Abstract
Childhood-onset schizophrenia (COS; defined as onset by age 12 years) is rare, difficult to diagnose, and represents a severe and chronic phenotype of the adult-onset illness. A study of childhood-onset psychoses has been ongoing at the National Institute of Mental Health (NIMH) since 1990, where children with COS and severe atypical psychoses (provisionally labeled “multidimensionally impaired” or MDI by the NIMH team) are studied prospectively along with all first-degree relatives. COS subjects have robust cortical gray matter (GM) loss during adolescence, which appears to be an exaggeration of the normal cortical GM developmental pattern and eventually mimics the pattern seen in adult-onset cases as the children become young adults. These cortical GM changes in COS are diagnostically specific and seemingly unrelated to the effects of medications. Furthermore, the cortical GM loss is also shared by healthy full siblings of COS probands suggesting a genetic influence on the abnormal brain development.
Keywords: childhood-onset schizophrenia, structural brain imaging
Introduction
Across medicine, childhood manifestation of a typical adult-onset illness usually shows a more severe phenotype and perhaps greater genetic influences.1 This also appears to be the case in childhood-onset schizophrenia (COS), which is a rare, severe phenotype of the typical adult-onset illness.2 Although the existence of childhood schizophrenia was recognized since early in the 20th century,2,3 “childhood psychosis” broadly included both the spectrum of behavioral disorders as well as autism4 until these were distinguished by Kolvin5 in 1971. Even today, high rates of misdiagnosis remain because transient psychotic symptoms can occur in healthy children6–8 and fleeting hallucinations are not uncommon in nonpsychotic pediatric patients,9,10 particularly in response to anxiety and stress.11 Fully developed psychotic disorders in children, however, are rare and tend to be more severe than their adult counterparts,1 and recent data from a large birth cohort study suggests that self-reported psychotic symptoms at age 11 years predicted a very high risk (odds ratio = 16.4, confidence interval = 3.9–67.8) of schizophreniform diagnosis at age 26 years suggesting that psychotic symptoms probably exist as a continuous phenotype rather than an all-or-none phenomenon.12
A study of COS was started at the National Institute o Mental Health (NIMH) in 1990 with the hypothesis that it would make it possible to study the illness with a more homogeneous phenotype, closer to the developmental roots, and also less confounded by environmental factors such as multiple hospitalizations or substance abuse. Over the past 15 years, children have been nationally recruited and diagnosis is made using unmodified Diagnostic and Statistical Manual of Mental Disorders, Third Revision/Fourth Edition (DSM-IIIR/IV), criteria for schizophrenia, with onset of psychosis by age 12 years, premorbid IQ of 70 or above, and absence of significant neurological disorder. In most cases, the diagnosis is confirmed after a complete medication washout followed by 1–3 weeks' drug-free inpatient observation.2,13,14 This has resulted in a unique homogeneous cohort of COS cases (n = 95 to date), where most patients resemble chronic, severe, treatment-refractory adult-onset cases. Earlier studies comprehensively showed the continuity between the early- and later onset schizophrenia through studies on premorbid risk factors,15–22 smooth pursuit eye-tracking function,23 familial schizophrenia-spectrum disorders,24,25 and neurocognitive measures.26
Structural brain abnormalities are an established feature of schizophrenia, characterized by decreased total gray matter (GM) volume reduction in cortex, hippocampus, and amygdala.27–30 The cortical GM loss appears progressive over time.31–34 Regional analyses of cortical GM, using either voxel-based morphometry or cortical thickness measures, show that most cortical GM deficits in schizophrenia are localized to prefrontal and superior temporal cortices in both first-episode and chronic adult patients.35–37 However, many questions regarding the GM findings remain unanswered; eg, whether the GM changes are genetically influenced, whether the GM loss could be considered a trait marker, what is the influence of medications on GM changes, whether the GM loss is functionally significant, and whether the GM changes in schizophrenia are diagnostically specific. The unique cohort of severe but homogeneous COS cases, along with their healthy as well as schizophrenia-spectrum full siblings, provide an important sample to gain further insights into some of these questions, which are discussed in this review.
Brain Imaging Studies in COS
The majority of the COS brain imaging studies have come from the NIMH cohort. Earlier cross-sectional imaging studies showed general agreement on findings of increased lateral ventricular volume, decreased total as well as regional GM volumes, and increased basal ganglia volume.38–44 Later on, prospective longitudinal brain magnetic resonance imaging (MRI) studies of the NIMH COS population showed increasing ventricular volume and decreasing total cortical, frontal, medial temporal, and parietal GM volumes at 2- to 6-year follow-up39,45 during adolescent years.
However, all these early studies were done using relatively crude whole-lobe volumetric measures that do not provide subregional specificity of GM findings. Recent advances in computational image analysis now permit regional GM density or cortical thickness measurements, which, when automated, can be applied to large samples, increasing statistical power46–48 and provide unprecedented anatomic detail of cortical GM change across the entire cortex, and across time.49 Using these novel brain mapping techniques, we now have mapped longitudinal GM developmental trajectories for COS probands contrasting them with matched groups of healthy children, healthy COS siblings, and children with pediatric bipolar illness to address some of the questions raised above.
What is the Pattern of Cortical GM Loss in COS? And How Does the GM Loss in COS Compare With the Normal GM Maturation Pattern?
Initial analyses on 12 COS patients and temporally matched controls with prospective scans over 5 years between showed a unique wave of “back-to-front” tissue loss with early parietal GM loss followed by frontal and temporal GM loss (parietofrontal and parietotemporal directions) during adolescence (ages 12–16 years),50 and in a top-down fashion on the medial cortical surface.51
In a recent analyses of 13 healthy children with 3–5 scans (n = 54 scans) over a 10-year period (ages 4–22 years),49 an overall similar pattern of GM maturation was seen also progressing in “back-to-front” or parietofrontal and parietotemporal directions. This suggests that GM loss in COS may reflect an exaggeration of normal maturational process of synaptic/dendritic pruning during adolescence,52,53 perhaps also supporting the excessive pruning (synaptic elimination) hypothesis proposed for GM loss in schizophrenia (figure 1).54
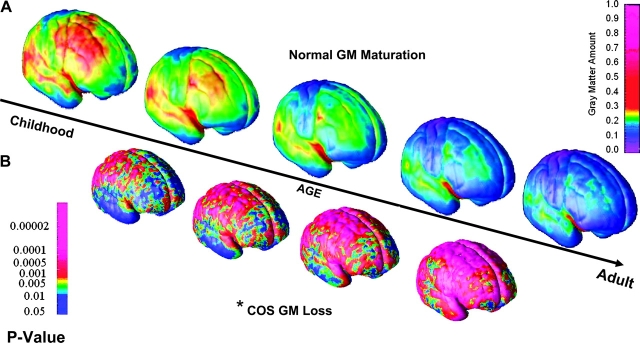
Fig. 1.
Comparison of the Patterns of Cortical Gray Matter (GM) Loss in Childhood-Onset Schizophrenia (COS) (Between Ages 12 and 16 Years) to That Seen in Normal Cortical Maturation (Between Ages 4 and 22 Years). A. Right lateral view of the dynamic sequences of cortical GM maturation in healthy children between ages 4 and 22 years (n = 13, 54 scans, upper panel) rescanned every 2 years. Scale bar shows GM amount at each of the 65 536 cortical points across the entire cortex represented using a color scale (red to pink—more GM, blue—GM Loss). Cortical GM maturation appears to progress in a “back-to-front” (parietotemporal) manner.49 B. Right lateral view of the dynamic sequence of cortical GM maturation in COS between ages 12 and 16 years compared with age- and sex-matched healthy controls (n = 12, 36 scans in each group), where children are rescanned every 2 years. Dynamic maps represent P values for the difference in GM amount between COS and controls at each of the 65 536 cortical points, and P values are represented using a color scale (eg, pink, P < .00002). Cortical GM loss in COS also appears to follow in a “back-to-front” direction on the lateral surface, thus suggesting that the COS pattern is an exaggeration of the normal GM maturation.50 Asterisk represents data on childhood schizophrenia only age 12–16 years. Adapted from Proc Natl Acad Sci USA 2004;101:8178 and Proc Natl Acad Sci USA 2001;98:11652.
Is the Cortical GM Loss More Striking for COS Than for the Adult-Onset Population?
Several adult schizophrenia studies using brain MRI have reported GM loss and ventricular enlargement, which are progressive.31–33,55–59 Because adolescence is a time for major brain reorganization, we hypothesized that the GM changes in COS will be more striking than seen in the adult population with the illness, which could be either the cause or result of the more severe COS phenotype. An effect size comparison of longitudinal anatomic MRI studies showed that the progressive brain changes in COS are indeed more robust than in the adult-onset schizophrenia (AOS) population.60 This may also indicate either a “pathological hit” in early childhood, or that in adult-onset patients, the disease process could have begun during adolescence, prior to the onset of psychosis, as suggested recently by Pantelis et al.61
Does the Cortical GM Loss in COS Mimic the Aadult-Onset Pattern After Adolescence?
If COS is continuous with AOS, as the children become young adults, the cortical GM pattern would take the pattern seen in AOS patients. Cortical thickness analyses in AOS show GM loss mostly in prefrontal and temporal cortices.36,46 In seeking whether COS eventually resembles the pattern seen in AOS, we analyzed GM development using prospective MRI scans of 70 COS subjects, ages 6–26 years, and 72 matched healthy controls (1–5 scans per subject; total scans = 330), using a fully automated cortical thickness analyses with mixed effect regression model statistics. The analyses show that as COS subjects mature, the robust and global GM loss during the adolescent years becomes limited to prefrontal and superior temporal cortices by age 24 years, mimicking a pattern seen in adult patients.35,62,63 These findings also establish the anatomic continuity between the 2 phenotypes (figure 2).
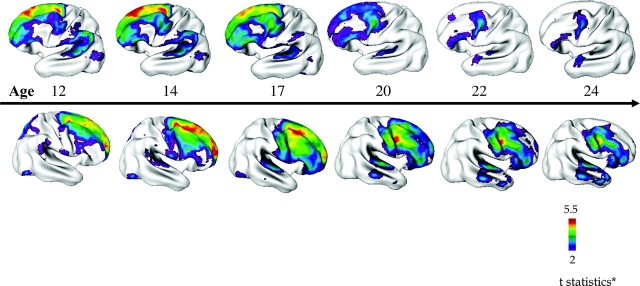
Fig. 2.
Progression of Cortical Gray Matter (GM) Loss in Childhood-Onset Schizophrenia (COS) (n = 70, 162 scans) Relative to Age-, Sex-, and Scan Interval–Matched Healthy Controls (n = 72, 168 Scans) From Adolescence to Young Adulthood (age 12–24 years). Analyses were done using mixed model regression statistics and covaried from mean cortical thickness. Side bar shows t statistic with threshold to control for multiple comparisons using the false discovery rate procedure with q = 0.05. Differences are from mixed model regression with age centered at approximate 3-year intervals for middle 80% of the age range, and colors represent areas of statistically significant thinning in COS.81 Adapted from J Child Psychol Psychiatry 2006;47:1007.
Is the Cortical GM Loss Medication Influenced and Diagnostically Specific?
How the effect of medications reflects on the pattern of GM loss in schizophrenia has been difficult to elucidate.35 Due to the severity of illness, medication-naive COS subjects are difficult to obtain. We have attempted to address this question using a cohort of atypical psychoses patients recruited and characterized during the course of the NIMH COS study.
The Multidimensionally Impaired Cohort.
A sizeable, heterogeneous group of children who were referred to the study with initial diagnosis of COS were ruled out as having COS after medication washout. These children could not be adequately characterized into any existing DSM-IV diagnoses13,64,65 (would probably be labeled psychosis not otherwise specified [NOS] or mood disorder NOS) and were labeled by the NIMH group as “multidimensionally impaired (MDI),” due to the following distinct features which were used as the “operational diagnostic criteria” by the NIMH group13,64: (1) brief, transient episodes of psychosis and perceptual disturbance usually in response to stress (as opposed to the pervasive hallucinations/delusions in COS); (2) nearly daily periods of emotional lability disproportionate to participitants, (3) impaired interpersonal skills despite the desire to initiate peer friendships (distinction from COS), (4) no thought disorder, and (5) high comorbidity with attention-deficit/hyperactivity disorder. These children were followed longitudinally along with the COS children with the thought that some of the MDI children may evolve into the schizophrenia spectrum,64 but at 2- to 10-year follow-up the MDI cohort appears to have a distinct long-term clinical course and none have progressed to schizophrenia.66 Due to their similar initial presentation and medication history, these children have provided a medication-matched contrast group to evaluate the specificity of brain findings in COS.
When progressive GM changes were mapped in a group of age-, sex-, and IQ-matched COS (n = 12) and “MDI” (n = 10) patients, it was clear that the cortical deficits seen in COS are not shared by the “MDI” group and thus are not a result of medication treatment.50 This is further supported by our recent quantitative analysis of a larger sample of matched COS (n = 23), “DI” (n = 19) and healthy community control (n = 38) groups, where the GM loss was specifically seen in COS group.67
At 4- to 8-year follow-up, 12 out of 32 MDI subjects (38%) developed a DSM-IV–defined manic episode and thus were diagnosed as Bipolar I. Nine of these 12 children had usable scans that were obtained before and after the first manic episode (Bipolar I diagnosis). We mapped the cortical brain development in pediatric bipolar illness before and after the onset of illness. These analyses show a pattern strikingly distinct from that seen in COS. The brain development in MDI children who became Bipolar I showed subtle, regionally specific, bilaterally asymmetrical cortical changes, with cortical GM increasing over the left temporal cortex and decreasing bilaterally in the anterior (and subgenual) cingulate cortex particularly after the illness onset.68 Thus, at least in pediatric samples, there appears to be no overlap between the brain changes in COS and bipolar illness.
Is the Increased GM Loss in COS Genetically Influenced and a Trait Marker?
Several studies have described structural brain abnormalities in families of schizophrenic patients.69–72 Our initial cross-sectional analysis with 15 healthy full siblings of COS (mean age 19.4 ± 5.9 years) showed GM reduction relative to 32 healthy controls (mean age18.7 ± 6.2 years), where the difference was most significant in the parietal GM (effect size = 0.3).73 This was recently explored further using automated cortical thickness analyses on an expanded sample of nonpsychotic siblings of COS probands(n = 52, 113 scans; age 8–28 years) contrasting with age-, sex-, and scan interval–matched controls (n = 52, 108 scans). In these analyses, younger healthy COS siblings showed significant GM deficits in left prefrontal and bilateral temporal cortices and smaller deficits in right prefrontal and inferior parietal cortices compared with the controls suggesting that the prefrontal and temporal GM deficits in COS may be familial/trait markers. However, the cortical deficits in siblings disappeared by age 20 years suggesting a plastic or restitutive brain response in these nonpsychotic, nonspectrum siblings (figure 3).75
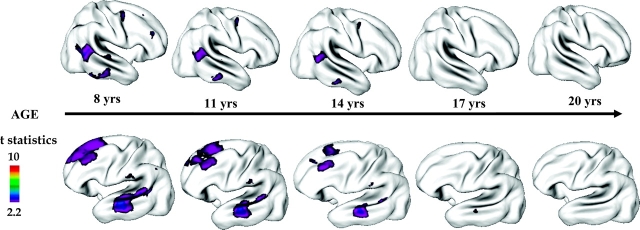
Fig. 3.
Cortical Gray Matter (GM) Thickness in Healthy Childhood-Onset Schizophrenia (COS) Siblings (n = 52, 110 scans) Compared With Age-, Sex-, and Scan Interval–Matched Healthy Controls (n = 52, 108 scans) Between Ages 8 Through 28 Years. Healthy COS siblings show significant GM deficits in left prefrontal and bilateral temporal cortices and smaller deficits in right prefrontal and inferior parietal cortices. These deficits in healthy siblings normalize with age with no abnormalities remaining by age 20 years. Side bar shows t statistic with threshold to control for multiple comparisons using the false discovery rate procedure with q = 0.05. Differences are from mixed model regression with age centered at approximate 3-year intervals for middle 80% of the age range, and colors represent areas of statistically significant thinning in COS siblings. Adapted from Arch Gen Psychiatry. 2007;64:774.
Numerous chromosomal abnormalities have been reported in schizophrenia.75,76 As has been seen in other pediatric onset diseases,1,77,78 the familial risk for schizophrenia-spectrum disorders appear higher for COS than for AOS.24,25 Candidate gene association studies and cytogenetic studies also show increased rates of abnormalities for the COS population, which are clearly higher than those seen for the AOS.79
We are beginning to explore the influence of individual risk alleles on GM developmental trajectories. For example, the gene for glutamate decarboxylase (GAD), a modulator enzyme for the inhibitory neurotransmitter γ-aminobutyric acid, influences the prefrontal cortical circuitry and postmortem studies have shown reduction in prefrontal cortical GM in schizophrenia patients with GAD risk gene.80,81 Our preliminary analyses using automated cortical thickness measures show that, in both COS subjects (n = 61, 149 scans) and their healthy siblings (n = 24, 50 scans), presence of GAD risk allele is associated with steeper slopes for GM loss in prefrontal and parietal cortices most prominently on the left side; while a similar analysis in the controls (n = 232, 500 scans) revealed no influence of the risk allele status (Dr Nitin Gogtay, unpublished data). These observations strongly suggest that the GM changes in COS are genetically influenced, possibly trait markers (at least in prefrontal and temporal regions), and influenced by risk allele status.
Is there a Relationship Between Brain Developmental Abnormalities and Functional Outcome?
The clinical and functional implications of cortical GM loss in schizophrenia have been unclear. We have recently studied this relationship in both COS probands and their healthy siblings. In healthy COS siblings, where the cortical GM deficits appear in early ages and normalize by 20 years, the process of deficit reduction appears to be correlated with overall functioning (GAS scores) at the last scan with children with higher GAS scores showing faster deficit reduction.74 Similarly, in our preliminary analyses, the COS probands who meet the criteria for remission at discharge (n = 19) have thicker cortical GM in prefrontal, temporal, as well as parietal cortical regions compared with the non remitted (n = 53) probands (Dr Deanna Greenstein, unpublished data). These findings suggest a relationship between brain plasticity and functional as well as clinical outcome in schizophrenia.
Summary
COS, which appears to represent more severe neurobiological and phenomenological counterpart of the adult-onset illness, follows a distinct course with more salient genetic factors. Advances in neuroimaging and genetic methodology, combined with the availability of longitudinal data, now allow further insights into the disease onset, course, and etiopathologic mechanisms in COS, which can be extended to the understanding of schizophrenia pathology at large. However, research in this field is still limited by small samples and few longitudinal and population-based studies stressing the need for continued future work in this area.
Acknowledgments
These studies were carried out at the NIMH intramural program.
References
- 1.Childs B, Scriver CR. Age at onset and causes of disease. Perspect Biol Med. 1986;29(Pt 1):437–460. doi: 10.1353/pbm.1986.0056. [DOI] [PubMed] [Google Scholar]
- 2.Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–1428. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- 3.Kraepelin E. Dementia Praecox and Paraphrenia. 1919 ed. Huntington, NY: Robert E Krieger; 1919. [Google Scholar]
- 4.Volkmar FR. Childhood and adolescent psychosis: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 1996;35:843–851. doi: 10.1097/00004583-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kolvin I. Studies in the childhood psychoses. I. Diagnostic criteria and classification. Br J Psychiatry. 1971;118:381–384. doi: 10.1192/bjp.118.545.381. [DOI] [PubMed] [Google Scholar]
- 6.Caplan R. Thought disorder in childhood. J Am Acad Child Adolesc Psychiatry. 1994;33:605–615. doi: 10.1097/00004583-199406000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Schreier HA. Hallucinations in nonpsychotic children: more common than we think? J Am Acad Child Adolesc Psychiatry. 1999;38:623–625. doi: 10.1097/00004583-199905000-00028. [DOI] [PubMed] [Google Scholar]
- 8.McGee R, Williams S, Poulton R. Hallucinations in nonpsychotic children. J Am Acad Child Adolesc Psychiatry. 2000;39:12–13. doi: 10.1097/00004583-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 9.McKenna K, Gordon CT, Rapoport JL. Childhood-onset schizophrenia: timely neurobiological research. J Am Acad Child Adolesc Psychiatry. 1994;33:771–781. doi: 10.1097/00004583-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lukianowicz N. Hallucinations in non-psychotic children. Psychiatr Clin (Basel) 1969;2:321–337. doi: 10.1159/000278582. [DOI] [PubMed] [Google Scholar]
- 11.Rothstein A. Hallucinatory phenomena in childhood. A critique of the literature. J Am Acad Child Psychiatry. 1981;20:623–635. doi: 10.1016/s0002-7138(09)61649-6. [DOI] [PubMed] [Google Scholar]
- 12.Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H. Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57:1053–1058. doi: 10.1001/archpsyc.57.11.1053. [DOI] [PubMed] [Google Scholar]
- 13.McKenna K, Gordon CT, Lenane M, Kaysen D, Fahey K, Rapoport JL. Looking for childhood-onset schizophrenia: the first 71 cases screened. J Am Acad Child Adolesc Psychiatry. 1994;33:636–644. doi: 10.1097/00004583-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kumra S, Briguglio C, Lenane M, et al. Including children and adolescents with schizophrenia in medication-free research. Am J Psychiatry. 1999;156:1065–1068. doi: 10.1176/ajp.156.7.1065. [DOI] [PubMed] [Google Scholar]
- 15.Asarnow JR, Ben-Meir S. Children with schizophrenia spectrum and depressive disorders: a comparative study of premorbid adjustment, onset pattern and severity of impairment. J Child Psychol Psychiatry. 1988;29:477–488. doi: 10.1111/j.1469-7610.1988.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 16.Watkins JM, Asarnow RF, Tanguay PE. Symptom development in childhood onset schizophrenia. J Child Psychol Psychiatry. 1988;29:865–878. doi: 10.1111/j.1469-7610.1988.tb00759.x. [DOI] [PubMed] [Google Scholar]
- 17.Russell A, Bott L, Sammons C. The phenomena of schizophrenia occurring in childhood. J Am Acad Child Adolesc Psychiatry. 1989;28:399–407. doi: 10.1097/00004583-198905000-00017. [DOI] [PubMed] [Google Scholar]
- 18.Green WH, Padron-Gayol M, Hardesty AS, Bassiri M. Schizophrenia with childhood onset: a phenomenological study of 38 cases. J Am Acad Child Adolesc Psychiatry. 1992;31:968–976. doi: 10.1097/00004583-199209000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Alaghband-Rad J, McKenna K, Gordon CT, et al. Childhood-onset schizophrenia: the severity of premorbid course. J Am Acad Child Adolesc Psychiatry. 1995;34:1273–1283. doi: 10.1097/00004583-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Hollis C. Child and adolescent (juvenile onset) schizophrenia. A case control study of premorbid developmental impairments. Br J Psychiatry. 1995;166:489–495. doi: 10.1192/bjp.166.4.489. [DOI] [PubMed] [Google Scholar]
- 21.Nicolson R, Lenane M, Singaracharlu S, et al. Premorbid speech and language impairments in childhood-onset schizophrenia: association with risk factors. Am J Psychiatry. 2000;157:794–800. doi: 10.1176/appi.ajp.157.5.794. [DOI] [PubMed] [Google Scholar]
- 22.Sporn AL, Addington AM, Gogtay N, et al. Pervasive developmental disorder and childhood-onset schizophrenia: comorbid disorder or a phenotypic variant of a very early onset illness? Biol Psychiatry. 2004;55:989–994. doi: 10.1016/j.biopsych.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 23.Sporn A, Greenstein D, Gogtay N, et al. Childhood-onset schizophrenia: smooth pursuit eye-tracking dysfunction in family members. Schizophr Res. 2005;73:243–252. doi: 10.1016/j.schres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch Gen Psychiatry. 2001;58:581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 25.Nicolson R, Brookner FB, Lenane M, et al. Parental schizophrenia spectrum disorders in childhood-onset and adult-onset schizophrenia. Am J Psychiatry. 2003;160:490–495. doi: 10.1176/appi.ajp.160.3.490. [DOI] [PubMed] [Google Scholar]
- 26.Gochman PA, Greenstein D, Sporn A, et al. Childhood onset schizophrenia: familial neuropsychological measures. Schizophr Res. 2004;71:43–47. doi: 10.1016/j.schres.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 27.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 28.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 29.Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 31.Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- 32.Gur RE, Cowell P, Turetsky BI, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman J, Chakos M, Wu H, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- 34.DeLisi LE. Regional brain volume change over the life-time course of schizophrenia. J Psychiatr Res. 1999;33:535–541. doi: 10.1016/s0022-3956(99)00028-x. [DOI] [PubMed] [Google Scholar]
- 35.Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- 36.Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- 37.Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 38.Rapoport JL, Giedd J, Kumra S, et al. Childhood-onset schizophrenia. Progressive ventricular change during adolescence. Arch Gen Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- 39.Rapoport JL, Giedd JN, Blumenthal J, et al. Progressive cortical change during adolescence in childhood-onset schizophrenia. A longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:649–654. doi: 10.1001/archpsyc.56.7.649. [DOI] [PubMed] [Google Scholar]
- 40.Sowell ER, Levitt J, Thompson PM, et al. Brain abnormalities in early-onset schizophrenia spectrum disorder observed with statistical parametric mapping of structural magnetic resonance images. Am J Psychiatry. 2000;157:1475–1484. doi: 10.1176/appi.ajp.157.9.1475. [DOI] [PubMed] [Google Scholar]
- 41.Frazier JA, Giedd JN, Hamburger SD, et al. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- 42.Jacobsen LK, Giedd JN, Vaituzis AC, et al. Temporal lobe morphology in childhood-onset schizophrenia [published erratum appears in Am J Psychiatry. 1996;153:851] Am J Psychiatry. 1996;153:355–361. doi: 10.1176/ajp.153.3.355. [DOI] [PubMed] [Google Scholar]
- 43.Matsumoto H, Simmons A, Williams S, et al. Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am J Psychiatry. 2001;158:1299–1304. doi: 10.1176/appi.ajp.158.8.1299. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto H, Simmons A, Williams S, Pipe R, Murray R, Frangou S. Structural magnetic imaging of the hippocampus in early onset schizophrenia. Biol Psychiatry. 2001;49:824–831. doi: 10.1016/s0006-3223(01)01073-3. [DOI] [PubMed] [Google Scholar]
- 45.Rapoport JL, Castellanos FX, Gogate N, Janson K, Kohler S, Nelson P. Imaging normal and abnormal brain development: new perspectives for child psychiatry. Aust N Z J Psychiatry. 2001;35:272–281. doi: 10.1046/j.1440-1614.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 46.Luders E, Narr KL, Thompson PM, et al. Mapping cortical gray matter in the young adult brain: effects of gender. Neuroimage. 2005;26:493–501. doi: 10.1016/j.neuroimage.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Thompson PM, Mega MS, Vidal C, Rapoport J, Toga A. 2082 Philadelphia, PA: Springer-Verlag; 2001. Detecting disease specific patterns of brain structure using cortical pattern matching and a population-based probabilistic brain atlas. IEEE Conference on Information Processing in Medical Imaging (IPMI), UC Davis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson PM, Giedd JN, Woods RP, MacDonald D, Evans AC, Toga AW. Growth patterns in the developing brain detected by using continuum mechanical tensor maps. Nature. 2000;404:190–193. doi: 10.1038/35004593. [DOI] [PubMed] [Google Scholar]
- 49.Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal CN, Rapoport JL, Hayashi KM, et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 52.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 53.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 54.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 55.DeLisi LE, Tew W, Xie S, et al. A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry. 1995;38:349–360. doi: 10.1016/0006-3223(94)00376-e. [DOI] [PubMed] [Google Scholar]
- 56.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 57.Lieberman JA, Alvir JM, Koreen A, et al. Psychobiologic correlates of treatment response in schizophrenia. Neuropsychopharmacology. 1996;14(suppl):13S–21S. doi: 10.1016/0893-133X(95)00200-W. [DOI] [PubMed] [Google Scholar]
- 58.Cahn W, Pol HEH, Lems EBTE, et al. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- 59.Ho B-C, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 60.Gogate N, Giedd J, Janson K, Rapoport JL. Brain imaging in normal and abnormal brain development: new perspectives for child psychiatry. Clin Neurosci Res. 2001;1:283–290. doi: 10.1046/j.1440-1614.2001.00900.x. [DOI] [PubMed] [Google Scholar]
- 61.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 62.Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psycol Psychiatry. Nov 2006;34(1):30–36. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]
- 63.Narr KL, Thompson PM, Szeszko P, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 64.Kumra S, Jacobsen LK, Lenane M, et al. “Multidimensionally impaired disorder”: is it a variant of very early-onset schizophrenia? J Am Acad Child Adolesc Psychiatry. 1998;37:91–99. doi: 10.1097/00004583-199801000-00022. [DOI] [PubMed] [Google Scholar]
- 65.Towbin KE, Dykens EM, Pearson GS, Cohen DJ. Conceptualizing “borderline syndrome of childhood” and “childhood schizophrenia” as a developmental disorder. J Am Acad Child Adolesc Psychiatry. 1993;32:775–782. doi: 10.1097/00004583-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Nicolson R, Lenane M, Brookner F, et al. Children and adolescents with psychotic disorder not otherwise specified: a 2- to 8-year follow-up study. Compr Psychiatry. 2001;42:319–325. doi: 10.1053/comp.2001.24573. [DOI] [PubMed] [Google Scholar]
- 67.Gogtay N, Sporn A, Clasen LS, et al. Comparison of progressive cortical gray matter loss in childhood-onset schizophrenia with that in childhood-onset atypical psychoses. Arch Gen Psychiatry. 2004;61:17–22. doi: 10.1001/archpsyc.61.1.17. [DOI] [PubMed] [Google Scholar]
- 68.Gogtay N, Ordonez A, Herman D, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psycol Psychiatry. 2007;48:852–862. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 69.Tepest R, Wang L, Miller MI, Falkai P, Csernansky JG. Hippocampal deformities in the unaffected siblings of schizophrenia subjects. Biol Psychiatry. 2003;54:1234–1240. doi: 10.1016/s0006-3223(03)00702-9. [DOI] [PubMed] [Google Scholar]
- 70.Staal WG, Hulshoff Pol HE, Schnack HG, Hoogendoorn ML, Jellema K, Kahn RS. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am J Psychiatry. 2000;157:416–421. doi: 10.1176/appi.ajp.157.3.416. [DOI] [PubMed] [Google Scholar]
- 71.Cannon TD, van Erp TG, Huttunen M, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- 72.van Haren NE, Picchioni MM, McDonald C, et al. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biol Psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 73.Gogtay N, A S, Clasen LS, et al. Structural brain MRI abnormalities in healthy siblings of patients with childhood-onset schizophrenia. Am J Psychiatry. 2003;160:569–571. doi: 10.1176/appi.ajp.160.3.569. [DOI] [PubMed] [Google Scholar]
- 74.Gogtay N, Greenstein D, Lenane M, et al. Cortical brain development in non-psychotic siblings of patients with childhood onset schizophrenia. Arch Gen Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- 75.Bassett AS. Chromosomal aberrations and schizophrenia. Autosomes. Br J Psychiatry. 1992;161:323–334. doi: 10.1192/bjp.161.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demirhan O, Tastemir D. Chromosome aberrations in a schizophrenia population. Schizophr Res. 2003;65:1–7. doi: 10.1016/s0920-9964(02)00504-2. [DOI] [PubMed] [Google Scholar]
- 77.St George-Hyslop PH. Genetic factors in the genesis of Alzheimer's disease. Ann N Y Acad Sci. 2000;924:1–7. doi: 10.1111/j.1749-6632.2000.tb05552.x. [DOI] [PubMed] [Google Scholar]
- 78.Bishop DT. BRCA1 and BRCA2 and breast cancer incidence: a review. Ann Oncol. 1999;10(suppl 6):113–119. [PubMed] [Google Scholar]
- 79.Rapoport JC, Addington AM, Frangou S. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:614. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 80.Lewis DA, Pierri JN, Volk DW, Melchitzky DS, Woo TU. Altered GABA neurotransmission and prefrontal cortical dysfunction in schizophrenia. Biol Psychiatry. 1999;46:616–626. doi: 10.1016/s0006-3223(99)00061-x. [DOI] [PubMed] [Google Scholar]
- 81.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Greenstein D, Lerch J, Shaw P, et al. Childhood onset schizophrenia: cortical brain abnormalities as young adults. J Child Psychol Psychiatry. 2006;47:1003–1012. doi: 10.1111/j.1469-7610.2006.01658.x. [DOI] [PubMed] [Google Scholar]