Abstract
Objective: The limbic structures in early-onset schizophrenia-spectrum illness (SZ) and bipolar disorder (BPD) were studied to discern patterns associated with diagnosis and sex. Methods: Thirty-five youths with DSM-IV BPD without psychosis, 19 with BPD with psychosis, 20 with SZ, and 29 healthy controls (HC), similar in age (6-17 years) and sex, underwent structured and clinical interviews, neurological examination, and cognitive testing. Structural magnetic resonance images (MRIs) were acquired on a 1.5 Tesla, General Electric Signa Scanner. Differences in subcortical brain volumes, including the amygdala and hippocampus, were evaluated using two-way (diagnosis, sex) univariate analyses covarying for total cerebral volume and age. Results: Youth with SZ and BPD showed no differences in amygdala and hippocampal volumes. However, boys with SZ had smallest left amygdala and girls with BPD had the smallest left hippocampal volumes. In exploratory analyses, SZ showed reduced thalamic volumes bilaterally and both BPD groups had larger right nucleus accumbens (NA) volumes relative to HC. Conclusion: There were no limbic volumetric differences between BPD and SZ. However, there were diagnosis-by-sex interactions in the amygdala and hippocampus, structures that are rich in sex hormone receptors. In addition, smaller thalamus was associated with SZ while larger right NA volumes were most related to BPD. This study underscores the importance of assessing diagnostic effects and sex effects on the brain in future studies and provides evidence that boys and girls with SZ and BPD may have differential patterns of neuropathology associated with disease expression.
Keywords: mood disorders, psychosis, brain imaging technique, child psychiatry
Introduction
Schizophrenia (SZ) and bipolar disorder (BPD) both typically onset in late adolescence or early adulthood.1–4 Neurodevelopmental models of both disorders posit subtle disease processes that affect critical circuits in the brain early in development that then manifest in disease around the time of puberty or shortly thereafter.4–6 Not only is there increasing support for the neurodevelopmental model for both disorders but there is also emerging evidence suggesting that these conditions might share some genetic susceptibility.7,8 Gene environment–mediated pre- and perinatal brain abnormalities set in motion a series of processes that increasingly reveal themselves in a diverse set of phenotypic signs as the various brain systems required for their expression-rich maturity.9 Abnormal brain development at one stage may hinder normal maturation of later developing structures and their functions.10,11
There are numerous neuroimaging studies of individuals with adult onset of both BPD and SZ that have investigated abnormal neural circuitry. There are far fewer neuroimaging studies of individuals with early onset (<18 years). The early-onset presentation is particularly important to study because these youths are close to the time of pubertal change and relatively free of confounds, such as extensive treatment, duration of illness, and history of electroconvulsive therapy, that are known to affect the brain.12,13 Moreover, studying early-onset groups provides information about whether the neural abnormalities seen in youth are consistent with or different from the “adult” expression of these disorders. Prior neuroimaging studies in both adults and children implicate the frontal-limbic networks in both BPD and SZ.12,14–20 For example, abnormal volumes have been found in both the amygdala and the hippocampus in early- and adult-onset forms of BPD and SZ.12,14–16,18,20,21 However, reduced amygdala volume is more consistently reported in early-onset BPD than in SZ, and reduced hippocampal volume is more consistently reported in early-onset SZ than in BPD.12,13,16,20 Other subcortical structures that have been reported to be abnormal in early-onset BPD include the right putamen and the nucleus accumbens (NA).16,22,23 In early-onset SZ, neuroimaging studies have reported that the thalamus is reduced.20,24 To date, there are no studies that directly compare early-onset BPD with SZ. Such a design may elucidate the specific neural structures differentially associated with BPD and SZ and would address a major research question, the answer to which may provide clues to explaining the nature of the differences and overlap between the disorders.
Neuroimaging studies of youths, though relatively unaffected by the use of exogenous confounds that effect brain anatomy and function, are challenging due to the fact that there are ongoing developmental events that influence brain maturation including age, sex, and pubertal status which need to be taken into consideration.13 Puberty is a time during which there are normally robust progressive (eg, myelination) and regressive (e.g., synaptic pruning) processes taking place in the brain.25,26 Given the number of changes associated with this epoch, neuromaturational processes may be particularly vulnerable to abnormality. In fact, there is mounting evidence to underscore that adolescence, marked by normative pubertal maturation and brain changes, is a critical developmental period for onset of mood and psychotic symptoms.3 The peripubertal surge in sex hormones impacts not only bodily development and sexual differentiation but also brain development.27 Sex-specific differentiation of neural systems occurs in brain regions laden with sex hormone receptors and may be responsible for at least some of the normal sex differences seen in cognition and affect.2,28 The amygdala, which is rich in androgen receptors, and the hippocampus, which is rich in estrogen receptors, have been of particular interest in studies of SZ and BPD due to their central role in affecting regulation and memory, respectively.29,30 In peripubertal onset of SZ and BPD, those brain regions that are undergoing neuromaturation and reorganization and are influenced by sex hormones around the time of disorder onset may be most vulnerable to abnormality or disruption in development.31,32 Despite the importance of puberty and sexual dimorphism, there are few studies that assess brain-sex effects in adults with these disorders,31 and to our knowledge there are no such studies in early-onset BPD and SZ.
In order to address several important gaps in the literature, we set out to compare youths with early-onset BPD without psychosis and BPD with psychosis to those with SZ to discern if there would be findings in the amygdala and the hippocampus that would differentiate these disorders. Based on prior neuroimaging studies of adults and children with mood and psychotic disorders, we hypothesized that reduced amygdala volume would more reliably be associated with BPD and reduced hippocampal volume with SZ. In the exploratory analysis of other subcortical structures, we predicted, based on previous reports, that youths with BPD would have abnormal NA volumes22,23 and that youths with SZ would have reduced thalamic volumes.24,33 We also predicted that the BPD with psychosis group would share features with the BPD without psychosis and the primary psychotic disorder groups. Finally, given what is known about normative brain development and sexual dimorphism, we predicted that there would be diagnosis-by-sex interactions in subcortical structures rich in sex hormone receptors, particularly in the amygdala and hippocampus.28,29,31
METHOD
The research protocols were approved by both the McLean Hospital and Cambridge Health Alliance Institutional Review Boards. All subjects signed assent forms, and their parents/legal guardians signed informed consent forms.
Subjects
Subjects from 3 different ongoing neuroimaging studies, using identical methodologies, were combined for the purposes of this data analysis. Two studies enrolled children and adolescents with unmodified Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), bipolar disorder (BPD I) (marked by elevated/expansive or irritable mood, with clear episodes and meeting full duration criteria) and healthy controls (HCs), and the third study enrolled youths with psychotic disorders (DSM-IV BPD with psychotic features, SZ, and schizoaffective disorder) as well as HCs. Youths aged 6–17 years, both male and female, and inpatient and outpatient were recruited through the McLean Hospital and Cambridge Health Alliance programs and through professional and patient advocacy groups. HCs, with no DSM-IV Axis-I diagnosis on semistructured and clinical interviews, were recruited through local advertisements. Exclusion criteria in both groups included presence of major sensorimotor handicaps; full-scale IQ < 70; presence of documented learning disabilities; history of claustrophobia, autism, anorexia, bulimia nervosa, alcohol or drug dependence or abuse (in the 2 months prior to the scan or total past history of 12 months or greater); active medical or neurological disease; presence of metal fragments or implants; history of electroconvulsive therapy; and current pregnancy or lactation.
All children, including the HCs, underwent diagnostic semistructured (Kiddie Schedule for Affective Disorders and Schizophrenia: Epidemiologic Version—KSADS-E)34 and clinical interviews by board-certified child psychiatrists. Each child received a physical and neurological examination (including Tanner Staging: a I–V scale of pubertal development).35,36 Additionally, parents were administered an indirect KSADS-E regarding their children (see Frazier et al12 for more detail). All raters achieved a high degree of interrater reliability; the mean kappa was 0.9, and all disorders achieved kappa coefficients of >0.82. In the case of BPD, the diagnosis was made if full DSM-IV diagnostic criteria were met BPD I (lifetime) with all subjects having clear cycles and meeting duration criteria. The diagnosis of psychosis (either with BPD or SZ) was based on lifetime history. Socioeconomic status was assessed using the Hollingshead Scale. Onset of the disorder, number of episodes, and duration of illness were also obtained. Measures of psychopathology were obtained using the Mania Rating Scale (MRS),37,38 including the psychosis items, and the Global Assessment of Functioning (GAF).39
Of the bipolar subjects (bipolar with and bipolar without psychosis n = 56), 43 were included in prior publications; of the 29 HCs, 20 were included in previous publications.12,40 None of the data on the youths with SZ have been published before.
Drug Exposure
In an effort to control for medication confounds, antipsychotic doses were converted to chlorpromazine equivalents following the equivalency estimates provided by both Woods41 and Stoll.42 This variable was used in correlation analyses with those subcortical volumes that showed significant group differences.
Magnetic Resonance Imaging Protocol
Images were acquired at the McLean Hospital Brain Imaging Center on a 1.5 Tesla General Electric Signa Scanner. Further details about the acquisition and image analysis are detailed in Frazier et al.12 Briefly, structural acquisitions included a conventional T1-weighted sagittal scout series, a proton density/T2-weighted interleaved double-echo axial series, and a three-dimensional inversion recovery-prepped spoiled grass coronal series. All scans were reviewed by a neuroradiologist to rule out clinically significant abnormalities.
Image Analysis
The regions of interest (ROIs) in this study consisted of the amygdala and hippocampus with all other subcortical structures included in an exploratory way. These segmentations were performed according to the anatomic boundaries described in Filipek et al43 and Frazier et al.12 In brief, structural scans were transferred to the Center for Morphometric Analysis-Charlestown Massachusetts General Hospital and coded and catalogued for blind analysis. Imaging analysis was done by 2 raters on Sun Microsystems, Inc (Mountainview, CA) workstations using Cardviews software.44 The datasets were positionally normalized to overcome variations in head position and then segmented into gray, white, and cerebrospinal fluid (CSF) tissue classes. The segmentation method uses a semiautomated intensity contour algorithm for external border definition and signal intensity histogram distributions for delineation of gray-white borders. Total cerebral volume (TCV) was defined as all tissue in the cerebrum, including CSF, and excluded cerebellum and brain stem.
Subcortical Segmentation
Segmentation of the thalamus traced the trajectory of the hypothalamic fissure in the sagittal plane to separate the thalamus proper from the ventral diencephalon. The structure was bounded medially by the third ventricle and laterally by the internal capsule. The superior border was the body of the lateral ventricle, and the inferior border was the hypothalamic fissure.
The amygdala and hippocampus were defined as a continuous gray matter structure in the primary segmentation. The hippocampus was then separated from the amygdala at the rostral-coronal plane, where the hippocampus first appears. The segmentation of the amygdala was performed manually in its entirety. The cross-referencing capability of Cardviews was used to outline the amygdala in axial and sagittal views, allowing a reliable preliminary separation of the amygdala from surrounding gray structures. The anterior portion of the amygdala was segmented because it appears beneath the medial temporal cortex. The choroidal fissure was used as the superior border of the amygdala along with the gray-white matter contrast between the amygdala and the surrounding white matter. The lateral border was defined using the gray-white matter contrast between the amygdala and the surrounding temporal white matter and the gray-CSF contrast between the amygdala and temporal horn of the lateral ventricle. The medial borders consisted of the parahippocampal cortex, the brain exterior at the inferior lip of the choroidal fissure, and partially the hippocampus. Finally, the inferior border consisted of the gray-white matter contrast between the amygdala and the surrounding temporal white matter and the alveus of the hippocampus and temporal horn of the lateral ventricle.12
The caudate was measured in its entirety (head, body, tail superior to ventricular trigone, and ventral striatum), defined superomedially by the interface with the lateral ventricles, inferiorly by the interface with the adjacent rostral peduncle of the thalamus (when present), and otherwise by the interface with adjacent white matter; putamen was defined medially by the external medullary lamina of the globus pallidus, laterally by the external capsule, and otherwise by adjacent white matter; globus pallidus was defined superomedially by the interface with the internal capsule, inferiorly by the anterior commissure, ansa lenticularis, or nucleus basalis, when present, and laterally by the external medullary lamina.43 The NA was separated from putamen and caudate superiorly by a segmentation line that connects the inferiormost tip of the lateral ventricle to the most ventral point of the internal capsule at the level of the ventral putamen. From this last point, a vertical line is drawn to define the lateral border with the putamen.45
For interrater reliability measurements, 10 scans from our dataset were selected at random and blindly segmented by 2 raters. The standard interrater intraclass correlation coefficients (ICCs) for the ROIs were (left, right) amygdala (0.81, 0.83), thalamus (0.96, 0.96), hippocampus (0.96, 0.92), NA (0.79, 0.57), caudate (0.95, 0.93), putamen (0.80, 0.78), and globus pallidus (0.84, 0.77). In addition to ICCs, interrater reliabilities were estimated as the volume of voxels where the labels of each structure were in agreement as a ratio to the average volume of the structure between the raters, also known as the percentage common voxel agreement (PCVA). For the ROIs considered here, the PCVAs were (left, right) amygdala (79.8, 80.5), thalamus (90.3, 91.3), hippocampus (85.7, 86.7), NA (76.9, 72.9), caudate (92.2, 92.4), putamen (89.7, 90.2), and globus pallidus (84.5, 86.6). The 2 reliability measures are comparable, apart from the right NA, in which the lower ICCs are in part due to the small size of the structure (0.6 cm3) in relation to the difference in volumes between raters, which was 0.1 cm3.
Data Analyses
Due to sparse numbers of left-handed subjects, making stratified analyses difficult, only data from right-handed subjects involved in the studies were included in these analyses. Differences in right and left subcortical brain volumes were evaluated using 2-way (diagnosis, sex) univariate analyses covarying for TCV and age. Similar models were also evaluated on the asymmetry index for each structure, which was calculated as (right volume−left volume)/(right volume + left volume)÷ 2. Post hoc between-group tests were corrected for multiple comparisons using the Tukey-Cramer honestly significant difference method. Differences in demographic and clinical variables between groups were assessed using analyses of variance for continuous variables and chi-square tests for categorical variables. In addition, within-group Pearson and Spearman correlations were performed on clinical variables and those structures which were found to be significantly different between diagnostic groups. These clinical variables included MRS and GAF scores, age at onset of illness, duration of illness, and chlorpromazine equivalents. In an effort to be conservative, we report only clinical correlations that reached significance on both Spearman and Pearson tests; the r and P value for the Pearson correlations are reported. Effect sizes were calculated and interpreted using Cohen d statistic. All statistical tests were 2 sided with alpha = .05. JMP 7 for Mac (SAS Institute, Cary, NC) was used for statistical analysis.
Results
This analysis includes data from ongoing neuroimaging studies at our site; all available segmented image sets on children that met the diagnostic and age criteria were included: 35 youths with BPD I without psychosis (mean age = 10.4 ± 3.0 years), 19 with BPD I with psychosis (mean age = 11.6 ± 2.6 years), 20 with SZ or schizoaffective disorder (mean age = 13.5 ± 2.9 years), and 29 HCs (mean age = 10.5 ± 2.9 years). The proportion of males in each group ranged from 47.4% to 58.8%. Demographic and clinical characteristics are included in tables 1 and 2.
Table 1.
Characteristics of Youths With Bipolar Disorder (BPD) With and Without Psychosis, Schizophrenia Spectrum, and Healthy Controls (HCs)
| Characteristic | BPD Without Psychosis (n = 35) | BPD With Psychosis (n = 19) | Schizophrenia Spectrum (n = 20) | HC (n = 29) | Statistical Significance of Group Difference |
| Age | 10.4 ± 3.0 | 11.6 ± 2.6 | 13.5 ± 2.9 | 10.5 ± 2.9 | F = 5.82; P = .001 |
| Males (%) | 55.6 | 47.4 | 58.8 | 57.1 | NS |
| Caucasian (%) | 97.1 | 100 | 90.0 | 85.2 | NS |
| Prepubertal (%) | 63.6 | 47.4 | 35.0 | 53.6 | NS |
| Low SES (Hollingshead III–V) (%) | 48.6 | 47.4 | 66.7 | 24.1 | NS (P = .1) |
Note: NS, not significant; SES, socioeconomic status.
Table 2.
Clinical and Treatment Characteristics
| Characteristic | Bipolar Without Psychosis (n = 35) | Bipolar With Psychosis (n = 19) | Schizophrenia Spectrum (n = 20) | Healthy Controls (n = 29) | Statistical Significance of Group Difference |
| Global Assessment of Functioning | 52.9 ± 6.4 | 48.6 ± 4.3 | 47.8 ± 4.8 | 68.3 ± 2.6 | F = 91.2, P < .001 |
| Mania Rating Scale (MRS) | 20.3 ± 9.2 | 21.9 ± 9.9 | 14.5 ± 11.1 | 1.7 ± 3.4 | F = 22.2, P < .001 |
| MRS psychosis subscale | 2.1 ± 2.1 | 4.6 ± 4.1 | 6.8 ± 5.4 | 0.5 ± 1.6 | F = 11.8, P < .001 |
| Age of onset (y) | 5.9 ± 3.8 | 7.4 ± 4.0 | 8.4 ± 4.1 | N/A | F = 2.5, P = .09 |
| Duration of illness (years) | 2.4 ± 3.1 | 3.0 ± 2.75 | 4.2 ± 3.4 | N/A | NS |
| History of hospitalizations (%) | 33.3 | 63.2 | 61.1 | N/A | χ2 = 5.8, P = .06 |
| Age at first hospitalization (y) | 7.7 ± 4.1 | 7.9 ± 3.5 | 9.8 ± 3.7 | N/A | NS |
| Chlorpromazine equivalents at entry | 146.5 ± 102.0 | 149.3 ± 73.2 | 393.7 ± 362.2 | N/A | F = 7.3, P = .002 |
| No. of psychoactive medications at entryb | 2.0 ± 1.0 | 2.8 ± 1.1 | 2.4 ± 1.5 | N/A | F = 3.0, P = .06 |
| Presence of attention-deficit hyperactivity disorder (%) | 63.2 | 62.9 | 0 | N/A | NS |
Note: All values are mean ± standard deviation, unless otherwise indicated. N/A, not applicable; NS= not significant.
Includes atypical antipsychotics, antidepressants, sedatives, mood stabilizers, stimulants, lithium, and others.
All diagnostic groups were significantly ill as measured by the GAF and MRS. Most had a history of hospitalizations and were being treated with multiple medications (see table 3 for detailed list).
Table 3.
Proportion of Youths on Medications in the Diagnostic Groups
| Bipolar Without Psychosis (n = 35) | Bipolar With Psychosis (n = 19) | Schizophrenia Spectrum (n = 20) | |
| Lithium | 8.8 | 42.1 | 16.7 |
| Stimulants | 20.6 | 21.1 | 10.0 |
| Mood stabilizers | 41.2 | 47.4 | 22.2 |
| Antidepressants | 26.5 | 36.8 | 27.8 |
| Atypical antipsychotics | 73.5 | 84.2 | 94.4 |
| Sedatives | 0 | 10.5 | 5.6 |
| Others | 17.6 | 21.1 | 5.6 |
Volumetric Measurements
Means and SDs of the raw volumes for the subcortical structures are presented in table 4.
Table 4.
Mean Volumes (cm3) and SDs of Subcortical Structures
| Region | Bipolar Without Psychosis (n = 35) | Bipolar With Psychosis (n = 19) | Schizophrenia Spectrum (n = 20) | Healthy Controls (n = 29) |
| Right cerebrum | 575.39 ± 57.74 | 563.19 ± 35.18 | 598.75 ± 73.48 | 605.10 ± 34.64 |
| Left cerebrum | 578.47 ± 57.53 | 564.26 ± 35.72 | 603.09 ± 73.89 | 609.19 ± 34.84 |
| Right hippocampus | 3.67 ± 0.46 | 3.65 ± 0.44 | 3.96 ± 0.59 | 3.99 ± 0.45 |
| Left hippocampus | 3.58 ± 0.44 | 3.61 ± 0.41 | 3.96 ± 0.65 | 3.87 ± 0.40 |
| Right amygdala | 1.56 ± 0.35 | 1.65 ± 0.25 | 1.51 ± 0.29 | 1.63 ± 0.33 |
| Left amygdala | 1.58 ± 0.37 | 1.61 ± 0.34 | 1.50 ± 0.38 | 1.60 ± 0.39 |
| Right thalamus | 7.78 ± 0.79 | 7.88 ± 0.50 | 7.81 ± 0.77 | 8.05 ± 0.69 |
| Left thalamus | 7.68 ± 0.79 | 7.82 ± 0.38 | 7.79 ± 0.80 | 7.95 ± 0.73 |
| Right caudate | 4.15 ± 0.56 | 4.15 ± 0.53 | 4.12 ± 0.68 | 4.19 ± 0.53 |
| Left caudate | 4.03 ± 0.59 | 3.99 ± 0.41 | 4.06 ± 0.65 | 4.03 ± 0.52 |
| Right pallidus | 1.67 ± 0.26 | 1.73 ± 0.20 | 1.66 ± 0.31 | 1.69 ± 0.26 |
| Left pallidus | 1.73 ± 0.24 | 1.80 ± 0.19 | 1.77 ± 0.34 | 1.77 ± 0.24 |
| Right putamen | 5.34 ± 0.61 | 5.28 ± 0.51 | 5.25 ± 0.69 | 5.32 ± 0.68 |
| Left putamen | 5.28 ± 0.65 | 5.25 ± 0.54 | 5.16 ± 0.71 | 5.27 ± 0.67 |
| Right nucleus accumbens | 0.67 ± 0.16 | 0.65 ± 0.14 | 0.64 ± 0.17 | 0.60 ± 0.11 |
| Left nucleus accumbens | 0.68 ± 0.16 | 0.69 ± 0.11 | 0.67 ± 0.17 | 0.66 ± 0.10 |
Univariate linear models for the right and left sides of each structure are summarized in detail below.
Diagnostic Differences
Significant diagnostic differences were seen in the left and right cerebral volumes in interaction with sex (right: F3,93 = 2.9, P = .04; left: F3,93 = 3.1, P = .04). Post hoc comparisons showed that both bipolar groups (with and without psychosis) had significantly smaller left and right cerebral volumes than HCs; this difference was even more marked in the female BPD groups. The SZ group did not differ significantly from the other groups.
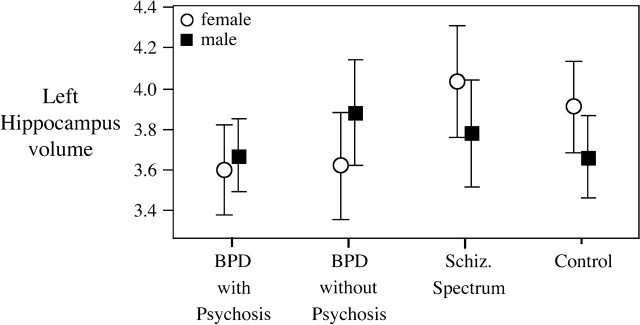
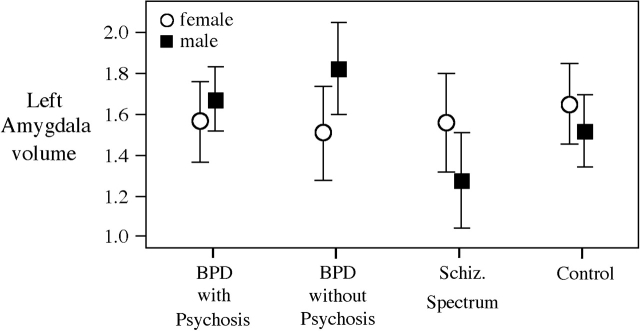
For the subcortical structures, the omnibus statistics showed no diagnostic differences in the hippocampus but did show a trend for diagnostic-by-sex differences in the left hippocampus (F3,93 = 2.3, P = .08); post hoc analyses showed that the diagnostic reduction was particularly marked in the female patient groups (please see figure 1; effect size range: 0.78–0.83). There were no between-group differences in the amygdala; however, there was significant diagnostic-by-sex interaction in the left amygdala (F3,93 = 3.0, P = .04). SZ males had the smallest left amygdala volume (effect size relative to other males = 0.65–1.23); this structure was actually enlarged relative to HC in the BPD groups, please see figure 2.
Fig. 1.
Diagnostic and Sex Differences in the Left Hippocampus. All figures plot mean volumes ± 95% confidence interval.
Fig. 2.
Diagnostic and Sex Differences in the Left Amygdala.
Exploratory analyses of other subcortical structures showed that youths with SZ had the smallest thalamic volumes, significantly so in the right (F3,93 = 3.1, P = .03) (see figure 3). The pairwise effect sizes for both hemispheres between the SZ group and the others were all moderate, suggesting overall diagnostic differences (Cohen d range: 0.64–0.74 [right]; 0.41–0.54 [left]). The right NA showed a significant diagnostic difference (F3,93 = 3.15, P = .03) and was found to be larger in both BPD groups relative to HC (effect sizes: 0.74–0.78); please see figure 3. Effects sizes between the BPD groups and SZ were 0.38 (right) and 0.42 (left), suggesting that there is a bilateral diagnostic difference in this structure.
Fig. 3.
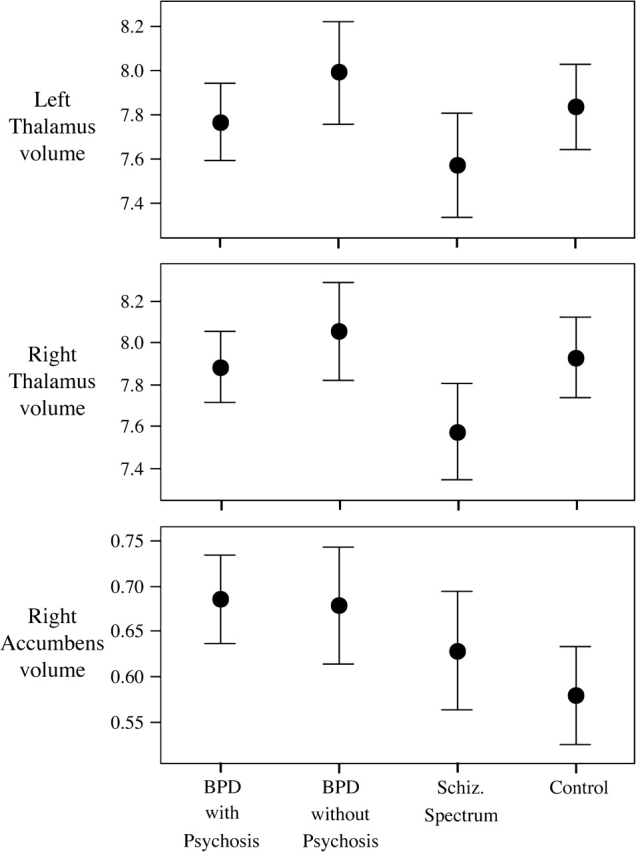
Diagnostic Differences in the Thalamus and Right Nucleus Accumbens.
The asymmetry indices for all structures also did not differ significantly between groups.
Sex Differences
Independently of diagnostic differences, significant sex differences were observed in bilateral cerebrum and pallidum volumes across groups, with females having significantly smaller volumes than males.
Clinical Correlates
HCs had increasing volumes with age in the thalamus (right: r = 0.38, P = .04; left: r = 0.36, P = .06). In addition, the right amygdala volume correlated with GAS scores in the HCs (r = 0.470, P = .01).
Youths with BPD without psychosis had a significant inverse correlation between the MRS score and amygdala volumes (right: r = –0.411, P = .02; left: r = –0.379, P = .004). No significant correlations were found in the BPD with psychosis group.
In the youths with SZ, there was a significant inverse correlation between GAS score and left amygdala volume (r = –0.634, P = .011). Also, there was a significant correlation between MRS scores and the right NA (r = 0.634, P = .03).
Discussion
This study investigated the volumetric differences in subcortical gray matter volumes, particularly the amygdala and the hippocampus, in early-onset BPD without psychosis, BPD with psychosis, and SZ in order to elucidate the diagnostic specificity of patterns of subcortical neural abnormality. In addition, this study evaluated diagnosis-by-sex interactions to understand the influence of sexual dimorphism on the limbic structures. We found that there were no volumetric differences between SZ and BPD in the amygdala and the hippocampus, which was not consistent with our a priori hypothesis. However, we found that youths with SZ had the smallest thalamic volumes bilaterally, with at least a moderate effect size when compared with the other 3 groups. In contrast, youths with BPD with and without psychosis had increased right and left NA volumes, with a moderate effect size compared with the other groups. Both the thalamic finding and the right NA finding were independent of sex effects. We also found that the youths with BPD with psychosis only shared findings with the BPD without psychosis and not with the primary psychotic disorder group. Taken collectively, these data suggest that specific subcortical brain regions are differentially affected in early-onset BPD and SZ, perhaps suggesting that these are disease-specific abnormalities. When diagnosis-by-sex interactions were evaluated, males with SZ had the smallest left amygdala and females with BPD had the smallest left hippocampus.
During normal brain development, there are clear patterns of growth that take place in limbic as well as other subcortical structures. For example, Giedd et al30 have reported that in healthy children, the left amygdala increases with age in males while the right hippocampus increases with age in females. In another study, school-age females were found to have larger hippocampal and smaller amygdala volumes, as a proportion of total cerebrum, than males.46 In our sample, we found that our HC girls had larger hippocampal volumes, but we did not find differences in the amygdala. The lack of an amygdala finding in our sample may in part be due to the fact that our sample included both children and adolescents. Finally, De Bellis et al47noted significant sex-by-age interactions for cerebral gray matter volumes (including both cortical and subcortical structures) in a sample of children and adolescents and that males had more prominent age-related gray matter decreases than females. In addition, these investigators found significant sex-by-Tanner stage (a measure of pubertal maturation) interactions in gray matter which supports the idea that hormones influence brain maturation and that they may play a role in abnormal brain structure and function in psychiatric illness.35,36,47 Such normative influences help lay the foundation for a neurodevelopmental model that associates puberty with onset of illness in BPD and SZ.
Anatomic neuroimaging studies of adults and children with both BPD and SZ generally have implicated frontal-limbic network abnormalities in both disorders.12,14,15,18 Although the limbic findings across studies are mixed, the most consistently reported finding in early-onset BPD is a reduced amygdala volume16 and in early-onset SZ, a decreased hippocampal volume.20,48 However, at least one study in early-onset SZ did not find a reduction in the hippocampal volumes but found that duration of illness negatively correlated with this structure, suggesting that a volumetric difference might be revealed with disease progression.49 In addition, there have been studies that have found a reduction in the hippocampus in BPD12,21,50 and a smaller amygdala in SZ.15,18 We did not find hippocampal or amygdalar volumetric differences in our study in either the SZ or the BPD groups. However, we did find that amygdala volumes in the BPD without psychosis group correlated inversely with the MRS, which suggests the involvement of this structure in the clinical phenotype. For example, higher MRS scores were associated with decreased right and left amygdala volumes in the BPD without psychosis group. In addition, we found that the right amygdala volume correlated with GAF scores in the SZ group. The mixed findings in these reports in the amygdala and hippocampus may be due to a number of factors, but given the findings in this study, age range of the sample, age of onset, and sample size need to be considered.
In our exploratory analyses of the other subcortical structure volumes for differences by diagnosis, we found decreased thalamic volumes bilaterally in SZ and not in BPD and an increased NA in BPD and not in SZ. These findings are consistent with prior reports. For example, volumetric studies of early-onset BPD have not found differences in the thalamus,16,51 whereas studies of SZ have reported a reduced size of this structure.24,33 The NA volume has been reported to be abnormal in early-onset BPD.22,23 To our knowledge, these are the only 2 anatomic neuroimaging studies in youths with BPD that have reported on this structure, which is of interest due to its role in the reward and motivation systems. Additionally, in one functional magnetic resonance imaging study, researchers found abnormal activity in a number of brain regions including the NA.52,53 Of note, we did find a correlation between MRS scores and the right NA in the SZ-spectrum disordered youth, which may provide further support for the role of the NA in manic symptoms regardless of diagnosis. In sharp contrast, studies in early-onset SZ have not found any difference in the NA.54 Our finding of a reduced thalamic volume in SZ and not BPD suggests that the thalamus may be more intimately involved in the SZ phenotype. This is of significant interest when exploring the neural circuits of SZ given that the thalamus is a central filter in the brain that helps individuals to appropriately process sensory information and integrate activity among forebrain regions. Finally, the increased NA in both the BPD without psychosis and the BPD with psychosis and not in the group with SZ suggests that this finding may be relatively specific to bipolar illness processes.
We assessed diagnosis-by-sex interactions in the limbic structures due to their involvement in affect and cognition and due to the fact that they are structures with a high concentration of sex hormone receptors. We found that males with SZ had smaller left amygdala volumes relative to other groups and that females with BPD had the smallest left hippocampus. These findings suggest that the proportion of males to females in anatomic neuroimaging studies is of critical importance and highlights the fact that the mixed findings in the limbic structures in prior reports in both SZ and BPD may be in part due to the sex ratio of the subjects included. These diagnosis-by-sex interactions in the amygdala and hippocampus may be secondary to abnormal hormone levels in males with SZ and females with BPD at critical developmental junctures or could be due to abnormal densities or function of the androgen receptors in the amygdala or estrogen receptors in the hippocampus and/or their interaction with stress, given the density of glucocorticoid receptors in both these structures.29 These structural abnormalities may account for some of the clinical differences seen in males and females affected by BPD or SZ.55–57
To our knowledge this is the first study to compare youths who suffer from BPD without psychosis, BPD with psychosis, and those with SZ. Strengths of the study include the well-characterized sample, the state of the art morphometric analysis, and the sufficient numbers of subjects to analyze limbic volume differences between groups and by sex. The limitations of this study include the fact that the NA, a structure with significant differences between psychiatric groups, is a structure that is small and difficult to measure. Our interrater reliabilities for this structure were not as strong as those of the other subcortical structures and thus may have attenuated power to detect reliable group differences. However, it is unlikely that the weaker reliability for this structure is responsible for the observed group differences because the image analysts were blind to diagnosis and any inconsistencies in measuring the NA would be expected to occur in all groups with equal probability. Another limitation of these data is that they are cross-sectional in nature making them vulnerable to cohort effects. We have learned from prior work that the longitudinal design is ultimately the most informative about the evolution of brain changes in healthy children and in early-onset psychiatric illness.29,58–63
However, these data do lay the foundation for future assessments of the trajectory of these brain changes in a longitudinal design. Such assessments are critical to tease out the complex interaction between normal developmental processes and the expression of disease, particularly in networks known to be involved in affect regulation, thought, and behavior.
Just as importantly, future studies should assess for sexually dimorphic abnormalities and their independent and/or interactive trajectories.63 De Bellis et al47 found sex-by-Tanner stage interactions in the brains of healthy children, and our data highlight that boys and girls with BPD and SZ may move to the expression of disease via divergent paths. Although the evaluation of the impact of pubertal status would yield even more information regarding the hormonal influences on brain anatomy peripubertally by sex, we could not pursue this line of inquiry here due to the relatively small numbers in our BPD with psychosis and SZ groups. Future investigations should include assessments of the association of pubertal status (and perhaps sex hormone levels), sex, and diagnosis to elucidate the various brain changes that occur with maturation and hormonal change. Ultimately, these methodologic approaches would highlight the important developmental periods during which brain changes emerge in these disorders and will contribute to determining the degree of continuity and etiological comparability between sex-specific adolescent and adult forms of disorder. Finally, such information may enhance our knowledge about critical periods during which interventions might be most helpful to prevent illness onset and progression for these diagnoses, perhaps in a sex-specific way.
Funding
This work was supported by research grants from the National Institute of Mental Health (K08 MH01573 to JAF and K01 MH01798 to CMM) as well as Private Family Foundation funds.
References
- 1.Lieberman JA, Perkins DO, Jarskog LF. Neuroprotection: a therapeutic strategy to prevent deterioration associated with schizophrenia. CNS Spectr. 2007;12:1–13. doi: 10.1017/s1092852900025906. [DOI] [PubMed] [Google Scholar]
- 2.Stevens JR. Schizophrenia: reproductive hormones and the brain. Am J Psychiatry. 2002;159:713–719. doi: 10.1176/appi.ajp.159.5.713. [DOI] [PubMed] [Google Scholar]
- 3.Walker E, Bollini AM. Pubertal neurodevelopment and the emergence of psychotic symptoms. Schizophr Res. 2002;54:17–23. doi: 10.1016/s0920-9964(01)00347-4. [DOI] [PubMed] [Google Scholar]
- 4.Blumberg HP, Kaufman J, Martin A, Charney DS, Krystal JH, Peterson BS. Significance of adolescent neurodevelopment for the neural circuitry of bipolar disorder. Ann N Y Acad Sci. 2004;1021:376–383. doi: 10.1196/annals.1308.048. [DOI] [PubMed] [Google Scholar]
- 5.Pantelis C, Yucel M, Wood SJ, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 6.Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: following a trail of evidence from cradle to grave. Dev Psychopathol. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- 7.Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32:9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14:47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 9.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71:405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Courchesne E, Yeung-Courchesne R, Egaas B. Methodology in neuroanatomic measurement. Neurology. 1994;44:203–208. doi: 10.1212/wnl.44.2.203. [DOI] [PubMed] [Google Scholar]
- 11.Steingard RJ, Schmidt C, Coyle JT. Basic neuroscience: critical issues for understanding psychiatric disorders. Adolesc Med. 1998;9:205–215. [PubMed] [Google Scholar]
- 12.Frazier JA, Chiu S, Breeze JL, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 13.Frazier JA, Ahn MS, DeJong S, Bent EK, Breeze JL, Giuliano AJ. Magnetic resonance imaging studies in early-onset bipolar disorder: a critical review. Harv Rev Psychiatry. 2005;13:125–140. doi: 10.1080/10673220591003597. [DOI] [PubMed] [Google Scholar]
- 14.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10:105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- 15.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 16.DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 17.Blumberg HP, Martin A, Kaufman J, et al. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- 18.Wright IC, Rabe-Hesketh S, Woodruff PW, David RS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 19.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 20.Frazier JA, Giedd JN, Hamburger SD, et al. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996;53:617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- 21.Blumberg HP, Kaufman J, Martin A, et al. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- 22.Ahn MS, Breeze JL, Makris N, et al. Anatomic brain magnetic resonance imaging of the basal ganglia in pediatric bipolar disorder. doi: 10.1016/j.jad.2007.04.015. [published online ahead of print May 25, 2007]. J Affect Disord. 2007. [DOI] [PubMed] [Google Scholar]
- 23.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–741. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 24.Kumra S, Giedd JN, Vaituzis AC, et al. Childhood-onset psychotic disorders: magnetic resonance imaging of volumetric differences in brain structure. Am J Psychiatry. 2000;157:1467–1474. doi: 10.1176/appi.ajp.157.9.1467. [DOI] [PubMed] [Google Scholar]
- 25.Yakolev PI, Lecours AR. The myclogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford, UK: Blackwell; 1967. pp. 3–70. [Google Scholar]
- 26.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg L. Risk taking in adolescence: what changes, and why? Ann N Y Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein JM, Seidman LJ, Horton NJ, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 29.Giedd JN, Clasen LS, Lenroot R, et al. Puberty-related influences on brain development. Mol Cell Endocrinol. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Giedd JN, Vaituzis AC, Hamburger SD, et al. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Goldstein JM, Seidman LJ, O'Brien LM, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- 32.Frazier JA, Alaghband-Rad J, Jacobsen L, et al. Pubertal development and onset of psychosis in childhood onset schizophrenia. Psychiatry Res. 1997;70:1–7. doi: 10.1016/s0165-1781(97)03062-x. [DOI] [PubMed] [Google Scholar]
- 33.McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 34.Orvaschel H, Puig-Antich J. Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic 4th Version. Fort Lauderdale, Fla: Center for Psychological Study, Nova University; 1987. [Google Scholar]
- 35.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fristad MA, Weller RA, Weller EB. The Mania Rating Scale (MRS): further reliability and validity studies with children. Ann Clin Psychiatry. 1995;7:127–132. doi: 10.3109/10401239509149039. [DOI] [PubMed] [Google Scholar]
- 38.Azorin JM, Kaladjian A, Akiskal HS, et al. Validation of a severity threshold for the Mania Rating Scale: a receiver-operating characteristic analysis. Psychopathology. 2007;40:453–460. doi: 10.1159/000107430. [DOI] [PubMed] [Google Scholar]
- 39.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 40.Frazier JA, Breeze JL, Makris N, et al. Cortical gray matter differences identified by structural magnetic resonance imaging in pediatric bipolar disorder. Bipolar Disord. 2005;7:555–569. doi: 10.1111/j.1399-5618.2005.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 42.Stoll AL. The Psychopharmacology Reference Card. 2001 [Google Scholar]
- 43.Filipek PA, Richelme C, Kennedy DN, Caviness VS., Jr The young adult human brain: an MRI-based morphometric study. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 44.Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: a volumetric analysis based on magnetic resonance images. Cereb Cortex. 1996;6:726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- 45.Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- 46.Caviness VS, Kennedy DN, Bates JF, Makris N. In: Thatcher RW, Lyon GR, and Krasnegor N, eds. Developmental Neuroimaging. The developing human brain: a morphometric profile. Mapping the Development of Brain and Behavior. New York: Academic Press;1996:3–14. [Google Scholar]
- 47.De Bellis MD, Keshavan MS, Beers SR, et al. Sex differences in brain maturation during childhood and adolescence. Cereb Cortex. 2001;11:552–557. doi: 10.1093/cercor/11.6.552. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen LK, Rapoport JL. Research update: childhood-onset schizophrenia: implications of clinical and neurobiological research. J Child Psychol Psychiatry. 1998;39:101–113. [PubMed] [Google Scholar]
- 49.Matsumoto H, Simmons A, Williams S, Pipe R, Murray R, Frangou S. Structural magnetic imaging of the hippocampus in early onset schizophrenia. Biol Psychiatry. 2001;49:824–831. doi: 10.1016/s0006-3223(01)01073-3. [DOI] [PubMed] [Google Scholar]
- 50.Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res. 2001;106:25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 51.Monkul ES, Nicoletti MA, Spence D, et al. MRI study of thalamus volumes in juvenile patients with bipolar disorder. Depress Anxiety. 2006;23(6):347–352. doi: 10.1002/da.20161. [DOI] [PubMed] [Google Scholar]
- 52.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- 53.Rich BA, Vinton DT, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci USA. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ballmaier M, Toga AW, Siddarth P, et al. Thought disorder and nucleus accumbens in childhood: a structural MRI study. Psychiatry Res. 2004;130:43–55. doi: 10.1016/j.pscychresns.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Castle D, Sham P, Murray R. Differences in distribution of ages of onset in males and females with schizophrenia. Schizophr Res. 1998;33:179–183. doi: 10.1016/s0920-9964(98)00070-x. [DOI] [PubMed] [Google Scholar]
- 56.Leibenluft E. Issues in the treatment of women with bipolar illness. J Clin Psychiatry. 1997;58(suppl 15):5–11. [PubMed] [Google Scholar]
- 57.Galdos P, van Os J. Gender, psychopathology, and development: from puberty to early adulthood. Schizophr Res. 1995;14:105–112. doi: 10.1016/0920-9964(94)00020-9. [DOI] [PubMed] [Google Scholar]
- 58.Nugent TF, 3rd, Herman DH, Ordonez A, et al. Dynamic mapping of hippocampal development in childhood onset schizophrenia. Schizophr Res. 2007;90:62–70. doi: 10.1016/j.schres.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 59.Vidal CN, Rapoport JL, Hayashi KM, et al. Dynamically spreading frontal and cingulate deficits mapped in adolescents with schizophrenia. Arch Gen Psychiatry. 2006;63:25–34. doi: 10.1001/archpsyc.63.1.25. [DOI] [PubMed] [Google Scholar]
- 60.Thompson PM, Vidal C, Giedd JN, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 62.Rapoport JL, Giedd J, Kumra S, et al. Childhood-onset schizophrenia. Progressive ventricular change during adolescence. Arch Gen Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- 63.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36:1065–1073. doi: 10.1016/j.neuroimage.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]