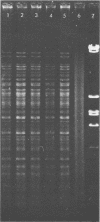
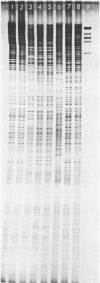
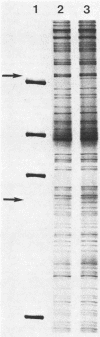
Abstract
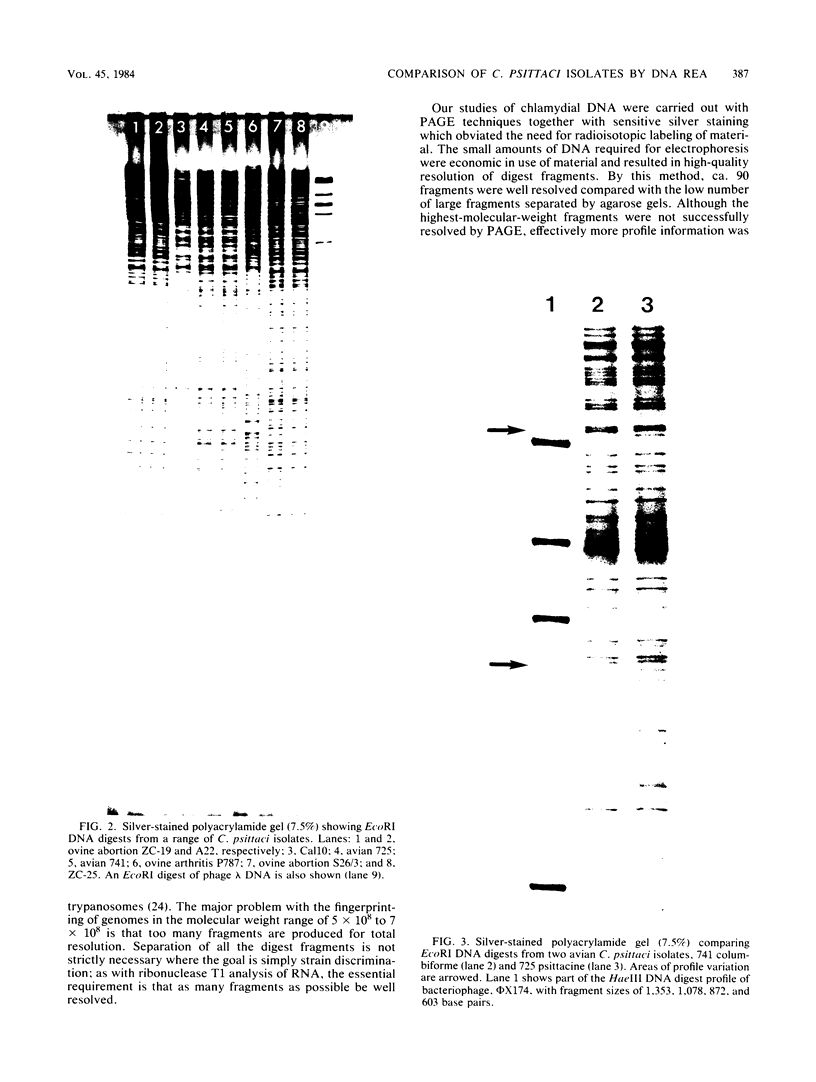
Preparations of DNA from 12 Chlamydia psittaci isolates and one Chlamydia trachomatis strain were compared by restriction endonuclease analysis. Polyacrylamide gel electrophoresis, followed by silver staining, resulted in optimal resolution of fragments generated by digestion. By this technique, four distinct electropherotypes were demonstrated when ovine abortion, ovine arthritis, and avian and Cal10 strains of C. psittaci were examined. Minor profile differences allowed the discrimination of avian isolates derived from psittacine and columbiforme species, and the Cal10 DNA electropherotype was shown to have features in common with these profiles. However, there were no detectable differences in the DNA patterns of eight ovine abortion isolates.
Full text
PDF

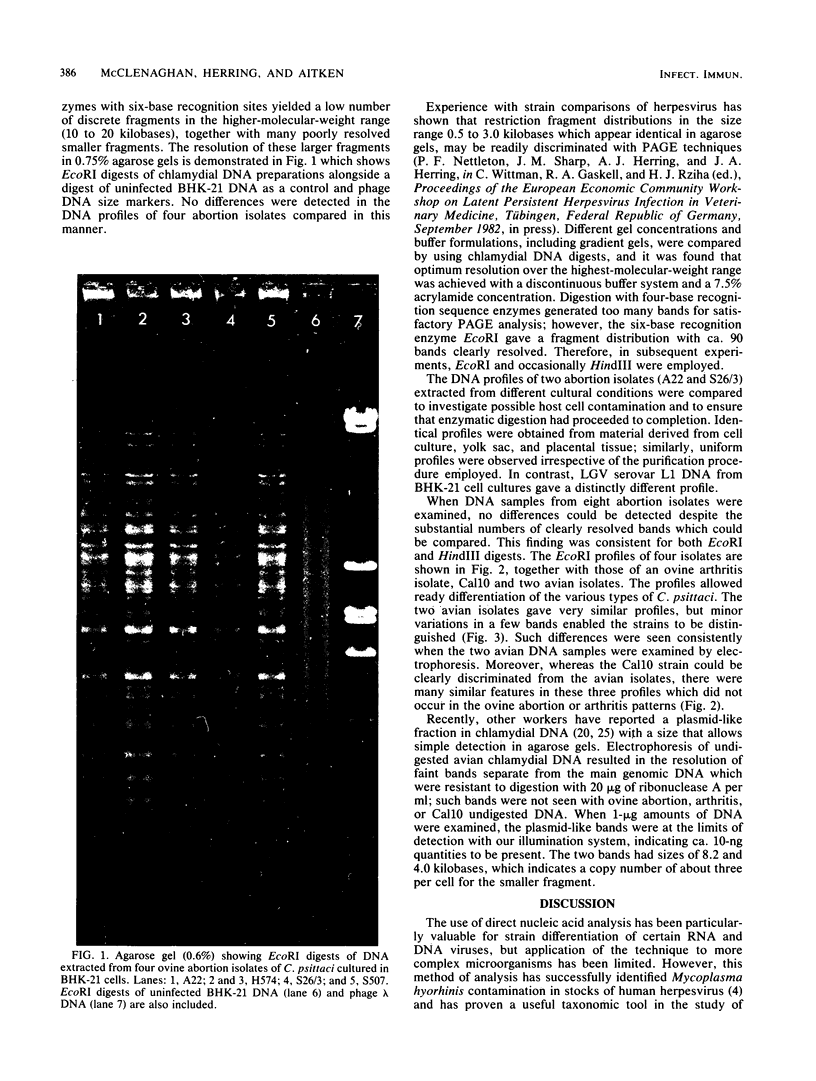


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan I., Pearce J. H. Amino acid requirements of strains of Chlamydia trachomatis and C. psittaci growing in McCoy cells: relationship with clinical syndrome and host origin. J Gen Microbiol. 1983 Jul;129(7):2001–2007. doi: 10.1099/00221287-129-7-2001. [DOI] [PubMed] [Google Scholar]
- Banks J., Eddie B., Sung M., Sugg N., Schachter J., Meyer K. F. Plaque reduction technique for demonstrating neutralizing antibodies for Chlamydia. Infect Immun. 1970 Oct;2(4):443–447. doi: 10.1128/iai.2.4.443-447.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darai G., Flügel R. M., Zöller L., Matz B., Kreig A., Gelderblom H., Delius H., Leach R. H. The plaque-forming factor for mink lung cells present in cytomegalovirus and herpes-zoster virus stocks identified as Mycoplasma hyorhinis. J Gen Virol. 1981 Jul;55(Pt 1):201–205. doi: 10.1099/0022-1317-55-1-201. [DOI] [PubMed] [Google Scholar]
- Eb F., Orfila J. Serotyping of Chlamydia psittaci by the micro-immunofluorescence test: isolates of ovine origin. Infect Immun. 1982 Sep;37(3):1289–1291. doi: 10.1128/iai.37.3.1289-1291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eb F., Orfila J. Typage de Chlamydia psittaci par la micromémethode en immunofluorescence. Ann Microbiol (Paris) 1981 Jan-Feb;132A(1):81–96. [PubMed] [Google Scholar]
- FRASER C. E., BERMAN D. T. TYPE-SPECIFIC ANTIGENS IN THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM GROUP OF ORGANISMS. J Bacteriol. 1965 Apr;89:943–948. doi: 10.1128/jb.89.4.943-948.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J. N. Use of solubilizable acrylamide disulfide gels for isolation of DNA fragments suitable for sequence analysis. Anal Biochem. 1981 Sep 1;116(1):146–151. doi: 10.1016/0003-2697(81)90337-7. [DOI] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkin H. M. Comparative lipid composition of psittacosis and trachoma agents. Am J Ophthalmol. 1967 May;63(5 Suppl):1087–1098. doi: 10.1016/0002-9394(67)94087-1. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T., Weiss E. Lack of deoxyribonucleic acid homology between species of the genus Chlamydia. J Bacteriol. 1968 Oct;96(4):1421–1423. doi: 10.1128/jb.96.4.1421-1423.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linklater K. A., Dyson D. A. Field studies on enzootic abortion of ewes in south east Scotland. Vet Rec. 1979 Oct 27;105(17):387–389. doi: 10.1136/vr.105.17.387. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANIRE G. P., MEYER K. F. The toxins of the psittacosis-lymphogranuloma group of agents; differentiation of strains by the toxin neutralization test. J Infect Dis. 1950 May-Jun;86(3):241–250. doi: 10.1093/infdis/86.3.241. [DOI] [PubMed] [Google Scholar]
- Mitzel J. R., Wright G. G., Swack N. S. Cross immunity among strains of Chlamydia psittaci. Proc Soc Exp Biol Med. 1970 Dec;135(3):944–946. doi: 10.3181/00379727-135-35176. [DOI] [PubMed] [Google Scholar]
- Morel C., Simpson L. Characterization of pathogenic trypanosomatidae by restriction endonuclease fingerprinting of kinetoplast DNA minicircles. Am J Trop Med Hyg. 1980 Sep;29(5 Suppl):1070–1074. doi: 10.4269/ajtmh.1980.29.1070. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., de la Maza L. M. Characterization of Chlamydia DNA by restriction endonuclease cleavage. Infect Immun. 1983 Aug;41(2):604–608. doi: 10.1128/iai.41.2.604-608.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo P., Vitu C., Lambert M., Giauffret A. Etude comparative de différentes souches de Chlamydia--resultats préliminaires. Comp Immunol Microbiol Infect Dis. 1979;2(1):75–85. doi: 10.1016/0147-9571(79)90061-4. [DOI] [PubMed] [Google Scholar]
- STAMP J. T., McEWEN A. D., WATT J. A. A., NISBET D. I. Enzootic abortion in ewes; transmission of the disease. Vet Rec. 1950 Apr 29;62(17):251–254. doi: 10.1136/vr.62.17.251. [DOI] [PubMed] [Google Scholar]
- Sarov I., Becker Y. Trachoma agent DNA. J Mol Biol. 1969 Jun 28;42(3):581–589. doi: 10.1016/0022-2836(69)90245-9. [DOI] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia: isolates of bovine origin. Infect Immun. 1975 May;11(5):904–907. doi: 10.1128/iai.11.5.904-907.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Caldwell H. D. Chlamydiae. Annu Rev Microbiol. 1980;34:285–309. doi: 10.1146/annurev.mi.34.100180.001441. [DOI] [PubMed] [Google Scholar]
- Skare J., Summers W. P., Summers W. C. Structure and function of herpesvirus genomes. I. comparison of five HSV-1 and two HSV-2 strains by cleavage their DNA with eco R I restriction endonuclease. J Virol. 1975 Apr;15(4):726–732. doi: 10.1128/jvi.15.4.726-732.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Biotyping of Chlamydia psittaci based on inclusion morphology and response to diethylaminoethyl-dextran and cycloheximide. Infect Immun. 1979 Apr;24(1):224–232. doi: 10.1128/iai.24.1.224-232.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears P., Storz J. Chlamydia psittaci: growth characteristics and enumeration of serotypes 1 and 2 in cultured cells. J Infect Dis. 1979 Dec;140(6):959–967. doi: 10.1093/infdis/140.6.959. [DOI] [PubMed] [Google Scholar]
- Storz J. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J Comp Pathol. 1966 Oct;76(4):351–362. doi: 10.1016/0021-9975(66)90055-7. [DOI] [PubMed] [Google Scholar]
- Treharne J. D., Forsey T., Thomas B. J. Chlamydial serology. Br Med Bull. 1983 Apr;39(2):194–200. doi: 10.1093/oxfordjournals.bmb.a071815. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am J Ophthalmol. 1970 Sep;70(3):367–374. doi: 10.1016/0002-9394(70)90096-6. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Koprowski H., Lonsdale D. M., Brown S. M., Subak-Sharpe J. H. The polypeptide and the DNA restriction enzyme profiles of spontaneous isolates of herpes simplex virus type 1 from explants of human trigeminal, superior cervical and vagus ganglia. J Gen Virol. 1979 Apr;43(1):151–171. doi: 10.1099/0022-1317-43-1-151. [DOI] [PubMed] [Google Scholar]
- Weiss E., Schramek S., Wilson N. N., Newman L. W. Deoxyribonucleic Acid Heterogeneity Between Human and Murine Strains of Chlamydia trachomatis. Infect Immun. 1970 Jul;2(1):24–28. doi: 10.1128/iai.2.1.24-28.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Lovett M. A. Expression in E. coli of Chlamydia trachomatis antigen recognized during human infection. Nature. 1982 Mar 4;296(5852):68–70. doi: 10.1038/296068a0. [DOI] [PubMed] [Google Scholar]
- Wilcke B. W., Jr, Newcomer C. E., Anver M. R., Simmons J. L., Nace G. W. Isolation of Chlamydia psittaci from naturally infected African clawed frogs (Xenopus laevis). Infect Immun. 1983 Aug;41(2):789–794. doi: 10.1128/iai.41.2.789-794.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger R. S., Salomon R., Dingman C. W., Peacock A. C. Role of base composition in the electrophoresis of microbial and crab DNA in polyacrylamide gels. Nat New Biol. 1972 Jul 19;238(81):65–69. doi: 10.1038/newbio238065a0. [DOI] [PubMed] [Google Scholar]