Abstract
Neurocognitive impairment is considered a core component of schizophrenia and is increasingly under investigation as a potential treatment target. On average, cognitive impairment is severe to moderately severe compared with healthy controls, and almost all patients with schizophrenia demonstrate cognitive decrements compared with their expected level if they had not developed the illness. Compared with patients with affective disorders, cognitive impairment in schizophrenia appears earlier, is more severe, and tends to be more independent of clinical symptoms. While the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, description of schizophrenia includes several references to cognitive impairment, neither the diagnostic criteria nor the subtypology of schizophrenia include a requirement of cognitive impairment. We forward for consideration a proposal that the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria include a specific criterion of “a level of cognitive functioning suggesting a consistent severe impairment and/or a significant decline from premorbid levels considering the patient's educational, familial, and socioeconomic background.” The inclusion of this criterion may increase the “point of rarity” with affective psychoses and may increase clinicians' awareness of cognitive impairment, potentially leading to more accurate prognosis and better treatment outcomes. Future research will need to address the validity of these possibilities. The reliable determination of cognitive impairment as part of a standard diagnostic evaluation may present challenges to diagnosticians with limited resources or insufficient expertise. Various cognitive assessment methods for clinicians, including brief assessments and interview-based assessments, are discussed. Given the current emphasis on the development of cognitive treatments, the evaluation of cognition in schizophrenia is an essential component of mental health education.
Keywords: schizophrenia, cognition, diagnosis
Introduction
Neurocognitive deficits of schizophrenia are profound and clinically relevant. Patients with schizophrenia perform 1.5 to 2.0 standard deviation below healthy controls on a variety of neurocognitive tasks. The most prominent of these deficits are memory, attention, working memory, problem solving, processing speed, and social cognition.1 These impairments exist prior to the initiation of antipsychotic treatment2 and are not caused by psychotic symptoms in patients who are able to complete cognitive testing, which includes the overwhelming majority of patients.3 The various cognitive deficits in schizophrenia have all been shown to be associated with functional outcomes such as difficulty with community functioning, difficulty with instrumental and problem-solving skills, reduced success in psychosocial rehabilitation programs,4 and the inability to maintain successful employment.5 In fact, cognitive deficits are better able to explain important functional outcomes, such as work performance and independent living,6 than positive or negative symptoms.
The importance of cognitive deficits in schizophrenia goes beyond their severity and relation to functional outcomes. Cognitive deficits appear to be present in some patients with schizophrenia prior to the onset of psychosis and are correlated with measurable brain dysfunction more than any other aspect of the illness. While the number of studies associating negative or positive symptoms with abnormal brain imaging results is small, the imaging literature in schizophrenia is filled with associations between cognitive deficits and structural and functional imaging results that differ from healthy controls. Perhaps most importantly, cognition is increasingly considered as a primary target for treatment.7–10
Despite the relevance of cognitive impairment to biology, function, and treatment in schizophrenia, it is not included in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria. It is noteworthy, however, that the first sentence of the description of schizophrenia in DSM-IV includes 4 references to cognitive disturbances: “the characteristics of schizophrenia involve a range of cognitive and emotional dysfunctions that include perception, inferential thinking, language and communication, behavioral monitoring, affect, fluency, and production of thought and speech, hedonic capacity, volition and drive, and attention.”11 Thus, it is clear that cognition is deemed important by diagnostic experts; however, a method for including this fundamental aspect of the illness in the diagnostic criteria for schizophrenia has not been determined. The current review will emphasize the importance of cognition in schizophrenia and forward a proposal for consideration that severe cognitive impairment should be part of the criteria for schizophrenia in Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V). A research agenda for determining the validity and usefulness of including cognitive impairment as part of the criteria for schizophrenia will be discussed.
Will Cognitive Impairment Help Distinguish the Diagnosis of Schizophrenia From Affective Disorders?
The first question that will be considered is whether adding some definition of cognitive impairment or cognitive decline to the criteria for schizophrenia will help define a “point of rarity” with affective psychoses.12 The ability of a diagnostic refinement to improve the distinction between 2 entities and thus create an increased nonoverlap between them is considered to be a crucial determinant for inclusion.
Diagnostic Differences in Severity of Cognitive Impairment
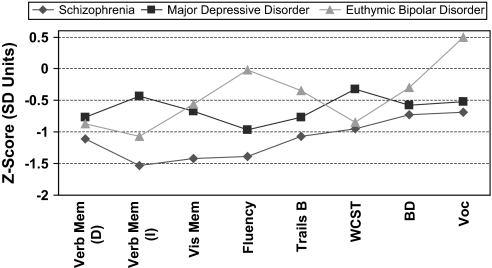
The conclusions from cognitive experts in the Measurement And Treatment Research to Improve Cognition in Schizophrenia (MATRICS) project were that “schizophrenia and schizoaffective disorder share a similar pattern of cognitive impairments, which is distinct from patterns in major depression, bipolar disorder, and Alzheimer's dementia.”10 This group of experts came to this conclusion based upon a series of studies indicating that patients with schizophrenia have a pattern of deficits that is more profound than those in major depression and bipolar disorder, more stable over the course of illness, and more related to clinical state. Meta-analyses of the cognitive profiles of patients with schizophrenia, major depression, and bipolar disorder are described in figure 1. Patients with schizophrenia have more cognitive impairment on all the cognitive tests that were measured in each of the diagnostic groups. While the pattern of deficits among these groups may not differ dramatically, it is well accepted that the deficits of schizophrenia are more profound than those in affective disorders.10,13 A recent meta-analysis comparing the performances of patients with schizophrenia and bipolar disorder concluded that patients with schizophrenia have cognitive deficits that are about 0.5 SD larger than those in patients with bipolar disorder. These deficits were found to be particularly profound on tests of verbal fluency, working memory, executive control, visual memory, mental speed, and verbal memory.13 Even when patients with schizophrenia and patients with bipolar disorder were matched on the severity of their clinical symptoms, the deficits of schizophrenia surpass those of patients with bipolar disorder by 0.5 SD.13
Fig. 1.
Cognitive Profiles in Schizophrenia, Major Depression, and Euthymic Bipolar Disorder From Published Meta-analyses.16 Data from Heinrichs and Zakzanis,44 Zakzanis et al,14 and van Gorp et al15 Healthy group mean = 0. Verb Mem (D), delayed verbal memory; Verb Mem (I), immediate verbal memory; Vis Mem, visual memory; Trails B, Trail Making Test, B; WCST, Wisconsin Card Sorting Test; BD, Wechsler Adult Intelligence Scale (WAIS) block design test; Voc, WAIS vocabulary. Reprinted with permission from Buchanan et al.10.
Studies of patients with first-episode schizophrenia and affective disorder appear to support the meta-analyses completed on more chronic patients. In an epidemiological study of all first-admission psychotic disorders in Suffolk County, NY, patients who received a diagnosis of schizophrenia at 24 months of follow-up (n = 148) were found to have significantly greater cognitive deficits compared with those first-episode psychotic patients who were diagnosed with bipolar disorder (n = 87) and depression (n = 56) 24 months later. Again, the differentiation between schizophrenia and affective psychoses were particularly profound with regard to memory, executive functions, and mental speed tasks (A. Reichenberg, PhD, unpublished data, 2007). These data suggest that cognitive information at first episode may aid in the determination of whether an individual's later diagnosis will be in the affective or schizophrenia spectrum.
Diagnostic Differences Regarding Relation of Cognitive Impairment to Clinical State
While patients with affective psychoses also have cognitive impairment, it appears as though these cognitive deficits are more strongly associated with clinical symptoms and state-related factors than in patients with schizophrenia.14,15 In a study of patients with schizophrenia or bipolar disorder who were assessed when psychotic at baseline and then 8 months later, patients who were psychotic at follow-up in both diagnostic groups had no difference in their cognitive impairment 8 months later. Among those patients whose psychosis had remitted 8 months later, schizophrenia patients also showed the same level of cognitive impairment. Only the bipolar patients whose psychosis had remitted at follow-up had improved in their cognitive performance.16 Similar data have been reported in first-episode samples. While first-episode patients with affective psychoses performed similarly to those with first-episode schizophrenia in 1 study, patients with nonpsychotic affective disorders performed significantly better than both psychotic groups.17 Thus, while the cognitive deficits of affective disorders may be profound in some cases, these cognitive deficits appear to be related to clinical symptoms. In contrast, cognitive impairment in schizophrenia patients has been repeatedly demonstrated to be uncorrelated with psychotic symptoms.3,18–20 Part of the explanation for these low correlations between cognitive deficits and symptoms in patients with schizophrenia is that while the symptoms of schizophrenia wax and wane in almost all patients, leading to low stability coefficients over time,21 the stability of cognitive deficits in all domains is very high, with test-retest coefficients ranging between 0.7 and 0.85 even in patients tested 1 year apart following their initial treatment for psychosis.21 Thus, while there are cognitive deficits in affective disorders, they fluctuate in parallel with clinical symptom changes. In schizophrenia, however, they may be the most stable aspect of the disorder.
Prevalence of Cognitive Impairment in Schizophrenia
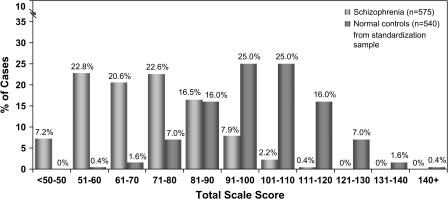
If cognitive impairment is to be considered as part of the diagnosis of schizophrenia, it will be important to demonstrate that its prevalence among patients with schizophrenia is high. Patients with schizophrenia and healthy controls both show normal distributions of scores on cognitive batteries such as the Repeatable Battery for Assessment of Neuropsychological Status22 (see figure 2). However, as has been frequently demonstrated, the distribution of a large number of patients with schizophrenia (n = 575) is shifted about 2 SDs below the 540 healthy controls from the standardization sample.22,23 While there is considerable overlap between these 2 distributions, it is noteworthy that there are very few healthy controls at the lower ends of this distribution and very few schizophrenia patients at the upper ends of this distribution. Traditional neuropsychological criteria for cognitive impairment would identify those individuals who performed better than 1 SD below the healthy control mean as “unimpaired”.24–26 By these criteria, about 20% of the patients in this study would be considered to have normal cognitive functions. However, it is possible that many of the individuals at the upper end of this schizophrenia distribution have demonstrated cognitive decline compared with what their cognitive functions would have been if they had never developed the illness. While this conjecture can never be proved, it is useful to investigate the relationship between antecedent factors, such as parental education and reading scores, and their relationship to current cognitive functions in patients with schizophrenia.
Fig. 2.
Distributions of Total Scores on the Repeatable Battery for the Assessment of Neuropsychological Status in Patients With Schizophrenia and Healthy Controls From Published Norms. Reprinted with permission from Randolph et al22 and Wilk et al.23
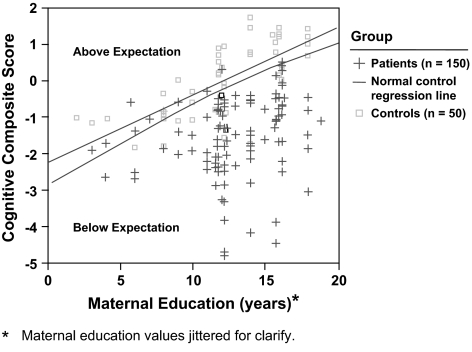
In healthy controls, current cognitive ability is strongly predicted by antecedent factors such as maternal education and reading score.27 As demonstrated in figure 3, the healthy controls whose mothers had greater education clearly had higher cognitive functions. The regression line in this figure describes the relationship between these 2 factors. Because of the natural variability of cognitive performance among healthy controls, about half of this distribution performs above expectation while half performs below expectations. However, an overwhelming majority of the patients with schizophrenia in this sample performed below the expectations established by antecedent factors, in this case, maternal education. Thus, it is likely that almost all schizophrenic patients have some measure of cognitive deficit compared with what their level of cognitive function would have been if they had never developed the illness.
Fig. 3.
Expected Neurocognitive Performance Based on Maternal Education of Healthy Controls. Reprinted with permission from Keefe et al.27
Early Cognitive Decline in Schizophrenia?
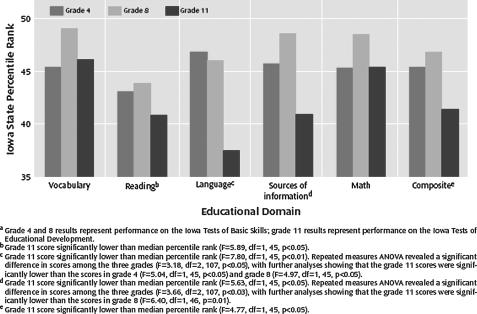
While most patients with schizophrenia appear to show some cognitive decline based upon what would have been predicted by antecedent factors, it is also important to note that on average patients with schizophrenia start out at a lower baseline prior to the onset of the illness. Children who will eventually develop schizophrenia show cognitive impairment compared with healthy controls and children who later develop affective disorders.28,29 However, individuals who will eventually develop schizophrenia also appear to show decline on scholastic measures between early childhood and late adolescence.29 The presence of cognitive deficits or cognitive decline during adolescence has been found to predict the conversion to schizophrenia in various samples.29–34 Thus, as depicted in figure 4, patients with schizophrenia appear to begin life with cognitive performance that is slightly worse than their peers. As childhood progresses, cognitive performance tends to worsen in those children who will eventually develop schizophrenia. By the time psychosis develops in late adolescence or early adulthood, patients perform substantially worse than their healthy peers. While patients with affective disorders also demonstrate cognitive impairment in adulthood, it appears as though these individuals do not show impairment until the adult onset of their disorders.28
Fig. 4.
Standardized Scholastic Test Performance in Grades 4, 8, and 11 Relative to State Norms for 70 Subjects Who Later Developed Schizophrenia. Reprinted with permission from Fuller et al.29
The literature review above supports the notion that the severity and longitudinal course of cognitive impairment in schizophrenia differ substantially from that found in patients with affective psychosis. Yet, the question of whether including a criterion of cognitive impairment or cognitive decline from healthy premorbid levels in the diagnosis of schizophrenia will help define a “point of rarity” with affective psychoses remains unanswered. Research studies and analyses of existing databases are needed that address this question in large numbers of psychotic individuals with schizophrenia and affective disorders assessed on measures of cognition and symptoms. These studies will help determine whether the inclusion of a criterion for cognitive impairment in the diagnosis of schizophrenia will increase the point of rarity between these diagnostic entities.
How Can Cognitive Impairment Be Assessed for Diagnostic Purposes?
While formal cognitive testing appears to be very sensitive to the cognitive impairment in schizophrenia, the resources required to complete neuropsychological evaluations are prohibitive in various treatment settings. In fact, the diagnoses of Alzheimer disease and attention deficit/hyperactivity disorder (ADHD), while being clearly cognitive disorders, do not require formal cognitive testing. Thus, it may not be realistic to expect that the diagnosis of schizophrenia would depend upon cognitive performance testing. It needs to be established how cognitive impairment will be assessed by clinicians who will diagnose schizophrenia so that the additional criterion will contribute sufficiently to diagnostic validity and treatment success. Recent work suggests that almost all the variance in cognitive composite scores can be accounted for by a small number of tests.3 Thus, clinicians may be able to develop the capacity to assess cognitive impairment in schizophrenia without overwhelming resource requirements. However, education and training on the use of standardized cognitive tests for clinicians will be essential to assure that the assessment procedures are completed in a manner that maintains test standardization. This aspect of training is usually included in the curriculum of clinical psychologists and neuropsychologists but is rarely a component of education for physicians, social workers, and nurses. A program for training in cognitive testing will be an essential step to increase the capacity for clinicians to assess the cognitive impairments of schizophrenia. It will take time to work this training into the traditional education of psychiatrists and other nonpsychologists. This training does not need to be limited to formal neuropsychological tests but may be better aimed toward the assessment of patients' ability on practical cognitive tasks, which may have stronger direct correlations with outcome.35
Interview-Based Assessments of Cognition
While the availability of formal test methods and trained testers may prohibit testing in many clinical environments, recent methodological advances have included the assessment of cognition in patients with schizophrenia with interview-based measures. Similar to ADHD assessment methods, which do not involve formal testing, these measures involve a series of questions directed toward the patient with schizophrenia and his or her relatives or caregivers. These questions address whether people with schizophrenia have cognitive deficits that impair fundamental aspects of their daily lives. For instance, some of the questions ask whether patients have difficulty remembering names, concentrating well enough to read a newspaper or book, being able to follow group conversations, and handling changes in daily routines.36,37 Interview-based assessments of cognition have historically been unreliable and have demonstrated low correlations with cognitive performance. However, these measures have generally relied upon the reports from patients and their treating clinicians, which have been notoriously unreliable and potentially invalid.38–41 A methodology that assesses cognition with interviews of patients and caregivers, such as relatives or caseworkers, may have improved reliability and validity. For example, the Schizophrenia Cognition Rating Scale (SCoRS) has been found to have excellent reliability and substantial correlations with cognitive performance and functional outcomes36 (M. F. Green, PhD, unpublished data, 2007). In fact, SCoRS global outcome measures have met several of the criteria for coprimary measures outlined by the MATRICS meeting for optimal designs for cognitive enhancement trials.10 One of the potential weaknesses of this methodology however, is that reports from patients have been found to have reduced reliability if patients are the only source of information. The relevance of this weakness is particularly important in the assessment of patients with schizophrenia because a substantial percentage of patients do not have an available informant who can provide information about the patient's cognitive deficits and how these deficits affect the patient's daily behavior. For example, in the MATRICS Psychometric and Standardization Study (PASS), test-retest reliability data over the course of 1 month was high when ratings were based upon a patient and informant as a source of information (intra class correlation [ICC] = 0 .82, goodness of fit [GF] = 123; P = .001); (M. F. Green, PhD, unpublished data, 2007). However, when patients were the only source of information, the test-retest reliability coefficient (ICC) was 0.60, (GF = 148, P < .001). While reliability is enhanced when information can be obtained from both sources, a considerable number of patients do not have an available informant. A more extensive series of questions, as found in the Clinical Global Impression of Cognition in Schizophrenia (CGI-CoGS),37 appears to improve the reliability of patient reports up to ICC = 0.80, but patients describe these longer interviews, which require up to 45 minutes per interview, as burdensome (M. F. Green, PhD, unpublished data, 2007). A shorter, less burdensome instrument that would not require an informant and could be completed on almost all patients would be ideal, although is not currently available. Future studies should focus on this methodology and must also determine whether interview-based assessments of cognition can contribute to the diagnostic separation between schizophrenia and affective psychoses.
Is the Clinical Importance of Cognition in Schizophrenia Sufficient to Include It in the Formal Criteria?
Even if including a definition of “cognitive impairment” in the criteria for schizophrenia does not increase the point of rarity between schizophrenia and other psychotic disorders, an additional consideration is whether it would be able to “provide useful information not contained in the definition of the disorder that helps in decisions about management and treatment.”42 The inclusion of cognitive impairment in the criteria for schizophrenia may increase psychiatrists' attention toward a neglected aspect of the core components of schizophrenia.7 Because cognition is rarely considered among psychiatrists as an important treatment target, inclusion of cognitive impairment in the criteria for schizophrenia may help to educate clinicians about the importance of cognition in their treatment options. Furthermore, representatives from the US Food and Drug Administration have indicated that the recognition of cognitive impairment in the diagnostic nomenclature would be an important step in approving a drug for a cognitive improvement indication.10 Many government agencies and pharmaceutical companies are currently involved in intense work to try to develop compounds that may improve cognition with schizophrenia. If successful, these compounds have the potential to alter the way that schizophrenia is currently treated. However, if the pathway is not established to allow these medications to be approved by FDA, and if clinicians are not trained to recognize cognitive improvement, this potential area of great benefit to patients may be missed. Inclusion of cognitive impairment in the diagnostic criteria for schizophrenia may be one of the steps that could be taken to help clinicians target and potentially improve cognition in patients with schizophrenia.
How Should Cognitive Impairment in Schizophrenia Be Defined?
We have presented an argument for the importance of cognitive impairment in schizophrenia and have suggested that cognitive impairment should be represented in the diagnosis for schizophrenia. However, there are several considerations regarding how it should be included. We propose that the following criterion should be considered for the diagnosis of schizophrenia: “a level of cognitive functioning suggesting a consistent severe impairment and/or a significant decline from premorbid levels considering the patient's educational, familial, and socioeconomic background.” Diagnosticians should consider all aspects of cognitive impairment in this definition but should be alerted that, in general, schizophrenia patients may have particularly severe deficits in the cognitive domains of memory, attention, working memory, reasoning and problem solving, processing speed, and social cognition.1 It is not uncommon for some aspects of cognition to be unimpaired in the context of severe impairments in other areas, with an overall level of impairment in the severe range. A statement that the assessment of cognitive function must consider the patients' background was included to avoid overdiagnosing schizophrenia in individuals whose environments deprive them of their ability to develop cognitive abilities. If in the event that DSM-V changes to a completely dimensional approach to the symptoms of psychosis,43 cognitive impairment should be one of the key dimensions.
This change in DSM will potentially increase the point of rarity with other psychoses. It is likely that some patients diagnosed with schizophrenia who have little or no cognitive impairment have treatment responses and courses of illness that are more consistent with a diagnosis of affective disorder. If this is the case, it will benefit clinicians to change their expectations based upon this revised diagnosis. On the other hand, some patients diagnosed with affective disorders and severe cognitive impairment may follow the longitudinal course and treatment response of patients with schizophrenia. One of the important research questions that will need to be addressed is whether patients whose diagnosis changes based upon the new criteria are more likely to have genetic and other biological indicators consistent with the new diagnosis.
Changing the DSM criteria for schizophrenia to include cognitive impairment will also force clinicians to consider the cognitive impairment of their patients, which has been largely ignored among clinical psychiatrists. This change would thus direct clinicians' attention toward the aspect of the disorder that is the largest determinant of long-term functioning. It may also help develop the pathway for new treatments to improve this fundamental component of the illness and force educational systems to teach clinicians how to recognize cognitive impairment and improvement.
However, the implementation of this change in DSM will present several challenges. If this criterion is included in the criteria for schizophrenia, it will be crucial to consider how cognition will be measured by clinicians and researchers making a diagnosis. It is unrealistic to expect that all patients with schizophrenia would receive formal neuropsychological testing by psychologists, which is time consuming and expensive. In most treatment settings, these costs are prohibitive. However, if cognitive paradigms were developed that were able definitively to separate diagnostic entities, a case could be made that this testing is essential to patient diagnosis and treatment planning. Unfortunately, as discussed above, we are not yet at this stage.
A second consideration regarding the use of cognitive impairment as a criterion for schizophrenia is that current cognitive performance is affected by factors unrelated to cognitive decline in patients with schizophrenia such as level of education and environments that are variably conducive to normal learning.23 Some patients may have very poor cognitive functioning due to factors unrelated to schizophrenia while other patients may have cognitive performance that is in the “normal range” despite significant decline from premorbid levels. How will diagnosticians determine how schizophrenia may interact with these factors to result in a patient's current cognitive levels? Since not all patients are defined as “impaired” on cognitive tests, it is important to emphasize that the criterion will be met if a patient's current cognitive performance represents a “decline from premorbid cognitive functioning”. On average, the longitudinal course of cognitive function in patients with schizophrenia appears to decline at least one full SD from childhood. During childhood and adolescence, patients who will eventually develop schizophrenia perform about 0.5 SDs below their peers who will not develop schizophrenia.28,29,31 Immediately prior to the onset of psychosis, patients who are about to develop schizophrenia demonstrate a worsened cognitive function, such that the average person at ultra high risk for schizophrenia disorders who will eventually convert to psychosis performs about 1 SD below healthy controls.30,34 It will be important in these cases for diagnosticians to determine whether there has been a decline in cognitive functions from expected cognitive levels based upon antecedent factors such as parental education, early school performance, and reading level. It will be essential for diagnosticians to collect a complete history on the cognitive performance of each patient, including how the patient's current cognitive performance compares to early school performance and any academic, intelligence, or cognitive testing that was performed during premorbid and prodromal periods. Further, a patient's level of cognitive performance will need to be compared with other members of the patient's family and sociocultural background, if available. In some cases, testing would benefit this assessment. In other cases, the amount of cognitive impairment in a patient would be clearly obvious and in direct contrast to early cognitive competence in an individual. Finally, because cognitive impairment in affective disorders in the context of clinical exacerbation may be difficult to distinguish from schizophrenia cross-sectionally, longitudinal assessment will be important for an accurate diagnosis. While this historical and longitudinal data collection may initially appear to add burden, if indeed the level and course of cognitive deficit is crucial not only to diagnosis but also to prognosis and treatment planning, it is likely that this “front-loading” of clinical care may actually reduce clinical burden in the form of improved treatment response and long-term functioning.
Third, while clinician judgment will be an important component of assessing cognition in schizophrenia, recent data suggest that clinicians cannot be the sole source of information for making this determination. The challenge that arises here is that many patients with schizophrenia will not have enough contact with other people for someone to be able to report reliably on their usual level of cognitive functioning. Patients without available informants will need to have additional assessments such as more extensive interviews or an actual cognitive assessment, which is the most informative method for collecting cognitive information about a patient.
As discussed above, if a patient is assessed during a period of clinical exacerbation, cognitive impairment may be very similar in patients with schizophrenia and those with affective psychoses.10,14,15 Thus, for the patient to meet the criterion of cognitive impairment, it will be important for the cognitive deficits to be stable throughout a long period of illness. This would help to differentiate the cognitive impairment found in schizophrenia from those in affective psychoses. However, it will also result in delays in definitive diagnoses in cases where cognitive impairment is present in the context of symptom exacerbation.
In sum, we have recommended for consideration that a criterion for consistent severe cognitive impairment be added to the DSM diagnosis of schizophrenia. There are several challenges for this suggestion to meet acceptance by the research and clinical communities. Research is needed to determine: if such a criterion will increase the point of rarity between schizophrenia and other diagnostic entities; if clinicians are able assess cognition reliably with brief formal assessment instruments or interview-based methods; and if the inclusion of such a criterion will improve the value of the diagnosis of schizophrenia for prognosis and treatment outcomes.
Acknowledgments
This article was generated from a meeting on “Deconstructing Psychosis” at the offices of the American Psychiatric Association in Alexandria, Virginia, on February 16–17, 2006. In that meeting, Dr Keefe presented many of the ideas discussed in this article, and they were commented on formally by Dr Fenton and informally by other panel participants. While Dr Fenton agreed to coauthor this article, he was not able to make comments on the manuscript before his tragic death on September 2, 2006. Dr Keefe discloses that he has received research funding from Eli Lilly and Pfizer through Duke University. He also owns a company that trains and certifies cognitive testers for clinical trials, and he consults and has received funding from various pharmaceutical companies, universities, and government agencies to carry out this work. He receives royalties through Duke University for cognitive measures developed in his laboratory, including the Brief Assessment of Cognition in Schizophrenia (BACS) and the BACS symbol coding subtest of the MATRICS Consensus Cognitive Battery, some of which are discussed here. Currently, there is no fee charged for the use of the SCoRS, copyrighted by Duke University, discussed in this article; however, in the future, a fee may be charged. Dr Keefe has devoted much of his career to research on cognitive impairment in schizophrenia. Thus, if the suggestions of this article are carried out, he stands potentially to benefit academically and financially. Courtney Kennel and Cathy Lefebvre provided editorial assistance for this article.
Richard Keefe: Potential financial conflicts for myself in this paper include: I receive research funding from Eli Lilly and Pfizer through Duke University. I also own a company that trains and certifies cognitive testers for clinical trials. I consult and have received funding from various pharmaceutical companies, universities and government agencies to carry out this work. I receive royalties through Duke University for cognitive measures developed in my lab, including the Brief Assessment.
References
- 1.Nuechterlein KH. Identification of separable cognitive factors in schizophrenia. Schizophr Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Saykin AJ, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 3.Keefe RSE, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- 4.Green MF. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 5.Bryson G. Initial and final work performance in schizophrenia: cognitive and symptom predictors. J Nerv Ment Dis. 2003;191:87–92. doi: 10.1097/01.NMD.0000050937.06332.3C. [DOI] [PubMed] [Google Scholar]
- 6.Harvey PD, et al. Symptoms, cognitive functioning, and adaptive skills in geriatric patients with lifelong schizophrenia: a comparison across treatment sites. Am J Psychiatry. 1998;155:1080–1086. doi: 10.1176/ajp.155.8.1080. [DOI] [PubMed] [Google Scholar]
- 7.Hyman SE. Medicine. What are the right targets for psychopharmacology? Science. 2003;299:350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 8.Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophr Res. 2004;72:21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Davidson M. Cognitive impairment as a target for pharmacological treatment in schizophrenia. Schizophr Res. 1995;17:123–129. doi: 10.1016/0920-9964(95)00037-m. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan RW, et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial designs for neurocognitive drugs for schizophrenia. Schizophr Bull. 2005;31:5–21. doi: 10.1093/schbul/sbi020. [DOI] [PubMed] [Google Scholar]
- 11.Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Arlington, VA: American Psychiatric Association, Inc; 2000. [Google Scholar]
- 12.Kendell R. Distinguishing between the validity and utility of psychiatric diagnoses. Am J Psychiatry. 2003;160:4–12. doi: 10.1176/appi.ajp.160.1.4. [DOI] [PubMed] [Google Scholar]
- 13.Krabbendam L. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80:137–149. doi: 10.1016/j.schres.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Zakzanis KK. On the nature and pattern of neurocognitive function in major depressive disorders. Neuropsychiatry Neuropsychol Behav Neurol. 1998;11:111–119. [PubMed] [Google Scholar]
- 15.van Gorp WG. Cognitive impairment in euthymic bipolar patients with and without prior alcohol dependence. Arch Gen Psychiatry. 1998;55:41–46. doi: 10.1001/archpsyc.55.1.41. [DOI] [PubMed] [Google Scholar]
- 16.Harvey PD. Cognitive deficits and thought disorder: II. An 8-month followup study. Schizophr Bull. 1990;16:147–156. doi: 10.1093/schbul/16.1.147. [DOI] [PubMed] [Google Scholar]
- 17.Albus M. Contrasts in neuropsychological test profile between patients with first-episode schizophrenia and first-episode affective disorders. Acta Psychiatr Scand. 1996;94:87–93. doi: 10.1111/j.1600-0447.1996.tb09830.x. [DOI] [PubMed] [Google Scholar]
- 18.Addington J. Neurocognitive and social functioning in schizophrenia: a 2.5 year follow-up study. Schizophr Res. 2000;44:47–56. doi: 10.1016/s0920-9964(99)00160-7. [DOI] [PubMed] [Google Scholar]
- 19.Hughes C, et al. Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr Res. 2003;59:137–146. doi: 10.1016/s0920-9964(01)00393-0. [DOI] [PubMed] [Google Scholar]
- 20.Strauss ME. Relations of symptoms to cognitive deficits in schizophrenia. Schizophr Bull. 1993;19:215–231. doi: 10.1093/schbul/19.2.215. [DOI] [PubMed] [Google Scholar]
- 21.Bilder RM, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 22.Randolph C. RBANS Manual-Repeatable Battery for the Assessment of Neuropsychological Status. Lutz, FL: PAR, Inc; 1998. [DOI] [PubMed] [Google Scholar]
- 23.Wilk CM. Brief cognitive assessment in schizophrenia: normative data for the repeatable battery for the assessment of neuropsychological status. Schizophr Res. 2004;70:175–186. doi: 10.1016/j.schres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Bryson GJ. Differential rate of neuropsychological dysfunction in psychiatric disorders: comparison between the Halstead-Reitan and Luria-Nebraska batteries. Percept Mot Skills. 1993;76:305–306. [PubMed] [Google Scholar]
- 25.Heinrichs RW. Neurocognitive subtypes of chronic schizophrenia. Schizophr Res. 1993;9:49–58. doi: 10.1016/0920-9964(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 26.Palmer BW, et al. Is it possible to be schizophrenic yet neuropsychologically normal? Neuropsychology. 1997;11:437–446. doi: 10.1037//0894-4105.11.3.437. [DOI] [PubMed] [Google Scholar]
- 27.Keefe RS. Defining a cognitive function decrement in schizophrenia. Biol Psychiatry. 2005;57:688–691. doi: 10.1016/j.biopsych.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Cannon M, et al. Evidence for early-childhood, pan-developmental impairment specific to schizophreniform disorder: Results from a longitudinal birth cohort. Arch Gen Psychiatr. 2002;59:449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- 29.Fuller R. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189. doi: 10.1176/appi.ajp.159.7.1183. [DOI] [PubMed] [Google Scholar]
- 30.Reichenberg A, et al. Elaboration on premorbid intellectual performance in schizophrenia: premorbid intellectual decline and risk for schizophrenia. Arch Gen Psychiatry. 2005;62:1297–1304. doi: 10.1001/archpsyc.62.12.1297. [DOI] [PubMed] [Google Scholar]
- 31.Davidson M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry. 1999;156:1328–1335. doi: 10.1176/ajp.156.9.1328. [DOI] [PubMed] [Google Scholar]
- 32.Brewer WJ, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- 33.Brewer WJ, et al. Memory impairments identified in people at ultra-high risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 34.Keefe RSE. A longitudinal study of neurocognitive function in individuals at-risk for psychosis. Schizophr Res. doi: 10.1016/j.schres.2006.06.041. In press. [DOI] [PubMed] [Google Scholar]
- 35.Bowie CR. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, function, functional capacity, and symptoms. Am J Psychiatry. 2006;163:418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 36.Keefe RSE. The Schizophrenia Cognition Rating Scale (SCoRS): interview-based assessment and its relationship to cognition, real-world functioning and functional capacity. Am J Psychiatry. 2006;163:426–432. doi: 10.1176/appi.ajp.163.3.426. [DOI] [PubMed] [Google Scholar]
- 37.Ventura J. Interview based measures of cognition in schizophrenia. Biol Psychiatry. 2006;59:1715. [Google Scholar]
- 38.Mortiz S. Memory and attention performance in psychiatric patients: lack of correspondence between clinician-rated and patient-rated functioning with neuropsychological test results. J Int Neuropsychol Soc. doi: 10.1017/S1355617704104153. In press. [DOI] [PubMed] [Google Scholar]
- 39.van den Bosch RJ. Causal mechanisms of subjective cognitive dysfunction in schizophrenic and depressed patients. J Nerv Ment Dis. 1998;186:364–368. doi: 10.1097/00005053-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Harvey PD, et al. The convergence of neuropsychological testing and clinical ratings of cognitive impairment in patients with schizophrenia. Compr Psychiatry. 2001;42:306–313. doi: 10.1053/comp.2001.24587a. [DOI] [PubMed] [Google Scholar]
- 41.Stip E. Exploring cognitive complaints in schizophrenia: the subjective scale to investigate cognition in schizophrenia. Compr Psychiatry. 2003;44:331–340. doi: 10.1016/S0010-440X(03)00086-5. [DOI] [PubMed] [Google Scholar]
- 42.Spitzer RL. Values and assumptions in the development of DSM-III and DSM-III-R: an insider's perspective and a belated response to Sadler, Hulgus, and Agich's “On values in recent American psychiatric classification”. J Nerv Ment Dis. 2001;189:351–359. doi: 10.1097/00005053-200106000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Carpenter WT. Schizophrenia: diagnostic class or domains of pathology. Schizophr Bull. 2007;33:203. [Google Scholar]
- 44.Heinrichs RW. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]