Abstract
Background: Injection of nerve growth factor (NGF) into the developing frontal cortex (FC) has been shown to produce adult-onset subcortical dopaminergic hyperactivity, impaired prepulse inhibition of the acoustic startle response, and several neuropathological features of schizophrenia. The present study was to determine whether such lesions would lead to impaired social interaction, a prominent negative feature of schizophrenia. Methods: Rat pups received daily injections of human recombinant NGF into the developing FC on postnatal days 1 and 2 to partially lesion subplate neurons. Lesioned rats were tested in similar-treatment pairings lasting 23.5 hours using the EthoVision behavioral monitoring system at 6 and 14 weeks of age. Brains were then perfusion fixed for histological analysis. Results: Lesioned rats showed significantly increased movement, relative to controls, during the light phase at 6 weeks of age. At 14 weeks, they maintained a significantly greater mean distance apart from one another, and engaged in significantly less approach and avoidance behavior during the dark phase, relative to controls. Histological changes were consistent with those described previously in this animal model. Conclusion: Results indicate that injections of NGF into the developing FC of neonatal rats result in reduced social interaction, which is consistent with behaviors observed in human schizophrenia patients.
Keywords: animal model, social withdrawal, EthoVision, mean distance apart, approach behavior, avoidance behavior
Introduction
Schizophrenia is a severe psychiatric disorder that disrupts language, thought, emotion, and perception and involves negative symptoms that reflect a loss or diminution of normal functioning, including deficits in emotional expression, speech, and goal-directed behaviour.1 Deficits in social interaction are also considered to be negative symptoms of schizophrenia.2,3 Negative symptoms, particularly impaired social behavior, can severely impact the quality of life of patients. Adult schizophrenia patients typically have both impaired social competence and impoverished social networks.4–9 Children with high risk for developing schizophrenia display reduced sociability in childhood prior to diagnosis, and reduced social competence in early adolescence.10–13
Animal models can be useful for investigating causal links between etiological factors, specific brain abnormalities, and the development of schizophrenia symptoms. Studies of social interaction in animal models show a reduction in active (within a prescribed interaction zone) but not passive (outside the interaction zone) interaction that is consistent with the results from human studies.14–16 Increased interanimal distance and decreased contact have also been found.14,17,18 Recently, Rajakumar19 found that injections of human recombinant nerve growth factor (NGF) into the developing frontal cortex (FC) of the neonatal rat caused partial ablation of subplate neurons of the developing FC, leading to adult-onset behavioral features indicating subcortical dopaminergic hyperactivity, similar to that described by us previously with neonatal injections of p75 receptor conjugated to saporin into the developing FC.20 As adults, these animals also showed enlarged lateral ventricles, loss of gray matter volume in the prefrontal cortex (PFC) and loss of γ-aminobutyric acid (GABA) transporter 1 immunoreactive chandelier neuronal terminals in upper layers of the PFC, abnormalities described in postmortem studies of schizophrenia.21–26 NGF is important in the development and survival of neurons, both during development and adulthood, and may mediate certain forms of synaptic plasticity.27 NGF binds to its high-affinity receptor, TrkA, as well as the low-affinity coreceptor, p75.27,28 Subplate neurons express both the p75 receptor and the TrkB receptor, but not TrkA.29 NGF has been shown to cause cellular apoptosis when binding to p75 receptors expressed on cells that do not express TrkA.28,30,31 The subplate is critical in forming connections between the cortex and the thalamus, and may be involved in the specification of cortical areas during development.29 Therefore, NGF-mediated apoptosis of subplate neurons may lead to axonal targeting errors in the FC.29,31
The present study investigated whether this model possesses decreases in social interaction, a feature often considered as a negative symptom in schizophrenia. Given that rats display a rich array of social behaviors, social interaction tests provide an excellent basis for modeling these symptoms.32 The EthoVision 3.0 behavioral monitoring and analysis system was used to objectively track, record, and analyze behavior throughout a 23.5-hour test period that included both light and dark phases to provide a more comprehensive assessment of social behavior. Technological developments of the EthoVision system now permit a more fine-grained analysis of “active social interaction” by quantifying approach and avoidance components that are important in characterizing decreases in social interaction relevant to human social behavior.
METHOD
Subjects
Pregnant Sprague-Dawley dams obtained from Charles River Canada (Saint-Constant, Quenec, Canada) gave birth to pups in our animal facility, and all pups were injected with either NGF or saline (control) on each of P1 and P2 (day of birth was considered as P0) as described below. Rats were weaned and sexed on P21, and were housed in polycarbonate cages (39 × 29 × 19 cm); each cage contained 2 NGF and 2 control same-sex littermates. Male rats (n = 16; 8 NGF and 8 control animals) were used in the current experiments for consistency with previous research.14,16,33–39 On P35, the rats were further separated into pairs (1 NGF and 1 control littermate) and housed in shoebox polycarbonate cages (26 × 48 × 21 cm). Rats were housed as dissimilar-treatment pairs to ensure similar social and environmental conditions in home cages for all rats. This avoided the possibility that housing rats in similar-treatment pairs might inflate the magnitude of any group difference found between the NGF and the control groups as adults, particularly if housing NGF rats together for an extended period of time might result in mutual deprivation of social contact thus increasing any social behavior impairment caused by the neonatal NGF treatment itself. There were no observed differences in body weight between the groups during development (data not shown). All cages contained Beta Chip bedding (Heat Treated Laboratory Bedding, Northeastern Products Corp., Warrensburg, NY), a paper towel, and a length of black plastic pipe. Rats were kept on a 12/12-hour light (0700–1900 hours)/dark (1900–0700 hours) phase, and were allowed access to food (ProLab RMH 3000, Ren's Feed and Supply Ltd, Oakville, Ontario, Canada) and water ad libitum. All procedures were in accordance with the guidelines of Canadian Council on Animal Care, and approved by the University of Western Ontario Animal Care Committee.
Neonatal Injections
A needle (30G) connected to a 10-μl Hamilton syringe by Teflon tubing was used to inject the rat pups. On P1, half of each litter was randomly selected and placed under a heat lamp; the other half remained with their mother. One by one, each selected pup received bilateral stereotaxic injections of 0.75 μl NGF (human recombinant NGF; 125 ng/μl in sterile saline; Cedarlane Laboratories, Hornby, ON) into the developing FC according to the coordinates referenced to bregma, which was visualized through the scalp, and were AP, +1.0 mm; ML, ±0.5 mm; DV, −1.5 mm.40 Injected pups were returned under the heat lamp, and all were returned to their mother when the final pup had been injected. Pups were not separated from their mother for more than 10 minutes, and none of the litter was culled. The untreated pups were similarly separated from their mothers and injected with the same volume of sterile physiological saline, and also received a cut to their left ear for identification. The injection procedure was repeated on P2.
Apparatus
Social interaction was tested in a circular arena (90 cm diameter; 40 cm high) painted light gray. A 150-ml water dish (8 cm diameter; 4 cm high) was fixed to the center of the arena. The floor of the arena was covered in Beta Chip bedding, on which several food pellets were randomly scattered. A color CD camera was placed above the centre of the arena, and 2 darkroom lamps attached to the ceiling above the arena illuminated the latter during the dark phase of testing. The camera was connected to a personal computer, and social interaction was recorded using the EthoVision 3.0.15 behavioral monitoring and analysis system (Noldus Information Technology, Leesburg, Va). This system tracks the x-y coordinates of each animal at 5.994 frames per second. The bedding, food, and water were removed after each experiment and replaced with fresh materials.
Procedure
Rats were tested at 6 weeks of age, prior to emergence of behavioral features of subcortical dopaminergic hyperactivity, and at 14 weeks of age.19 At 6 weeks of age, rats were paired with a similarly treated and unfamiliar conspecific (n = 4 NGF and n = 4 control pairs). Such pairings were used to detect differences in the mean distance apart and proximity measures that are calculated for the pair, rather than the individual. Rats in each pair were dyed using black beard dye (Combe Inc., White Plains, NY) at least 1 day prior to testing. The whole dorsal surface of one rat of each pair was dyed, while only half of the dorsal surface of the other rat was dyed, which allowed EthoVision system to continuously track and distinguish the rats. Experiments began at the beginning of the dark phase (1900 hours) and ended 23.5 hours later (1830 hours). Rats were carried into the testing room in their home cages 15 minutes prior to 1900 hours, and placed in the arena 5 minutes prior to the start of the experiment. Once the dark phase began, the experiment was started, and EthoVision system began recording the rats' movements. Rats were undisturbed for the 23.5-hour testing period except for brief periodic checks to ensure that the rats were being tracked properly. Entry into the experimental room was made quietly through a door distant from the arena to avoid disturbing the rats. After the experiment, the rats were returned to their home cages with their original cage mate. At 14 weeks of age, the rats were retested in the same arena, with their conspecific from the 6-week testing phase using the same procedure.
Behavioural Data Analysis
The characterization of social interaction made use of the objective measures described below, which were developed for the EthoVision system by Noldus Information Technology (2003), and are similar to those reported by Sams-Dodd33–38:
Movement: percentage of time that an individual rat was engaged in locomotion defined as reaching a speed greater than 2 cm/s. A particular bout of movement was considered to have ended if the rat's speed dropped below 1.75 cm/s. Measure 1 yielded 8 data points per group.
Mean distance apart: mean distance between the rats over a 30-minute interval.
Proximity: percentage of time over the 30-minute interval that the rats were within 20 cm from one another. Measures 2 and 3 yielded 4 data points per group.
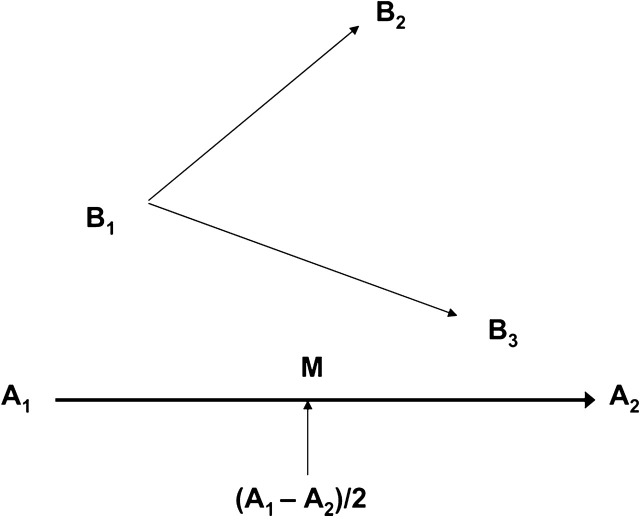
Approach and avoidance: these behaviors were scored separately and indicated relative movement between the rats, as defined by the EthoVision 3.0.15 software, that compared the trajectory of Rat B with the midpoint of the trajectory of Rat A to compute relative motion (see figure 1). The computations were performed for behavior within a 20-cm inter-rat interaction distance to allow comparison with earlier research.38,41 Each measure reflects the percentage of time that each rat displayed the specified behavior and yielded 8 data points per group.
Fig. 1.
Calculation of the relative movement measures. EthoVision detects whether Rat B is moving toward or away from Rat A by comparing the trajectory of Rat B with the midpoint of the trajectory of Rat A (M). The distance from M to B1 is less than the distance from M to B2; therefore, Rat B is avoiding Rat A. The distance from M to B1 is greater than the distance from M to B3; therefore, Rat B is approaching Rat A. (Figure and caption adapted from Noldus Information Technology, 2003.)
For most statistical analyses, data were collapsed into 30-minute time bins. However, for clarity of presentation data were collapsed into 2-hour time bins for most graphs. For the mean distance apart and proximity measures, rats were analyzed as a pair. However, for the movement measure, rats were analyzed as individuals, as in Sams-Dodd.38,41 For the approach and avoidance measures, the data were collapsed into 2-hour intervals for statistical analysis because there was occasionally missing data resulting from the rats spending an entire 30-minute interval outside of the 20-cm interaction zone. Analyses were run separately for approach and avoidance behavior.
Two-way analysis of variances were conducted for each age group separately, with treatment as a between-subjects factor and trial number as a within-subjects factor. Post hoc t tests were strictly limited to pairs of data points with nonoverlapping error bars contained within the dark phase of testing and the first 4 hours of the light phase of testing for the mean distance apart measure. Since directionality of any potential difference was hypothesized a priori, a 1-tailed analysis was used for the treatment effect. A 2-tailed analysis was used to test the treatment × trials interaction. Given the difference in ambient conditions and behavior during the dark and light phases, each phase was analyzed separately. The Huyndt-Feldt correction was applied to the degrees of freedom. However, for clarity of presentation, standard degrees of freedom are reported. Statistics were calculated using SPSS 13 for Windows. The α level used was P < .05.
Histology
Rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and perfused transcardially with 100 ml of physiological saline followed by 500 ml of solution containing freshly depolymerized 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4).42 Brains were removed and placed in a 15% buffered sucrose solution (pH 7.4) at 4°C. Frozen sections through the forebrain were cut at 40 μm, mounted on chrome alum–subbed slides and air dried. Sections were stained with Neutral Red, dehydrated in graded series of alcohol, cleared in xylene, and cover glassed using Entalan (Sigma). Sections were viewed using an Axiophot microscope (Ziess) under bright-field illumination. Digitized areas of the FC and the lateral ventricles were measured in coronal sections taken at 2.2 and 1.0 mm, respectively, rostral to bregma using “Image J” software (National Institutes of Health). Areas are presented as number of pixels (px).
Results
Histology
NGF-injected animals showed decreased gray matter thickness in anterior cinguate, prelimbic, and infralimbic cortices bilaterally compared with saline-treated rats (figure 2A,B). Thickness of gray matter in the sensorimotor cortex appeared comparable between both the groups. NGF rats showed significantly decreased PFC area compared with control rats (NGF, 258778.5 ± 2014.2 px [mean ± standard error of the mean]; control, 281823.6 ± 1059.0 px; t(14) = 10.13, P < .0001), and the range of values did not overlap (NGF, 249683–266407 px; control, 277135–286347 px). As shown in figure 2C,D, the NGF rats also had significantly larger lateral ventricles at the level of the decussation of the anterior commissure compared with controls (NGF, 173461.3 ± 3088.2 px; control, 140928.8 ± 1814.9 px; t(14) = 9.08, P < .0001), and the range of values did not overlap (NGF, 159382–184210 px; control, 135927–147278 px).
Fig. 2.
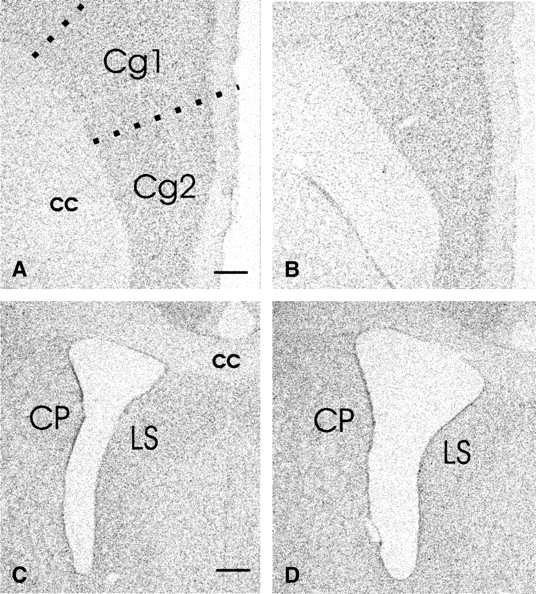
Bright-field images of neutral red-stained coronal sections of adult rats that received neonatal infusions of saline (A, C) or NGF (B, D) showing the thickness of cingulate cortex (Cg1, Cg2) (A, B) and the size of lateral ventricles (C, D). Sections A and B, and C and D were taken through the same rostrocaudal plane. Note that the thickness of Cg1 and Cg2 areas appears smaller in NGF-lesioned rat (B) compared with the control rat (A). The surface area of the lateral ventricle in NGF-lesioned rat (D) appears larger than that of the control (C); cc, corpus callosum, CP, caudate-putamen, LS, lateral septum (bar, A, B = 650 μm; C, D = 300 μm).
Similar Pairs, Age 6 Weeks
Movement
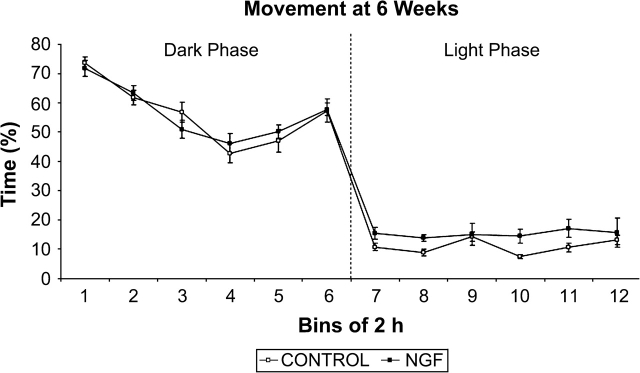
As expected, rats were generally more active during the dark phase than during the light phase of the cycle (figure 3). During the light phase, NGF rats were more active than controls (F(1, 14) = 3.289, P < .05). There was a trial × treatment interaction for light phase data (F(22, 308) = 2.690, P < .01), and post hoc t tests revealed that for 8 of the 30-minute light phase time bins there was a significant group difference, with NGF rats showing greater movement during 7 of the time bins and control rats showing greater movement during 1 time bin. The groups did not differ on any other measure.
Fig. 3.
Percent time moving at 6 weeks of age. There was a significant group difference during the light phase, in which NGF rats engaged in greater movement. Data points represent group mean percent of time moving analyzed for time bins of 2 hours, except for bin 12, which is 1.5 hours in duration. Error bars in all figures represent ± 1 standard error of the mean.
Similar Pairs, Age 14 Weeks
Movement, Mean Distance Apart, and Proximity
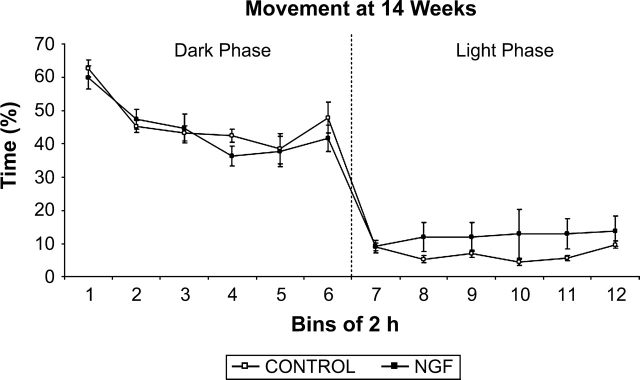
As expected, rats were again more active during the dark phase than during the light phase of the cycle (figure 4), but there was no group difference and no interaction for either phase.
Fig. 4.
Percent time moving at 14 weeks of age. There was no significant treatment effect in either the dark or the light phases. Data points represent groups mean percent of time moving analyzed for time bins of 2 hours, except for bin 12, which is 1.5 hours in duration.
As shown in figure 5, NGF rats consistently maintained a greater mean distance apart than controls during the dark phase of the cycle (F(1, 6) = 5.752, P < .05). When the individual 30-minute time bins were analyzed, 1-tailed t tests showed significant treatment effects for trials 1 (t(6) = 2.264, P < .05), 9 (t(6) = 2.039, P < .05), 11 (t(6) = 2.377, P < .05), and 19 (t(6) = 2.119, P < .05). Consistent with this finding, there was a nonsignificant trend for the NGF rats to spend less time within 20 cm of each other during the dark phase (F(1, 6) = 2.656, P = .077). During the entire dark phase, NGF rats spent 16.39% of the time, as compared with the controls who spent 18.19% of their time, within the 20-cm interaction zone. During the entire light phase, NGF rats spent 61.59% of their time, as compared with the controls who spent 57.58% of their time, within the 20-cm interaction zone. There were no trial × treatment interactions during either phase for either measure.
Fig. 5.
Mean distance apart at 14 weeks of age. There was a significant treatment effect during the dark phase, in which NGF rats maintained a greater mean distance apart. Data points represent group mean distance apart in centimeter averaged for time bins of 2 hours, except for bin 12, which is 1.5 hours in duration.
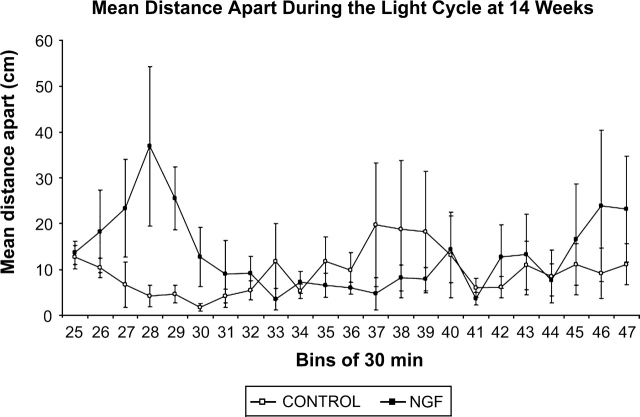
NGF rats maintained a greater mean distance apart during the dark phase of testing (figure 5). Further examination of data in figure 5 suggested that this effect persisted during the transition from the dark phase to the first part of the light phase. To further investigate this possibility, mean distance apart data for the individual 30-minute bins of the light cycle (30-minute bins 25–47) were separately graphed (figure 6). The light cycle was arbitrarily divided into 3 approximately equal parts: the first 4 hours (bins 25–32), the second 4 hours (bins 33–40), and the last 3.5 hours (bins 41–47). Separate analysis of data in each part revealed that the NGF rats maintained a greater mean distance apart during the first 4 hours (F(1, 6) = 7.81, P < .05), but that there were no group differences for the other parts of the light phase. During the first 4 hours of the light cycle, post hoc analyses revealed a significant difference during trial 29 (t(6) = 2.937, P < .05).
Fig. 6.
Mean distance apart during the light phase at 14 weeks. There is a significant treatment effect during the first 4 hours of the light phase (bins 25–32), in which NGF rats maintain a greater mean distance apart. There are no significant treatment effects during the second 4 hours or the last 3.5 hours. Data points represent group mean distance apart in centimeter averaged for time bins of 30 minutes.
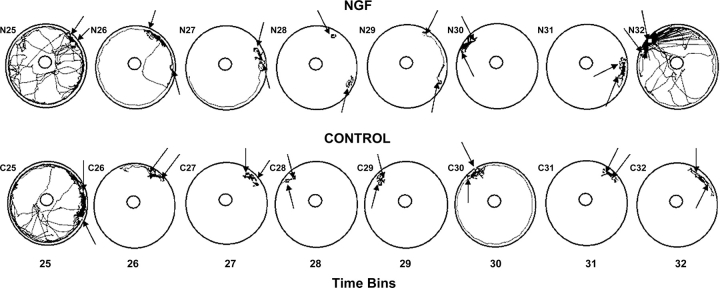
Given that there were no effects of treatment on movement at 14 weeks of age, movement (locomotion) differences per se cannot account for group differences in mean distance apart. Therefore, data for the first 4 hours of the light phase, when rats are less active and often sleeping, were further analyzed to evaluate individual animal movements and positions. For this purpose, digitized movement paths of individual rats were generated by EthoVision for each 30-minute period during the first part of the light phase. Plots of the movements and the most common positions of representative rats are shown in figure 7 that contain data from the rat pairs whose mean distance apart during this part of the light phase was closest to the mean distance apart of their respective groups.
Fig. 7.
Digitized movement paths of control and NGF rat pairs for time bins 25–32. Each time bin represents 30 minutes. The rat pairs whose data appear in this figure are those whose mean distance apart data for time bins 25–32 were closest to the mean values of their respective groups. The squares embedded in the paths represent the most common position of the rats for each time bin. The squares are indicated by arrows for clarity.
During the first 30-minute period (bin 25), both rat pairs engaged in considerable movement (figure 7). By bin 28 there was very little movement and a clear difference in the most common positions of the rats became apparent, with the positions of the NGF rats considerably farther apart than the positions of the control rats. This corresponds to the clear separation of the group data at bin 28 in figure 6, and suggests that the NGF rats were sleeping apart but the control rats were sleeping huddled together, as would be expected for normal rats. The same pattern was apparent in bin 29, again corresponding to the separation of group data at bin 29 in figure 6. The tendency for differential nesting positions disappeared after bin 29.
Approach and Avoidance
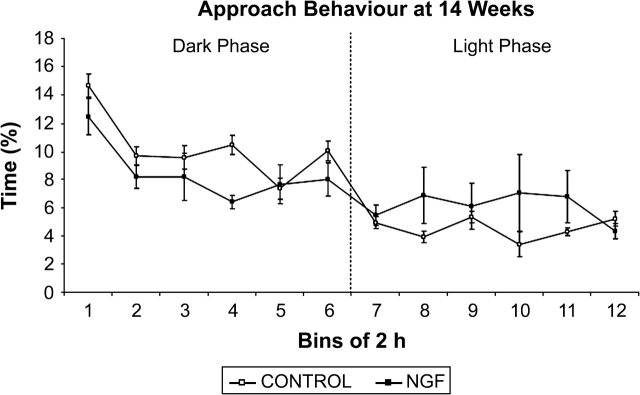
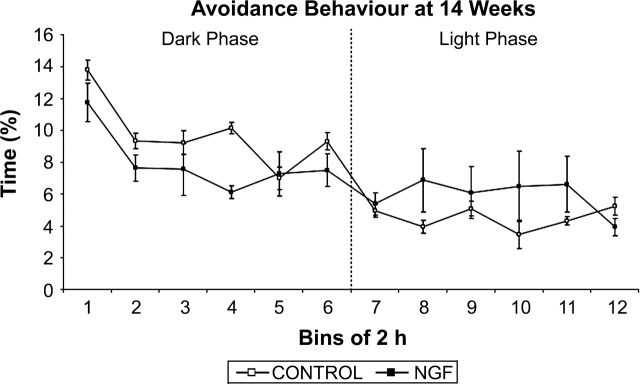
As shown in figures 8 and 9, NGF rats spent less time displaying approach and avoidance behavior during the dark phase (approach: F(1, 14) = 12.236, P < .05; avoidance: F(1, 14) = 4.093, P < .05). There were no trial × treatment interactions during either phase for approach or avoidance. We also examined whether approach and avoidance differed within an interaction zone of 20–40 cm. Neither approach nor avoidance behavior was significantly different between the groups during the dark or the light phases.
Fig. 8.
Approach behavior at 14 weeks of age. There was a significant effect of treatment during the dark cycle, in which NGF rats engaged in less approach behavior. Data points represent group mean distance apart in centimeter averaged for time bins of 2 hours, except for bin 12, which is 1.5 hours in duration.
Fig. 9.
Avoidance behavior at 14 weeks of age. There was a significant treatment effect during the dark cycle, in which NGF rats engaged in less avoidance behavior. Data points represent group mean distance apart in centimeter averaged for time bins of 2 hours, except for bin 12, which is 1.5 hours in duration.
Discussion
The data reported here suggest that neonatal injections of NGF into developing rat FC result in a decrease in social interaction in adulthood but not adolescence. At 6 weeks there was no treatment effect on measures of mean distance apart, proximity, or approach and avoidance behavior. Our failure to find social behavior differences at 6 weeks is consistent with most animal models of schizophrenia,14,18 although one article reported such differences.43 However, we did find that NGF rats spent more time moving than controls at 6 weeks, suggesting that the NGF rats were hyperactive, which is consistent with the hyperactivity reported in children who later developed schizophrenia.44
Evidence for a decrease in social interaction emerged at 14 weeks of age, with NGF rats maintaining a greater mean distance apart during the dark phase and the first 4 hours of the light phase. Rats are nocturnal animals and display active behavior throughout the dark phase; therefore, behavior during this phase is most comparable to human behavior during the daytime hours. The greater mean interanimal distance seen in the NGF rats during this phase is consistent with data obtained from human schizophrenia patients.15 The tracking data shown in figure 7 suggest that the greater mean distance apart during the first 4 hours of the light phase likely resulted from NGF rats nesting farther apart than controls, which is consistent with findings from a genetically engineered schizophrenia mouse model.45 Post hoc analyses revealed several time points during both the dark and light phases, which significantly differed from one another. Although the small sample size of 4 pairs per group may limit the number of time bins that are found to be significant, despite the variability in the data, graphical representations consistently show the NGF rats maintaining a greater mean distance apart than controls.
The proximity measure was used to further characterize the social interaction deficit by assessing the amount of time the rats spent within a 20-cm interaction zone. At 14 weeks there was a nonsignificant trend for NGF rats to spend less time than controls within 20 cm of each other, which is consistent with the findings of Flagstad et al.14 The proximity data are also consistent with the mean distance apart measure, in that rats that maintain a greater mean distance apart would be expected to spend less time in close proximity of one another. However, this finding is also consistent with the possibility that NGF rats may spend almost the same amount of time in close proximity as controls, but that they maintain a greater distance apart when not in close proximity.
Both approach and avoidance behavior of NGF rats were significantly decreased at 14 weeks of age relative to controls during the dark phase. While this may seem contradictory, if NGF rats were engaged in less approach behavior than controls they would be expected to have fewer occasions to display avoidance behavior. Hence, the reduction in approach behavior is consistent with the idea that NGF rats were interacting with one another less than controls were interacting during the active phase. These results are also consistent with the frequent finding that schizophrenia rat models engage in less active social interaction.14,16,33,34,36 The fact that NGF and control groups did not differ during the light phase may be related to their low level of activity during this phase.
At 14 weeks there was no treatment effect in the movement measure. This is consistent with the symptomology of human schizophrenia patients that does not include hyperactivity as one of the diagnostic criteria. Some rat models of schizophrenia have reported increased activity in adulthood.33,34,36 However, these studies used mean distance traveled, as opposed to movement, as a measure of locomotor activity. As defined by EthoVision, movement may be the more appropriate analysis of locomotor activity because slight changes in the animal's position (ie, head movements or postural shifts) are not counted as movement. Instead, the rat must reach a certain minimal speed before activity is considered to be movement (Noldus Information Technology, 2003). Hence, it is possible that the measure of distance traveled used in the earlier studies inadvertently inflated locomotor activity by considering slight changes in position as locomotion. In any case, as hyperactivity is not a symptom of human schizophrenia in adulthood, the lack of a difference in this measure may add to the construct validity of the NGF model. The lack of differences in movement between NGF and control rats suggests that increases in mean distance apart and reductions in approach and avoidance behavior are unlikely to be due to group differences in locomotion.
Histological Findings and Relation to Schizophrenia
Although no formal examination of neonatal brain tissue was made, previous work using the same treatments indicated little or no observable tissue damage at the injection site 1 week after injection, and similar appearance in NGF and saline pups19 (unpublished observations, N. Rajakumar). The histological findings from the NGF rats in the present study included enlarged lateral ventricles and decreased thickness of anterior cinguate, prelimbic, and infralimbic cortex relative to controls, with no overlap in the ranges of measured areas in the 2 groups. This is consistent with both previous findings with this model19 and with neuropathological abnormalities described in postmortem brains of schizophrenia patients.21,23,25 Previous research with the NGF model has found evidence of positive schizophrenia symptoms such as adult-onset subcortical dopaminergic hyperactivity and impaired prepulse inhibition of startle.19 The decrease in social interaction reported here for NGF rats is consistent with social interaction deficits found in human schizophrenia patients and in previous research on animal models,14,16,17,45 and is consistent with the presence of negative symptoms in the NGF model of schizophrenia. The modeling of brain abnormalities and both positive19 and negative symptoms increase the construct validity of the NGF model.
Although confirmation of elevated NGF during neurodevelopment is not available from developing human schizophrenia brain, reduced levels of brain-derived neurotrophic factor (BDNF), the ligand for TrkB, have been found in the CSF of adult schizophrenics,46,47 and reduced levels of both NGF and BDNF have been implicated in the pathogenesis of schizophrenia.48 This suggests that neurotrophic dysregulation may be a preexisting condition that could cause similar damage, by neurotrophic deprivation, to subplate cells as the present experimental manipulation.49 Thus, exogenous NGF administered in the present study might mimic aspects of the neurotrophic dysregulation that is hypothesized to be a factor in the damage to human subplate cells seen in human schizophrenia.49,50 The present experimental technique might be a means of producing frontal subplate abnormalities relevant to an animal model of schizophrenia, much like the widely used ventral-hippocampal lesion that is used to replicate impaired hippocampal influence on FC development.16,17,43,51
Advantages and Disadvantages of the NGF Model
To our knowledge, this is the first study of a rat model of schizophrenia to employ uninterrupted collection of social behavior data throughout both dark and light phases of a diurnal cycle. An advantage of both the NGF and ventral hippocampal lesion models19,43,52 is that the behavioral manifestations are persistent, unlike models based on short-term drug effects, and thus can be measured throughout one or more diurnal cycles. In the present study, this allowed the consistency of the group difference in the mean distance apart measure to be established for the dark phase, and the continuation of the group difference into the first part of the light phase to be documented. It also allowed specific times of interest to be identified, during which decreases in social interaction were maximal. This information allows researchers to employ short duration tests involving large numbers of subjects to assess the predictive validity of the model by reversing the social interaction decrease in NGF rats using atypical antipsychotics35,38,53 or other treatments.
An unavoidable disadvantage of collecting data throughout a full diurnal cycle that is not present with a brief, focused period of data collection33,34,37 is that animals engage in different behaviors at different times during a particular phase of the cycle (eg, eating, drinking, exploring, sleeping) and make transitions from one phase to the next at different times. Therefore, considerable variability in behavioral measures can be expected, and this was clearly evident in the data reported here, particularly in comparison to earlier studies that employed much shorter periods of behavioral data collection that did not involve consummatory behaviors or transitions between active and sleep states33,34,36,37. Although the group differences reported here were statistically reliable and are consistent with previously reported data in the context of neonatal frontal NGF injection as a rat model of schizophrenia,19 conclusions from these data should be made cautiously.
A limitation of this study is that EthoVision cannot distinguish between specific kinds of social behavior. Previous studies using phencyclidine injections or ventral hippocampal lesions have reported increased aggression in model animals.17,39,52 Although EthoVision was unable to detect such behavior here, there was no evidence of aggression between the rats during our limited direct observations of their interactions. Further, there was no evidence of injury to the rats when they were removed from the test arena, suggesting that rat-rat aggression was not a factor in this study. This conclusion seems consistent with the fact that in studies of the effects of phencyclidine injections or ventral hippocampal lesions on social behavior there was an overall decrease in social interaction despite an increase in aggressive behavior.17,39,52
Although the present experiments were primarily conducted to further evaluate the validity of the NGF model of schizophrenia, the findings have additional implications that extend to the neurobiology of social interaction. Kolb and Whishaw54 have noted that lesions of the FC in nonhuman primates lead to inappropriate vocal and gestural behaviors, and inappropriate responses to vocalizations and gestures made by conspecifics. These researchers concluded that social interaction in humans might be mediated by the FC.54 Findings of both FC abnormality and decreased social interaction in the NGF model are consistent with this idea.
Acknowledgments
We would like to thank Drs B. Gardner, E. Hampson, and J. Neufeld for their advice and assistance during the preparation of this manuscript. We are grateful for the technical assistance of Mr F. Boon, Ms B. Rajakumar, Mr R. Cantrup, Ms K. Dyer, Ms E. Lazar, and Ms M. Fudge for technical support. This research was supported by grants from the Social Sciences and Humanities Research Council of Canada (N.L.L.), the Ontario Ministry of Training, Colleges and Universities (N.L.L.), the Ontario Mental Health Foundation (N.R.), and the Natural Sciences and Engineering Research Council of Canada (D.P.C.).
Conflict of Interest
Currently, one of the authors (N.R.) has a patent pending on the methods used to generate the NGF model of schizophrenia.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental. 4th ed. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- 2.Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci U S A. 2000;97:8104–8109. doi: 10.1073/pnas.97.14.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;43:114–124. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 4.Bediou B, Franck N, Saoud M, et al. Effects of emotion and identity on facial affect processing in schizophrenia. Psychiatry Res. 2005;133:149–157. doi: 10.1016/j.psychres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Bellack AS, Morrison RL, Wixted JT, Mueser KT. An analysis of social competence in schizophrenia. Br J Psychiatry. 1990;156:809–818. doi: 10.1192/bjp.156.6.809. [DOI] [PubMed] [Google Scholar]
- 6.Halford WK, Hayes RL. Social skills in schizophrenia: assessing the relationship between social skills, psychopathology and community functioning. Soc Psychiatry Psychiatr Epidemiol. 1995;30:14–19. doi: 10.1007/BF00784429. [DOI] [PubMed] [Google Scholar]
- 7.Penn DL, Corrigan PW, Bentall RP, Racenstein JM, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121:114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 8.Penn DL, Ritchie M, Francis J, Combs D, Martin J. Social perception in schizophrenia: the role of context. Psychiatry Res. 2002;109:149–159. doi: 10.1016/s0165-1781(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 9.Walker E, Davis D, Baum K. Social withdrawal. In: Costello CG, editor. Symptoms of Schizophrenia. Toronto, Ontario: John Wiley & Sons, Inc; 1993. pp. 227–260. [Google Scholar]
- 10.Dworkin RH, Bernstein G, Kaplansky LM, et al. Social competence and positive and negative symptoms: a longitudinal study of children and adolescents at risk for schizophrenia and affective disorder. Am J Psychiatry. 1991;148:1182–1188. doi: 10.1176/ajp.148.9.1182. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg JO, Schmidt LA. Shyness, sociability, and social dysfunction in schizophrenia. Schizophr Res. 2001;48:343–349. doi: 10.1016/s0920-9964(00)00143-2. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman J, Walker E, Ekstrom M, Schulsinger F, Sorensen H, Mednick S. Childhood videotaped social and neuromotor precursors of schizophrenia: a prospective investigation. Am J Psychiatry. 2004;161:2021–2027. doi: 10.1176/appi.ajp.161.11.2021. [DOI] [PubMed] [Google Scholar]
- 13.Watt NF, Lubensky AW. Childhood roots of schizophrenia. J Consult Clin Psychol. 1976;44:363–375. doi: 10.1037//0022-006x.44.3.363. [DOI] [PubMed] [Google Scholar]
- 14.Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative symptoms and alters amphetamine-induced dopamine release in the nucleus accumbens. Neuropsychopharmacology. 2004;29:2052–2064. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- 15.Nechamkin Y, Salganik I, Modai I, Ponizovsky AM. Interpersonal distance in schizophrenic patients: relationship to negative syndrome. Int J Soc Psychiatry. 2003;19:166–174. doi: 10.1177/00207640030493002. [DOI] [PubMed] [Google Scholar]
- 16.Sams-Dodd F, Lipska BK, Weinberger DR. Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology. 1997;132:303–310. doi: 10.1007/s002130050349. [DOI] [PubMed] [Google Scholar]
- 17.Becker A, Grecksch G, Bernstein HG, Hollt V, Bogerts B. Social behaviour in rats lesioned with ibotenic acid in the hippocampus: quantitative and qualitative analysis. Psychopharmacology. 1999;144:333–338. doi: 10.1007/s002130051015. [DOI] [PubMed] [Google Scholar]
- 18.Futamura T, Kakita A, Tohmi M, Sotoyama H, Takahashi H, Nawa H. Neonatal perturbation of neurotrophic signaling results in abnormal sensorimotor gating and social interaction in adults: implication for epidermal growth factor in cognitive development. Mol Psychiatry. 2003;8:19–29. doi: 10.1038/sj.mp.4001138. [DOI] [PubMed] [Google Scholar]
- 19.Rajakumar N, Rajakumar B. A mild disruption of subplate function in the developing prefrontal cortex is sufficient to cause multiple neuropathological features of schizophrenia [Google Scholar]
- 20.Rajakumar N, Leung LS, Ma J, Rajakumar B, Rushlow W. Altered neurotrophin receptor function in the developing prefrontal cortex leads to adult-onset dopaminergic hyperresponsivity and impaired prepulse inhibition of acoustic startle. Biol Psychiatry. 2004;55:797–803. doi: 10.1016/j.biopsych.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Andreasen NC, Ehrhardt JC, Swayze VW. Magnetic resonance imaging of the brain in schizophrenia: the pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry. 1990;47:35–44. doi: 10.1001/archpsyc.1990.01810130037006. [DOI] [PubMed] [Google Scholar]
- 22.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology. 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 23.Kelsoe JR, Cadet JL. Quantitative neuroanatomy in schizophrenia: a controlled magnetic resonance imaging study. Arch Gen Psychiatry. 1988;45:533–541. doi: 10.1001/archpsyc.1988.01800300029003. [DOI] [PubMed] [Google Scholar]
- 24.Schleimer SB, Hinton T, Dixon G, Johnston GA. GABA transporters GAT-1 and GAT-3 in the human dorsolateral prefrontal cortex in schizophrenia. Biol Psychiatry. 2004;50:226–230. doi: 10.1159/000079975. [DOI] [PubMed] [Google Scholar]
- 25.Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- 26.Volk DW, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 27.Bonhoeffer T. Neurotrophins and activity-dependent development of the neocortex. Curr Opin Neurobiol. 1996;6:119–126. doi: 10.1016/s0959-4388(96)80017-1. [DOI] [PubMed] [Google Scholar]
- 28.Carter BD, Lewin GR. Neurotrophins live or let die: dose p75NTR decide? Neuron. 1997;18:187–190. doi: 10.1016/s0896-6273(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 29.Allendoerfer KL, Shatz CJ. The subplate, a transient neocortical structure: its role in the development of connections between thalamus and cortex. Annu Rev Neurosci. 1994;17:185–218. doi: 10.1146/annurev.ne.17.030194.001153. [DOI] [PubMed] [Google Scholar]
- 30.Cabelli RJ, Allendoerfer KL, Radeke MJ, Welcher AA, Feinstein SC, Shatz CJ. Changing patterns of expression and subcellular localization of TrkB in the developing visual system. J Neurosci. 1996;16:7965–7980. doi: 10.1523/JNEUROSCI.16-24-07965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dechant G, Barde YA. Signaling through the neurotrophin receptor p75NTR. Curr Opin Neurobiol. 1997;7:413–418. doi: 10.1016/s0959-4388(97)80071-2. [DOI] [PubMed] [Google Scholar]
- 32.Whishaw IQ, Kolb B, Sutherland RJ. The analysis of behavior in the laboratory rat. In: Robinson TE, editor. Behavioral Approaches to Brain Research. Oxford, England: Oxford University Press; 1981. pp. 141–210. [Google Scholar]
- 33.Sams-Dodd F. Distinct effects of d-amphetamine and phencyclidine on the social interaction of rats. Behav Pharmacol. 1995;6:55–65. [PubMed] [Google Scholar]
- 34.Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in the rat: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- 35.Sams-Dodd F. Effect of novel antipsychotic drugs in phencyclidine-induced stereotyped behaviour and social isolation in the rat social interaction test. Behav Pharmacol. 1997;8:196–215. [PubMed] [Google Scholar]
- 36.Sams-Dodd F. A test of the predictive validity of animal models of schizophrenia based on phencyclidine and d-amphetamine. Neuropsychopharmacology. 1998;18:293–304. doi: 10.1016/S0893-133X(97)00161-9. [DOI] [PubMed] [Google Scholar]
- 37.Sams-Dodd F. Effects of continuous d-amphetamine and phencyclidine administration on social behaviour, stereotyped behaviour, and locomotor activity in rats. Neuropsychopharmacology. 1998;19:18–25. doi: 10.1016/S0893-133X(97)00200-5. [DOI] [PubMed] [Google Scholar]
- 38.Sams-Dodd F. Effects of diazepam, citalopram, methadone and naloxone on PCP-induced stereotyped behaviour and social isolation in the rat social interaction test. Neurosci Biobehav Rev. 1998;23:287–293. doi: 10.1016/s0149-7634(98)00030-x. [DOI] [PubMed] [Google Scholar]
- 39.Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Ashwell KWS, Tork I. Atlas of the Developing Rat Nervous System. San Diego, Calif: Academic Press; 1994. [Google Scholar]
- 41.Sams-Dodd F. Effects of dopamine agonists and antagonists on PCP-induced stereotyped behaviour and social isolation in the rat social interaction test. Psychopharmacology. 1998;135:182–193. doi: 10.1007/s002130050500. [DOI] [PubMed] [Google Scholar]
- 42.Rajakumar N, Rushlow W, Naus CC, Elisevich K, Flumerfelt BA. Neurochemical compartmentalization of the globus pallidus in the rat: an immunocytochemical study. J Comp Neurol. 1994;346:337–348. doi: 10.1002/cne.903460303. [DOI] [PubMed] [Google Scholar]
- 43.Lipska BK, Jaskiw GE, Weinberger DR. Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology. 1993;9:67–75. doi: 10.1038/npp.1993.44. [DOI] [PubMed] [Google Scholar]
- 44.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from the cradle to the grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 45.Miyakawa T, Leiter LM, Gerber DJ, et al. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc Natl Acad Sci U S A. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. doi: 10.1016/j.neulet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 47.Toyooka K, Asama K, Watanabe Y, et al. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 48.Aloe L, Iannitelli A, Angelucci F, Bersani G, Fiore M. Studies in animal models and humans suggesting a role of nerve growth factor in schizophrenia-like disorders. Behav Pharmacol. 2000;11:235–242. doi: 10.1097/00008877-200006000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Angelucci F, Brene S, Mathe AA. BDNF in schizophrenia, depression and corresponding animal models. Mol Psychiatry. 2005;10:345–352. doi: 10.1038/sj.mp.4001637. [DOI] [PubMed] [Google Scholar]
- 50.Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 51.Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 52.Flores G, Silva-Gomez AB, Ibanez O, Quirion R, Srivastava LK. Comparative behavioral changes in postpubertal rats after neonatal excitotoxic lesions of the ventral hippocampus and the prefrontal cortex. Synapse. 2005;56:147–153. doi: 10.1002/syn.20140. [DOI] [PubMed] [Google Scholar]
- 53.Burton S. Symptom domain of schizophrenia: the role of atypical antipsychotic agents. J Psychopharmacol. 2006;20:6–19. doi: 10.1177/1359786806071237. [DOI] [PubMed] [Google Scholar]
- 54.Kolb B, Whishaw IQ. Fundamentals of Human Neuropsychology. 5th ed. New York, NY: Worth Publishers; 2003. [Google Scholar]