9. [DOI] [PubMed] [Google Scholar]
- 42.Pantelis C, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 43.Job D. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25:1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Johnstone EC. Predicting schizophrenia—findings from the Edinburgh High Risk Study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- 45.Velakoulis D, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 46.Job DE. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Psychiary. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stanley JA. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings–part I. Biol Psychiatry. 2000;48:357–368. doi: 10.1016/s0006-3223(00)00949-5. [DOI] [PubMed] [Google Scholar]
- 48.Keshavan MS. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings–part II. Biol Psychiatry. 2000;48:369–380. doi: 10.1016/s0006-3223(00)00940-9. [DOI] [PubMed] [Google Scholar]
- 49.Urenjak J. Proton nuclear magnetic resonance spectroscopy unambiguously identifies different neural cell types. J Neurosci. 1993;13:981–989. doi: 10.1523/JNEUROSCI.13-03-00981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudkin TM. Proton magnetic resonance spectroscopy for the diagnosis and management of cerebral disorders. Arch Neurol. 1999;56:919–926. doi: 10.1001/archneur.56.8.919. [DOI] [PubMed] [Google Scholar]
- 51.Baslow MH. Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem. 2000;75:453–459. doi: 10.1046/j.1471-4159.2000.0750453.x. [DOI] [PubMed] [Google Scholar]
- 52.Lim KO. Proton magnetic resonance spectroscopic imaging of cortical gray and white matter in schizophrenia. Arch Gen Psychiatry. 1998;55:346–352. doi: 10.1001/archpsyc.55.4.346. [DOI] [PubMed] [Google Scholar]
- 53.Tsai G. N-acetylaspartate in neuropsychiatric disorders. Prog Neurobiol. 1995;46:531–540. doi: 10.1016/0301-0082(95)00014-m. [DOI] [PubMed] [Google Scholar]
- 54.Steen RG. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 55.Ende G, et al. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Res. 2000;41:389–395. doi: 10.1016/s0920-9964(99)00089-4. [DOI] [PubMed] [Google Scholar]
- 56.Yildiz-Yesiloglu A. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 57.Yildiz-Yesiloglu A. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25. doi: 10.1016/j.pscychresns.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 58.Callicott JH, et al. Hippocampal N-acetyl aspartate in unaffected siblings of patients with schizophrenia: a possible intermediate neurobiological phenotype. Biol Psychiatry. 1998;44:941–950. doi: 10.1016/s0006-3223(98)00264-9. [DOI] [PubMed] [Google Scholar]
- 59.Tibbo P. 3-T proton MRS investigation of glutamate and glutamine in adolescents at high genetic risk for schizophrenia. Am J Psychiatry. 2004;161:1116–1118. doi: 10.1176/appi.ajp.161.6.1116. [DOI] [PubMed] [Google Scholar]
- 60.Jessen F, et al. Proton magnetic resonance spectroscopy in subjects at risk for schizophrenia. Schizophr Res. 2006;87:81–88. doi: 10.1016/j.schres.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Wood SJ, et al. Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophr Bull. 2003;29:831–843. doi: 10.1093/oxfordjournals.schbul.a007049. [DOI] [PubMed] [Google Scholar]
- 62.Pettegrew JW, et al. Alterations in brain high-energy phosphate and membrane phospholipid metabolism in first-episode, drug-naive schizophrenics. A pilot study of the dorsal prefrontal cortex by in vivo phosphorus 31 nuclear magnetic resonance spectroscopy. Arch Gen Psychiatry. 1991;48:563–568. doi: 10.1001/archpsyc.1991.01810300075011. [DOI] [PubMed] [Google Scholar]
- 63.Stanley JA, et al. An in vivo study of the prefrontal cortex of schizophrenic patients at different stages of illness via phosphorus magnetic resonance spectroscopy. Arch Gen Psychiatry. 1995;52:399–406. doi: 10.1001/archpsyc.1995.03950170073010. [DOI] [PubMed] [Google Scholar]
- 64.Fukuzako H. Changes in levels of phosphorus metabolites in temporal lobes of drug-naive schizophrenic patients. Am J Psychiatry. 1999;156:1205–1208. doi: 10.1176/ajp.156.8.1205. [DOI] [PubMed] [Google Scholar]
- 65.Jensen JE, et al. Focal changes in brain energy and phospholipid metabolism in first-episode schizophrenia: 31P-MRS chemical shift imaging study at 4 Tesla. Br J Psychiatry. 2004;184:409–415. doi: 10.1192/bjp.184.5.409. [DOI] [PubMed] [Google Scholar]
- 66.Berger GE. Implications of lipid biology for the pathogenesis of schizophrenia. Aust N Z J Psychiatry. 2002;36:355–366. doi: 10.1046/j.1440-1614.2001.01021.x. [DOI] [PubMed] [Google Scholar]
- 67.Klemm S, et al. Cerebral phosphate metabolism in first-degree relatives of patients with schizophrenia. Am J Psychiatry. 2001;158:958–960. doi: 10.1176/appi.ajp.158.6.958. [DOI] [PubMed] [Google Scholar]
- 68.Keshavan MS, et al. Prolonged untreated illness duration from prodromal onset predicts outcome in first episode psychoses. Schizophr Bull. 2003;29:757–769. doi: 10.1093/oxfordjournals.schbul.a007045. [DOI] [PubMed] [Google Scholar]
- 69.Kato T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord. 1993;27:53–59. doi: 10.1016/0165-0327(93)90097-4. [DOI] [PubMed] [Google Scholar]
- 70.Wong DF, et al. Positron emission tomography reveals elevated D2 dopamine receptors in drug-naive schizophrenics. Science. 1986;234:1558–1563. doi: 10.1126/science.2878495. [DOI] [PubMed] [Google Scholar]
- 71.Farde L. No D2 receptor increase in PET study of schizophrenia. Arch Gen Psychiatry. 1987;44:671–672. doi: 10.1001/archpsyc.1987.01800190091013. [DOI] [PubMed] [Google Scholar]
- 72.Andreasen NC, et al. Workshop on schizophrenia, PET, and dopamine D2 receptors in the human neostriatum. Schizophr Bull. 1988;14:471–484. doi: 10.1093/schbul/14.3.471. [DOI] [PubMed] [Google Scholar]
- 73.Zakzanis KK. Dopamine D2 densities and the schizophrenic brain. Schizophr Res. 1998;32:201–206. doi: 10.1016/s0920-9964(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 74.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- 75.Erritzoe D. Positron emission tomography and single photon emission CT molecular imaging in schizophrenia. Neuroimaging Clin N Am. 2003;13:817–832. doi: 10.1016/s1052-5149(03)00089-3. [DOI] [PubMed] [Google Scholar]
- 76.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(Suppl 1) doi: 10.1017/S1461145704004110. S1–S5. [DOI] [PubMed] [Google Scholar]
- 77.Cropley VL. Molecular imaging of the dopaminergic system and its association with human cognitive function. Biol Psychiatry. 2006;59:898–907. doi: 10.1016/j.biopsych.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 78.Cannon DM, et al. Serotonin transporter binding in bipolar disorder assessed using [11C]DASB and positron emission tomography. Biol Psychiatry. 2006;60:207–217. doi: 10.1016/j.biopsych.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Cannon DM, et al. Reduced muscarinic type 2 receptor binding in subjects with bipolar disorder. Arch Gen Psychiatry. 2006;63:741–747. doi: 10.1001/archpsyc.63.7.741. [DOI] [PubMed] [Google Scholar]
- 80.Talbott PS. The role of in vivo molecular imaging with PET and SPECT in the elucidation of psychiatric drug action and new drug development. Eur Neuropsychopharmacol. 2002;12:503–511. doi: 10.1016/s0924-977x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 81.Tauscher J. Choosing the right dose of antipsychotics in schizophrenia: lessons from neuroimaging studies. CNS Drugs. 2001;15:671–678. doi: 10.2165/00023210-200115090-00001. [DOI] [PubMed] [Google Scholar]
- 82.Gur RE. Neuroimaging in schizophrenia: linking neuropsychiatric manifestations to neurobiology. In: Kaplan HI, editor. Comprehensive Textbook of Psychiatry/VIII. Philadelphia, Pa: Lippincott Williams & Wilkins; 2005. pp. 1396–1407. [Google Scholar]
- 83.Zakzanis KK. Schizophrenia and the frontal brain: a quantitative review. J Int Neuropsychol Soc. 1999;5:556–566. doi: 10.1017/s1355617799566101. [DOI] [PubMed] [Google Scholar]
- 84.Hill K. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr Scand. 2004;110:243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- 85.Davidson LL. Quantification of frontal and temporal lobe brain-imaging findings in schizophrenia: a meta-analysis. Psychiatry Res. 2003;122:69–87. doi: 10.1016/s0925-4927(02)00118-x. [DOI] [PubMed] [Google Scholar]
- 86.Achim AM. Episodic memory-related activation in schizophrenia: meta-analysis. Br J Psychiatry. 2005;187:500–509. doi: 10.1192/bjp.187.6.500. [DOI] [PubMed] [Google Scholar]
- 87.Glahn DC, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davis CE. Review of longitudinal functional neuroimaging studies of drug treatments in patients with schizophrenia. Schizophr Res. 2005;78:45–60. doi: 10.1016/j.schres.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 89.MacDonald AW, III, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. doi: 10.1176/appi.ajp.162.3.475. [DOI] [PubMed] [Google Scholar]
- 90.Zakzanis KK. Searching the schizophrenic brain for temporal lobe deficits: a systematic review and meta-analysis. Psychol Med. 2000;30:491–504. doi: 10.1017/s0033291799002172. [DOI] [PubMed] [Google Scholar]
- 91.Friston KJ. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- 92.Stephan KE. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Uhlhaas PJ, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uhlhaas PJ. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 95.Whalley HC. Neural correlates of enhanced genetic risk for schizophrenia. Neuroscientist. 2005;11:238–249. doi: 10.1177/1073858404274111. [DOI] [PubMed] [Google Scholar]
- 96.Spence SA, et al. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry. 2000;176:52–60. doi: 10.1192/bjp.176.1.52. [DOI] [PubMed] [Google Scholar]
- 97.Whalley HC, et al. Functional disconnectivity in subjects at high genetic risk of schizophrenia. Brain. 2005;128:2097–2108. doi: 10.1093/brain/awh556. [DOI] [PubMed] [Google Scholar]
- 98.Whalley HC, et al. Functional imaging as a predictor of schizophrenia. Biol Psychiatry. 2006;60:454–462. doi: 10.1016/j.biopsych.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Wolf DH, et al. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. 2007;154:221–232. doi: 10.1016/j.pscychresns.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wexler BE. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157:1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- 101.Wykes T, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy: functional magnetic resonance imaging in schizophrenia. Br J Psychiatry. 2002;181:144–152. doi: 10.1017/s0007125000161872. [DOI] [PubMed] [Google Scholar]