Abstract
Bipolar disorder is one of the most debilitating and common illnesses worldwide. Individuals with bipolar disorder frequently present to clinical services when depressed but are often misdiagnosed with unipolar depression, leading to inadequate treatment and poor outcome. Increased accuracy in diagnosing bipolar disorder, especially during depression, is therefore a key long-term goal to improve the mental health of individuals with the disorder. The attainment of this goal can be facilitated by identifying biomarkers reflecting pathophysiologic processes in bipolar disorder, namely impaired emotion regulation, impaired attention, and distractibility, which persist during depression and remission and are not common to unipolar depression. In this critical review, we examine the feasibility of identifying biomarker of bipolar disorder by discussing existing findings regarding functional abnormalities in neural systems underlying emotion processing (amygdala centered), working memory, and attention (dorsolateral prefrontal cortex centered) that persist through bipolar depression and remission and are bipolar specific rather than common to unipolar depression. We then focus on future research goals relating to major clinical problems in bipolar disorder, including, the identification of biomarkers allowing detection of individuals at risk of subsequent development of the disorder. Bipolar disorder is a common, debilitating, and potentially fatal disorder. Current and future research in bipolar disorder should focus on identification of disorder biomarkers to improve diagnostic accuracy and the mental heath of those with the disorder.
Keywords: pathophysiology, mood disorder, amygdala, prefrontal cortex, neuroimaging
Introduction
Bipolar disorder remains one of the 10 most debilitating illnesses worldwide,1 with a prevalence of at least 1%. Bipolar disorder type I (BPI), characterized by the presence of episodes of mania and depression, in particular is associated with a poor clinical and functional outcome, a high suicide rate,2 and a huge societal cost.3 One reason for the poor prognosis is the frequent misdiagnosis or late diagnosis of the disorder,4,5 leading to delays in the initiation of appropriate treatment. Indeed, while depression is a more common presentation and a cause of greater disruption of occupational, family, and social functioning than mania in individuals with bipolar disorder,6 bipolar depression continues to be frequently misdiagnosed and inappropriately treated as unipolar depression in individuals without a clear previous history of manic episodes.7–10 Increased accuracy in diagnosing bipolar disorder in individuals when they present during depression therefore remains a key goal to help improve the mental health, treatment, and clinical and functional outcomes of individuals with all subtypes of the disorder.
The recent research agenda for Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V), has emphasized a need to translate basic and clinical neuroscience research findings into a new classification system for all psychiatric disorders based upon pathophysiologic and etiological processes.11–14 These pathophysiologic processes involve complex relationships between genetic variables, abnormalities in brain systems, and related neuropsychological function and behavior and may be represented as biomarkers of a disorder.15 Abnormalities that are persistent rather than episodic or state features of a disorder can be more readily used to identify those individuals with the disorder.16 Measurement in individuals with bipolar disorder of brain system abnormalities underlying characteristic behavioral impairments that are “common to remission and depression” may therefore help to identify future biomarkers of the disorder,17,18 as will examination of brain system abnormalities that are “specific to bipolar disorder” and not common to unipolar depression. These studies will facilitate future increases in accuracy of diagnosis of bipolar disorder and subsequent treatment improvements in depressed individuals presenting without a clear history of mania.
There may be several different symptom domains in the traditional BPI.14 One important symptom domain is mood instability leading to variability in depression and/or hypomanic/manic states as well as other aspects of mood variability which might be expressed as irritability or sadness. This may be related to impaired processing of emotionally salient information in the environment. A second major symptom domain is impaired cognitive control and executive dysfunction, which includes symptoms such as the inability to concentrate, difficulty in decision making, and memory difficulties. Together, these 2 symptom domains may confer an inability to regulate emotional states in any given context, as individuals are unable to employ appropriate cognitive control processes, including reappraisal, suppression, or inhibitory processes,19 either with or without overt awareness, to regulate and inhibit the generation of inappropriate emotional states. Subsyndromal levels of these symptom domains persist during remission in individuals with the disorder20 and may underlie the vulnerability to subsequent severe mood episodes.21 Thus, examination of activity in neural systems associated with (1) initial identification and generation of emotional states in response to emotionally salient material and (2) covert and overt cognitive control processes that may be linked with the ability to regulate emotional states22 is a first stage toward the longer term goal of identifying biomarkers of bipolar disorder.
We therefore next describe experimental paradigms which can be employed in neuroimaging studies to measure activity in neural systems that are associated with these 2 major symptom domains in bipolar disorder. These include neural systems underlying (1) emotion processing, specifically, neural systems implicated in the initial identification and generation of emotional states in response to emotionally salient material and (2) cognitive control processes, including attention, working memory, inhibitory control, strategy development, and cognitive flexibility.23 Abnormal function in these 2 neural systems may be linked, respectively, with the mood instability and impaired cognitive control processes that are commonly observed in bipolar disorder. We therefore subsequently describe the functional abnormalities in these neural systems that have been reported in bipolar disorder using paradigms designed specifically to examine activity in these neural systems and the extent to which these abnormalities may be specific to bipolar disorder rather than being common to unipolar depression.
Paradigms Measuring Neural Responses During Emotion Processing, Working Memory, Attention, and Emotion Regulation
Paradigms to examine activity associated with the first symptom domain, mood instability, that may be associated with impaired processing of emotionally salient information have included displays of facial expressions. These stimuli are highly salient social signals of emotional states, the correct recognition of which is crucial for social interaction. Facial expression identification tasks have therefore been widely used in the examination of emotion-processing abilities in healthy and psychiatric populations.21,24 In healthy individuals, findings from neuroimaging studies have implicated a network of subcortical, predominantly anterior limbic regions. In response to presentations of different facial expressions, including ventral striatum, amygdala, anterior hippocampus, and anterior insula,24–28 numerous other types of emotional stimuli have been employed in the examination of neural systems implicated in emotion processing. These include emotional scenes, emotional words, and emotional material presented in different sensory modalities.21
The second symptom domain, impaired cognitive control and executive dysfunction, maps to dysfunction in a lateral prefrontal cortical system, comprising dorsolateral and ventrolateral prefrontal cortex (DLPFC and VLPFC), which is important for cognitive and executive function (eg, Monchi et al29), and the hippocampus, important for memory. One commonly employed task of working memory and attention is the digit-sorting task. This task requires the sorting of digits into numerical order and memorization of the digit with the middle value. The performance of this task has been reliably associated with DLPFC activity in healthy individuals.30 Numerous studies employing attentional tasks, including the Stroop interference task, in which individuals selectively attend to the color ink in which a color word is written rather than the color word per se, have further implicated the DLPFC,31 dorsal regions of the anterior cingulate gyrus,32–35 and ventral prefrontal cortex (VPFC)36 during performance of these tasks in healthy individuals.
Fewer studies have specifically focused on examination of neural systems underlying regulation of emotion. Recent studies have implicated dorsal prefrontal cortical regions both in the suppression of arousal to emotive stimuli37 and reappraisal of emotive scenes38,39 during attempts to reduce emotional experience. Another method of examining emotion regulatory processes less confounded by interindividual differences in emotion regulatory strategies is to employ paradigms measuring the impact of emotional contexts upon subsequent performance of executive control or attentional tasks. This method has previously been employed in healthy individuals and those with unipolar depression,30 with findings indicating reciprocal relationships between amygdala and dorsolateral prefrontal cortical responses during the attentional component of such tasks. Clearly, further study is required in healthy individuals of the nature of neural systems that are specifically implicated in the different cognitive control processes implicated in emotional state regulation.
In the following sections, evidence is presented for abnormalities in neural response during performance of these tasks in remitted individuals with bipolar disorder compared with healthy individuals. Findings are then described from studies examining neural responses during these tasks in depressed individuals with bipolar disorder compared with healthy individuals, and in depressed individuals compared with remitted individuals with bipolar disorder, to examine the extent to which such abnormalities are common in remission and depression.
Functional Abnormalities in Neural Systems Underlying Emotion Processing and Cognitive Control Processes in Bipolar Remission
The few existing studies examining neural responses to emotional stimuli have indicated increased amygdala and ventral striatal activity to mild happy40 and intense fearful expressions40,41 in remitted, and increased amygdala activity to happy expressions in a mixed group of remitted and unwell,42 individuals with bipolar disorder. Findings also show decreased DLPFC activity to fearful expressions39 in remitted individuals with bipolar disorder (predominantly the bipolar I subtype) compared with healthy individuals. No significant relationship between subsyndromal depression severity and amygdala responses to happy and fearful facial expressions has been observed in remitted individuals with bipolar disorder.40 Interestingly, other studies have demonstrated widespread decreases in prefrontal cortical and subcortical neural activity to emotional words in remitted individuals with BPI.43,44 It is therefore possible that emotional facial expressions are processed as particularly significant in individuals with bipolar disorders during remission.
During performance of attentional tasks, findings in remitted, euthymic individuals with bipolar disorder compared with healthy individuals have indicated reduced activity in dorsal and ventral prefrontal cortical regions36 and reduced activity within dorsal regions of the anterior cingulate gyrus, although increased DLPFC activity,45 during a Stroop interference task. Other studies have demonstrated reduced DLPFC activity in euthymic individuals with bipolar disorder during working memory and verbal encoding tasks.46,47 Increases in activity within subcortical regions associated with emotion processing rather than working memory or attention have also been demonstrated in remitted, euthymic individuals with bipolar disorder during performance of a continuous performance task48 and a working memory task49 and in adolescents with bipolar disorder during performance of a Stroop attentional task.50
These findings suggest “increased amygdala and subcortical” activity but predominantly “decreased DLPFC” activity during emotion-processing and cognitive control tasks in bipolar remission. There are some inconsistencies that may relate to the nature of the emotional stimuli employed in these tasks. Findings indicate that facial expressions may be processed as particularly salient stimuli in remitted individuals with bipolar disorder. We next describe findings from studies examining neural responses during these tasks in bipolar depression.
Are There Functional Abnormalities in Neural Systems Common to Bipolar Remission and Depression?
Findings indicate increased subcortical activity to negative scenes during the generation of emotional states in bipolar depressed individuals (including rapid cycling) compared with healthy individuals,51 and decreased activity in medial prefrontal cortex during sad mood induction in remitted52 more than depressed bipolar individuals.53 One study has reported relative increases in activity in a number of subcortical regions to happy facial expressions in bipolar depressed compared with manic individuals and healthy individuals,54 but further study focused upon amygdala and prefrontal cortical responses is required in larger numbers of bipolar depressed and remitted individuals. Increased amygdala activity has been demonstrated in both bipolar depressed and remitted individuals (approximately 50% type I) compared with healthy individuals at rest.55 During a sustained attention task, findings have indicated decreased absolute prefrontal cortical and increased subcortical metabolism, with negative and positive correlations between metabolism in these prefrontal cortical and subcortical regions, respectively, and depression severity in bipolar depressed (predominantly rapid cycling) compared with healthy individuals.56
Of the few studies directly comparing neural activity during performance of attentional tasks in remitted or euthymic vs depressed individuals with bipolar disorder, relative increases in ventrolateral prefrontal cortical activity have been reported in bipolar depressed compared with euthymic individuals during performance of a Stroop task.36 Similarly, during performance of a Stroop attentional task, depression severity correlated negatively with the magnitude of the ventral prefrontal cortical decreases in individuals with bipolar disorder.57 These findings suggest common functional abnormalities in subcortical and prefrontal cortical regions during bipolar depression and remission compared with healthy individuals. They also indicate further depression-related abnormalities, in particular, relative increases in prefrontal cortical activity during attentional tasks in bipolar depressed compared with remitted individuals. Further study is required to identify abnormal neural responses during emotion-processing, attentional, and working memory tasks and tasks involving emotion regulatory processes, which persist and are therefore common to remission and depression in bipolar disorder. Current data comparing bipolar depressed and remitted individuals suggest a positive association between increased prefrontal cortical activity and increased depression severity during attentional tasks.
There is a lack of studies specifically examining the relationship between change in depression severity over time in individuals with bipolar disorder and change in the nature and magnitude of abnormal neural activity during emotion and cognitive challenge tasks. This seriously limits current understanding of the neural mechanisms associated with change in depression severity over time in bipolar depression. Longitudinal examination of the relationship between depression severity and abnormal neural activity is therefore required to better understand these neural mechanisms. Another problem is the paucity of studies comparing neural activity during emotion and cognitive challenge tasks in bipolar and unipolar depressed populations. This limits understanding of the extent to which abnormalities observed in bipolar depression are bipolar specific or depression related and therefore common to both bipolar and unipolar populations. We next describe findings from studies which have employed these tasks in unipolar depressed compared with healthy individuals and the few findings from studies that have directly compared neural activity in bipolar and unipolar populations.
The link With Mania: Are There Similar Functional Neural Abnormalities Evident in Mania, Depression, and Remission in Bipolar Disorder?
A clear history of mania indicates a diagnosis of bipolar disorder rather than unipolar depression. To fully understand the pathophysiologic mechanisms underlying bipolar disorder, it is important to consider the nature of functional abnormalities in neural systems that may persist across mania, depression, and remission. Few studies have examined activity in neural systems associated with the 2 symptom domains in individuals during mania. To date, studies have reported in manic individuals relative to healthy individuals increased amygdala,58 insula,59 and subcortical activity per se54 to negative emotional facial expressions and to negative scenes,60 increased ventral striatal activity at rest61 and during motor tasks,62 and decreased ventral prefrontal cortical activity during performance of a variety of different cognitive control tasks.63–66Together with findings from functional neuroimaging studies of depressed and remitted individuals with bipolar disorder, these data suggest patterns of increased amygdala and subcortical activity to emotional—at least negative emotional—stimuli and decreases in activity in prefrontal cortical regions implicated in cognitive control processes that may be common to all 3 phases of bipolar illness. More study is required, however, examining the nature of functional abnormalities in emotion-processing neural systems to different categories of emotional stimuli (eg, positive vs negative emotional stimuli) and during different cognitive control tasks in individuals during mania.
Abnormal Neural Responses in Unipolar Depressed Individuals
The majority of functional neuroimaging studies of unipolar depressed individuals pre- and postremission after treatment with pharmacological and psychological interventions have been performed during resting state and not during performance of specific emotion-processing or attentional tasks.67–76 There are discrepant findings from these studies. Some studies report increases in dorsal and ventral prefrontal cortical activity67,68,72,76,77 or decreases in subcortical and ventral prefrontal cortical responses74 in unipolar depressed individuals and in mixed groups of individuals with unipolar and bipolar depression after depression improvement with pharmacological intervention. Other studies suggest decreases in dorsolateral and ventrolateral prefrontal cortical activity after successful psychological and pharmacological interventions69,71,75,78 or relative increases only in subcortical, limbic regions after both types of intervention in unipolar depressed individuals.74 Studies have also reported an inverse relationship between depression severity and dorsal prefrontal cortical and anterior cingulate gyral activity in unipolar depressed individuals at rest.67,79 Regarding neural responses during performance of emotion-processing tasks, abnormal increases in amygdala or ventral striatal activity have been demonstrated by unipolar depressed individuals to negative emotional expressions,80–82 and similar patterns of decreased ventromedial prefrontal activity during sad mood induction compared with healthy individuals have been reported in unipolar remitted and depressed individuals.83 Decreased activity in left DLPFC relative to healthy individuals has been reported in unipolar depressed individuals during working memory trials following negative stimuli30 and during working memory and attention.84–86 In the majority of studies, an amelioration of the abnormal pattern of neural response during depression has been demonstrated in unipolar depressed individuals after remission. For example, abnormal increases in amygdala or ventral striatal activity to negative emotional expressions in unipolar depressed individuals80–82 significantly reduce in remission after treatment with antidepressant medication.80,81 Increases after remission in insular and anterior cingulate gyral activity to negative vs neutral scenes have also been reported.87
In summary, while findings regarding neural responses during at rest studies are somewhat discrepant in unipolar depressed individuals, findings from studies employing emotional challenge paradigms suggest that, similar to individuals with bipolar disorder, unipolar depressed individuals show increased amygdala and subcortical activity to emotional stimuli relative to healthy individuals. Unlike individuals with bipolar disorder, however, in unipolar depressed individuals this abnormal pattern of neural activity is predominantly negative rather than positive emotional stimuli. Furthermore, these abnormalities appear to be depression dependent in unipolar depressed individuals rather than abnormalities common throughout depression and remission. Only 1 study to date has directly compared neural activity in bipolar and unipolar individuals. Here, we showed increases in amygdala and subcortical activity predominantly not only to mild happy expressions but also to fearful facial expressions, vs neutral expressions in remitted bipolar relative to unipolar depressed individuals.40 There is thus clearly a need for studies comparing neural activity in bipolar and unipolar populations during emotional and cognitive challenge paradigms, but data to date suggest that examination of patterns of subcortical neural activity to negative and positive stimuli may help distinguish individuals with bipolar disorder from those with unipolar depression. Furthermore, there is a need to examine the extent to which relationships between changes in depression severity over time and changes in neural activity differentiate bipolar and unipolar populations.
A Neural Model of Bipolar Disorder
Current findings require replication in larger numbers of participants but suggest that bipolar disorder can be modeled as dysfunction in 2 neural systems that are implicated in 2 major symptom domains in bipolar disorder: (1) abnormally increased activity in an amygdala- and subcortical-centered system underlying emotion processing that may be linked with the mood instability commonly observed in individuals with bipolar disorder and (2) abnormally decreased activity in a prefrontal cortical neural system comprising predominantly DLPFC and VPFC underlying cognitive control processes, including attention, working memory, inhibitory control, strategy development, and cognitive flexibility,21,23,29 that may be linked with impaired cognitive control and executive dysfunction observed in bipolar disorder (figure 1). The former (1) may underlie the emotional lability, the latter (2) the impaired attention and distractibility, and the combination of both abnormalities, the inability to employ cognitive control strategies, either with covert or overt awareness, to successfully regulate emotional states that are common clinical features of bipolar disorder. While functional regulation of the amygdala may be directly mediated by ventromedial prefrontal cortex,88 functional abnormalities in these 2 neural systems are suggested by the few functional neuroimaging studies to date in bipolar disorder. Findings suggest that abnormalities described in (1) to emotional stimuli may occur both in remitted40 and depressed54 bipolar individuals, but no studies have directly compared neural activity during emotion-processing paradigms in these 2 populations. Findings further suggest relative increases in activity in the prefrontal cortical-centered system in bipolar depression compared with bipolar remission during attention tasks.36,55 In unipolar depression, unlike bipolar disorder, findings suggest that abnormalities described in (1) above may occur to some negative, but not positive, emotional stimuli and may thus distinguish bipolar from unipolar populations40,70 (table 1). To date, only 1 study has directly compared bipolar and unipolar individuals40 and provides some support for this potential distinction between bipolar remitted and unipolar depressed individuals. Clearly, there is a need for far more research in this burgeoning area of clinical neuroscience. Specifically, future studies should focus on the employment of experimental paradigms to examine the nature of functional abnormalities in the 2 neural systems described above that map closely to common symptom domains in bipolar disorder and the extent to which these abnormalities may help distinguish individuals with bipolar disorder, especially when presenting during depressed episode, from individuals with unipolar depression.
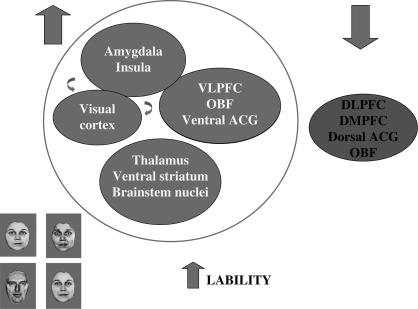
Fig. 1.
This Depicts a Schematic Model for the Neural Basis of the Affective Instability in Individuals with Bipolar Disorder. In bipolar disorder, it is postulated that to many emotional stimuli, including the different categories of facial expression depicted here, although not consistently to emotional words, a pattern of increased activity occurs in an amygdala- and subcortical-centered neural system important for the identification of emotional information and the generation of emotional states (depicted in dark gray). This, together with reduced activity in a DLPFC- and VLPFC-centered system important for cognitive control processes involved in the regulation of behavioral responses to emotional stimuli (depicted in light gray), may lead to impaired emotion regulation and increased lability of mood frequently observed in individuals with bipolar disorder. VLPFC, ventrolateral prefrontal cortex; DLPFC, dorsolateral prefrontal cortex; DMPFC, dorsomedial prefrontal cortex; ACG, anterior cingulate gyrus; OBF, orbitofrontal cortex.
Table 1.
Neural Activity During Emotion and Cognitive Challenge Tasks in Bipolar Disorder and Unipolar Depression
| Bipolar Remitted | Bipolar Depressed | Bipolar Mania | Unipolar Depressed | |
| Emotion processing | Increased amygdala and ventral striatal activity to positive and negative stimuli | Increased amygdala and ventral striatal activity to positive and negative stimuli | Increased amygdala activity and ventral striatal to negative emotional stimuli | Increased amygdala activity to negative, but not positive, stimuli |
| Decreased subcortical activity to emotional words | ||||
| Cognitive control tasks | Decreased DLPFC and VPFC activity | Increased DLPFC and VPFC activity relative to bipolar remitted | Decreased VPFC activity | Decreased DLPFC and VPFC activity |
Note: DLPFC, dorsolateral prefrontal cortex; VPFC, ventral prefrontal cortex.
The Effect of Structural Volume Abnormalities, Medication, and Other Clinical Variables Upon Functional Neural Abnormalities
Here, we describe other factors which may impact upon measurements of functional neural response during task performance in individuals with bipolar disorder (some findings including other bipolar subtypes), but which remain relatively unexamined. These include effects of regional structural neural volume abnormalities, psychotropic medication, and other clinical variables, including illness duration, subsyndromal symptoms of depression and mania in remitted individuals with bipolar disorder comorbid anxiety, history of psychotic symptoms, and history of alcohol and illicit substance abuse. Regarding regional structural volume abnormalities, findings have indicated amygdala volume increases in adult individuals with bipolar disorder,89–94 although decreases in adolescent individuals with BPI.95–101 Other studies have reported increased ventral striatal (caudate nucleus and putamen) volumes93,99,101 and decreased anterior thalamic volumes.102 In contrast, smaller amygdala (and hippocampal) volumes have been more consistently reported in unipolar depressed individuals.103–105 Findings regarding prefrontal cortical volumes have indicated decreased volume and gray matter density in anterior cingulate and subgenual cingulate gyri106–109 and dorsal prefrontal cortex and VPFC 92,108,110–112 and reduced density in the right subgenual anterior cingulate and adjacent white matter in individuals with bipolar disorder compared with healthy individuals.113 Recent findings further indicate gray matter volume reductions in the lateral orbitofrontal cortex of medicated individuals with bipolar subtypes I and II compared with healthy individuals.114 There have also been reports of no significant differences in prefrontal cortical volumes between individuals with bipolar disorder and healthy individuals, however.115,116 Similar patterns of reduced prefrontal cortical volume have been shown in unipolar depressed individuals,103,117–119 particularly elderly unipolar depressed individuals.120 Overall, while findings indicate structural volume abnormalities in amygdala and prefrontal cortical volumes,121 a recent meta-analysis has indicated that the most consistent structural abnormality is an increase in right ventricular volume in individuals with bipolar disorder.122
Mood-stabilizing medications, including divalproex sodium and lithium, have been reported as either decreasing prefrontal cortical blood flow or having no effect. In healthy individuals, benzodiazepine dose inversely correlates with amygdala response to facial expressions,123 while acute administration of selective serotonin reuptake inhibitor antidepressant medication has been associated with decreased amygdalar response to fearful facial expressions and aversive scenes124–126 and a suppressed electrophysiological response in frontal and occipital cortices to unpleasant scenes.127 Administration of the atypical neuroleptic sultopride has been associated with decreased amygdalar response to aversive compared with neutral scenes in healthy individuals.125 In bipolar depressed individuals, antidepressant medication has been associated with relative increases in prefrontal cortical metabolism at rest.67 Mood stabilizer medication has been associated with relative decreases in amygdala activity in remitted individuals with bipolar disorder (50% type I) at rest55 and decreases in amygdala activity in a mixed group of remitted and unwell individuals with bipolar disorder to emotional facial expressions.42 Other studies have shown a significant positive correlation between neuroleptic medication dose (in chlorpromazine equivalents) and activity in rostral anterior cingulate gyrus and DLPFC in remitted, euthymic individuals with BPI45 and increased DLPFC activity in medicated compared with unmedicated euthymic individuals with bipolar disorder128 during Stroop task performance, although no significant effect of any psychotropic medication was reported in individuals with bipolar disorder during a working memory task.49 Long-term psychotropic medication use114 and antidepressant exposure110 have been associated with relative decreases in ventral prefrontal cortical gray matter volume in individuals with bipolar disorder, but long-term effects of psychotropic medication upon regional structural volumes remain unclear.128,129 The effect of medication upon neural responses during task performance in bipolar populations therefore requires further study.
Together, these findings indicate structural abnormalities in prefrontal cortical and subcortical regions that are components of neural systems implicated in 2 symptom domains of bipolar disorder. Furthermore, psychotropic medications that are commonly taken by individuals with bipolar disorder may impact activity, at least in part, in these neural regions of interest in individuals with bipolar disorder. As there is so little study of the nature of structural-functional relationships in these neural regions or the effect of psychotropic medication upon activity in these regions in bipolar study, these remain important areas for future research.
Future Research: Can We Identify Biomarkers of Risk for Bipolar Disorder?
Elucidation of neural system abnormalities that are persistent and bipolar disorder specific remains a main focus of research aiming to identify biomarkers of bipolar disorder. A subsequent stage will be the examination of the extent to which these abnormalities are shared with bipolar subtypes other than the traditional bipolar I subtype.130,131 Another goal for longer term, future research in bipolar disorder that reflects major clinical problems associated with the disorder is the identification of biomarkers that allow us to predict the degree of risk of subsequent development of bipolar disorder in individuals who are at risk for, but as yet undiagnosed with, the disorder.
Findings from studies examining neural system abnormalities in bipolar disorder will ultimately lead to future studies examining the extent to which such neural system abnormalities exist as potential biomarkers of risk for bipolar disorder. Thus, future research should focus on examination of neural system abnormalities that are common to individuals with bipolar disorder and those as yet undiagnosed with the disorder, for example, individuals presenting with depression but yet to develop a manic or hypomanic episode, and individuals at high genetic risk for the disorder, for example offspring and as yet unaffected siblings of individuals with bipolar disorder. Only one study to date has examined functional neural abnormalities in individuals with bipolar disorder and their healthy siblings.52 In this study, the authors measured regional cerebral blood flow (rCBF) with [15O] water positron emission tomography after induction of transient sadness in 9 euthymic individuals with bipolar disorder who had responded to lithium and 9 healthy siblings. Common to both groups and a group of individuals with bipolar disorder who had responded to sodium valproate were rCBF increases in the dorsal/rostral anterior cingulate and anterior insula and decreases in the orbitofrontal and inferior temporal cortices. The authors noted that changes in rCBF during sadness induction were not seen previously in healthy subjects without a family history of mood disorder. The study's findings are therefore a first stage toward the identification of biomarkers of risk for bipolar disorder. Another study has demonstrated an association between genetic risk for bipolar disorder (in healthy siblings of adults with bipolar disorder) with gray matter volume deficits specifically within the right anterior cingulate gyrus and ventral striatum.132
To date, there have been no studies examining neural system abnormalities in healthy offspring of individuals with bipolar disorder. One study has, however, examined neurochemical abnormalities in offspring diagnosed with mood disorders (but not bipolar disorder) of adults with bipolar disorder using proton magnetic resonance spectroscopy.133 Similar to findings in adults with bipolar disorders, neurochemical abnormalities were demonstrated within frontal cortex and cerebellar vermis in these offspring. There is clearly much scope for future research to employ different functional neuroimaging techniques to examine potential biomarkers of risk for bipolar disorder.
Conclusion
The recent research agenda for DSM-V highlights a need to translate basic and clinical neuroscience research findings into a new classification system for all psychiatric disorders based upon pathophysiologic and etiological processes. Furthermore, identification of neural system abnormalities in individuals with bipolar disorder is of critical importance for the advance in diagnosis and subsequent treatment of this frequently misdiagnosed disorder, particularly in individuals presenting with depression without a clear history of mania. A first stage toward the identification of biomarkers of bipolar disorder is the examination of functional abnormalities in neural systems directly related to common symptom domains of bipolar disorder that are common to depression and remission, rather than remission- or depression specific, and those abnormalities that are specific to bipolar disorder rather than common to unipolar depression. Such common symptom domains include mood instability, linked with impaired emotion processing, and impaired cognitive control processes, linked with cognitive dysfunction, that together may underlie the inability to regulate emotional states in individuals with bipolar disorder. Findings from a small number of studies indicate increased amygdala activity to mild happy and fearful facial expressions and decreased DLPFC activity to fearful expressions, although no consistent pattern of emotion identification deficits, in remitted individuals with bipolar disorder. There are more consistent findings indicating impaired performance on working memory and attentional tasks in remitted individuals with bipolar disorder. Findings also indicate decreased prefrontal cortical, in particular ventrolateral and dorsal anterior cingulate gyral, activity, but also increased subcortical activity, during attentional task performance in these individuals. Data suggest relative increases in prefrontal cortical activity during attentional task performance in depressed compared with remitted individuals with bipolar disorder. There are limited data examining abnormalities that are common to remitted and depressed individuals with bipolar disorder—or even examining abnormalities that may persist throughout mania—in addition to depression and remission. Very few neuroimaging studies have directly compared bipolar and unipolar populations. Similarly, the relationship between structural and functional neural abnormalities and effects of psychotropic medication upon patterns of abnormal neural responses also remain unclarified in bipolar disorder. Current research should focus upon elucidation of neural system abnormalities that can be identified as biomarkers of bipolar disorder to help improve diagnostic accuracy in individuals in earlier stages of illness using paradigms. Major future goals are then to identify biomarkers that reflect risk for subsequent development of bipolar disorder and biomarkers that enable us to predict treatment response in individuals with the disorder.
References
- 1.Murray CJL. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Disease, Injuries and Risk Factors in 1990 and Project to 2020. Cambridge, Mass: Harvard School of Public Health, Harvard University Press; 1996. On behalf of the World Health Organization and the World Bank. [Google Scholar]
- 2.Baldessarini RJ. Suicide risk and treatments for patients with bipolar disorder. JAMA. 2003;290:1517–1519. doi: 10.1001/jama.290.11.1517. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt RJ. An economic evaluation of manic-depressive illness—1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30:213–219. doi: 10.1007/BF00789056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowden CL. Strategies to reduce misdiagnosis of bipolar depression. Psychiatr Serv. 2001;52:51–55. doi: 10.1176/appi.ps.52.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Bowden CL. A different depression: clinical distinctions between bipolar and unipolar depression. J Affect Disord. 2005;84:117–125. doi: 10.1016/S0165-0327(03)00194-0. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese JR. Impact of depressive symptoms compared with manic symptoms in bipolar disorder: results of a U.S. community-based sample. J Clin Psychiatry. 2004;65:1499–1504. doi: 10.4088/jcp.v65n1109. [DOI] [PubMed] [Google Scholar]
- 7.Ghaemi SN. Bipolar spectrum disorder: a pilot study. Psychopathology. 2004;37:222–226. doi: 10.1159/000080717. [DOI] [PubMed] [Google Scholar]
- 8.Hirschfeld RM. Perceptions and impact of bipolar disorder: how far have we really come? Results of the National Depressive and Manic-Depressive Association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161–174. [PubMed] [Google Scholar]
- 9.Lish JD. The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 10.Manning JS. Bipolar disorder in primary care. J Fam Pract. 2003;3(suppl 1):S6–S9. [PubMed] [Google Scholar]
- 11.Charney DS, et al. Neuroscience research agenda to guide development of a pathophysiologically based classification system. In: Kupfer DJ, editor. A Research Agenda for DSM-V. Washington DC: American Psychiatric Association; 2002. pp. 31–38. [Google Scholar]
- 12.Hasler G. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60:93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Hasler G. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 14.Phillips ML. Redefining bipolar disorder—toward DSM-V. Am J Psychiatry. 2006;163:7. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 15.Kraemer HC. Biomarkers in psychiatry: methodological issues. Am J Geriatr Psychiatry. 2002;10:653–659. [PubMed] [Google Scholar]
- 16.Kraemer HC. Can state and trait variables be disentangled? A methodological framework for psychiatric disorders. Psychiatry Res. 1994;52:55–69. doi: 10.1016/0165-1781(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 17.Kupfer DJ. The increasing medical burden in bipolar disorder. JAMA. 2005;293:2528–2530. doi: 10.1001/jama.293.20.2528. [DOI] [PubMed] [Google Scholar]
- 18.Swann A. What is bipolar disorder? Am J Psychiatry. 2006;163:177–179. doi: 10.1176/appi.ajp.163.2.177. [DOI] [PubMed] [Google Scholar]
- 19.Ochsner KN. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Clark L. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry. 2005;57:183–187. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Phillips ML. The neurobiology of emotion perception II: implications for understanding the neural basis of emotion perceptual abnormalities in schizophrenia and affective disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 22.Phillips ML. The neurobiology of emotion perception I: towards an understanding of the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 23.Stuss DT. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 24.Calder AJ. Neuropsychology of fear and loathing. Nat Rev Neurosci. 2001;2:352–363. doi: 10.1038/35072584. [DOI] [PubMed] [Google Scholar]
- 25.Morris JS, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- 26.Phillips ML, et al. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- 27.Surguladze SA, et al. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 28.Hariri AR, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 29.Monchi O. Wisconsin card sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–7741. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegle GJ. Can't shake that feeling: fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51:693–707. doi: 10.1016/s0006-3223(02)01314-8. [DOI] [PubMed] [Google Scholar]
- 31.Ridderinkhof KR. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Botvinick MM. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Garavan H. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 34.Kerns JG. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- 35.van Veen V. Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn. 2004;56:267–276. doi: 10.1016/j.bandc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Blumberg HP, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 37.Levesque J, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- 38.Ochsner KN. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Phan KL. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Lawrence N. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Yurgelun-Todd DA. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disord. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- 42.Blumberg HP, et al. Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology. 2005;183:308–313. doi: 10.1007/s00213-005-0156-7. [DOI] [PubMed] [Google Scholar]
- 43.Malhi GS. An emotional Stroop functional MRI study of euthymic bipolar disorder. Bipolar Disord. 2005;7(suppl 5):58–69. doi: 10.1111/j.1399-5618.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 44.Malhi GS. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J Affect Disord. 2007;97:109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Gruber SA. Decreased activation of the anterior cingulate in bipolar patients: an fMRI study. J Affect Disord. 2004;82:191–201. doi: 10.1016/j.jad.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 46.Deckersbach T, et al. Impaired recruitment of the dorsolateral prefrontal cortex and hippocampus during encoding in bipolar disorder. Biol Psychiatry. 2006;559:138–146. doi: 10.1016/j.biopsych.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 47.Monks PJ, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6:550–564. doi: 10.1111/j.1399-5618.2004.00147.x. [DOI] [PubMed] [Google Scholar]
- 48.Strakowski SM. A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–1740. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 49.Adler CM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6:540–549. doi: 10.1111/j.1399-5618.2004.00117.x. [DOI] [PubMed] [Google Scholar]
- 50.Blumberg HP, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 51.Malhi GS, et al. Cognitive generation of affect in bipolar depression: an fMRI study. Eur J Neurosci. 2004;19:741–754. doi: 10.1111/j.0953-816x.2003.03159.x. [DOI] [PubMed] [Google Scholar]
- 52.Kruger S. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. Am J Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- 53.Kruger S. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry. 2003;54:1274–1283. doi: 10.1016/s0006-3223(03)00691-7. [DOI] [PubMed] [Google Scholar]
- 54.Chen C-H, et al. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Drevets WC. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71:431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 56.Ketter TA, et al. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biol Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- 57.Kronhaus DM, et al. The ventral prefrontal cortex in bipolar disorder: distinguishing trait and depression-related abnormalities. Bipolar Disord. 2006;8:28–39. doi: 10.1111/j.1399-5618.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 58.Altshuler LL, et al. Blunted activation in orbitofrontal cortex during mania: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:763–769. doi: 10.1016/j.biopsych.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Lennox BR. Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med. 2004;34:795–802. doi: 10.1017/s0033291704002557. [DOI] [PubMed] [Google Scholar]
- 60.Malhi GS. Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord. 2004;6:271–285. doi: 10.1111/j.1399-5618.2004.00123.x. [DOI] [PubMed] [Google Scholar]
- 61.Blumberg HP, et al. Increased anterior cingulate and caudate activity in bipolar mania. Biol Psychiatry. 2000;48:1045–1052. doi: 10.1016/s0006-3223(00)00962-8. [DOI] [PubMed] [Google Scholar]
- 62.Caligiuri MP, et al. An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res. 2003;123:171–182. doi: 10.1016/s0925-4927(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 63.Altshuler L, et al. Increased amygdala activation during mania: a functional magnetic resonance imaging study. Am J Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- 64.Blumberg HP, et al. Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry. 1999;156:1986–1988. doi: 10.1176/ajp.156.12.1986. [DOI] [PubMed] [Google Scholar]
- 65.Elliott R. Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry. 2004;55:1163–1170. doi: 10.1016/j.biopsych.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Rubinsztein JS, et al. Decision-making in mania: a PET study. Brain. 2001;124(pt 12):2550–2563. doi: 10.1093/brain/124.12.2550. [DOI] [PubMed] [Google Scholar]
- 67.Baxter LR, Jr, et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry. 1989;46:243–250. doi: 10.1001/archpsyc.1989.01810030049007. [DOI] [PubMed] [Google Scholar]
- 68.Mayberg HS, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 69.Brody AL, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 70.Martin SD. Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry. 2001;58:641–648. doi: 10.1001/archpsyc.58.7.641. [DOI] [PubMed] [Google Scholar]
- 71.Goldapple K, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;6:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- 72.Kennedy SH, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 73.Goodwin GM, et al. State changes in brain activity shown by the uptake of 99mTc-exametazime with single photon emission tomography in major depression before and after treatment. J Affect Disord. 1993;29:243–253. doi: 10.1016/0165-0327(93)90014-b. [DOI] [PubMed] [Google Scholar]
- 74.Holthoff VA, et al. Changes in brain metabolism associated with remission in unipolar major depression. Acta Psychiatr Scand. 2004;110:184–194. doi: 10.1111/j.1600-0447.2004.00351.x. [DOI] [PubMed] [Google Scholar]
- 75.Tutus A, et al. Changes in regional cerebral blood flow demonstrated by single photon emission computed tomography in depressive disorders: comparison of unipolar vs. bipolar subtypes. Psychiatry Res. 1998;83:169–177. doi: 10.1016/s0925-4927(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 76.Vlassenko A. Cerebral perfusion response to successful treatment of depression with different serotoninergic agents. J Neuropsychiatry Clin Neurosci. 2004;16:360–363. doi: 10.1176/jnp.16.3.360. [DOI] [PubMed] [Google Scholar]
- 77.Martinot JL. Left prefrontal glucose hypometabolism in the depressed state: a confirmation. Am J Psychiatry. 1990;147:1313–1317. doi: 10.1176/ajp.147.10.1313. [DOI] [PubMed] [Google Scholar]
- 78.Saxena S, et al. Differential cerebral metabolic changes with paroxetine treatment of obsessive-compulsive disorder vs major depression. Arch Gen Psychiatry. 2002;59:250–261. doi: 10.1001/archpsyc.59.3.250. [DOI] [PubMed] [Google Scholar]
- 79.Kimbrell TA, et al. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- 80.Fu CH, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 81.Sheline YI. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 82.Surguladze S, et al. A differential pattern of neural response towards sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 83.Liotti M. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 84.Elliott R, et al. Prefrontal dysfunction in depressed patients performing a complex planning task: a study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- 85.Goethals I, et al. Blunted prefrontal perfusion in depressed patients performing the Tower of London task. Psychiatry Res. 2005;139:31–40. doi: 10.1016/j.pscychresns.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 86.Okada G. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology. 2003;47:21–26. doi: 10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- 87.Davidson RJ. The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry. 2003;160:64–75. doi: 10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- 88.Price JL. Comparative aspects of amygdala connectivity. Ann N Y Acad Sci. 2003;985:50–58. doi: 10.1111/j.1749-6632.2003.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 89.Altshuler LL. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- 90.Altshuler LL, et al. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- 91.Brambilla P, et al. MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- 92.Frangou S. The Maudsley bipolar disorder project. Epilepsia. 2005;46(suppl 4):19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- 93.Strakowski SM, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 94.Benabarre A, et al. The somatics of psyche: structural neuromorphometry of bipolar disorder. Psychother Psychosom. 2002;71:180–189. doi: 10.1159/000063642. [DOI] [PubMed] [Google Scholar]
- 95.Blumberg HP, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 96.Blumberg HP, et al. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang K. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- 98.Chen BK, et al. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 99.DelBello MP. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 100.Dickstein DP, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 101.Aylward EH, et al. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- 102.McIntosh AM, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 103.Hastings RS. Volumetric analysis of the prefrontal cortex, amygdala, and hippocampus in major depression. Neuropsychopharmacology. 2004;29:952–959. doi: 10.1038/sj.npp.1300371. [DOI] [PubMed] [Google Scholar]
- 104.Sheline YI. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160:1516–1518. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 105.Siegle GJ. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003;985:481–484. doi: 10.1111/j.1749-6632.2003.tb07105.x. [DOI] [PubMed] [Google Scholar]
- 106.Bruno SD. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain. 2004;127:2433–2440. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- 107.Lochhead RA. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55:1154–1162. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 108.Lyoo IK, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 109.Sassi RB, et al. Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry. 2004;56:467–475. doi: 10.1016/j.biopsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 110.Lopez-Larson MP. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 111.Sax KW. Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- 112.Sharma V. An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord. 2003;77:167–171. doi: 10.1016/s0165-0327(02)00109-x. [DOI] [PubMed] [Google Scholar]
- 113.Adler CM, et al. Abnormal frontal white matter tracts in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2004;20:197–203. doi: 10.1111/j.1399-5618.2004.00108.x. [DOI] [PubMed] [Google Scholar]
- 114.Nugent AC, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 115.Brambilla P, et al. Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology. 2002;27:792–799. doi: 10.1016/S0893-133X(02)00352-4. [DOI] [PubMed] [Google Scholar]
- 116.Sanches M, et al. Subgenual prefrontal cortex of child and adolescent bipolar patients: a morphometric magnetic resonance imaging study. Psychiatry Res. 2005;138:43–49. doi: 10.1016/j.pscychresns.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 117.Caetano SC, et al. Smaller cingulate volumes in unipolar depressed patients. Biol Psychiatry. 2004;59:702–706. doi: 10.1016/j.biopsych.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 119.Kanner AM. Structural MRI changes of the brain in depression. Clin EEG Neurosci. 2004;35:46–52. doi: 10.1177/155005940403500111. [DOI] [PubMed] [Google Scholar]
- 120.Lavretsky H. Sex differences in brain structure in geriatric depression. Am J Geriatr Psychiatry. 2004;12:653–657. doi: 10.1176/appi.ajgp.12.6.653. [DOI] [PubMed] [Google Scholar]
- 121.Strakowski SM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 122.McDonald C, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 123.Paulus MP. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 124.Del-Ben CM, et al. The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology. 2005;30:1724–1734. doi: 10.1038/sj.npp.1300728. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi H, et al. Effects of dopaminergic and serotonergic manipulation on emotional processing: a pharmacological fMRI study. Neuroimage. 2005;27:991–1001. doi: 10.1016/j.neuroimage.2005.05.039. [DOI] [PubMed] [Google Scholar]
- 126.Harmer CJ. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59:816–821. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 127.Kemp AH. Augmentation of serotonin enhances pleasant and suppresses unpleasant cortical electrophysiological responses to visual emotional stimuli in humans. Neuroimage. 2004;22:1084–1096. doi: 10.1016/j.neuroimage.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 128.Strakowski SM. Abnormal FMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162:1697–1705. doi: 10.1176/appi.ajp.162.9.1697. [DOI] [PubMed] [Google Scholar]
- 129.Manji HK. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 130.Vieta E. Differential features between bipolar I and bipolar II disorder. Compr Psychiatry. 1997;38:98–101. doi: 10.1016/s0010-440x(97)90088-2. [DOI] [PubMed] [Google Scholar]
- 131.Torrent C, et al. Cognitive impairment in bipolar II disorder. Br J Psychiatry. 2006;189:254–259. doi: 10.1192/bjp.bp.105.017269. [DOI] [PubMed] [Google Scholar]
- 132.McDonald C, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61:974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 133.Cecil KM. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]