Abstract
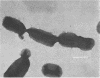
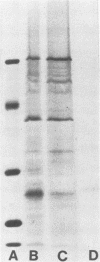
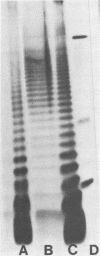
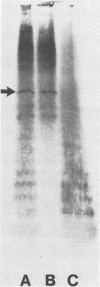
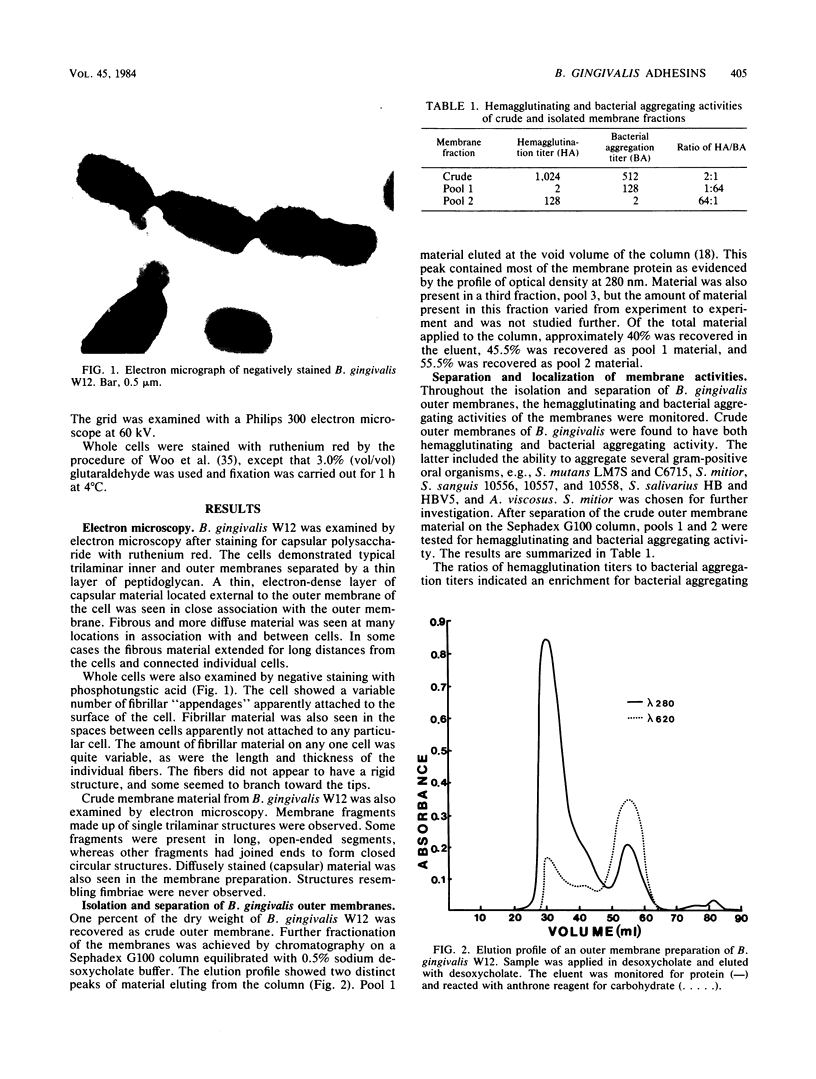
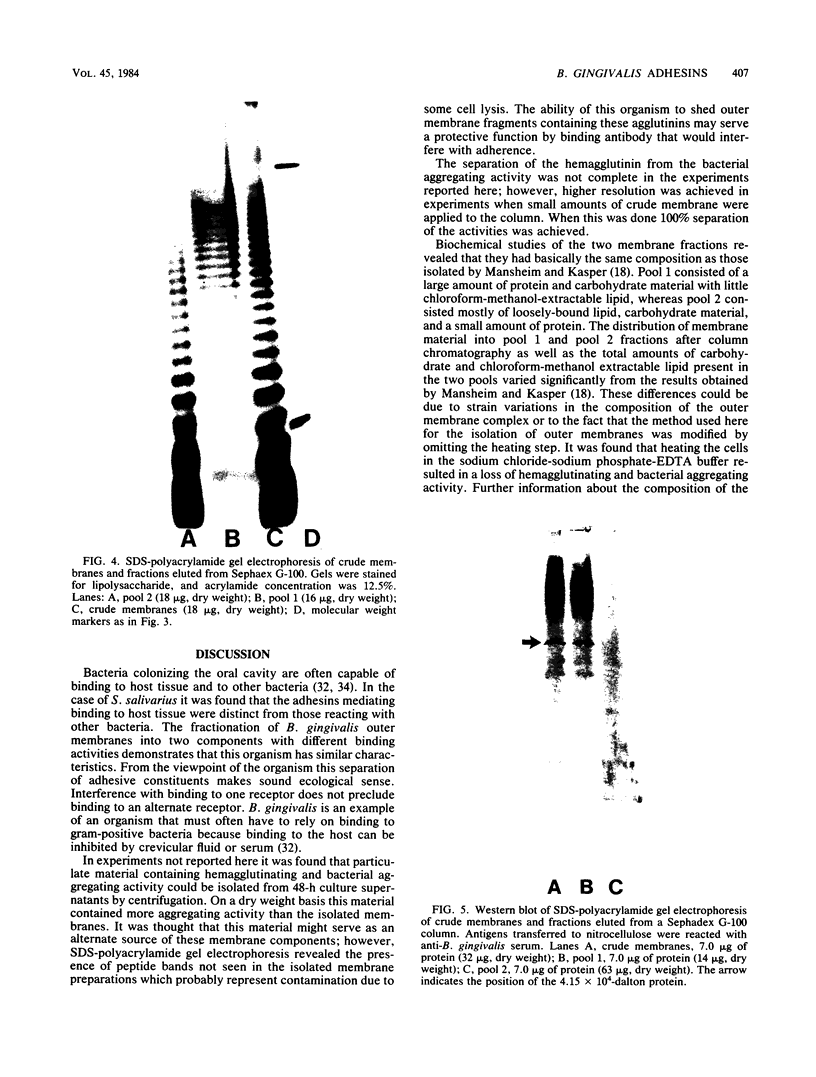
An outer membrane complex containing hemagglutinating and bacterial aggregating activity has been isolated from Bacteroides gingivalis. Examination of the membrane material by biochemical analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and immunological means revealed that the crude outer membrane preparation contained three major proteins and a lipopolysaccharide population that displayed size heterogeneity. At least two membrane proteins as well as the lipopolysaccharide were found to be antigenically active by immunoblot analysis. With gel chromatography and a lipopolysaccharide disaggregating buffer the membrane material was separated into two fractions. An accompanying separation of the two adherence activities was observed. The first membrane fraction, containing mostly protein and carbohydrate material, was found to contain the bacterial aggregating activity. This fraction also contained a high-molecular-weight lipopolysaccharide population. The second membrane fraction, consisting of low-molecular-weight lipopolysaccharide, protein, and loosely bound lipid was found to contain the hemagglutinating activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Jann B., Reske K., Jann K. Heterogeneity of lipopolysaccharides. Analysis of polysaccharide chain lengths by sodium dodecylsulfate-polyacrylamide gel electrophoresis. Eur J Biochem. 1975 Dec 1;60(1):239–246. doi: 10.1111/j.1432-1033.1975.tb20996.x. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M., MCCARTY M. Studies on the chemical structure of the streptococcal cell wall. I. The identification of a mucopeptide in the cell walls of groups A and A-variant streptococci. J Exp Med. 1961 Jul 1;114:127–140. doi: 10.1084/jem.114.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D. L., Seiler M. W. Immunochemical characterization of the outer membrane complex of Bacteroides fragilis subspecies fragilis. J Infect Dis. 1975 Oct;132(4):440–450. doi: 10.1093/infdis/132.4.440. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Kuzio J., Angus B. L., Hancock R. E. Chemical and chromatographic analysis of lipopolysaccharide from an antibiotic-supersusceptible mutant of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Feb;21(2):310–319. doi: 10.1128/aac.21.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Dur A., Chaby R., Szabó L. Isolation of two protein-free and chemically different lipopolysaccharides from Bordetella pertussis phenol-extracted endotoxin. J Bacteriol. 1980 Jul;143(1):78–88. doi: 10.1128/jb.143.1.78-88.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Coleman S. E. Immunochemical differences between oral and nonoral strains of Bacteroides asaccharolyticus. Infect Immun. 1980 Feb;27(2):589–596. doi: 10.1128/iai.27.2.589-596.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansheim B. J., Kasper D. L. Purification and immunochemical characterization of the outer membrane complex of Bacteroides melaninogenicus subspecies asaccharolyticus. J Infect Dis. 1977 May;135(5):787–799. doi: 10.1093/infdis/135.5.787. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Onderdonk A. B., Kasper D. L. Immunochemical characterization of surface antigens of Bacteroides melaninogenicus. Rev Infect Dis. 1979 Mar-Apr;1(2):263–277. doi: 10.1093/clinids/1.2.263. [DOI] [PubMed] [Google Scholar]
- Mansheim B. J., Solstad C. A., Kasper D. L. Identification of a subspecies-specific capsular antigen from Bacteroides melaninogenicus subspecies asaccharolyticus by immunofluorescence and electron microscopy. J Infect Dis. 1978 Dec;138(6):736–741. doi: 10.1093/infdis/138.6.736. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer membrane of Salmonella. Isolation of protein complex that produces transmembrane channels. J Biol Chem. 1976 Apr 10;251(7):2176–2178. [PubMed] [Google Scholar]
- Nakamura K., Mizushima S. Effects of heating in dodecyl sulfate solution on the conformation and electrophoretic mobility of isolated major outer membrane proteins from Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1411–1422. doi: 10.1093/oxfordjournals.jbchem.a131414. [DOI] [PubMed] [Google Scholar]
- Nixdorff K., Fitzer H., Gmeiner J., Martin H. H. Reconstitution of model membranes from phospholipid and outer membrane proteins of Proteus mirabilis. Role of proteins in the formation of hydrophilic pores and protection of membranes against detergents. Eur J Biochem. 1977 Nov 15;81(1):63–69. doi: 10.1111/j.1432-1033.1977.tb11927.x. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Haemagglutinating activity of Bacteroides melaninogenicus. Arch Oral Biol. 1974 May;19(5):415–416. doi: 10.1016/0003-9969(74)90184-8. [DOI] [PubMed] [Google Scholar]
- Okuda K., Takazoe I. Immunological study of the pili of Bacteroides melaninogenicus. Bull Tokyo Dent Coll. 1978 May;19(2):93–95. [PubMed] [Google Scholar]
- ROE J. H. The determination of sugar in blood and spinal fluid with anthrone reagent. J Biol Chem. 1955 Jan;212(1):335–343. [PubMed] [Google Scholar]
- Reed M. J., Slots J., Mouton C., Genco R. J. Antigenic studies of oral and nonoral black-pigmented Bacteroides strains. Infect Immun. 1980 Aug;29(2):564–574. doi: 10.1128/iai.29.2.564-574.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandermann H., Jr, Strominger J. L. Purification and properties of C 55 -isoprenoid alcohol phosphokinase from Staphylococcus aureus. J Biol Chem. 1972 Aug 25;247(16):5123–5131. [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Weerkamp A. H., McBride B. C. Characterization of the adherence properties of Streptococcus salivarius. Infect Immun. 1980 Aug;29(2):459–468. doi: 10.1128/iai.29.2.459-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo D. D., Holt S. C., Leadbetter E. R. Ultrastructure of Bacteroides species: Bacteroides asaccharolyticus, Bacteroides fragilis, Bacteroides melaninogenicus subspecies melaninogenicus, and B. melaninogenicus subspecies intermedius. J Infect Dis. 1979 May;139(5):534–546. doi: 10.1093/infdis/139.5.534. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Takahashi M., Takamori K., Sasaki T. Ultrastructure of the outer membrane surface of black-pigmented Bacteroides isolated from human oral cavity. Bull Tokyo Dent Coll. 1982 Feb;23(1):47–60. [PubMed] [Google Scholar]