Abstract
The deficit syndrome is thought to characterize a pathophysiologically distinct subgroup of patients with schizophrenia. Supporting this notion, prior research examining the neuropsychological correlates of the deficit syndrome has suggested the presence of a differential impairment in frontal and parietal functions. This article reports findings from 2 studies attempting to replicate and extend previous reports of a differential neuropsychological impairment in deficit schizophrenia. In the first study, we administered a comprehensive neuropsychological battery to 20 deficit and 25 nondeficit patients with schizophrenia and 25 normal healthy controls. In the second study, a meta-analysis was conducted of 13 separate studies examining the neuropsychology of the deficit syndrome. There was little evidence from either of the present studies that the deficit syndrome is associated with a selective impairment in frontal and parietal lobe functions. The first study failed to find significant differences in frontal or parietal abilities for deficit vs nondeficit patients. The meta-analytic findings revealed that deficit patients were globally more neuropsychologically impaired than nondeficit patients (effect size [ES] = 0.41). Relative to nondeficit patients, deficit patients performed poorest on tests of olfaction (ES = 1.11), social cognition (ES = 0.56), global cognition (ES = 0.52), and language (ES = 0.51). The neuropsychological impairments associated with the deficit form of schizophrenia do not follow an obvious anatomically defined pattern of impairment. The question of whether deficit patients exhibit a unique cognitive impairment profile will require a more sophisticated and rigorous examination of the neuropsychology of the deficit syndrome.
Keywords: schizophrenia, negative symptoms, cognition, processing speed
Introduction
The deficit syndrome is theorized to be a pathophysiologically distinct manifestation of schizophrenia characterized by enduring and idiopathic negative symptoms. Support for the construct validity of the deficit syndrome as a separate disease within schizophrenia has been marshaled from findings that patients with the deficit syndrome differ from nondeficit patients on a number of key epidemiological, clinical, treatment response, and biological variables (see Kirkpatrick1 for a review). A critical issue in this regard concerns distinguishing how the pathophysiology of the deficit syndrome differs from that of nondeficit schizophrenia. Buchanan et al2 proposed that disrupted frontal and parietal cortical functions were involved in the production of deficit symptomatology and presented neuropsychological evidence consistent with this hypothesis.2–4 Subsequent neuroimaging studies have provided support for this hypothesis because deficit patients have shown differential reductions in frontal and parietal regional cerebral blood flow compared with nondeficit patients.5,6 Moreover, over a dozen studies have examined the neuropsychological correlates of the deficit syndrome.2,7–17
In these studies of neuropsychological performance, deficit patients have consistently performed more poorly than healthy controls. Similarly, deficit patients have performed similarly to or more poorly than nondeficit patients on most neuropsychological measures; very rarely do they perform better than nondeficit patients. With respect to whether deficit patients show differential impairments on tests of frontal-parietal functioning, the findings are more complicated. Nearly all studies examining this issue claim to support the differential frontal-parietal hypothesis2,7–14,16 but seeTiryak et al.9 However, several of these studies present evidence that is inconsistent with this claim. For example, 3 studies found that deficit patients had significant differential impairment on tests related to temporal lobe functions10,13,16 and 4 studies failed to find significant deficit/nondeficit group differences on frontal-parietal tests.2,7,9,14 These findings raise questions about the extent to which selective impairment in frontal-parietal abilities characterizes deficit schizophrenia.
The present report includes 2 studies designed to clarify this issue. In the first study, performance on a neuropsychological battery designed to assess behaviors associated with frontal, parietal, and temporal lobe dysfunction was administered to patients with the deficit form of schizophrenia and carefully matched patients with the nondeficit form of schizophrenia and normal healthy controls. The second study employed a meta-analysis of 13 studies examining the neuropsychology of the deficit syndrome. We consider this analytic approach particularly valuable given that this literature has many studies with small sample sizes and, thus, limited statistical power.
Study I: New Empirical Study
In our previous study examining the neuropsychological correlates of the deficit syndrome, we reported that deficit patients had selective impairments on 2 measures of frontal lobe function: the Stroop Color-Word Interference and Trails B tests and one measure of parietal lobe function: the Mooney Faces Closure test.2 There were no significant group differences on any of the temporal lobe measures. Both patient groups performed more poorly than the normal control comparison group. The current study was designed to replicate these results in an independent sample of deficit and nondeficit patients and normal controls.
Methods
Participants.
Forty-five outpatients meeting Diagnostic and Statistical Manual for Mental Disorders (DSM-IV; American Psychiatric Association18) criteria for schizophrenia were recruited for study participation. Diagnoses were confirmed using a best estimate diagnostic approach, utilizing information from direct assessments, family informants, and medical records. Patients with documented brain damage (eg, trauma, stroke), mental retardation, a history of severe head trauma, or history of drug abuse or dependence were excluded from the study. The patient group included 20 patients with the deficit syndrome and 25 nondeficit patients. Twenty-five healthy controls were recruited from the general population via newspaper advertisements and flyers placed in the Baltimore/Washington DC area. Controls did not have a past or current DSM-IV axis I or axis II disorder, as determined by the Structural Clinical Interview for DSM-IV, or a history of central nervous system disease, mental retardation, or severe head trauma. All participants gave written informed consent prior to participating in the study and were judged to be clinically stable. None of the participants were included in our previous publications.2,11 This study was approved by the Institutional Review Board.
Assessments.
The Schedule for the Deficit Syndrome (SDS; Kirkpatrick et al19) was used to categorize patients with schizophrenia into deficit or nondeficit subgroups. The SDS is a semi-structured interview designed to assess 6 enduring (lasting >1 year) and idiopathic negative symptoms, including restricted affect, diminished emotional range, poverty of speech, curbed interests, diminished sense of purpose, and diminished social drive. To meet criteria for the deficit syndrome, an individual must demonstrate a moderate or higher level of severity on at least 2 of these symptoms. Deficit ratings were made by one of 2 of the SDS authors (Kirkpatrick and Buchanan) who have shown acceptable levels of interrater reliability (Kappa = 0.73).
The Brief Psychotic Rating Scale (BPRS; Overall and Gorham20) was used to measure psychiatric symptoms. BPRS scales were organized into separate positive (including the grandiosity, suspiciousness, unusual thought content, and hallucinatory behavior items), negative (including the blunted affect, uncooperativeness, emotional withdrawal, and motor retardation items), and disorganized (the tension, disorientation, excitement, conceptual disorganization, and odd mannerisms items) syndromes based on the findings of the most comprehensive factor analysis of the 18-item BPRS to date.21 BPRS ratings were made by trained raters, who have shown acceptable levels of interrater reliability (eg, Intraclass Correlation Coefficients for total BPRS scores >0.80). Patients were also assessed using the Simpson-Angus Extrapyramidal Symptom Rating Scale (EPRS; Simpson and Angus22). Total EPRS scores are reported here.
A battery of tests was selected to measure a diverse set of frontal, parietal, and temporal lobe functions. Classifications were made based on a team consensus approach led by 2 of the authors who both have extensive experience with neuropsychology (Buchanan and Gold), based on consultation with neuropsychological textbooks,23,24 previously published studies on the classifications of these tests (eg, Buchanan et al2 and Bilder et al25), as well as MEDLINE and PsycINFO literature searches. Measures included tests sensitive to frontal lobe functions: the Stroop Color-Word interference score,26 CFL phonemic fluency total number of words,23 Trails B time to completion,27 and Wisconsin Card Sorting Test (WCST) perseverative errors28; parietal lobe functions: 3D Block Design total correct,29 Wechsler Adult Intelligence Scales Revised (WAIS-R) Block Design test total score,30 and Judgment of Line Orientation total correct31; and temporal lobe functions: Benton's Facial Recognition total correct,32 Mooney's Faces total correct33 and Figural Memory, Logical Memory I and II, Visual Pairs I and II, Verbal Pairs I and II, and Visual Reproduction I and II tests from the Wechsler Memory Scale—Revised (WMS-R; Wechsler34). The scores from the immediate and delayed WMS-R memory tests were averaged together for data reduction purposes. Additionally, several speeded performance tests (Trails A time to completion, Grooved Pegboard total time,27 and Letter Cancellation total time23) were administered and collectively referred to as a “nonspecific” domain because there was no obvious lobe-based assignment. Test categorizations are summarized in table 1.23,30,34
Table 1.
A Summary List of Neuropsychological Tests Examined in This Report and Their Various Categorizations
| Test Name and Citation Reference | Lobe | Speeded Performance | Neuropsychological Process |
| 3D Block Design | Parietal | No | Visual spatial |
| Boston Naming | Temporal | No | Language |
| Card Sorting Tests | Frontal | No | Reasoning |
| Constructional Praxis | Parietal | No | Visual spatial |
| CPT Auditory | Nonspecific | No | Sustained attention |
| CPT Ax | Frontal | No | Sustained attention |
| CPT Degraded | Nonspecific | No | Sustained attention |
| Digit Distraction | Frontal | No | Working Memory |
| Facial Recognition | Temporal | No | Visual object |
| Finger Tapping | Nonspecific | Yes | Motor |
| Grooved Pegboard | Nonspecific | Yes | Motor |
| Judgment Of Line Orientation | Parietal | No | Visual spatial |
| Letter Cancellation | Nonspecific | Yes | Processing speed |
| MMSE | — | — | Global cognition |
| Mooney Faces Closurea | Temporal | No | Visual object |
| Picture Memory | Temporal | No | Visual memory |
| Social Cognition | — | No | Social cognition |
| Span Of Attention 12 Letter | Nonspecific | No | — |
| Stroop Color | Temporal | Yes | Motor |
| STROOP Interference | Frontal | Yes | Set shifting |
| Trails A | Nonspecific | Yes | Processing speed |
| Trails B | Frontal | Yes | Set shifting |
| Smell Identification | Frontal | No | Olfactory discrimination |
| Verbal Fluency | Frontal | Yes | Verbal fluency |
| Verbal Learning Test | Temporal | No | Verbal memory |
| WAIS-R Arithmetic | Parietal | No | Working memory |
| WAIS-R Block Design | Parietal | No | Visual spatial |
| WAIS-R Comprehension | Nonspecific | No | Language |
| WAIS-R Digit Span | Parietal | No | Working memory |
| WAIS-R Digit Symbol | Nonspecific | Yes | Processing speed |
| WAIS-R Full Scale Performance | — | — | Global cognition |
| WAIS-R Full Scale IQ | — | — | Global cognition |
| WAIS-R Full Scale Verbal IQ | — | — | Global cognition |
| WAIS-R Information | Temporal | No | Language |
| WAIS-R Object Assembly | Parietal | No | Visual spatial |
| WAIS-R Picture Arrangement | Temporal | No | — |
| WAIS-R Picture Completion | Temporal | No | Visual object |
| WAIS-R Similarities | Temporal | No | Language |
| WAIS-R Vocabulary | Temporal | No | Language |
| WMS-R Figural Memory | Temporal | No | Visual memory |
| WMS-R Logical Memory | Temporal | No | Verbal memory |
| WMS-R Verbal Pairs | Temporal | No | Verbal memory |
| WMS-R Visual Reproduction | Temporal | No | Visual memory |
Note: “—”Indicates that the test was not included in these analyses; WMS-R,Wechsler Memory Scale—Revised; MMSE, Mini-Mental State Examination; WAIS-R, Wechsler Adult Intelligence Scales Revised; CPT, Continuous Performance Test.
The classification of the Mooney's Faces test was changed from “parietal” in Buchanan et al2 to “temporal” for the present study.
To evaluate the presence of differential neuropsychological impairments within the deficit patients, we used SAS PROC MIXED to compare within-group test performance across the various categories. A mixed model for incomplete repeated measures was used to compare the magnitude of the deficit-nondeficit cognitive z scores with the model
where the group × domain term tested homogeneity of deficit vs nondeficit differences across domains. Because all patients did not have complete data on all domains, the Kenward-Rogers approximation was used to calculate degrees of freedom. Benjamini and Hochberg's 35 procedure for controlling the false discovery rate (FDR) was used to adjust P values from these tests to insure that the probability of a false positive differences discovered was not greater than 0.05.
Results
The deficit, nondeficit, and healthy control groups were compared on demographic, medication, and clinical characteristics. There were no statistically significant group differences in age or ethnicity, but the control subjects had significantly more female participants and more years of education than both patient groups. Deficit patients had more severe negative symptoms than nondeficit patients, but the patient groups did not differ statistically on any of the other demographic, clinical, or medication variables. Accordingly, no attempt to control for demographic or clinical variables was made in this study.
Means and SDs of the individual neuropsychological tests were separately computed for the deficit, nondeficit, and control subjects (see table 3). We also computed lobe-domain scores by summing the z-transformed scores from the tests in each common domain, after applying corrections so that increasing scores reflected better performance. The patient groups both performed significantly worse than controls on each neuropsychological test except for the WAIS-R Block Design test, where deficit but not nondeficit patients performed statistically worse than controls. Deficit patients did not differ from nondeficit patients on any individual test identified as having a prominent frontal or parietal lobe demand, although both frontal (effect size [ES] = 0.46) and parietal (ES = 0.14) domain scores were lower for deficit vs nondeficit patients. The ES for the temporal lobe measures was 0.52, in favor of the nondeficit group, although this group difference was not statistically significant. Compared with both controls and nondeficit patients, deficit patients showed significantly poorer performance on one test ascribed to temporal lobe functioning (Facial Recognition), 2 of the 3 nonspecific domain tests, and the overall nonspecific domain score. The overall test for homogeneity of differences between domain scores was not significant (F3,450 = 1.13, P > .05 for group × domain interaction). This failure to detect a group by domain interaction, coupled with the nonsignificant contrasts on the frontal and parietal domain scores, are evidence against an association of deficit pathology with selective frontal-parietal impairment. A global cognitive index was computed as the average of the 4 domain scores. Deficit patients showed nonsignificantly poorer global performance than nondeficit patients (t[40] = –1.85, P < .10; ES = 0.56).
Table 3.
Neuropsychological Performance Compared between the Deficit (n = 20), Nondeficit (n = 25), and Control (n = 25) Subjects, with Domain Scores
| Deficit | Nondeficit | Controls | Omnibus F | |
| Frontal | ||||
| Stroopa | 28.11 ± 9.26 | 33.12 ± 10.15 | 42.28 ± 9.25 | 0.48 |
| Phonemic fluencyb | 25.55 ± 8.99 | 32.08 ± 11.66 | 39.80 ± 10.55 | 10.25***,c |
| Trails Bb,d (¥) | 166.05 ± 102.42 | 120.21 ± 95.99 | 57.44 ± 14.11 | 8.27**,c |
| WCSTb,d (¥) | 39.90 ± 28.22 | 32.21 ± 24.29 | 11.12 ± 10.15 | 10.88***,c |
| Frontal domaine | –0.42 ± 0.68 | –0.11 ± 0.68 | 0.48 ± 0.39 | 13.25***,c |
| Parietal | ||||
| WAIS Block Designb | 7.90 ± 2.45 | 8.36 ± 3.33 | 10.25 ± 3.10 | 3.82f |
| 3D block designa | 30% max score | 32% max score | 62% max score | χ2 = 9.85*,c |
| Judgement of line orientationa | 20.30 ± 5.69 | 21.44 ± 5.43 | 24.00 ± 4.33 | 3.13+ |
| Parietal domaine | –0.31 ± 0.94 | –0.19 ± 0.80 | 0.43 ± 0.69 | 5.75**,c |
| Temporal | ||||
| Facial recognitiona | 40.35 ± 5.43 | 44.04 ± 5.00 | 47.24 ± 3.56 | 12.36***,g |
| Mooney's facesa | 19.75 ± 2.00 | 19.20 ± 3.11 | 20.56 ± 1.73 | 2.06 |
| Figural memorya | 5.50 ± 1.67 | 6.00 ± 2.29 | 7.68 ± 1.60 | 8.49**,c |
| Logical memorya | 25.90 ± 13.55 | 31.68 ± 15.06 | 58.16 ± 14.69 | 33.08***,c |
| Visual pairsa | 12.45 ± 6.17 | 15.72 ± 5.74 | 20.48 ± 4.20 | 12.78***,c |
| Verbal pairsa | 20.70 ± 6.61 | 22.84 ± 5.81 | 28.60 ± 3.06 | 13.93***,c |
| Visual reproductiona | 45.90 ± 20.08 | 53.72 ± 18.86 | 65.68 ± 7.39 | 8.63***,c |
| Temporal domaine | –0.52 ± 0.67 | –0.19 ± 0.60 | 0.61 ± 0.34 | 26.46***,c |
| Nonspecific | ||||
| Trails Ab,d, ¥ | 52.20 ± 22.44 | 38.38 ± 14.29 | 25.08 ± 7.02 | 22.24***, g |
| Letter cancellationa (¥) | 173.15 ± 48.17 | 138.76 ± 38.24 | 107.36 ± 16.40 | 18.84***, g |
| Grooved pegboardh (¥) | 113.59 ± 41.11 | 97.48 ± 50.47 | 63.94 ± 8.81 | 22.55***,c |
| Nonspecific domaine, (¥) | –0.82 ± 0.80 | –0.07 ± 0.71 | 0.71 ± 0.30 | 33.15***,g |
Note: Post hoc test = Scheffe.Means and SDs reported here are the original, unprocessed scores. Unless noted by “¥,” increasing scores reflect better test performance. Domain scores were computed as the average of the summed z scores for each of the variables in that measure. WAIS Block design and Grooved pegboard were excluded from the domain scores to minimize the impact of missing test data.
Missing data for 1 deficit patient.
Missing data for 2 deficit patients.
Deficit = nondeficit < controls.
Missing data for 1 nondeficit patient.
Deficit, n = 18; nondeficit, n = 24; and control = 25.
Deficit < controls.
Deficit < nondeficit < controls.
Deficit, n = 17 and nondeficit n = 22.
P < .10;
P < .05;
P < .01;
= P < .001.
The finding that deficit patients showed significantly poorer performance on Trails A and Letter Cancellation tests suggests that deficit patients may show differential speeded performance impairments. Post hoc ANOVAs were conducted to investigate this issue. Speeded performance and nonspeeded performance domains were derived by summing the z-transformed scores of each test in the respective domains (see "Methods" section and table 2), and the 3 groups were then compared using a multivariate analysis of variance. The Stroop and Grooved Pegboard measures were excluded from these analyses to minimize the impact of missing test data. The omnibus F value was significant for group (Wilk Lamda F118 = 13.15, P < .01), and between subjects comparisons suggested that the 3 groups differed on the speeded (F2,60 = 27.20, P < .01) and nonspeeded (F2,60 = 19.34, P < .01) performance domain scores. Planned follow-up analyses, conducted using Scheffe tests, indicated that the deficit patients performed significantly more poorly (P < .05) on speeded performance tests (mean ± SD = –0.70 ± 0.72) than both nondeficit patients (mean ± SD = –0.09 ± 0.69) and controls (mean ± SD = 0.64 ± 0.30). Nondeficit patients also performed significantly worse than controls. With respect to the nonspeeded performance tests, deficit patients (mean ± SD = –0.44 ± 0.72) and nondeficit patients (mean ± SD = –0.18 ± 0.58) were not significantly different (P > .20) from each other, but both groups performed significantly more poorly than controls (mean ± SD = 0.57 ± 0.35; all P values < .01). However, the test for differences in ES between speeded and nonspeeded cognitive measures was not significant (F1,40 = 2.75. P > .05 for group × processing speed interaction).
Table 2.
Demographic, Descriptive Medication and Clinical Scores Compared between the Deficit (n = 20), Nondeficit (n = 25), and Control (n = 25) Subjects
| Deficit | Nondeficit | Controls | |
| Age | 40.80 ± 6.13 | 38.64 ± 6.22 | 37.85 ± 6.94 |
| % Female | 10 | 16 | 42 |
| % Caucasian | 70 | 72 | 73 |
| Age of onset | 20.72 ± 6.66 | 19.39 ± 4.29 | — |
| Education | 11.40 ± 2.44 | 12.67 ± 1.97 | 13.96 ± 1.57 |
| Antipsychotic medication dataa | |||
| % Being prescribed clozapine | 40 | 21 | — |
| % Being prescribed atypicals (not clozapine) | 20 | 42 | — |
| % Being prescribed conventional | 35 | 33 | — |
| Extrapyramidal Rating Scale—totalb | 2.29 ± 2.34 | 2.36 ± 2.54 | — |
| Brief Psychiatric Rating Scale—total | 34.15 ± 6.90 | 34.67 ± 12.29 | — |
| Positive syndrome | 9.45 ± 4.43 | 10.00 ± 5.33 | — |
| Negative syndrome | 8.95 ± 2.78 | 6.29 ± 2.18 | — |
| Disorganization syndrome | 7.90 ± 4.70 | 8.71 ± 5.33 | — |
Missing data for 1 deficit and 1 nondeficit subjects.
Missing data for 2 deficit subjects.
Study II: Meta-analysis Study
The results of the new empirical study failed to replicate the findings from our original investigation of the neuropsychology of the deficit syndrome.2 However, the results did suggest that deficit patients may be characterized by selective impairments on measures of processing speed. In light of previous studies that have claimed to find selective frontal and parietal impairments in deficit patients2,7–17 and the results from the current empirical study, we decided to perform a meta-analytic study to determine whether the broader literature supports the presence of selective neuropsychological impairments in the deficit form of schizophrenia.
Methods
Search Strategy for the Meta-analysis.
In order to identify relevant studies for the meta-analysis, we conducted a combined MEDLINE and PsychINFO search for studies published between 1987 and 2005 having one or more of the following terms: “deficit syndrome,” “deficit schizophrenia,” or “nondeficit” and a word base of either “cog” or “neuropsychol.” Additionally, we reviewed all published articles citing Buchanan et al2 using a PsychINFO citation function. Our inclusion criteria included the following: (1) the study reports bivariate correlation values or group means and SDs, (2) the study includes both deficit and nondeficit patients (one was excluded for not including a nondeficit control group36), (3) the study is published in a peer-reviewed journal (2 excluded for being unpublished dissertations37,38), (4) the article was written or translated in English (2 studies excluded39,40), (5) the dependent variable measures a basic neuropsychological process (1 study excluded for measuring prediction ability41), and (6) a measure of the deficit syndrome with published psychometric properties was used to determine deficit/nondeficit group status (one excluded42). Five studies were identified that reported data for olfaction discrimination performance in overlapping samples.7,43–46 Malaspina and Coleman45 was chosen to represent this sample for this neuropsychological measure because it had the largest sample size of the studies reporting group means. Our search strategy yielded 12 independent studies (see table 4). Additionally, we included the data from study I in the meta-analysis.
Table 4.
A Summary List of Studies Included in the Present Meta-analyses, Including Information Regarding the Neuropsychological Measures, Sample Size and Procedure for Diagnosing the Deficit Syndrome
| Authors and Publication Date | Deficit Instrument | Sample Size | Number of Neuropsychological measures | Deficit Diagnosis Procedure and Reliability Statistics |
| Seckinger et al (2004) | SDS | 13 Deficit | 14 | Independent raters were trained by the SDS authors. No reliability statistics included. |
| 33 Nondeficit | ||||
| Bryson et al (2001) | SDS | 33 Deficit | 9 | Independent raters were used. Kappa for deficit diagnosis computed from 20 interviews = 0.91. |
| 57 Nondeficit | ||||
| Tiryaki et al (2003) | Turkish version of the SDS | 19 Deficit | 10 | Independent raters were used. Cronbach α = .85. Range of Kappa values for individual SDS items = 0.51–0.61. |
| 43 Nondeficit | ||||
| Galderisi et al (2002) | Italian version of the SDS | 58 Deficit | 10 | Independent raters were used with consultation from the SDS authors. Range of ICC values for individual symptoms = 0.62–0.81. |
| 54 Nondeficit | ||||
| 26 Controls | ||||
| Buchanan et al (1994) | SDS | 18 Deficit | 12 | Consensus procedure between 2 of the SDS authors was used. No reliability statistics reported. |
| 21 Nondeficit | ||||
| 30 Controls | ||||
| Buchanan et al (1997) | SDS | 20 Deficit | 2 | Independent raters were 2 of the SDS authors. Kappa = 0.73. |
| 56 Nondeficit | ||||
| 27 Controls | ||||
| Horan et al (2003) | SDS | 15 Deficit | 9 | Consensus procedure was used. Questionable cases were excluded. Kappa based on videotape training = 0.90. |
| 30 Nondeficit | ||||
| 41 Controls | ||||
| Brazo et al (2002) | French version of the SDS | 12 Deficit | 11 | Not described. Deficit patients had no overt positive or disorganized symptomatology. |
| 23 Nondeficit | ||||
| 35 Controls | ||||
| Cohen et al (2004) | PDS based on the BPRS | 40 patients | 7 | PDS was used dimensionally. Range of ICC values for BPRS ratings were > 0.69. |
| Bryson et al (1998) | SDS | 19 Deficit | 2 | Independent raters were used. Kappa = 0.91 and range of ICC for individual symptoms = 0.68–0.95. |
| 50 Nondeficit | ||||
| Putnam et al (2000) | PDS, based on the PANSS | 119 Deficit | 16 | Deficit grouping was assigned to patients with a high PDS value at baseline and at 1 y follow-up. Range of ICC values for the PANSS = 0.86–1.0. |
| 114 Nondeficit | ||||
| Malaspina and Coleman (2003) | SDS | 19 Deficit | 1 | Independent raters, trained to reliability by SDS authors (Kappa = 0.71) categorized patients by reviewing records. |
| 51 Nondeficit | ||||
| 68 Controls |
Note: SDS, Schedule for Deficit Syndrome; ICC, Intraclass Correlation Coefficient; PDS, Proxy for Deficit syndrome Scale; BPRS, Brief Psychotic Rating Scale; PANSS, Positive and Negative Syndrome Scale.
Kirkpatrick et al1 have raised concerns that lack of intergroup reliability in deficit diagnostic procedures may complicate interpretation of findings across studies. We address these concerns through the following procedures. First, we excluded all studies from the meta-analysis that used a deficit diagnostic procedure lacking published reliability and validity data. Second, as suggested in Kirkpatrick et al1, we evaluated whether the samples from the meta-analyses exhibited clinical characteristics commonly associated with deficit schizophrenia [eg, more severe negative symptoms]. For each study, deficit vs nondeficit patients showed equivalent or more severe negative symptoms and equivalent or less severe positive symptoms. One study8 reported that deficit patients had significantly more severe disorganization symptoms, but these deficit patients also had significantly more severe negative symptoms and nonsignificantly less severe positive and hostility symptoms. Finally, we recomputed the meta-analyses including only those studies where SDS authors were reportedly active in the diagnostic procedure. As noted in table 1, 6 of the 13 studies examined in this report [including study I] explicitly state that the authors of the SDS made the diagnoses, trained the raters, or were consulted by the individual authors regarding categorization issues. The recomputed ES values were within 10 points of the original values. These steps provide assurance that the deficit patients examined in the meta-analyses share a common pathological process.
Categorizing Neuropsychological Tests.
As in study I, we grouped the neuropsychological measures by putative lobe-domain (ie, frontal, parietal, temporal, and nonspecific) and speeded and nonspeeded performance. Although potentially useful, grouping tests by putative lobe functions is an obvious simplification as performance on nearly every test involves a much more widely distributed neural system. To account for this limitation, we also grouped tests in terms of the primary cognitive function or process that is captured in the dependent measure used for analysis. To this end, each test was grouped into one of 15 processes, including: verbal fluency, reasoning, verbal memory, sustained attention, set shifting, visual spatial, working memory, processing speed, visual object, visual memory, social cognition, motor speed, olfactory discrimination, language, and global cognitive ability. The picture arrangement and the span of attention tests did not readily fit into this scheme and were excluded from this set of analyses. The results of this categorization process are summarized in table 1.
Meta-analytic Procedure.
Meta-analyses were conducted using MetaWin software.47 We used fixed-effects models except when the Q-within statistic was suggestive of within-group heterogeneity, thus dictating the use of random-effects models. ES for each neuropsychological measure from each study were independently computed. When one study reported data for multiple measures, which we had classified in a similar domain or process (defined below), the ES for each of these measures was averaged together so that specific domains or processes were not represented more than once for each study. In order to control for differences in sample size across studies, studies were weighted according to the ES variance scores. The rationale for this weighting procedure is that the closer a sample's ES approximates the population's ES, the smaller the variance of the ES will be (see Rosenberg47 for further discussion of this technique). Many of the studies examined here (6 of 13) did not employ a control sample, so we limited the present meta-analyses to deficit vs nondeficit patients. A QTotal statistic47 was computed to determine whether ES values significantly differed between categories.
Results
The findings for the meta-analysis examining neuropsychological performance by lobe domain are presented in table 5. The ES for the specific lobe domains were each positive, with the parietal domain having the lowest ES (but above the 0.20 criteria for a small effect) and the other effects approaching the 0.50 criteria for a medium ES. The ES for the between-group Q-statistic was not significant, suggesting that the frontal, parietal, temporal, and nonspecific scores were not demonstrably different from each other. Findings from this set of analyses offer little support for a differential frontal or parietal impairment in deficit schizophrenia but are strongly suggestive of impairment in deficit patients across neuropsychological domains.
Table 5.
Meta-analytic Findings: ES Computed by Neuropsychological Domain and Speeded Performance Categories for Deficit vs Nondeficit Patients
| Total Number of Studies | Total Number of Measures | Total Number of Subjects | Mean Weighted ES | 95% CI | Q Between | Q Error | |
| Domain | 8.89 | ||||||
| Frontal | 9 | 31 | 533 | 0.42 | 0.23–0.62 | 12.89 | |
| Parietal | 7 | 16 | 540 | 0.22 | 0.01–0.44 | 1.30 | |
| Temporal | 9 | 44 | 533 | 0.40 | 0.19–0.60 | 4.81 | |
| Nonspecific | 7 | 15 | 463 | 0.42 | 0.21–0.64 | 5.53 | |
| Performance speed | .17 | ||||||
| Speeded | 8 | 19 | 467 | 0.46 | 0.26–0.67 | 4.95 | |
| Nonspeeded | 13 | 90 | 719 | 0.42 | 0.26–0.57 | 13.03 |
The results for the meta-analysis examining neuropsychological performance grouped into speeded and nonspeeded performance tests (see table 2) are presented in table 5. The test for differences between speeded performance and nonspeeded performance domains was not significant. Thus, findings from the first study regarding differential speeded performance impairments were not replicated across studies.
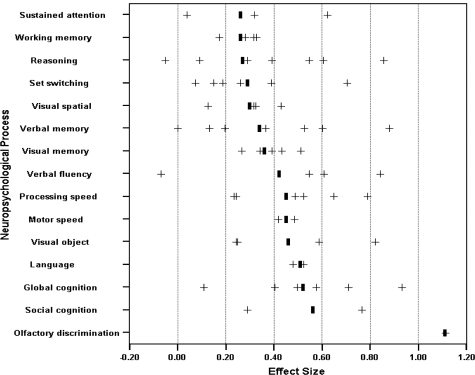
The findings for the meta-analysis examining individual neuropsychological processes are presented in Figure 1. All 15 of the individual ES values were at least in the “small” range, with 4 in the medium range: language (ES = 0.51; 95% confidence interval [CI] = –1.81–2.83), global cognition (ES = 0.52; 95% CI = 0.23–0.82), social cognition (ES = 0.56; 95% CI = –2.09–3.21), and olfactory discrimination (ES = 1.11; 95% CI = NA). With the possible exception of the olfactory discrimination test, it is noteworthy that none of these “medium” effect-size tests preferentially involved frontal or parietal lobe functions. Moreover, neither the between- or within-group statistical tests assessing for differential impairment across the 15 constructs were significant (Qtotal[61] = 53.82, P > .05; Qwithin[48] = 47.08, P > .05), suggesting that the variability in ES across process categories was not substantial.
Fig. 1.
ES of neuropsychological performance by process for deficit and nondeficit patients. Positive ES means performance was poorer for deficit patients. “+,”Individual ES by study and “|,” weighted mean of ES.
Discussion
The purpose of the present studies was to evaluate the hypothesis that deficit schizophrenia is associated with differential impairment on neuropsychological tests that are sensitive to frontal and parietal lobe functions. Consistent with the broader literature on the neuropsychology of schizophrenia (eg, Heinrichs and Zakzanis48), findings from study I revealed that patients as a group were more impaired than nonpatient controls on nearly every neuropsychological function. When deficit and nondeficit patients were compared, deficit patients performed more poorly on 16 of 17 measures, with these differences reaching statistical significance on 3 measures. However, little support for a frontal-parietal differential impairment was generated from either of the present studies. While deficit patients showed relatively greater impairments compared with nondeficit patients across both frontal and parietal functions, they also showed greater impairments in temporal functions. Greater variability in performance across tests was observed when they were organized by neuropsychological process. However, the results of these analyses also did not support the hypothesis that deficit patients exhibit selective frontal or parietal lobe dysfunction. For example, differences in working memory ability, presumably demanding frontal lobe function, showed some of the smallest ES seen in this study, while differences in language ability, presumably drawing on temporal lobe structures, were among the largest.
Several factors are important to consider when attempting to resolve the present null findings in light of prior studies which have suggested a differential frontal-parietal lobe neuropsychological impairment in deficit schizophrenia.2,7–14,16 First, the selection and classification of neuropsychological measures varies markedly across these studies, and it is possible that some of the claims of focal frontal-parietal involvement in the deficit syndrome might reflect a more localizationist interpretation than can really be supported by the measures used in these studies. Second, the combination of a wide array of neuropsychological measures based on presumed anatomical correlates, as was done in the meta-analysis, could obfuscate finding true group differences in the pattern of neuropsychological impairment. This did not appear to be the case because the findings from study I, which examined performance on individual tests, were generally consistent with the meta-analytic findings in associating deficit schizophrenia with relatively global cognitive impairment. Third, most of the studies included in the meta-analysis were underpowered for detecting group differences at a small to medium ES level. Thus, conclusions regarding differential impairments drawn from prior studies may have been prone to type I error. Finally, nondeficit schizophrenia is a heterogeneous mix of patients, so variable findings across studies could reflect sample differences in the nondeficit group. This appeared to be the case in study I. Compared to the sample from Buchanan et al,2 the current cohort was older and had greater overall neuropsychological impairment.
Interpreting the pattern of ES from Figure 1 is potentially informative for understanding whether the deficit syndrome is a separable disease within schizophrenia or whether it merely reflects a more severe subgroup of the larger schizophrenia population. If the latter were true, we might expect deficit patients to demonstrate a neuropsychological profile that is similar to nondeficit patients but that is uniformly exaggerated in the magnitude of impairment. That is, deficit patients would show pronounced impairments in neuropsychological abilities that have previously been shown to most reliably differentiate patients with schizophrenia from normal controls (eg, Saykin et al49 and Bilder et al25). This did not appear to be the case in the present studies because the smallest ES were generally seen in processes that are typically observed to be impaired in schizophrenia (ie, sustained attention, working memory, and set switching; Heinrichs and Zakzanis48 Nuechterlein et al50). In contrast, larger ES were seen on measures that could be considered less fundamental to schizophrenia psychopathology (eg, language,51 social cognition52,53). In other words, the most severe relative impairments in deficit patients were outside the prototypical set of schizophrenia neuropsychological impairments, thus suggesting the presence of neuropathology beyond, and perhaps even different from what is characteristic of nondeficit schizophrenia. Alternatively, one might view such “excess” or “extra” impairment to be evidence of greater illness severity: the illness has spread further, impacting abilities that are not typically impaired. The similar level of impairment on attention and working memory processes argues, in part, against such an interpretation.
A significant obstacle in elucidating the neuropathology of the deficit syndrome involves reconciling the seemingly discordant findings from functional imaging and behavioral studies. Despite the lack of evidence for a differential frontal-parietal neuropsychological impairment in deficit schizophrenia, data from imaging studies suggests hypoactivity in the frontal/parietal,5,6 but not temporal cortical4 or hippocampal5 regions, during rest or while engaging in task performance conditions. Frontal and parietal cortical circuitry is responsible for a wide range of behaviors so that the failure of deficit patients to appropriately activate these regions may explain their poorer performance across a wide range of neuropsychological abilities. Alternatively, deficit patients may be characterized by relative frontal and parietal cortical circuitry dysfunction, which requires deficit patients to recruit other brain regions to compensate for this dysfunction. Functional imaging procedures could be used to examine both of these hypotheses.
The present results suggest that clinical neuropsychological methods are unlikely to yield clear evidence of localized dysfunction in the deficit syndrome. Nonetheless, neuropsychological tests could be an important tool for understanding the behavioral impairments associated with deficit schizophrenia, particularly, given recent efforts to develop cognitive rehabilitation programs for patients. Whether the application of more refined methods emerging from the cognitive neuroscience literature will produce more interpretable results has yet to be tested.
Several limitations warrant mention. First, the present studies utilized clinical assessment techniques that, for the most part, are not matched in sensitivity. Thus, it could be the case that the pattern of differences (or lack thereof) between the patient groups reflected variability in instrument psychometric properties.52 Future studies can address this concern by using more rigorously matched tests. Second, there was variability in how the deficit syndrome was defined across the studies included in the meta-analysis, and it is possible that this obscured true differential neuropsychological impairments. It is encouraging that findings from study I, for which the SDS authors made diagnoses, and study II were consistent with each other. Moreover, none of the studies from the meta-analysis appeared overtly problematic in their diagnostic procedures. Third, we were unable to account for individual differences in variables such as gender, medications, or severity of illness in the meta-analyses. It is unclear the degree to which a third variable may be responsible for any group differences in neuropsychological functioning. Generally speaking, most studies reported here have not found significant group differences in such variables, so it would seem that the impact of demographic and clinical variables would be relatively small. Nonetheless, this is an important issue to consider.
In the time between the conduct of the study and publication of this article, the following manuscript has been electronically published.54 In this study, the authors found a large effect for the following frontal and parietal measures: WCST and Trails B. However, they also found a significant large effect for the temporal lobe vocabulary measure and for overall IQ. The pattern of results is similar to what we found in our meta-analyses and is unlikely to alter the conclusions of these analyses.
In summary, the findings from this report are important for understanding the heterogeneity of schizophrenia. Evidence that the deficit syndrome reflects a distinct disease within schizophrenia has come, in part, from findings that deficit patients show a differential pattern of neuropsychological impairments. The present findings failed to confirm the hypothesis of differential frontal-parietal neuropsychological impairment but are not inconsistent with the notion that deficit schizophrenia is characterized by a differential pattern of neuropsychological impairment, which may be considerably more complicated than previously thought. A call for more sophisticated and rigorous examination of the behavioral pathology of the deficit syndrome is warranted.
Acknowledgments
This project was supported by grants MH48225 and P30 MH068580 (Buchanan; Principal Investigator). Buchanan had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors wish to thank Dr McMahon for his assistance analyzing the data.
References
- 1.Kirkpatrick B. A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry. 2001;58:165–171. doi: 10.1001/archpsyc.58.2.165. [DOI] [PubMed] [Google Scholar]
- 2.Buchanan RW. Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry. 1994;51:804–811. doi: 10.1001/archpsyc.1994.03950100052005. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan RW. Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry. 1990;147:290–294. doi: 10.1176/ajp.147.3.290. [DOI] [PubMed] [Google Scholar]
- 4.Tamminga CA, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- 5.Heckers S. Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry. 1999;56:1117. doi: 10.1001/archpsyc.56.12.1117. [DOI] [PubMed] [Google Scholar]
- 6.Lahti AC. Abnormal patterns of regional cerebral blood flow in schizophrenia with primary negative symptoms during an effortful auditory recognition task. Am J Psychiatry. 2001;158:1797. doi: 10.1176/appi.ajp.158.11.1797. [DOI] [PubMed] [Google Scholar]
- 7.Seckinger RA, et al. Olfactory identification and WAIS-R performance in deficit and nondeficit schizophrenia. Schizophr Res. 2004;69:55. doi: 10.1016/S0920-9964(03)00124-5. [DOI] [PubMed] [Google Scholar]
- 8.Bryson G. Memory and executive function impairments in deficit syndrome schizophrenia. Psychiatry Res. 2001;102:29–37. doi: 10.1016/s0165-1781(01)00245-1. [DOI] [PubMed] [Google Scholar]
- 9.Tiryaki A. Reexamination of the characteristics of the deficit schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2003;253:221. doi: 10.1007/s00406-003-0434-5. [DOI] [PubMed] [Google Scholar]
- 10.Galderisi S, et al. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am J Psychiatry. 2002;159:983. doi: 10.1176/appi.ajp.159.6.983. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan RW. Attentional impairments in deficit and nondeficit forms of schizophrenia. Am J Psychiatry. 1997;154:363–370. doi: 10.1176/ajp.154.3.363. [DOI] [PubMed] [Google Scholar]
- 12.Horan WP. Neurocognitive, social, and emotional dysfunction in deficit syndrome schizophrenia. Schizophr Res. 2003;65:125–137. doi: 10.1016/s0920-9964(02)00410-3. [DOI] [PubMed] [Google Scholar]
- 13.Brazo P. Cognitive patterns in subtypes of schizophrenia. European Psychiatry. 2002;17:155. doi: 10.1016/s0924-9338(02)00648-x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen AS. Negative vs. deficit syndrome: prediction of attentional impairment. Schizophr Bull. 2004;30:827–835. doi: 10.1093/oxfordjournals.schbul.a007135. [DOI] [PubMed] [Google Scholar]
- 15.Bryson G. Affect recognition in deficit syndrome schizophrenia. Psychiatry Res. 1998;77:113–120. doi: 10.1016/s0165-1781(97)00140-6. [DOI] [PubMed] [Google Scholar]
- 16.Putnam KM. Cognitive impairment and enduring negative symptoms: a comparative study of geriatric and nongeriatric schizophrenia patients. Schizophr Bull. 2000;26:867. doi: 10.1093/oxfordjournals.schbul.a033501. [DOI] [PubMed] [Google Scholar]
- 17.Malaspina D. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Kirkpatrick B. The Schedule for the deficit syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 20.Overall JE. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799. [Google Scholar]
- 21.Mueser KT. Factor structure of the Brief Psychiatric Rating Scale in schizophrenia. Psychological Assess. 1997;9:196. [Google Scholar]
- 22.Simpson GM. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand. 1970;45(suppl 212):11–19. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- 23.Lezak MD. Neuropsychological Assessment. 3rd ed. London, England: Oxford University Press; 1995. [Google Scholar]
- 24.Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 25.Bilder RM, et al. Neuropsychology of first-episode schizophrenia: initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- 26.Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, IL: Stoelting Co; 1978. [Google Scholar]
- 27.Boll TJ. The Halstead-Reitan neuropsychological battery. In: Filskov SB, editor. Handbook of Clinical Neuropsychology. New York: Wiley-Interscience.; 1981. pp. 577–607. [Google Scholar]
- 28.Heaton RK. Wisconsin Card Sorting Test Manual—Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 29.Benton AL. Contributions to Neuropsychological Assessment: A Clinical Manual. New York, NY: oxford university press; 1983. [Google Scholar]
- 30.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corperation; 1981. [Google Scholar]
- 31.Benton A. Visual perception of line direction in patients with unilateral brain disease. Neurology. 1975;25:907. doi: 10.1212/wnl.25.10.907. [DOI] [PubMed] [Google Scholar]
- 32.Benton A. Test of Facial Recognition Manual. Iowa City, IA: Benton Laboratory of Neuropsychology; 1978. [Google Scholar]
- 33.Mooney CM. Closure as affected by viewing time and multiple visual fixation. Can J Psychol. 1957;11:21. doi: 10.1037/h0083687. [DOI] [PubMed] [Google Scholar]
- 34.Wechsler D. Wechsler Memory Scale-Revised. New York, NY: Psychological Corporation; 1987. [Google Scholar]
- 35.Benjamini Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. J Educ Behav Stat. 2000;25:60. [Google Scholar]
- 36.Vaiva G, et al. SPECT—imaging, clinical features, and cognition before and after low doses of amisulpride in schizophrenic patients with deficit syndrome. Psychiatry Res. 2002;115:37. doi: 10.1016/s0925-4927(02)00031-8. [DOI] [PubMed] [Google Scholar]
- 37.Limpert CA. Diminished Emotional Experience and Its Relationship to Poor Insights in Schizophrenia: Dissertation Abstracts International: Section b: The Sciences and Engineering. Ann Arbor, MI: University Microfilms International; 1999. [Google Scholar]
- 38.Marcinko L. Gender Differences in Schizophrenia: Dissertation Abstracts International: Section b: The Sciences and Engineering. Ann Arbor, MI: University Microfilms International; 1999. [Google Scholar]
- 39.Torres CA. Deficit cognitivo y esquizofrenia. Actas Esp Psiquiatr. 2001;29:1. [PubMed] [Google Scholar]
- 40.Kahn JP. Tolerance aux neuroleptiques, deficit et fonctions cognitives. Encephale. 1996;22:49. [PubMed] [Google Scholar]
- 41.Ludewig K. Behavioural dysregulation of decision-making in deficit but not nondeficit schizophrenia patients. Psychiatry Res. 2003;119:293. doi: 10.1016/s0165-1781(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 42.Wagman AM. Deficit and nondeficit forms of schizophrenia: Neuropsychological evaluation. Psychiatry Res. 1987;22:319. doi: 10.1016/0165-1781(87)90111-9. [DOI] [PubMed] [Google Scholar]
- 43.Goudsmit N. A brief smell identification test discriminates between deficit and non-deficit schizophrenia. Psychiatry Res. 2003;120:155. doi: 10.1016/s0165-1781(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 44.Goudsmit N. Trail making and olfaction in schizophrenia: implications for processing speed. CNS Spectr. 2004;9:344. doi: 10.1017/s1092852900009329. [DOI] [PubMed] [Google Scholar]
- 45.Malaspina D. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- 46.Malaspina D, et al. Odor identification, eye tracking and deficit syndrome schizophrenia. Biol Psychiatry. 2002;51:809. doi: 10.1016/s0006-3223(01)01319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg MS. MetaWin: Statistical Software for Meta-analysis. Sunderland, Mass: Sinauer Associates, Inc.; 2000. [Google Scholar]
- 48.Heinrichs RW. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 49.Saykin AJ, et al. Neuropsychological deficits in neuroleptic naive patients with first-episode schizophrenia. Arch Gen Psychiatry. 1994;51:124–131. doi: 10.1001/archpsyc.1994.03950020048005. [DOI] [PubMed] [Google Scholar]
- 50.Nuechterlein KH. Information-processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatr Scand Suppl. 1994;384:71–79. doi: 10.1111/j.1600-0447.1994.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 51.Friedman MS. Perceptual asymmetries in schizophrenia: subtype differences in left hemisphere dominance for dichotic fused words. Am J Psychiatry. 2001;158:1437. doi: 10.1176/appi.ajp.158.9.1437. [DOI] [PubMed] [Google Scholar]
- 52.Green MJ. Visual scanpaths to threat-related faces in deluded schizophrenia. Psychiatry Res. 2003;119:271–285. doi: 10.1016/s0165-1781(03)00129-x. [DOI] [PubMed] [Google Scholar]
- 53.Davis PJ. Recognition of posed and genuine facial expressions of emotion in paranoid and nonparanoid schizophrenia. J Abnorm Psychol. 2000;109:445–450. [PubMed] [Google Scholar]
- 54.Wang X. Psychopathology and neuropsychological impairments in deficit and nondeficit schizophrenia of Chinese origin. Biological Psychiatry. doi: 10.1016/j.psychres.2006.09.007. In press. [DOI] [PubMed] [Google Scholar]