Abstract
Increased latency of stimulus encoding is presented as a central deficit in schizophrenia cognition. Encoding, here, entails the internal representation of presenting stimuli in a format facilitating their implementation in other cognitive processes, such as those taking place in working memory. Historical roots of suspected encoding debility in schizophrenia briefly are reviewed, and its singular empirical robustness is described. More recently, this deficit has been subjected to stochastic mathematical modeling, resulting in its decomposition into discrete cognitive functions. A nonmathematical exposition of this account is provided, and substantial behavioral study support is illustrated. Implications for clinical assessment of individuals and of treatment regimens, with respect to encoding-related cognitive efficiency, are noted. Finally, because stochastic dynamic trajectories of process duration are modeled, times of measurement interest, complementing neuroanatomical regions of interest, become available for enhanced temporal navigation of event-related fMRI. Results from recent implementations of such process-defined events are described.
Keywords: encoding deficit, clinical mathematical modeling, clinical stochastic modeling, cognitive neurocircuitry, clinical assessment technology
If breakdown occurs at the data-processing level, then all subsequent operations will be adversely affected (just as an intact motor car engine will function inefficiently if it receives an inadequate supply of fuel)1 (p. 271).
The prominent experimental psychologist and clinical scientist, Aubrey Yates, cogently illustrated his hypothesis regarding schizophrenia cognition, originally presented some 7 years earlier, with the above scenario.2 Yates' “data processing” referred to so-called basic data assembly,3 meaning cognitive operations whereby “… incoming data is organized (or coded) for presentation to the highest cortical (mediational or cognitive) centers ….1” A central part of the disorder, in other words, was thought to comprise slowness in constructing something meaningful from incoming stimuli; much in the way of troubles encountered by schizophrenia patients, in turn, allegedly stemmed from this central problem.
During this same period, Checkosky,4 applying Saul Sternberg's now famous memory search task to the study of cognitive psychopathology, observed that scanning for the presence of a presented alphanumeric item (probe item) within a memorized set of items (memory set) proceeded with normal speed and accuracy in patients with schizophrenia. Higher than normal latencies nevertheless were obtained specifically for the intercept of Sternberg's “linear reaction time function.” This intercept was deemed to express the effects of both probe-item encoding as well as response selection and registration. A subsequent pair of studies by the present author indicated the agent responsible for intercept elevation among schizophrenia patients entailed the first process, probe-item encoding.5,6 Moreover, this deficit evidently was present in cognitive tasks other than the Sternberg paradigm, where task performance was encoding dependent.7
In his poignant mathematical analysis of memory search and related performance, James Townsend8 chastised clinical scientists studying schizophrenia cognition (including the present author) for failing to exploit pertinent developments in quantitative cognitive psychology. Appropriation of contemporary cognitive science indeed has spurred new avenues of empirical study, measurement, and explanation and has impinged in potentially important ways on collateral lines of investigation (eg, high-field strength fMRI). Much emphasis in such formal modeling of schizophrenia cognition has addressed specifically the nature and consequences of encoding abnormalities.
Encoding, here, stipulates cognitive conversion of raw stimulation into a format facilitating collateral task transactions, principally those of working memory. Note that the current reference to “encoding” should be distinguished from its frequent use in the “two-stage learning retrieval9,10” and related literature11,12 prominent in clinical cognitive science. During the learning or study stage of learning retrieval paradigms, items are presented for memorial storage with retrieval being evaluated during a subsequent “test stage.” To be sure, encoding subserving rehearsal and storage comes under the present encoding rubric. The encoding process, as treated in the current developments, however, entails task-facilitative operations in general. Rehearsal/storage-facilitative transactions in the 2-stage learning retrieval paradigm are considered to be a case in point.
The above verbal delineation of the current use of “encoding” has been augmented quantitatively, and with empirical correlates; discernment of encoding in task-performance data, evidence of its deficit in schizophrenia, and mathematical signatures of the key parameters of its quantitative modeling have been detailed in other venues.13–19
In keeping with the theme issue mandate, the remainder of this paper is devoted to elaborating upon the composition of schizophrenia encoding deficit and drawing out potential clinical significance with respect to certain symptomatology, clinical measurement, and selected aspects of cognitive neuroscience.
Robustness of Findings on Encoding Protraction
Increased latency on the Sternberg memory search and related cognitive tasks has been compatible with an “encoding-delay interpretation,” as defined above.5,6,20–24 Performance patterns on a variety of other tasks as well have lent themselves to translation as expressions of protracted encoding. Forms of encoding invoked in the service of task performance have included the following: locating verbal stimuli on dimensions of semantic similarity7,16,25,26; carrying out judgments of facial affect16,17; assembling dimensions of schematic faces and geometric forms for dichotomous classifications7,27; organizing item attributes underlying correct card sorting, resembling that of the Wisconsin Card Sorting Test28; and predicting the position of a moving target for smooth-pursuit eye tracking.29–31
It is safe to say that Yates' singling out of encoding as pivotal to cognitive dysfunction in schizophrenia appears to have been prescient. Moreover, contemporary empirical studies continue to be compatible with, if not patently supportive of, this position32–38; dissection of tasks, whereby such inferences are tendered, has been illustrated by Neufeld and Williamson.18
Prolonged Stimulus Encoding in Light of Stochastic Models of Processing Times
Comment on Stochastic Modeling
Mathematical research on encoding abnormalities in schizophrenia has exploited developments in the stochastic modeling of response times in human cognitive performance39,40 (for a clinical science perspective, see Carter, Neufeld, and Benn41; Neufeld14). Doob42 has aptly defined a stochastic model as “… the mathematical abstraction of an empirical process whose development is governed by probabilistic laws (p. v).” Stochastic mathematical modeling of mentation thus incorporates as a fundamental property, an aspect of indeterminacy.
As empirical latencies for process completion are randomly distributed, empirical targets of model prediction entail distribution properties, such as the mean of multiple information-processing trials.43,44 The empirical latency distribution, derived from a large number of trial repetitions, is modeled in terms of distribution parameters. Such parameters are substantively significant in that they convey selected aspects of information processing itself.
For example, the encoding process is thought to be made up of constituent operations or dissociable constituent stages (subprocesses). In the memory search task described above, for instance, encoding an alphanumeric probe item, for template matching to items in the memory set, tenably requires the encrypting of curves, lines, and intersections of the probe character. Similarly, the preparatory representation of a written statement, for purposes of its verification against a referent object, ostensibly demands the assembly of the statement's linguistic properties.45 The number of such “subprocesses” can be expressed as a model parameter, conveniently labeled k. Rate of completion or the number of subprocesses completed per unit time (eg, milliseconds) can be expressed as another model parameter, v. If completions were invariant and occurred in a determinist fashion in every task trial, each encoding trial would take precisely k/v units of time. For that matter, any one trial under the governance of k and v would be sufficient to tell the full story about encoding duration.
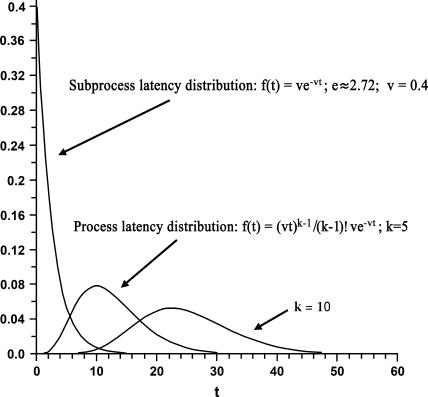
Because ambient influences come into play, however, variation in process latencies inevitably occur, and stochastic models attempt to account for these variations. It would be impossible to track down such influences exhaustively, but the collective effects of these variations can be summarized using appropriate mathematical expressions. Quantitative expression of the relative frequencies, out of a large number of trials, of process completions across time, is such a summary [technically, this is the distribution's density function, denoted f(t)]. A tenable expression of the distribution of completions of individual subprocesses, transacted with rate v, is known as the “exponential” with rate parameter v. (Recall that a parameter is “an arbitrary constant” whose value affects the specific nature, but not the formal properties of a mathematical expression …; p. 435.46) An example of this distribution, with v = 0.4, is depicted in figure 1. Also presented in the figure are distributions of latencies for completion of all the subprocesses assembled into the in toto (encoding) process. The total latency is a sum of these cognitive processes.40 In one example presented in figure 1, there are 5 subprocesses (k = 5), and in the other, there are 10 (k = 10). Mean latency of such distributions (named Erlang distributions43), is k/v, with an intertrial latency variance of k/v2. Note that for these latter curves, each subprocess is assumed to have an exponential distribution, with an unchanging rate of 0.4 throughout. Model developments described in this paper, however, are not necessarily limited to these assumptions.18,19
Fig. 1.
Latency distributions of processes comprising different numbers of subprocesses and of one of their constituent subprocesses. The height of a curve f(t) represents the relative frequency of (sub)process completion at time t; area under each curve is constrained to be 1.0, as t goes from 0 to infinity. Note that for the subprocess distribution, f(t) necessarily equals v (0.4), its maximum value, at t = 0. Shape of this distribution essentially conveys that relative frequencies of completion are greater during earlier time intervals.
From their roles in the density function (specified in figure 1) and the modeled mean and intertrial variance, it is apparent then that cognition-significant parameters—in the present case, namely the number of subprocesses, or “process stages,” and speed of completion, or “process-wise cognitive capacity”—are part and parcel of the latency dispersion summaries. This example thus illustrates the model format brought to bear on estimated encoding latencies of individuals with schizophrenia.
Epistemic Aspects of Modeling Cognitive Pathology and Findings on Encoding Abnormality
Stochastic modeling of cognitive pathology starts with selecting models of normal cognitive task performance and using these models to analyze performance of the process of interest among clinical samples. The model can then be adjusted to account for the observed patterns of deviation in the performance of the clinical group.
Valid application of this procedure, of course, requires multiple precautions.39,47–49 A major experimental challenge, in the present case, involves ferreting out encoding from other information-processing stages, notably scanning for memory-held information, and other “working-memory” operations, along with response processes. Several tacks to addressing this challenge have been recruited. One takes the form of dissecting response latency “curves,” as plotted against memory-scanning load, into separate components. Recall, eg, that in a memory-scanning task, described in the Introduction, the intercept of the “linear reaction time function” is considered to be affected by both encoding and response processes. When timing of a trial commences with the presentation of encoding requirements, duration of their completion is registered on the intercept. This duration is not registered on the intercept, however, when the required encoding is arranged to take place before actual timing of the trial commences. Schizophrenia–control group intercept differences, present under the first condition, have disappeared under the second condition, where the encoding contribution essentially has been removed.5,6
Second, separate estimates of response processes involved in the present type of cognitive tasks, especially movement time, have been obtained to ascertain that values of schizophrenia and control groups are comparable.16,17 Moreover, intercept differences have remained present, following allowance for such response-process estimates.19 Third, response requirements have been systematically varied, while holding encoding requirements constant and establishing that group differences also have remained constant.28
Encoding duration, then, has been deciphered in these ways. The model parameters adjusted to accommodate its elongation among schizophrenia subjects, however, rest on testing resulting predictions against configurations of the educed encoding latencies (elaborated upon under Symptom Significance of Modeled Extended Encoding, below).
Once the model has been adjusted to accommodate deviant performance, it can be used to determine which aspects of cognitive performance are aberrant in the clinical group. Cognitive faculties that are represented by aspects of the original model that remain unchanged in the revised model are deemed as spared in the disorder; model features that have been altered represent cognitive faculties that are affected by the disorder.13 Results from application to reliable deviations in latency estimates among schizophrenia patients18,19 have suggested that extended encoding observed in schizophrenia is due to the occurrence of additional subprocesses during encoding in these patients, rather than to the rate of subprocess transaction50 or to changes in the architecture of the encoding system, meaning the organization of subprocesses, such as their serial (consecutive) or parallel (concurrent) arrangement.14,15,51
The shape of these findings is analogous to the following depiction of a railway train traveling through a crossing; the railway cars pass at normal speed, but because additional cars have been marshaled, total time of passage is increased.
Since its inception, further support for the above characterization of elongated encoding has taken several forms. These have involved predictions from developments integrating encoding of multidimensional stimuli with memory trace theory,16 and tests of neuroconnectionist simulations summoning additional encoding steps as the agent distinguishing schizophrenia performance.17 Moreover, the theorized presence of additional encoding stages has been coherent with findings on cognitive task performance of assorted formats, as described in Robustness of Findings on Encoding Protraction section, above.
Cognitive-Behavioral Mechanisms of Added Encoding Steps
Deciphering the exact cognitive-behavioral underpinnings of additional steps in the encoding process remains a work in progress. This state of mechanism identification, however, should not be interpreted as undermining the quantitative formulation. Indeed, there is much precedent in science for “mathematical necessity” to precede any pinpointing of associated mechanisms. (Various perspectives on this important property of formal theory can be accessed.52–56) For that matter, proffered mechanisms tenably are judged according to conformity with quantitative statements, rather than vice versa; no one would take seriously a proposed physical mechanism of gravity, eg, if it did not conform to Newton's mathematically stated universal law of gravitation. Meanwhile, formal formulations themselves can be held up to the standards of “aesthetic appeal.” A major criterion of the latter involves a formulation's apparent simplicity, pending unveiling of its theoretical and empirical ramifications.57
This said, potential cognitive-behavioral candidates nevertheless suggest themselves from extant findings of clinical cognitive science. One apparent candidate involves enhanced “priming” or “activating” of the processing system, which may entail orienting or other activities preliminary to encoding operations themselves.58 Another candidate is failure to tag encoding subprocesses that have been completed59,60 or to store and recognize redundancy characteristics of constituent encoding operations.61,62 An additional prospect is negative priming deficit.63 Here, the usual tendency to inhibit distractors, in favor of selective attention to target stimuli, is impaired. This possibility relates to the present context, not so much as a debility in detecting previousness but rather as a failure to edit it out (although whether negative priming deficit may be a cause or consequence of surfeit subprocess, becomes debatable when assayed analytically15). Electrophysiological evidence of stimulus gating deficits64 also appear compatible with the above composition of encoding delay. Specifically, diminished attenuation of effects of successive stimulation may point to the incursion of stimulus impingements normally filtered out, and such impingements may be part and parcel of the added subprocesses under consideration.
The term “binding” has been used in the cognitive neuroscience literature to signify formation of a Gestalt of otherwise disparate stimulus features.65 As stated above, evidence of compromised processing architecture in schizophrenia has been negative. Paradigms for quantifying the presence of Gestalt processing architecture have been available from quantitative cognitive science for some 2.5 decades.66 Clinical science adaptations67 have led to rejection of hypotheses addressed to adoption of alternate architectures, such as inefficient serial processing. Once more, when called for, encoding presenting stimulation into a “perceptual Gestalt” format takes place no less than it does among controls; increased duration, however, again can be understood as the recruiting of additional subprocesses to Gestalt formation (D. Vollick and R. W. J. Neufeld, unpublished data, and Vollick68). From this perspective, increased cognitive entropy is attributable to the undermining of collateral operations by excess encoding subprocesses.16,17 Although not supplying a behavioral candidate underlying forestalled encoding completion, “binding impairment” viewed as secondary to this delayed finalization, nevertheless, is a cognitive-behavioral observation consistent with the proffered formulation.
Suffice it to say that on balance, although it cannot be stated with certainty at this point what constitutes the precise cognitive-behavioral underpinnings of extended-encoding duration, its quantitative expression arguably is in good standing when it comes to pertinent experimental results. In a similar vein, its biological basis awaits identification; however, provocative evidence recently has been supplied by high-field fMRI effective-connectivity analyses (below). These analyses point to an additional possible source of this formally modeled deficit.
Symptom Significance of Modeled Extended Encoding
An advantage to theorizing afforded by quantitative models is the ready availability of model predictions that occur when selected model properties are perturbed. Changes on the input side convey disorder-affected functions, as deciphered above; changes on the output side take the form of model predictions, potentially providing angles on symptomatology sui generis to a formal platform. Such theoretical extension has the merit of being constrained by an axiomatic system, thereby making explicit the linkage between input—tweaking of model properties—and consequences—in this case, symptom significant changes in speed, and/or accuracy of the encoding process. Furthermore, identifying these relations within a formal investigative environment can proceed quite economically and without imposition on clinical resources.
Another advantage of a formal theoretical infrastructure is the requirement that its assumptions be spelled out (eg, distributions, such as those of figure 1, must be defined). Assumptive frameworks of course virtually never are fully correct but at least they are laid bare. They even may be such poor approximations of the actual workings of target phenomena that they yield grossly erroneous inferences. It can be counter argued, however, that at least we know explicitly of what the assumptions are composed.69,70 Formally stated assumptions that are fatally flawed can be clearly flagged as such and replaced or improved upon. Indeed, their formally imposed precision can implicate particular routes to redressing frailties. This format also bolsters efficiency of theorizing in that there need be no mistake as to “what hasn't worked,” thereby discouraging the retracing of blind alleys.
The above is a necessarily brief description of the context of the developments to follow. A further exposition of the fabric and utility of formal clinical cognitive science is available in other venues.47,71 Finally, note that the present version of modeling is “analytic” in that its emphasis is on derivations; this version contrasts “computational modeling” with its emphasis on computer simulation.
With the above qualifications and caveats in hand, potential symptom significance of encoding elongation can be drawn out. In the case of encoding consequences, the parameter to be manipulated is subprocess quantity. This portion of the development thus marks the transition from model-based identification of disorder-affected cognitive faculties to model-based inferential outgrowths.
If additional subprocesses infiltrate the encoding process, probability of process completion within a particular time interval would be predicted to be compromised. This expectation is borne out upon examination of computed probabilities of encoding-process completion at times t. The relevant aspect of stochastic model distributions now becomes the cumulative probability distribution function F(t), which is the probability of process completion at or before time t. For each of the 2 right-hand curves presented in figure 1, F(t) corresponds to its area to the left of a designated value of t on the horizontal axis. (Strictly speaking, this statement also applies to the subprocess distribution considered in isolation.) Difference in this area for the curve where k = 10 versus that where k = 5, exemplifies compromised probabilities of completion attributable to an elevation in subprocess amount. The point can be elaborated by plotting F(t) itself as a function of increasing subprocesses.
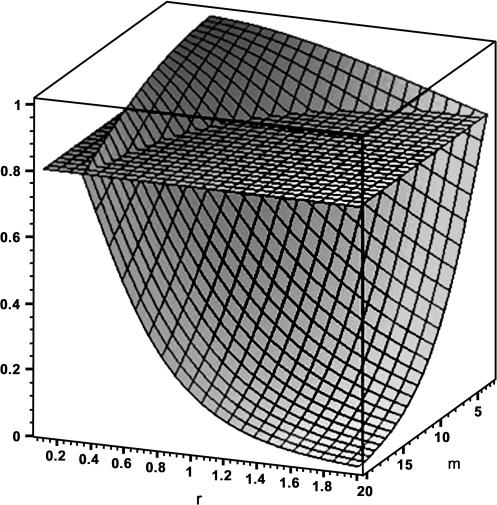
Such a plot is presented in figure 2, where t is set to 3 for illustration. Observe that the number of subprocesses is expressed on the depth axis, increasing from back to front, whereas the rate of dispatching them is expressed on the horizontal axis, increasing from right to left. Note that now k of the Erlang distribution (figure 1) is replaced with m and v is (inversely) replaced with r. These replacements denote that values plotted in figure 2 allow for the realistic possibility that both k and v vary randomly across processing trials (or episodes) and/or individuals. Computed values of F(t) instantiate this possibility by implementing stochastic distributions of k and v—“mixing distributions of k and v”—random values of which come into play for any single instance of F(t)—a single instance of F(t) corresponding to that of a “base distribution of t.” As m increases, therefore, the distribution of k moves upward, expressing the tendency associated with schizophrenia, and as r increases, the distribution of v moves downward, summarizing certain stimulus features or conditions of processing common to schizophrenia and control subjects alike (to be elaborated upon, below). Computational details of this extended distribution structure, labeled a “mixture model,” are available.13,41 Such an extension is invoked for the sake of increased comprehensiveness and arguably generality. The idea is to acknowledge the element of indeterminacy as a fundamental property of the addressed cognitive phenomenon, both on the “input and output sides of the model.”
Fig. 2.
Probability of encoding completion at or before time t as a function of average subprocess number m and rate-affecting stimulus degradation r (curved response surface). Horizontal surface marks an arbitrary “benchmark probability” of .80. (Technical specifications: probability distribution function F(t) for t = 3, of an Erlang base distribution, whose intensity parameter v is gamma distributed, the latter with shape hyperparameter K = 4, and intensity hyperparameter r, which varies along the horizontal axis; and whose shape parameter k is Poisson distributed, the latter with hyperparameter m, which varies along the depth axis; see text.)
As expected, the resulting 3-dimensional plot shows that process completion within the allotted time suffers with elevation in number of subprocesses. An even greater toll would be taken if the interval t were shorter, as with quickly changing scenes or normally rapid speech (although to expedite exposition, the current emphasis is on the visual modality).
The risk of noncompletion is exacerbated by sources of increased r. Increased r conveys a reduction in the speed of completing individual subprocesses and hence the process as a whole. It is identified with reduced stimulus salience or reduced discriminability. Reduction in processing rate with stimulus degradation of this nature has considerable empirically supported precedent in quantitative cognitive science.8,72 As noted above, processing rates parsimoniously are regarded as equal between schizophrenia and control subjects. Processing rate nevertheless is brought into play because it can vary for other reasons and when combined with encoding-process magnitude connects with selected symptomatology in potentially important ways (deferred to a later portion of this section). The point to be made for the moment is that completion of a stimulus subjected to encoding, already adversely affected by an increase in subprocess number, becomes even more at risk with diminished salience. The horizontal plane in figure 2 represents an arbitrary benchmark of a .80 probability of process completion. The region demarcated by m and r equaling or exceeding this benchmark becomes increasingly eroded as the values of m and r increase. Separability of m and r can be illustrated by describing experimental manipulations selectively targeting one or the other of these parameters.
An increase in m occurs with diagnostic status as “schizophrenia” versus “control.” It occurs, as well, with an increase in encoding requirements entailing additional operations, as follows. Where material to be encoded entails a short sentence, eg, its complexity can be increased in a way that demands the cognitive representation of more linguistic properties.5,6 Alternatively, in a case where nonverbal properties of presented items are required (eg, their “real-life item size”), item names can be presented in one condition and item drawings in another.19,20 In contrast to item drawings, item names are considered to engage more steps in accessing one's store of nonverbal item properties.
Increase in r (identified with reduced rate of subprocess completion), on the other hand, can be affected through degradation of the to-be-encoded stimulation (eg, by diminishing its intensity or clarity8,37 or possibly locating it in the peripheral visual field50).
Selective sensitivity of m and r to the above exemplary treatments can be shown to render specific statistical predictions. For example, in a research design combining control vs schizophrenia diagnostic status with a manipulation inducing 2 levels of encoding load (each of these factors hypothesized to affect m, above) and another comprising the presence vs absence of stimulus degradation (hypothesized to affect r, above), the following results are predicted for response latencies: significant main effects of diagnostic status, and of encoding load; a significant main effect of presence–absence of stimulus degradation, and of its interaction both with diagnostic status and with the encoding-load manipulation; along with no second-order interaction among the above 3 factors.
Returning to figure 2, if reduced stimulus salience exacerbates adverse effects of elevated subprocesses on the likelihood of process completion, what might be its sources outside the experimental setting? Two empirically grounded candidates come to the fore. The first is peripheral vs central status within the individual's attentional domain. Attentional periphery may be attributable to physical properties, such as marginal location in the visual field or attenuated vividness (resembling the experimental treatments, above). Furthermore, to this first source of reduced stimulus salience, the agent of peripheral status may be psychological in nature, as follows. Some features of a stimulus array stand to be downplayed because of the information processor's deployment of attentional resources. Such functional gradations in salience of parts of the stimulus constellation can be seen experimentally for example in the unequal rates of processing attending separate portions of the visual field that differentially bear on successful task completion.50,73,74
By extension—with characteristics of thought-content disorder (delusions and thematic hallucinations) borne in mind—degraded portions of the attentional field tenably are those imparting objective significance to the broader stimulus composite. In the workplace setting, eg, a pair of fellow employees, quietly planning a hunting expedition, may have ready to hand an equipment catalogue and topographical map. If these items are peripheral in the attentional domain, they may not be picked up during a limited encoding interval, and more the case if the encoding process is protracted. Whatever is successfully encoded—say the occurrence of quiet conversation—may be construed as personally significant, even conspiratorial.
This scenario is integrated with figure 2 as follows. Items that are more attentionally central—hypothetically represented in this example by the quiet conversation—are encoded at a faster rate, corresponding to a lower range of r. Conversely, items that are more attentionally peripheral—in this example, the equipment catalogue and topographical map—are encoded at a slower rate, corresponding to a higher range of r. Protracted encoding, represented in figure 2 as increased m, is seen in that figure to exacerbate the reduced likelihood of encoding the attentionally peripheral items. In these ways, material that stands a greater chance of being successfully encoded also risks being unaccompanied by cues that are critical to an informed interpretation of the encoded material. Any number of such scenarios could serve to “illustrate” the proposed fractional processing of a presenting stimulus complex.
A necessary consequence of the postulated marginalization of interpretation-grounding items is their disproportionally greater risk of being missed during available encoding-time windows. If so, then that which successfully is taken in stands in jeopardy of being conceptually dislodged from its contextual moorings. In keeping with this argument, Dobson and Neufeld28 observed experimentally that reduced influence of contextual constraints on information processing was identified specifically with protracted encoding of the constraints. These findings, together with the propensity in schizophrenia to overinfer the presence of stimuli or stimulus properties,75 may contribute to delusional content. The potential upshot is that evaluating the corpus of encoded material may proceed without the usual benefit of contextual guideposts. As stated by George and Neufeld,76 “While retarded encoding does not necessarily reduce the amount of perceptual input initially entering the information-processing system, it tends to render unavailable the context required in subsequent stages to make that input meaningful; p. 269.” This line of theoretical reasoning is in the spirit of Brendan Maher's77 writings on delusional beliefs: considering the information at the disposal of the “logician,” syllogistic reasoning and other rules of deduction yield superficially defensible and coherent conclusions—conclusions, in other words, that bear verisimilitude, but are not veridical.
Question might be raised as to why delusional content produced by mental operations applied to incomplete or fractional information might so often take on an oppressive, persecutory, or jealous bent. It can be reasonably conjectured that this inferential tendency may convey “survival value,” as follows. Given partial information, the net cost of erroneously ascribing malevolent intent to others may be substantially less than that of falsely assuming benevolence. That is to say, an excessively defensive posture may impart a degree of physical or social protection in this way.77,78
The idea of “context deficit” remains current in theory and research on schizophrenia cognitive performance.79,80 Reduction or removal of its centering influence on information manipulation in working memory can be seen as a higher level version of “binding impairment.” Diminished influence of context is viewed here as a natural outgrowth of generally forestalled encoding-process completion, in the company of inherent degradation of context bestowing stimulation. To be sure, inherent degradation of context bestowing stimulation remains an assumption within the present formulation—one that lends coherence to the formulation but is yet to be empirically validated.
Certain qualifications to the parsimony lending assumption of shared processing rates among schizophrenia and control subjects may be in order as follows. As preoccupation with delusional content (and possibly thematic hallucinations) increases, the rate of encoding objective-significance-endowing stimulation may be further eroded.81 Where certain stimulation is decidedly illness-relevant, attentional resources conceivably are drawn away from other parts of the attentional domain. In this way, the topography of attention deployment, expressed in associated processing rates, may come to deviate from that of controls—even if processing capacity at large is not assuaged.
Note as well that although emphasis here has been on thought-content symptomatology, associated with paranoid schizophrenia, subprocess-related encoding retardation, albeit less extreme, is not absent in nonparanoid schizophrenia.16,17,21,82 Specific mechanisms whereby additional subprocesses evince formal thought disorder, developed from the standpoint of stochastic modeling, currently are underway.
A second source of stimulus degradation that comes to the fore is psychological stress. Psychological stress (for definition, see Neufeld83,84) has been shown to “absorb” processing capacity, as estimated through a stochastic modeling platform of the class presented in this paper.50,67 A lowering of processing rate, over and above that already described, is in keeping with empirical relations between stress and symptomatology.85,86 Inspection of the 3-dimensional response surface in figure 2 illustrates how preexisting damage to the economy of subprocess production (increased m) is poised to compound adverse effects on encoding-process completion of stress-induced shrinkage in process-wise capacity (increased r).
Finally, the above developments intersect with selected forms of coping with stress, a prominent representative being “decisional control.87,88” Decisional control is a cognition-intensive version of coping, where predictive judgments surrounding available choices for handling environmental demands and stresses play a key role.89,90 Effective implementation of decisional control leans heavily on encoding of choice-relevant properties of the environment. A viable mechanism of stress negotiation deficit therefore is encoding debility. Stress, moreover, compromises its own resolution to the extent that it adds to consequences of this debility by impinging on processing rate.50,67
Clinical Measurement Implications
It is to be hoped that if stimulus encoding holds a central role in schizophrenia cognition, it lends itself to clinical assessment of individuals. There is good reason to be sanguine about this prospect because cognitive stochastic mixture models readily lend themselves to Bayesian measurement technology.91 The fundamental ingredients of Bayesian-based measurement are 2-fold: Bayesian priors and individual likelihood functions. Bayesian priors correspond directly with the parameter mixing distributions belonging to mixture models as described in this paper (random distributions of k and v). Second, individual likelihood functions are produced by computationally combining a performance sample of encoding latency, obtained from the individual at hand, with the density function supplied by the base-distribution latency model (figure 1; the likelihood function for an individual base distribution, being probability of the encoding-latency sample, given a specific pair of values for k and v).
Computational specifics apropos of the present context are beyond the scope and mandate of this paper but are amply provided elsewhere.13–15 Available are individualized estimates of base-distribution parameters and custom-modeled base distributions of encoding latencies. Even with modest performance specimens (to be welcomed when assessing distressed participants), estimates, nevertheless, can be made with decent precision, essentially because of the sharpening influence of information lodged in prior (mixing) distributions.
Also via Bayes' theorem (above sources), methods are forthcoming for monitoring individual encoding efficiency over the course of treatment. Such cognitive assessment moreover is entrenched in quantitative cognitive science because performance is placed squarely within stochastic cognitive process models whose parameter values are randomly distributed according to tenable “Bayesian priors.” This asset presumably is at a premium, considering contemporary emphasis on cognitive efficiency in evaluating pharmacotherapy.13,14,92 Extensions include time-course evaluation of entire regimens, using similar cognitive science– and statistical science–principled techniques.14 Finally, Bayesian procedures usher in unique methods for affirming mixture model validity.15 It is apparent they are ideally suited to integrative psychological science (or, more informally, “bench to bedside research”), a prominent initiative of NIMH.
Neurocircuitry of Extended Encoding
Estimation of the neuronal activation patterns identified with abnormal encoding duration has invoked high magnetic field (4.0 Tesla) fMRI. Investigation has appropriated 2 paradigmatic strategies. The first strategy seeks to isolate through experimental control the process of interest (eg, encoding) to the exclusion of collateral processes (eg, memory scanning and response operations). The second strategy essentially leaves the multiprocess constellation intact and seeks to delineate those aspects of neuronal activation patterns that map onto a target process. Functional MRI studies addressing schizophrenia encoding have used both these strategies. It has extended the second strategy—“event-related fMRI”—so as to exploit chronometric properties of stochastic modeling (below).
The first strategy, entailing “selected-process paradigms,” can simplify both interpretation of cognitive performance and estimated mapping to neuronal systems.93,94 Pursuant to this tack, schizophrenia patients and controls were required to encode visually presented consonants in terms of their lexical properties by affiliating them with consonant-commencing words.32 Task performance by first-episode, never-treated schizophrenia patients was accompanied by diminished right anterior cingulate (ACC) activation. Furthermore, effective connectivity (essentially time-series covariance distinguishing the above encoding activity from silent reading95) between the ACC and left inferior temporal region was apparent among controls but not the schizophrenia subjects. Note that coactivation of these regions is consistent with long-term auditory-lexical memory processes. Clearly evident instead was diffuse connectivity of the ACC to multiple brain regions (notably prefrontal and parietal regions; for consideration of this finding, from the perspective of final common pathway neuronal systems, see P. C. Williamson, this issue).
Apropos of findings from the second strategy note that event-related fMRI typically seeks to align monitored patterns of activation with observed (usually manipulated) changes in the appropriated cognitive or perceptual paradigm (eg, shift from a stationary to moving visual target, neutral to task-relevant stimulation, and so on96). The “events” of interest nevertheless presumably are not variations in the experimental paradigm per se, so much as the cognitive-perceptual functions to which these variations give rise (eg, altered visual scanning). In compliance with this goal, stochastic models convey probabilistic dynamical trajectories of their modeled processes, rendering among other useful quantities, statistical confidence intervals for process occurrence. In so doing, in principle they contribute times of interest available to complement regions of interest, in calibrating space-time coordinates of fMRI measurements. Consequently, events of interest are demarcated by epochs within task-performance trials rather than paradigmatic transitions in and of themselves.13,14
Maintaining a target process within the context of collateral processes (eg, probe-item encoding conjoint with memory scanning and response processes) preserves the integrity of the target process with respect to its ecologically valid format. Ensuring the presence of stimulus encoding in the service of memory scanning and ultimately correct responding, for instance, in principle mandates accompaniment by the latter 2 processes. Avoided is the extrication of the target process from its setting of functional significance. To use an ecological metaphor, dismantling of the multiprocess task risks distortion of its constituent processes by removing them from their “natural habitat of operation.” Leaving component processes within the multiprocess constellation, however, throws into relief reliance on valid dynamical performance models to estimate key times of target-process occurrence.
Accordingly, stochastic modeling results were used in conjunction with a Sternberg memory-search task (described in the Introductory section of this paper) to estimate trial epochs associated with encoding of the (alphanumeric) probe item (that was to be compared with members of the memory set). As in the above study of never-treated first-episode subjects, relative to controls, in-treatment schizophrenia subjects displayed more diffuse (left) ACC effective connectivity mainly to midbrain regions notably during the period estimated to accentuate probe-item encoding (K. Boksman, J. Miller, P. Williamson et al, unpublished data). Taken together, these studies offer up the provisional inference that diminished economy of encoding subprocesses is paralleled by a less consolidated, seemingly more entropic system of neuronal coactivation. Note that provision was made for equality of performance levels—word production,32 and response accuracy (K. Boksman, J. Miller, P. Williamson et al, unpublished data)—in each of the above studies. (For an enumeration of imaging neuroconnectivity findings, entailing cognitive tasks implicating encoding as a constituent, along with related spectroscopy and structural findings, see Boksman et al32 Wolf et al97).
A candidate source of prolonged encoding latency and associated effective connectivity, above, takes the following form. Anomalous fMRI-monitored functional connectivity during maintenance, so-called resting phases of mental activity, has been evident among schizophrenia participants (R. Bluhm, J. Miller, R. Lanius et al, this issue, and Liang et al98). Neuroconnectivity attending “on-hold” cognitive functioning has been deemed to evince a default system, possibly operative pending cognitive challenge. Lending default system resources to the encoding process99 tenably is impeded if deviations in its circuitry renders the system less poised to convey such resources upon encounter of the to-be-encoded stimulus or stimulus complex.
The developments presented here illustrate that extension of contemporary quantitative cognitive science can prescribe fMRI paradigms and inform direction and interpretation of MRI measurements. Because of potential symptom significance of encoded functions (described above), formal cognitive paradigms moreover hold out the promise of brokering relations between monitored neuronal activation patterns and symptomatology.41
The above observations on the neurocircuitry of extended encoding of course in no way exhaust electrophysiological and other biological findings potentially bearing on this formally modeled deficit. These observations focus on selected fMRI studies most directly linked to the quantitative specification of the abnormality as described in this paper. This specification arguably aligns most closely with the “computational level of analysis,” as mapped onto the influential taxonomy of levels of analysis put forth by vision theorist, David Marr.100 Computational (eg, stochastic modeling), algorithmic (neuroconnectionist), and implementational (biological) levels of analysis are deemed isomorphic, each being a coextensive to the other. Current algorithmic extensions (involving outpatient samples), of the computational-level formalisms (established among inpatients),16,17 and the above fMRI findings indicate that such isomorphic relations may be tenable in the present case. Note that each level of analysis defensibly contributes information unique to that level,100 and one level does not inherently trump the others in importance. As indicated in the Cognitive-Behavioral Mechanisms of Added Encoding Steps section, it behooves observations emanating from algorithmic and implementational levels of analysis to accord with the parameterized version of encoding depicted at the computational level, if such observations are offered as coextensions of the tendered computational-level signatures.
Cognitive-Behavioral Final Common Pathway
Delayed completion of stimulus encoding due to insertion into the encoding process of additional subprocesses has been put forth as a central and potentially coalescing account of schizophrenia cognition. This account provides a quantitative explanation of longer encoding latencies reported in the clinical cognitive science literature for performance conditions where time restrictions are not imposed. It also specifies diminished probabilities of encoding completion reported for performance taking place under time limits. Cognitive-behavioral correlates of the mathematically stipulated additional subprocesses are forthcoming from clinical cognitive science studies.
Consequences of encoding prolongation in the absence of time limits include undermining the integrity of extant task-relevant information whose retention is contingent on the efficient encoding of other task-relevant information. Consequences of reduced likelihoods of completion under limited time intervals comprise the loss of stimulus information whose function is to set a context for stimulation inherently more resistant to encoding deficit. This combination has been deductively integrated with thought-disorder symptomatology.
The quantitative representation of the encoding process and its extended duration with schizophrenia spawns cognitive science– and statistical science–principled clinical assessment techniques that fit well with current emphases on integrative psychological science. Ushered in are Bayesian statistical methods for monitoring individual progress in cognitive efficiency over time and for evaluating treatment regimens with respect to their progressive impact on cognitive functioning.
Stochastic modeling of the encoding process also provides potentially unique guidance on times of measurement interest, augmenting regions of interest, for cerebrovascular and electrophysiological studies of neuronal activation correlates. Recent applications of model-endowed stochastic dynamical trajectories of the encoding process, entailing the extraction of probabilistic confidence intervals for the presence of encoding, have yielded reliable findings from high-field fMRI research.
Direction for promising lines of future investigation are embedded in these developments. Included are the assembly of parametrically homogeneous subjects for fMRI and other investigations, using the “Bayesian posterior” values described under Clinical Measurement Implications section. Included as well is the extension of construct validity of individual Bayesian posterior estimates of encoding parameters (k and v) through their correlations with measures of related cognitive faculties.41,101
Differential degradation of context-endowing stimulation, described under Symptom Significance of Modeled Extended Encoding section, also lends itself to empirical study. Methodologically, formal methods of evaluating apportionment of attention to demarcated sections of a stimulus complex present themselves (eg, Townsend and Ashby,40 Chapter 5; for clinical science applications, see Neufeld et al50).
Finally, methods of clinical assessment addressing dynamical changes in individual encoding efficiency and applicable to evaluation of overall treatment regimens, above, lend themselves to preliminary “field testing” and potential application in the clinical setting. In these ways, the parametric expression of delayed encoding completion sets the stage for synthesizing diverse observations in clinical cognitive science, embodies specific directions for future research, and potentially provides a rigorous explanatory framework for its findings.
Acknowledgments
Preparation of this article was supported by an operating grant from the Social Sciences and Humanities Research Council of Canada to Neufeld. Studies involving high-field fMRI, reported herein, were supported by the Canadian Institutes of Health Research, in the form of an operating grant (Neufeld, coinvestigator), and a Doctoral Fellowship to Kristine Boksman. Thanks are extended to Robyn Bluhm, Derek Mitchell, and Kristine Boksman for their comments on an earlier version of this manuscript.
References
- 1.Yates A. Abnormalities of psychomotor functions. In: Eysenck HJ, editor. Handbook of Abnormal Psychology. San Diego, Calif: R.R. Knapp; 1973. pp. 261–283. [Google Scholar]
- 2.Yates A. Psychological deficit. Annu Rev Psychol. 1966;17:111–144. doi: 10.1146/annurev.ps.17.020166.000551. [DOI] [PubMed] [Google Scholar]
- 3.Yates A. Speed of perceptual functioning in chronic nonparanoid schizophrenics. J Abnorm Psychol. 1970;76:452–461. doi: 10.1037/h0030281. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg S. High-speed scanning in human memory. Science. 1966;153:652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 5.Neufeld RWJ. Components of processing deficit among paranoid and nonparanoid schizophrenics. J Abnorm Psychol. 1977;86:60–64. doi: 10.1037//0021-843x.86.1.60. [DOI] [PubMed] [Google Scholar]
- 6.Neufeld RWJ. Paranoid and nonparanoid schizophrenics' deficit in the interpretation of sentences: an information-processing approach. J Clin Psychol. 1978;34:333–339. doi: 10.1002/1097-4679(197804)34:2<333::aid-jclp2270340214>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 7.Neufeld RWJ. Simultaneous processing of multiple stimulus dimensions among paranoid and nonparanoid schizophrenics. Multivariate Behav Res. 1976;4:425–442. doi: 10.1207/s15327906mbr1104_4. [DOI] [PubMed] [Google Scholar]
- 8.Townsend JT. Uncovering mental processes with factorial experiments. J Math Psychol. 1984;28:363–400. [Google Scholar]
- 9.Batchelder WH. Using multinomial processing tree models to measure cognitive deficits in clinical populations. In: Neufeld RWJ, editor. Advances in Clinical Cognitive Science: Formal Modeling of Processes and Symptoms. Washington, DC: American Psychological Association; 2007. pp. 19–50. [Google Scholar]
- 10.Chechile RA. A model-based storage-retrieval analysis of developmental dyslexia. In: Neufeld RWJ, editor. Advances in Clinical Cognitive Science: Formal Modeling of Processes and Symptoms. Washington, DC: American Psychological Association; 2007. pp. 51–80. [Google Scholar]
- 11.Atkinson RC. Human memory: a proposed system and its control processes. In: Spence KW, editor. The Psychology of Learning and Motivation. Vol. 2. New York, NY: Academic Press; 1968. pp. 742–775. [Google Scholar]
- 12.Atkinson RC. Multiprocess models for memory with applications to a continuous presentation task. J Math Psychol. 1967;4:277–300. [Google Scholar]
- 13.Neufeld RWJ. Application of stochastic modelling to group and individual differences in cognitive functioning. Psychol Assess. 2002;14:279–298. [PubMed] [Google Scholar]
- 14.Neufeld RWJ. Composition and uses of formal clinical cognitive science. In: Shuart B, editor. Modeling Complex Systems: Nebraska Symposium on Motivation. Lincoln, Neb: University of Nebraska Press; [PubMed] [Google Scholar]
- 15.Neufeld RWJ, et al. A mathematical process account of group and individual differences in memory-search facilitative stimulus encoding, with application to schizophrenia. In: Neufeld RWJ, editor. Advances in Clinical Cognitive Science: Formal Modeling and Assessment of Processes and Symptoms. Washington, DC: American Psychological Association; 2007. pp. 147–177. [Google Scholar]
- 16.Carter JR. Cognitive processing of multidimensional stimuli in schizophrenia: formal modeling of judgment speed and content. J Abnorm Psychol. 1999;108:633–654. doi: 10.1037//0021-843x.108.4.633. [DOI] [PubMed] [Google Scholar]
- 17.Carter JR. Cognitive processing of facial affect: neuro-connectionist modeling of deviations in schizophrenia. J Abnorm Psychol. doi: 10.1037/0021-843X.116.2.290. In press. [DOI] [PubMed] [Google Scholar]
- 18.Neufeld RWJ. Neuropsychological correlates of positive symptoms: delusions and hallucinations. In: Pantelis C, editor. Schizophrenia: A Neuropsychological Perspective. London, England: John Wiley & Sons; 1996. pp. 205–235. [Google Scholar]
- 19.Neufeld RWJ. Stochastic modelling of stimulus encoding and memory search in paranoid schizophrenia: clinical and theoretical implications. In: Cromwell RL, editor. Schizophrenia: Origins, Processes, Treatment, and Outcome: The Second Kansas Series in Clinical Psychology. Oxford, England: Oxford University Press; 1993. pp. 176–196. [Google Scholar]
- 20.George L. Imagery and verbal aspects of schizophrenic informational-performance. Br J Clin Psychol. 1984;23:9–18. doi: 10.1111/j.2044-8260.1984.tb00621.x. [DOI] [PubMed] [Google Scholar]
- 21.George L. Attentional resources and hemispheric functional asymmetry in schizophrenia. Br J Clin Psychol. 1987;26:35–45. doi: 10.1111/j.2044-8260.1987.tb00721.x. [DOI] [PubMed] [Google Scholar]
- 22.Marusarz TZ. Contextual effects on the short-term memory retrieval of schizophrenic young adults. J Abnorm Psychol. 1980;89:683–696. doi: 10.1037//0021-843x.89.6.683. [DOI] [PubMed] [Google Scholar]
- 23.Russell PN. Visual and memory search in process schizophrenics. J Abnorm Psychol. 1980;89:109–114. doi: 10.1037//0021-843x.89.2.109. [DOI] [PubMed] [Google Scholar]
- 24.Wishner J. Stages of information processing in schizophrenia: Sternberg's paradigm. In: Wynne L, editor. The Nature of Schizophrenia: New Approaches to Research and Treatment. New York, NY: Wiley & Sons; 1978. pp. 233–243. [Google Scholar]
- 25.Koh SD. Remembering in schizophrenia. In: Schwartz S, editor. Language and Cognition in Schizophrenia. Hillsdale, NJ: Erlbaum; 1978. pp. 384–399. [Google Scholar]
- 26.Neufeld RWJ. Multidimensional scaling of schizophrenics' and normals' perceptions of verbal similarity. J Abnorm Psychol. 1975;84:498–507. doi: 10.1037/h0077122. [DOI] [PubMed] [Google Scholar]
- 27.Neufeld RWJ. Relationship between conceptual judgments and stimulus dimensions among schizophrenics and normals. Br J Soc Clin Psychol. 1976;15:85–91. doi: 10.1111/j.2044-8260.1976.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 28.Dobson D. Paranoid-nonparanoid schizophrenic distinctions in the implementation of external conceptual constraints. J Nerv Ment Dis. 1982;170:614–621. doi: 10.1097/00005053-198210000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Mather JA. Schizophrenic performance in line bisection: no simple defects. J Psychiatr Res. 1990;24:185–190. doi: 10.1016/0022-3956(90)90058-x. [DOI] [PubMed] [Google Scholar]
- 30.Neufeld RWJ. Multivariate structure of eye-movement dysfunction distinguishing schizophrenia. Multivariate Exp Clin Res. 1995;11:1–21. [Google Scholar]
- 31.O'Donnell BF. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–692. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 32.Boksman K, et al. A 4.0 Tesla fMRI study of brain connectivity during word fluency in first episode schizophrenia. Schizophr Res. 2005;75:247–263. doi: 10.1016/j.schres.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 33.Elkins IJ. Span of apprehension in schizophrenic patients as a function of distractor masking and laterality. J Abnorm Psychol. 1992;101:53–60. doi: 10.1037//0021-843x.101.1.53. [DOI] [PubMed] [Google Scholar]
- 34.Fuller RL. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- 35.Fuller RL. Working memory consolidation is abnormally slow in schizophrenia. J Abnorm Psychol. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- 36.Kieffaber P. Switch and maintenance of task set in schizophrenia. Schizophr Res. 2006;84:345–358. doi: 10.1016/j.schres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Kieffaber PD. Event related brain potential evidence for preserved attentional set switching in schizophrenia. Schizophr Res. doi: 10.1016/j.schres.2007.03.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold JM. Intact attentional control of working memory encoding in schizophrenia. J Abnorm Psychol. 2006;115:658–673. doi: 10.1037/0021-843X.115.4.658. [DOI] [PubMed] [Google Scholar]
- 39.Luce RD. Response Times: Their Role in Inferring Elementary Mental Organization. New York, NY: Oxford University Press; 1986. [Google Scholar]
- 40.Townsend JT. Stochastic Modelling of Elementary Psychological Processes. New York, NY: Cambridge University Press; 1983. [Google Scholar]
- 41.Carter JR. Application of process models in assessment psychology: potential assets and challenges. Psychol Assess. 1998;10:379–395. [Google Scholar]
- 42.Doob JL. Stochastic Processes. New York, NY: John Wiley & Sons; 1953. [Google Scholar]
- 43.Evans M. Statistical Distributions. 3rd ed. New York, NY: Wiley & Sons; 2000. [Google Scholar]
- 44.Neufeld RWJ. Introduction. Special section on psychological assessment. Psychol Assess. 1998;10:396–398. [Google Scholar]
- 45.Carpenter PA. Sentence comprehension: a psycholinguistic processing model of verification. Psychol Rev. 1975;82:45–73. [Google Scholar]
- 46.Borowski EJ. The Harper Collins Dictionary of Mathematics. 2nd ed. New York, NY: Harper Collins; 1989. [Google Scholar]
- 47.Neufeld RWJ. Introduction. In: Neufeld RWJ, editor. Advances in Clinical Cognitive Science: Formal Modeling and Assessment of Processes and Symptoms. Washington, DC: American Psychological Association Publications; 2007. pp. 3–18. [Google Scholar]
- 48.Neufeld RWJ. Data aggregation in evaluating psychological constructs: multivariate and logical-deductive considerations. J Math Psychol. 1990;34:276–296. [Google Scholar]
- 49.Pachella R. The interpretation of reaction time in information processing research. In: Kantowitz BH, editor. Human Information Processing: Tutorials in Performance and Cognition. Hillsdale, NJ: Erlbaum; 1974. pp. 41–82. [Google Scholar]
- 50.Neufeld RWJ. Quantitative response time technology for measuring cognitive-processing capacity in clinical studies. In: Neufeld RWJ, editor. Advances in Clinical Cognitive Science: Formal Modeling and Assessment of Processes and Symptoms. Washington, DC: American Psychological Association; 2007. pp. 207–238. [Google Scholar]
- 51.Townsend JT. Assessment of mental architecture in clinical/cognitive research. In: Treat TA, editor. Psychological Clinical Science: Papers in Honor of Richard M. McFall. Hillsdale, NJ: Erlbaum; 2007. pp. 223–258. [Google Scholar]
- 52.Braithwaite RB. Scientific Explanation. London, England: Cambridge University Press; 1968. [Google Scholar]
- 53.Kline M. Mathematics and the Search for Knowledge. Oxford, England: Oxford University Press; 1985. [Google Scholar]
- 54.Penrose A. The Road to Reality. London, UK: Alfred A. Knopf Publishers; 2004. [Google Scholar]
- 55.Smolin L. The Trouble With Physics: The Rise of String Theory, The Fall of a Science, and What Comes Next. Boston, MA: Houghton Mifflin Company; 2006. [Google Scholar]
- 56.Thorne KS. Black Holes and Time Warps: Einstein's Outrageous Legacy. New York, NY: Norton; 1994. [Google Scholar]
- 57.Polkinghorne J. Plenary Lecture: Veritas Forum. London, Ontario, Canada: University of Western Ontario; 2003. Friendship of Science and Religion. [Google Scholar]
- 58.Russell PN. Performance of process schizophrenics on tasks involving visual search. J Abnorm Psychol. 1977;86:16–26. doi: 10.1037//0021-843x.86.1.16. [DOI] [PubMed] [Google Scholar]
- 59.Hemsley DR. Perception and cognition in schizophrenia. In: Cromwell RL, editor. Schizophrenia: Origins, Processes, Treatment and Outcome. New York, NY: Oxford University Press; 1993. pp. 135–150. [Google Scholar]
- 60.Hemsley DR. Perceptual and cognitive normality as the basis for schizophrenic symptoms. In: David AS, editor. The Neuropsychology of Schizophrenia. Hillsdale, NJ: Erlbaum; 1994. pp. 97–118. [Google Scholar]
- 61.Steffy RA. Relation between latency and redundancy-associated deficit in schizophrenic reaction time performance. J Abnorm Psychol. 1980;89:419–427. doi: 10.1037//0021-843x.89.3.419. [DOI] [PubMed] [Google Scholar]
- 62.Steffy RA. Schizophrenics' reaction time: North star or shooting star? In: Cromwell RL, editor. Schizophrenia: Origins, Processes, Treatment, and Outcome. New York, NY: Oxford University Press; 1993. pp. 111–134. [Google Scholar]
- 63.MacQueen GM. Impaired distractor inhibition in patients with schizophrenia on a negative priming task. Psychol Med. 2003;33:121–129. doi: 10.1017/s0033291702006918. [DOI] [PubMed] [Google Scholar]
- 64.Meincke U. Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis—a longitudinal study. Eur Arch Psychiatry Clin Neurosci. 2004;254:415–421. doi: 10.1007/s00406-004-0523-0. [DOI] [PubMed] [Google Scholar]
- 65.Treisman A. The binding problem. Curr Opin Neurobiol. 1996;6:171–178. doi: 10.1016/s0959-4388(96)80070-5. [DOI] [PubMed] [Google Scholar]
- 66.Snodgrass JG. Comparing parallel and serial models: theory and implementation. J Exp Psychol Hum Percept Perform. 1980;6:330–354. [Google Scholar]
- 67.Neufeld RWJ. A formal analysis of stressor and stress-proneness effects on basic information processing. Br J Math Stat Psychol. 1994;47:193–226. doi: 10.1111/j.2044-8317.1994.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 68.Vollick D. PhD Dissertation. London, Canada: Department of Psychology, Faculty of Graduate Studies, University of Western Ontario; 1994. Stochastic Models of Encoding-Latency Means and Variances in Paranoid Schizophrenia. [Google Scholar]
- 69.Staddon JER. Social learning theory and the dynamics of interaction. Psychol Rev. 1984;91:502–507. [Google Scholar]
- 70.Staddon JER. “The distemper of learning….” A review of S.B. Klein and R.R. Mowrer (Eds.) contemporary learning theories: instrumental conditioning theory and the impact of biological constraints on learning. Contemp Psychol. 1991;36:506–507. [Google Scholar]
- 71.Neufeld RWJ. Advances in Clinical Cognitive Science: Formal Modeling and Assessment of Processes and Symptoms. Washington, DC: American Psychological Association Publications; 2007. [Google Scholar]
- 72.McGill WJ. The general-gamma distribution and reaction times. J Math Psychol. 1965;2:1–18. [Google Scholar]
- 73.Hockey GRJ. Effect of loud noise on attention selectivity. Q J Exp Psychol. 1970;22:28–36. [Google Scholar]
- 74.Hockey GRJ. Signal probability and spatial location as possible bases for increased selectivity in noise. Q J Exp Psychol. 1970;22:37–42. [Google Scholar]
- 75.Broga MI. Multivariate cognitive performance levels and response styles among paranoid and nonparanoid schizophrenics. J Abnorm Psychol. 1981;90:495–509. doi: 10.1037//0021-843x.90.6.495. [DOI] [PubMed] [Google Scholar]
- 76.George L. Cognition and symptomatology in schizophrenia. Schizophr Bull. 1985;11:264–285. doi: 10.1093/schbul/11.2.264. [DOI] [PubMed] [Google Scholar]
- 77.Maher B. Delusions as the product of normal cognitions. In: Oltmanns TF, editor. Delusional Beliefs. New York, NY: John Wiley & Sons; 1988. pp. 333–336. [Google Scholar]
- 78.Neufeld RWJ. Memory in paranoid schizophrenia. In: Magaro P, editor. The Cognitive Bases of Mental Disorders: Annual Review of Psychopathology. Vol 1. Newbury Park, Calif: Sage; 1991. pp. 231–261. [Google Scholar]
- 79.Cohen JD. Schizophrenic deficits in the processing of context: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- 80.Kerns JG. The relationship between formal thought disorder and executive functioning component processes. J Abnorm Psychol. 2003;112:339–352. doi: 10.1037/0021-843x.112.3.339. [DOI] [PubMed] [Google Scholar]
- 81.Johnson-Laird PN. A hyper-emotion theory of psychological illnesses. Psychol Rev. 2006;113:822–841. doi: 10.1037/0033-295X.113.4.822. [DOI] [PubMed] [Google Scholar]
- 82.Highgate-Maynard S. Schizophrenic memory-search performance involving nonverbal stimulus properties. J Abnorm Psychol. 1986;95:67–73. doi: 10.1037//0021-843x.95.1.67. [DOI] [PubMed] [Google Scholar]
- 83.Neufeld RWJ. Methodological aspects of laboratory studies of stress. In: Neufeld RWJ, editor. Advances in the Investigation of Psychological Stress. New York, NY: John Wiley & Sons; 1989. pp. 71–132. [Google Scholar]
- 84.Neufeld RWJ. Coping with stress, coping without stress, and stress with coping: on inter-construct redundancies. Stress Med. 1990;6:117–125. [Google Scholar]
- 85.Nicholson IR. A dynamic vulnerability perspective on stress and schizophrenia. Am J Orthopsychiatry. 1992;62:117–130. doi: 10.1037/h0079307. [DOI] [PubMed] [Google Scholar]
- 86.Norman RMG. Stressful life events and schizophrenia, I: a review of the literature. Br J Psychiatry. 1993;162:161–166. doi: 10.1192/bjp.162.2.161. [DOI] [PubMed] [Google Scholar]
- 87.Averill JR. Personal control over aversive stimuli and its relationship to stress. Psychol Bull. 1973;80:286–303. [Google Scholar]
- 88.Neufeld RWJ. Dynamic differentials of stress and coping. Psychol Rev. 1999;106:385–397. [Google Scholar]
- 89.Morrison MS. The economy of probabilistic stress: interplay of controlling activity and threat reduction. Br J Math Stat Psychol. 1988;41:155–177. doi: 10.1111/j.2044-8317.1988.tb00894.x. [DOI] [PubMed] [Google Scholar]
- 90.Lees MC. Decision-theoretic aspects of stress arousal and coping propensity. J Pers Soc Psychol. 1999;77:185–208. [Google Scholar]
- 91.Berger JO. Statistical Decision Theory and Bayesian Analysis. 2nd ed. New York, NY: Springer; 1985. [Google Scholar]
- 92.Neufeld RWJ. Mount Sinai Workshop on Cognition in Schizophrenia: International Congress on Schizophrenia Research. British Columbia, BC: Whistler; 2001. Formal Models in Explanation and Measurement of Cognitive Psychopathology. [Google Scholar]
- 93.Fabiani M. The learning of complex task performance. Acta Psychol. 1989;71:259–300. [Google Scholar]
- 94.Yechiam E. Cognitive models for evaluating basic decision processes in clinical populations. In: Neufeld RWJ, editor. Advances in Clinical Cognitive Science: Formal Modeling and Assessment of Processes and Symptoms. Washington, DC: American Psychological Association; 2007. pp. 81–111. [Google Scholar]
- 95.Friston KJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 96.Friston KJ. Models of brain functioning in neuroimaging. Annu Rev Psychol. 2005;56:57–87. doi: 10.1146/annurev.psych.56.091103.070311. [DOI] [PubMed] [Google Scholar]
- 97.Wolf DH, et al. Alterations of fronto-temporal connectivity during word encoding in schizophrenia. Psychiatry Res. doi: 10.1016/j.pscychresns.2006.11.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang M, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 99.Braver TS. Extracting core components of cognitive control. Trends Cogn Sci. 2006;12:529–532. doi: 10.1016/j.tics.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 100.Marr D. Vision. New York, NY: Freeman; 1982. [Google Scholar]
- 101.Wallsten TW. Cognitive modeling of a sequential risk-taking task. Psychol Rev. 2005;112:862–880. doi: 10.1037/0033-295X.112.4.862. [DOI] [PubMed] [Google Scholar]