Abstract
Cognitive adaptation training (CAT) is a psychosocial treatment that uses environmental supports such as signs, checklists, alarms, and the organization of belongings to cue and sequence adaptive behaviors in the home. Ninety-five outpatients with schizophrenia (structured clinical interview for diagnosis, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) were randomly assigned to (1) Full-CAT (CAT focused on many aspects of community adaptation including grooming, care of living quarters, leisure skills, social and role performance, and medication adherence), (2) Pharm-CAT (CAT focused only on medication and appointment adherence), or (3) treatment as usual (TAU). Treatment lasted for 9 months, and patients were followed for 6 months after the withdrawal of home visits. Medication adherence (assessed during unannounced, in-home pill counts) and functional outcomes were assessed at 3-month intervals. Results of mixed-effects regression models indicated that both CAT and Pharm-CAT treatments were superior to TAU for improving adherence to prescribed medication (P < .0001). Effects on medication adherence remained significant when home visits were withdrawn. Full-CAT treatment improved functional outcome relative to Pharm-CAT and TAU (P < .0001). However, differences for functional outcome across groups decreased following the withdrawal of home visits and were no longer statistically significant at the 6-month follow-up. Survival time to relapse or significant exacerbation was significantly longer in both CAT and Pharm-CAT in comparison to TAU (.004). Findings indicate that supports targeting medication adherence can improve and maintain this behavior. Comprehensive supports targeting multiple domains of functioning are necessary to improve functional outcomes. Maintenance of gains in functional outcome may require some form of continued intervention.
Keywords: medication adherence, cognitive deficits, cognitive rehabilitation, medication compliance, cognitive adaptation training, environmental supports
Introduction
Psychosocial treatments designed to remediate or to bypass cognitive impairments are important to pursue in maximizing outcomes for individuals with schizophrenia.1–7 Cognitive adaptation training (CAT) is an intervention that utilizes compensatory strategies and supports such as pill containers with alarms, organization of belongings, and activity checklists to prompt and sequence adaptive behaviors in an individual's home environment.2,8,9 CAT strategies are tailored to the specific cognitive impairments and behavioral approaches to goal-directed activity exhibited by each participant. These customized strategies have been found to decrease levels of symptomatology and to improve social and occupational functioning for individuals with schizophrenia.2,9 While the entire package of environmental supports provided in CAT has been found to improve community outcomes, it is important to demonstrate that individualized supports targeting specific behavioral outcomes are able to improve those specific behaviors.
Arguably, one of the most important behavioral targets for individuals with schizophrenia is adherence to antipsychotic medications. Medications may help to form a foundation upon which the process of functional recovery can proceed. The role of antipsychotic medications in preventing symptom exacerbation and rehospitalization has been firmly established.10 Even small gaps in the time during which medication is available to patients can increase the risk of hospitalization.11 More than 50% of patients with schizophrenia do not take medications as prescribed.12–18 The adherence problem contributes substantially to poor outcomes and high health care costs for individuals with this diagnosis.
Using environmental supports to cue and reinforce taking medication have been found to be among the most effective strategies for individuals with physical illnesses.19–22 CAT provides a way to customize these supports directed at adherence behavior for individuals with schizophrenia.
In the present study, we investigated the efficacy of environmental supports for adherence to antipsychotic medications and functional outcome in patients with schizophrenia. We hypothesized that both Full-CAT (CAT focused on many aspects of community adaptation including grooming, independent living skills, social and role performance, and medication adherence) and Pharm-CAT (a subset of CAT treatment in which participants received environmental supports individually tailored for cognitive impairments and overt behaviors that were focused only on medication and appointment adherence) would improve adherence to antipsychotic medication as compared with a group receiving only standard outpatient medication follow-up. Moreover, because medication alone is not enough to substantially improve functional outcomes for individuals with schizophrenia, we hypothesized that functional outcomes would be better for participants in Full-CAT treatment in comparison to those in the other treatment groups. Secondary hypotheses included that patients in both CAT and Pharm-CAT would demonstrate lower levels of symptomatology and decreased rates of relapse relative to those in standard treatment.
METHODS
Study Design
Subjects were outpatients with a diagnosis of schizophrenia or schizoaffective disorder who were being seen at a community mental health center for medication follow-up. Participants received a baseline assessment and were then randomized into one of 3 treatment conditions for a period of 9 months: (1) Full-CAT treatment, (2) Pharm-CAT treatment (CAT treatment addressing only issues of medication and appointment adherence), and (3) treatment as usual (TAU)—no additional treatment other than standard medication follow-up provided by the community outpatient clinic. Randomization was stratified by recruitment site (hospital vs community clinic), gender, and age.
Treatment lasted for 9 months, and then subjects were followed for an additional 6 months after the end of formal treatment. Note that environmental supports (eg, signs, pill containers) in CAT and Pharm-CAT were not taken away at the end of treatment visits.
Assessments of symptomatology and functioning were conducted at baseline and every 3 months. Medication adherence was assessed each 3 months following randomization to treatment (no baseline).
Subjects
Subjects were outpatients recruited in 2 streams. Ninety-nine were recruited at the time of discharge from an inpatient psychiatric facility and followed for 3 months after discharge prior to baseline assessments and randomization into treatment. Fifty-seven were recruited as outpatients from one of 3 public clinics run by the same mental health authority. As shown in figure 1, of the 156 patients signing consent, 105 were randomized. Of these 105, there were 95 subjects with baseline and follow-up data for data analyses. Reasons for drops are listed in figure 1.
Fig. 1.
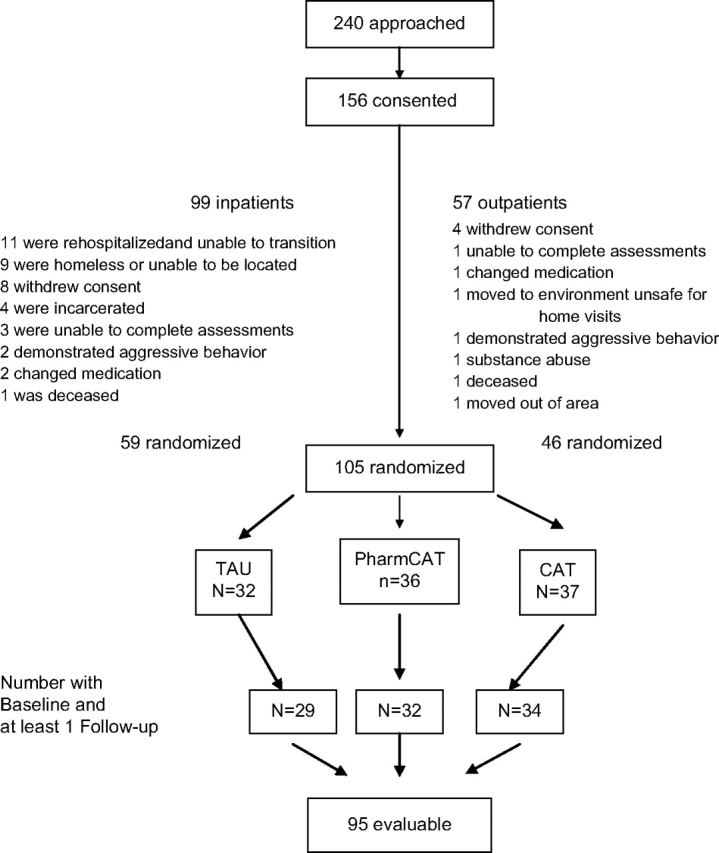
Subject Recruitment and Retention.
Subjects were identified through chart reviews by research staff credentialed at participating sites in accordance with Health Insurance Portability and Accountability Act requirements. All participants signed a written consent form approved by an Institutional Review Board, and procedures were consistent with internationally recognized standards for ethical conduct of human research.
Diagnoses were confirmed utilizing the Structured Clinical Interview for Diagnosis.23 In addition to meeting diagnostic criteria, subjects were required to (1) be between the ages of 18 and 60 years, (2) be receiving treatment with an oral atypical antipsychotic with the treating physician's recommendation to continue the medication and participate in follow-up at the Center for Health Care Services, (3) have primary responsibility for taking their own medications, (4) have a stable residence, and (5) be able to understand and complete rating scales and neuropsychological testing. Individuals were excluded if they were on clozapine or depot medication, had a documented history of significant head trauma, seizure disorder, or mental retardation, had a history of substance abuse or dependence in the past month, or had a history of violence in the past 6 months.
Fifty-four subjects were male, and 41 were female. Thirty-five were Hispanic, 35 were Anglo, 20 were African American, and 5 were from other or mixed ethnic groups. Mean age of participants was 39.0 years (SD = 10.7). Seventy-five percent of patients (n = 71) were on risperidone or olanzapine with the remaining 25% on aripiprazole (n = 9) or a combination of 2 antipsychotics (n = 15). Just over 70% of patients (n = 67) were on concomitant medications for side effects, mood, or anxiety. At baseline, symptoms of psychosis as rated from the Brief Psychiatric Rating Scale (BPRS) psychosis factor were in the mild range on average (M = 2.59; SD = 1.38). There were no significant differences in demographics for participants not making it to randomization vs those randomized to treatment (all P′s > .20). There were no differences in baseline variables for participants in the 2 different recruitment streams (all P′s > .20) suggesting that by the time of randomization, the outpatients recruited from the inpatient site were similar to those recruited from the outpatient clinics on baseline measures.
Treatment Groups
Cognitive Adaptation Training
CAT is a series of manual-driven compensatory strategies and environmental supports designed to improve multiple domains of adaptive functioning including adherence to medication, grooming, and activities of daily living in patients with schizophrenia.2,8,9,24,25 Environmental supports in Full-CAT treatment were based upon a comprehensive assessment of neurocognitive function, behavior, adaptive functioning, and the environment. Interventions for each functional deficit were based on 2 dimensions, (1) level of impairment in executive functions (as determined by scores on a set of neurocognitive tests) and (2) whether the overt behavior of the individual during performance of goal-directed activity was characterized more by apathy (poverty of speech and movement and the inability to initiate and follow through on behavioral sequences), disinhibition (distractibility and behavior which is highly cue driven), or a combination of these styles (based upon scores from the Frontal Systems Behavior Scale).26–28
According to the CAT model, individuals with poor executive functioning need high levels of structure and more obviously placed environmental cues, while those with somewhat better executive functioning need less structure and more subtle cues.2 Individuals with apathetic behavior benefit from environmental supports that cue and sequence behavior, those with disinhibition benefit most from the removal of distracting stimuli, and those with mixed behavior benefit from a combination of these strategies. Assessment results yield one of 6 CAT classifications for which interventions can be targeted (ie, apathy/poor executive function, apathy/fair executive function; disinhibited/poor executive function, disinhibited/fair executive function; mixed/poor executive function; mixed/fair executive function).
Once an individual's CAT classification was determined, strategies for specific functional problems (medication adherence, laundry, and leisure activity) were chosen from the manual. CAT interventions were established, trained, and maintained in the home during weekly visits from a CAT therapist/trainer.
Problems with dressing/grooming and taking medication are described, and examples of CAT interventions for each of these problems are presented in table 1 below. Examples describe interventions for some of the 6 possible CAT classifications.
Table 1.
Problems in Adaptive Function and Examples of Cognitive Adaptation Training (CAT) Interventions
| General Problem and CAT Classification Based on Comprehensive Assessment | Specific Behavior | CAT Interventions |
| Dressing disinhibited behavior—poor executive functioning | Wears 3 shirts at once because they are hanging in the closet and wears clothing that is inappropriate for the weather (eg, jackets in 90° heat). | Place complete outfits (shirt, pants, underwear, socks) in separate plastic containers labeled with the day of the week. No clothes are hanging in the closet so there is no cue to put on additional clothing. Remove clothing inappropriate for the weather to be stored elsewhere. |
| Grooming apathetic behavior—poor executive functioning | Unkempt appearance, shirt not tucked in, hair not combed, etc. Difficulty initiating each step in a multiple step task. | Place a full-length mirror in an obvious location. Attach a comb to the mirror with a string and a sign, “Is my hair combed? Is my shirt tucked in?” |
| Taking medication disinhibited behavior—fair executive functioninga | Takes too much medication, uses old medications, and combines them with over-the-counter medications without checking with physician. | Clear out all old prescriptions and over-the-counter medications to be stored elsewhere (eg, in a container under the bed). Place a sign on the box “Do not take without checking with Dr X at 555-5555.” Assist patient in placing current medicines in daily use containers, labeled with date and time. |
| Apathetic behavior—fair executive functioning | Forgets to take medication. Would not get out of bed to take AM medications and misses 50% of prescribed doses due to this. | Use a voice alarm recorded in the patient's own voice reminding “It's time to take medication.” If no children are present or visit, place AM medications on a stand by the bed with bottled water. |
These interventions are available in both Full-CAT (CAT focused on many aspects of community adaptation including grooming, care of living quarters, leisure skills, social and role performance, and medication adherence) and Pharm-CAT (CAT focused only on medication and appointment adherence).
Pharm-CAT
Pharm-CAT, a subset of the Full-CAT program, is a manual-driven treatment utilizing environmental supports such as signs, checklists, and electronic cuing devices to improve medication and treatment adherence. Prior to treatment, patients received the same set of assessments as those in Full-CAT. Interventions in Pharm-CAT are individualized in the same manner as those in Full-CAT treatment. However, only interventions that specifically target adherence are used. Additional issues such as transportation are addressed only if they relate to taking medication (eg, picking up prescriptions at a pharmacy) or making it to clinic appointments (eg, bus passes).
Patients in Full-CAT and Pharm-CAT were seen once weekly for 30–45 minutes. Visits in Pharm-CAT were necessarily shorter in duration because the focus of treatment was circumscribed around the issue of adherence.
CAT and Pharm-CAT therapists were individuals with bachelor's or master's degrees in psychology or related fields trained using a combination of didactic and in vivo strategies. Fidelity checks to ensure therapist adherence to the model were made using the CAT fidelity scale (available from D.I.V.) by an independent CAT specialist who reviewed 30% of tape recordings of home visits and made home visits to examine the extent to which the supports established were indicated. Scores range from 0 to 100. Mean fidelity scores for a total of 18 therapists (trained in CAT and Pharm-CAT) were 90.66 (SD = 5.14) with a range from 75.15 to 98.0.
Assessments
Diagnosis
The Structured Clinical Interview for the Diagnostic and Statistical Manual for Mental Disorders was utilized to make Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, diagnoses. Prior to administering this interview, all raters were trained to a reliability of 0.95 kappa statistic for a diagnosis of schizophrenia or schizoaffective disorder vs all other diagnoses.
Medication Adherence
Our primary measure was percent adherence derived from unannounced pill counts conducted in participants' homes twice during each 3 months during the study. Asking patients to bring pills into clinic appointments biases data by collecting of more complete data on subjects with greater adherence.29 The researcher making the visit requested that he or she be given all pills available to be counted. An adherence percent was (the number of pills missing from the bottle and assumed taken/number of pills prescribed) × 100. Assessment of medication adherence using unannounced, in-home pill counts is innovative in schizophrenia research. The method required some initial preparation including asking patients to save all empty medication bottles (providing a sign and empty box) and bagging and stapling old bottles of medication so that the researcher would know when pills were taken from multiple bottles. Specific procedures to address multiple bottles, samples of medication, and other problems associated with pill counts are available from D.I.V. Pill counts could not be blinded due to the obvious nature of environmental supports.
Pharmacy records were examined as a secondary measure of adherence that would not be impacted by the obvious nature of environmental supports in the participants' homes. Pharmacy percent adherence [(number of pills supplied/number days in period) × 100] was calculated. Pharmacy records that overlapped assessment periods were included in the assessment period in which the majority of prescription days fell.
Symptom, Relapse, and Functional Assessment
Symptom and functional assessments were administered by bachelor's, master's, or doctoral level psychologists required to reach a criterion of 0.80 intraclass correlation coefficient on a combination of video-recorded and live interviews for each assessment prior to making ratings. In addition, all raters were observed administering multiple assessments to ensure standardized and competent administration of all scales. Checks on rater competency were conducted throughout the study, and regular meetings were held to prevent rater/scorer drift.30
Symptomatology
Symptoms were assessed using the expanded version of the BPRS.31 The psychosis factor score, calculated as the mean of items assessing hallucinations, unusual thought content, suspiciousness, and conceptual disorganization, was utilized as a measure of positive symptoms.32,33 Higher scores indicate higher levels of symptomatology.
Relapse
Hospital use is influenced by social, economic, administrative, and service delivery issues.34,35 Therefore, based upon the work of Nuechterlein et al36 and Schooler,35 we developed an index for relapse in remitted/partially remitted patients. For subjects to be counted as at least partially remitted, scores on 3 of the 4 BPRS psychosis items had to be 4 or lower indicating moderate symptoms only. A relapse was scored if scores on any of the 4 items increased a minimum of 2 points to a score of 5 or higher, if the patient was suicidal, if the patient was hospitalized, or if the patient was unable to care for themselves without continual supervision.
Functional Outcome
Global functioning was assessed using the Social and Occupational Functioning Scale (SOFAS).23 This instrument assesses the overall level of functioning on a scale from 1 to 100 based upon social, school, and work functioning. Higher scores indicate better adaptive function. The SOFAS score was based upon all information obtained about adaptive and social functioning during a lengthy semistructured interview adapted from multiple validated functional measures covering independent living skills and social and role functioning. To increase the validity of ratings, collateral information was obtained from caregivers and relatives.
Treatment Blinds
In an effort to maintain treatment blinds, all subjects and collaterals were asked at the beginning of each assessment neither to divulge information about any visits made by staff of the research project nor to refer to any items they may have received as part of the study. If blinds were broken, alternative raters blind to group assignment completed the remaining assessments.
Data Analysis
We examined distributions for normality and homogeneity of variance and used transformations where necessary to meet the assumptions of the statistical models. We examined group differences in medication adherence (pill counts and pharmacy refill data), symptomatology (BPRS positive symptom score), and functional outcome (SOFAS score) over time (3, 6, 9, 12, 15 months) by treatment group (CAT, Pharm-CAT, TAU) using mixed-effects regression with repeated measures (SAS PROC MIXED).37 A random subject effect was included modeling intercept, linear, and quadratic trend components. As described by Kraemer and Blasey,38 we utilized centering to ensure that the regression coefficient tested was relevant to the research question. When baseline scores were available (eg, functional outcome, symptoms), these were used as covariates in the model. Group differences were tested by estimating group means at each of the assessment points from the model using an overall F test and pairwise t tests at points of interest. Graphs of outcome variables depict lines generated from random regression model estimates. Specific data points at each time period are plotted around these lines. For time to relapse, 3-month intervals were examined utilizing a proportional hazard regression model with DISCRETE option to deal with ties. The graph was prepared utilizing LifeTest.
To investigate whether statistically significant effects were clinically meaningful, effect sizes were calculated utilizing the SD for the outcome variable in the control group pooled over time. In addition, for significant effects, we report number needed to treat to reach a clinically meaningful difference for the control vs each of the treatment groups.
Results
Table 2 presents the demographic and baseline variables by treatment group. There were no statistically significant group differences with respect to demographic or baseline data. The numbers of subjects available at each assessment point differed slightly by measure. For symptom and functional measures, the number of subjects with data at each time point was as follows: baseline n = 95; 3-month n = 94, 6-month n = 87, 9-month n = 81, 12-month n = 67, and 15-month n = 61. For pill count and pharmacy records, respectively, 90 and 83 subjects had available data at baseline and at least one follow-up. We examined differential dropout by treatment group using a proportional hazards regression model. Individuals in Pharm-CAT were more likely to drop out of the study (χ(2)2 = 14.01; P < .0009). During the active treatment phase, 2 individuals dropped in CAT treatment and TAU (6% and 7%, respectively), while 9 individuals (28%) in the Pharm-CAT group dropped from study.
Table 2.
Baseline Characteristics by Treatment Group
| Baseline Characteristics | Treatment As Usual (n = 29) | Pharm-CAT (n = 32) | CAT (n = 34) |
| % Male | 62.07 | 43.75 | 64.71 |
| % Hispanic | 27.59 | 38.71 | 29.41 |
| % Non-Hispanic White | 44.83 | 38.71 | 29.41 |
| Age, y | 39 (SD = 22.1) | 40 (SD = 11.1) | 37 (SD = 9.4) |
| Education | 11.7 (SD = 2.3) | 11.3 (SD = 2.6) | 12.1 (SD = 2.3) |
| BPRS psychosis factor | 2.7 (SD = 2.34) | 2.6 (SD = 1.47) | 2.5(SD = 1.34) |
| Social and occupational functioning scale | 45.6 (SD = 8.07) | 45.5 (SD = 8.90) | 45.9 (SD = 8.19) |
| Baseline medication | |||
| % Risperidone | 41.4 | 21.9 | 38.2 |
| % Olanzapine | 34.5 | 46.9 | 41.2 |
| % Other | 24.1 | 31.2 | 20.6 |
Note: No significant group differences (all P′s with one exception >.40; trend for fewer males in Pharm-CAT [CAT focused only on medication and appointment adherence] P < .08). CAT, cognitive adaptation training; BPRS, Brief Psychiatric Rating Scale.
Medication Adherence
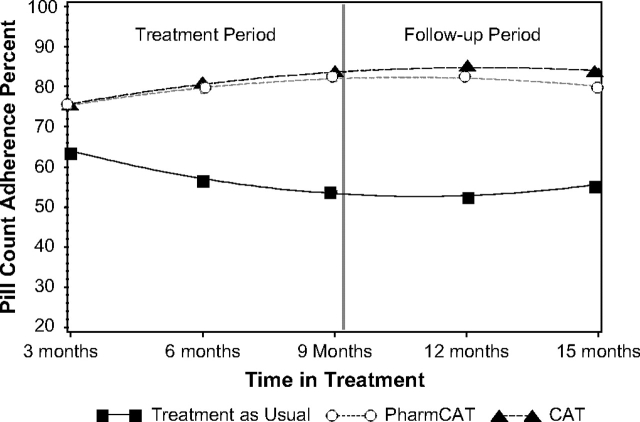
With respect to pill count adherence, the mixed-effects regression model yielded a significant quadratic by group interaction and a trend for linear by group interaction indicating that the effects of the treatment differed over time. Although the size of the treatment effect varied, both CAT and Pharm-CAT were significantly better than standard treatment at all time points throughout formal treatment and the follow-up period. Differences between CAT and Pharm-CAT treatments were not significant at any time point (all P′s > .85). Figure 2 depicts estimated means derived from the regression model at specified time points by treatment group. P values of the differences between experimental and standard treatments are shown for each time point.
Fig. 2.
Adherence Derived From Unannounced Pill Counts in the Participants' Homes Over Time by Treatment Group. Main effect of group—F2,138 = 23.51; P < .0001; visits by group (linear) —F2,264 = 2.85; P < .06; visits by visits by group (quadratic)—F2,251 = 3.46p P < .033). P values for cognitive adaptation training (CAT) vs standard were .04 at 3 months and .001 for all other time periods. For Pharm-CAT (CAT focused only on medication and appointment adherence) vs standard were .05 at 3 months and .002 for 15 months. All other time points had P values of .0001. No differences between CAT and Pharm-CAT (CAT focused only on medication and appointment adherence) were found at any time point.
Averaged across the time points, the effect sizes for CAT and Pharm-CAT compared with TAU were 1.09 and 1.05, respectively, values that correspond to number needed to treat of 1.79 and 1.84, respectively.39 For specific time points, these effects sizes varied from 0.53 at 3 months to 1.43 at 12 months. Beginning with 6 months of treatment and for the remainder of the study, all effect sizes for CAT or Pharm-CAT vs standard treatment were over 1. According to Cohen's conventions,40 these are large treatment effects.
As a secondary measure of treatment adherence, we examined prescription refill rates. The mixed-effects regression model yielded a significant main effect of group (F2,105 = 3.93, P < .02). There were neither significant effects of time nor significant interactions. Patients in CAT were significantly more adherent than those in standard treatment, and there was a nonsignificant trend for patients in the Pharm-CAT group to be more adherent than those in standard treatment (F2,98 = −2.85 P < .006 and F2,102 = −1.81, P < .07, respectively). Averaged across time points, the effect sizes for CAT and Pharm-CAT vs the control for refill adherence were 0.51 and 0.33, respectively. According to Cohen's conventions,40 these are moderate and small effect sizes, respectively.
Symptomatology
Results of a mixed-effects regression model with the baseline symptom scores used as covariates yielded no significant main effects or interactions (all P′s > .09).
Relapse
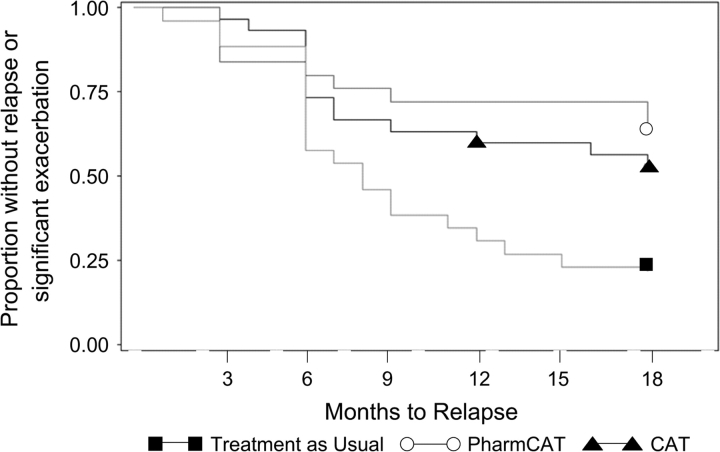
Complete a total of 69 participants met criteria for remission at baseline (21/29 for standard, 23/32 for Pharm-CAT, and 25/34). There were no significant differences in the numbers of patients remitted in the 3 treatment groups at baseline (χ(2)2 = 0.24; P < .89). The time to relapse differed by treatment group based on the proportional hazards regression model (χ(2)2 = 11.09; P < .004). Differences between CAT and standard treatment and Pharm-CAT and standard treatment were significant (χ(1)2 = 8.29; P < .004 and (χ(2)2 = 8.20; P < .005, respectively). Over 65% of patients in CAT and Pharm-CAT treatments survived the 15 months without a relapse vs only 19% of individuals in the standard treatment group. There were no differences between active treatments. The survival curves for each treatment group over time are presented in figure 3.
Fig. 3.
Time to Relapse or Significant Exacerbation by Treatment Group. Proportional hazards regression model for time to relapse (χ(2)2 = 11.09; P < .004).
Functional Outcome
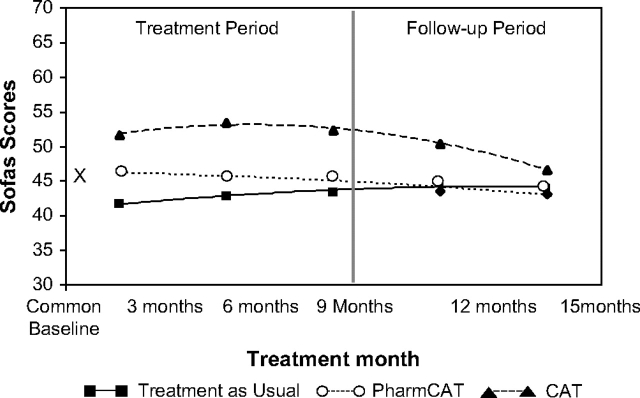
For SOFAS scores, results of a mixed-effects regression model yielded a significant group effect (F1,147 = 22.01; P < .0001) and significant group by linear and group by quadratic interactions (F1,303 = 4.85; P < .009; and F2,290 = 3.51; P < .04, respectively). Figure 4 depicts estimates and means at each time point. Pharm-CAT differed significantly from standard treatment at 3 and 6 months (P′s < .05) but not at any time point thereafter. CAT was significantly better than standard treatment at all assessment points during the treatment period (all P′s < .0001). CAT was significantly better than TAU in the first 3 months of follow-up (P < .0001), but there was only a nonsignificant trend for patients in CAT to do better than those in standard treatment by the end of 6-month follow-up (P < .07). CAT was significantly better than Pharm-CAT at all time points (P′s < .0004) with the exception of the 6-month follow-up. The effect size for CAT vs TAU varied from 1.47 after 6 months of treatment to 0.50 at the 6-month follow-up following withdrawal of home visits. During the treatment phase, the effect size for CAT vs standard treatment remained above 1.0. According to Cohen's conventions40 this is a large effect size during treatment that decreases to a moderate effect size 6 months after withdrawal of home visits. The Number Needed to Treat (NNT) for CAT vs standard treatment was less than 1.4 for all points during treatment and was 1.8 and 2.3 during follow-up. The effect size for Pharm-CAT varied from 0.42 and 0.44 at 3 and 6 months of treatment to 0.22 at 6-month follow-up.
Fig. 4.
Social and Occupational Functioning Scale Score (SOFAS) Over Time by Treatment Group. Main effect of group—F2,147 = 113.38; P < .0001; visits by group (linear)—F2,202 = 4.85; P < .009; visits by visits by group (quadratic)—F2,290 = 3.51; P < .032. P values for cognitive adaptation training (CAT) vs standard treatment were .0001 for all time periods but P < .07 for 15 months. P values for Pharm-CAT (CAT focused only on medication and appointment adherence) vs standard treatment were <.014 at 3 months, .043 at 6 months, and nonsignificant thereafter. P values for CAT vs Pharm-CAT were .004, .0001, .0001, .0004, and .37 for 3,6,9,12, and 15 months, respectively.
Discussion
Environmental supports targeted at global functional outcome improved global community functioning in individuals with schizophrenia. Environmental supports targeted at medication adherence alone improved adherence to antipsychotic medications in schizophrenia as assessed by unannounced, in-home pill counts. Data support that individually tailored environmental supports improve targeted behaviors.
This is the first randomized trial to demonstrate that the systematic application of individually tailored environmental supports in CAT and Pharm-CAT improve adherence to oral antipsychotic medication in individuals with schizophrenia. Adherence was significantly higher in CAT and Pharm-CAT as compared with TAU during treatment and 6 months after home visits were withdrawn. The average level of medication adherence in CAT and Pharm-CAT across a 15-month period remained close to 80%. CAT/Pharm-CAT treatment and perhaps the continued use of these previously provided supports after the withdrawal of home visits can sustain an important behavior change that has major public health implications.11 It is important to note that supports improved adherence to medication whether or not they were embedded in the context of a CAT program that targeted multiple additional areas of functional outcome. Maintenance of medication adherence gains in CAT and Pharm-CAT may result from the formation of habit behavior with the repeated use of environmental cues.
Adherence data obtained from unannounced, in-home pill counts suggested more robust treatment effects than data obtained from pharmacy records. Differences may reflect that filling a prescription only ensures that medication is available to be taken. Larger samples may be needed when using pharmacy refill data to examine the efficacy of interventions. This study provides important support for the feasibility of conducting in-home pill counts and their sensitivity to change with targeted behavioral interventions.
While there were not significant differences in positive symptoms across treatment groups, rates of relapse utilizing a composite measure as suggested by Schooler et al35 were significantly lower with CAT and Pharm-CAT treatment compared with TAU. This finding corresponds to published data indicating that poor adherence is a significant predictor of relapse.11 Moreover, medication adherence has been characterized as a foundation upon which the rehabilitation and recovery process is built.41
CAT improved functional outcome as compared with TAU and to Pharm-CAT. This finding replicates what was found in our previous trials. The number needed to treat to improve functional outcome compared with TAU was 1.4 during treatment. This is important in that CAT is a fairly labor intensive treatment. A low NNT indicates that the intensity of treatment may be justified by clinically important outcomes. Surprisingly, statistically significant improvement in functioning was noted for Pharm-CAT over TAU. While Full-CAT treatment made a far more robust and longer standing impact upon functional outcome, it is possible that better medication adherence in the Pharm-CAT group may have been a platform which allowed minimal functional improvement to occur even in the absence of a comprehensive rehabilitation program. However, results clearly indicate that the Full-CAT program has significant benefits for functional outcomes over a program targeting only medication adherence. It is important to determine whether limiting the goals of treatment to improving medication adherence and preventing relapse is enough. More individuals in the Pharm-CAT group began to drop during the last part of treatment. It may be that to keep individuals involved in intensive treatment, functional goals important to the individual need to be targeted. Medication in Full-CAT treatment can be seen as a means to help the individual achieve a broader range of goals. Moreover, when an individual is resistant to working on medication adherence, working on other issues can keep them engaged in treatment so that adherence can be approached again at a later point. Such individuals are likely to drop out of a treatment targeting adherence only. Because home visits can be labor intensive, it may make more sense to get more out of each visit by targeting the broad range of adaptive behaviors. This approach is likely to produce the best results with little additional financial burden.
Functional gains in CAT were sustained throughout the first 3 months after the withdrawal of home visits but the CAT group was no longer significantly better than TAU or Pharm-CAT by the 6-month follow-up. Environmental supports for complex functional behaviors may need more individual tailoring, adjustment, and reinforcement in a changing environmental context than a relatively circumscribed behavior such as adherence. It is a common practice to examine whether behavioral improvements with psychosocial treatments are maintained when the treatment is withdrawn. This is not a standard applied to medication treatments, and is likely most applicable to psychosocial treatments that focus on systematic teaching of skills which are anticipated to be sustained after treatment is withdrawn. For a treatment such as CAT which is expected to bypass cognitive problems rather than restore cognitive functioning, it is unclear whether treatment should be withdrawn. It may make more sense after an initial treatment period to decrease the frequency of visits in an attempt to maintain gains while controlling the costs of treatment, or to train family members or other caregivers to establish and alter environmental supports. Future research on sustaining treatment gains and comparing the costs and benefits of CAT with skills training programs would be important to pursue.
Contrary to the results of previous studies, mean levels of psychosis did not significantly improve in the CAT and Pharm-CAT groups in comparison to those in TAU. These findings may suggest that in effect, taking medication as prescribed prevented relapse but did not result in a statistically significant reduction in symptoms. There are several possible interpretations of this finding.
Symptoms of psychosis were mild on average at baseline. Further improvement may have been difficult to demonstrate with this starting point. It could also be true that lack of accurate information on adherence available to treating psychiatrists may make it difficult for them to optimize medication regimens to produce the best outcomes in terms of symptomatology. In a recent study, we found that among partially adherent outpatients with schizophrenia, adherence as measured with Medication Event Monitoring (MEMS; caps that record day and time opened) was not correlated with either symptoms or symptom improvement over a 12-week period.42 These findings raise interesting questions. What is the right amount of medication for an individual patient? How can the right dose be determined in the context of limited or no information about the patient's adherence? How adherent is adherent and over what time period? Future studies that feed adherence data back to providers in an effort to optimize dose may be needed.
It is important to note that symptom scores did not worsen in CAT even though patients were more involved in social and role functioning. Increased social and role demands may increase stress. Stress in turn can worsen symptoms. Better functional status in the context of stable psychotic symptoms translates to a positive outcome for CAT patients.
Pharm-CAT is a new treatment that improves adherence to medication and reduces rates of relapse. Pharm-CAT and CAT add to the growing number of interventions targeting this problem for individuals with schizophrenia. Clinical experience suggests that Pharm-CAT is likely to be best suited for individuals who are willing to take medication but who may miss doses due to distraction, memory problems, poor planning and problem solving, chaotic environments, and negative symptoms. For individuals with more ambivalence toward medication, CAT treatment may be able to help them to connect medication adherence to improvements in functional outcomes. For individuals refusing medications altogether, interventions such as cognitive behavior therapy,43 family therapy,44 or compliance therapy45may be important precursors to Pharm-CAT. Willingness to take medication may be a necessary but not sufficient condition to improve actual adherence to medication. Combining treatments focusing on changing attitudes and insight with the use of environmental supports to change behavior may lead to improved adherence outcomes for a broader range of individuals.
These results must be examined within the context of the study's methodological limitations. A significant number of inpatient recruits did not make it to the point of randomization into treatment for this outpatient treatment study. Results can only apply to those individuals treated as outpatients or inpatients who have successfully negotiated the transition from inpatient to outpatient status. Participants in the study had been ill on average for more than a decade. The extent to which these techniques would be helpful to individuals with a more recent onset of schizophrenia should be examined in future research. In future studies including a baseline period of assessment for medication adherence prior to randomization would be important. In-home pill counts could not be conducted by blinded observers given the nature of environmental supports. However, pharmacy records were not subject to observer bias. It is also likely that more adherent individuals agreed to participate in the study; a common problem in adherence research. Moreover, assessments of medication adherence involve error that may overestimate or underestimate actual adherence. Methodological problems with pill counts and pharmacy records are outlined elsewhere.29 The fact that medication adherence improved, while psychotic symptoms did not suggests that our notions about adequate adherence and optimal dosing may have been formed in the ambiguous environment of partial adherence or negotiation and may need to be altered to maximize the outcomes of our patients.
Despite these methodological limitations, the study provides strong support for the benefits of CAT and Pharm-CAT with respect to improving medication adherence and for the benefits of CAT for improving functional outcomes.
Funding
National Institutes of Health (R01 MH62850, R01 MH61775 to D.I.V.).
Acknowledgments
We wish to thank the participants and staff from the San Antonio State Hospital (Superintendent: Robert Arizpe) and the Center for Health Care Services (Executive Director: Leon Evans) for their ongoing support of our research program. We wish to thank Nina Schooler for consulting on this research.
References
- 1.Bell MD, Fiszdon J, Bryson G, Wexler BE. Effects of neurocognitive enhancement therapy in schizophrenia: normalisation of memory performance. Cognit Neuropsychiatry. 2004;9:199–211. doi: 10.1080/13546800344000084. [DOI] [PubMed] [Google Scholar]
- 2.Velligan DI, Bow-Thomas CC, Huntzinger CD, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157:1317–1323. doi: 10.1176/appi.ajp.157.8.1317. [DOI] [PubMed] [Google Scholar]
- 3.Wykes T, Reeder C. Cognitive Remediation Therapy for Schizophrenia: An Introduction. New York, NY: Brunner-Routledge; 2005. [Google Scholar]
- 4.Medalia A, Revheim N, Casey M. The remediation of problem-solving skills in schizophrenia. Schizophr Bull. 2001;27:259–267. doi: 10.1093/oxfordjournals.schbul.a006872. [DOI] [PubMed] [Google Scholar]
- 5.Medalia A, Revheim N, Casey M. Remediation of memory disorders in schizophrenia. Psychol Med. 2000;30:1451–1459. doi: 10.1017/s0033291799002913. [DOI] [PubMed] [Google Scholar]
- 6.Silverstein SM, Hatashita-Wong M, Solak BA, et al. Effectiveness of a two-phase cognitive rehabilitation intervention for severely impaired schizophrenia patients. Psychol Med. 2005;35:829–837. doi: 10.1017/s0033291704003356. [DOI] [PubMed] [Google Scholar]
- 7.Kern RS, Green MF, Mitchell S, Kopelowicz A, Mintz J, Liberman RP. Extensions of errorless learning for social problem-solving deficits in schizophrenia. Am J Psychiatry. 2005;162:513–519. doi: 10.1176/appi.ajp.162.3.513. [DOI] [PubMed] [Google Scholar]
- 8.Velligan DI, Bow-Thomas CC. Two case studies of cognitive adaptation training for outpatients with schizophrenia. Psychiatr Serv. 2000;51:25–29. doi: 10.1176/ps.51.1.25. [DOI] [PubMed] [Google Scholar]
- 9.Velligan DI, Prihoda TJ, Ritch JL, Maples N, Bow-Thomas CC, Dassori AA. Randomized single-blind pilot study of compensatory strategies in schizophrenia outpatients. Schizophr Bull. 2002;28:283–292. doi: 10.1093/oxfordjournals.schbul.a006938. [DOI] [PubMed] [Google Scholar]
- 10.Davis JM, Janicak PG, Singla A, Sharma RP. Maintenance antipsychotic medication. In: Barnes TRE, editor. Antipsychotic Drugs and Their Side Effects. New York, NY: Academic Press; 1993. pp. 183–203. [Google Scholar]
- 11.Valenstein M, Copeland L, Owen R, Blow FC, Visnic S. Adherence assessments and the use of depot antipsychotics in patients with schizophrenia. J Clin Psychiatry. 2001;62:545–551. doi: 10.4088/jcp.v62n07a08. [DOI] [PubMed] [Google Scholar]
- 12.Serban G, Thomas A. Attitudes and behaviors of acute and chronic schizophrenic patients regarding ambulatory treatment. Am J Psychiatry. 1974;131:991–995. doi: 10.1176/ajp.131.9.991. [DOI] [PubMed] [Google Scholar]
- 13.Sackett DL, Haynes RB. Compliance with Therapeutic Regimens. Baltimore, Md: Johns Hopkins University Press; 1976. [Google Scholar]
- 14.Sullivan G, Wells KB, Morgenstern H, Leake B. Identifying modifiable risk factors for rehospitalization: a case-control study of seriously mentally ill persons in Mississippi. Am J Psychiatry. 1995;152:1749–1756. doi: 10.1176/ajp.152.12.1749. [DOI] [PubMed] [Google Scholar]
- 15.Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159:103–108. doi: 10.1176/appi.ajp.159.1.103. [DOI] [PubMed] [Google Scholar]
- 16.Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, Weiden P. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51:216–222. doi: 10.1176/appi.ps.51.2.216. [DOI] [PubMed] [Google Scholar]
- 17.Velligan DI, Dicocco M, Castillo D, et al. Obstacles in assessing adherence to oral antipsychotic medications. Schizophr Res. 2003;60S:330. [Google Scholar]
- 18.Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161:692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 19.Epstein LH, Cluss PA. A behavioral medicine perspective on adherence to long-term medical regimens. J Consult Clin Psychol. 1982;50:950–971. doi: 10.1037//0022-006x.50.6.950. [DOI] [PubMed] [Google Scholar]
- 20.Chen A. Noncompliance in community psychiatry: a review of clinical interventions. Hosp Community Psychiatry. 1991;42:282–287. doi: 10.1176/ps.42.3.282. [DOI] [PubMed] [Google Scholar]
- 21.Boczkowski J, Zeichner A, DeSanto N. Neuroleptic compliance among chronic schizophrenic outpatients: an intervention outcome report. J Consult Clin Psychol. 1985;53:666–671. doi: 10.1037//0022-006x.53.5.666. [DOI] [PubMed] [Google Scholar]
- 22.Young JL, Zonana HV, Shepler L. Medication noncompliance in schizophrenia: codification and update. J Am Acad Psychiatry Law. 1986;14:105–122. [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.Diamond PM, Zeber J, Maples NJ, et al. Environmental supports and outcomes in schizophrenia. Schizophr Bull. 2007;33:56. doi: 10.1093/schbul/sbm111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velligan DI, Mahurin RK, True J, Lefton R, Flores CV. Preliminary evaluation of cognitive adaptation training to compensate for cognitive deficits in schizophrenia. Psychiatr Serv. 1996;47:415–417. doi: 10.1176/ps.47.4.415. [DOI] [PubMed] [Google Scholar]
- 26.Grace J, Stout JC, Malloy PF. Assessing frontal lobe behavioral syndromes with the Frontal Lobe Personality Scale. Assessment. 1999;6:269–284. doi: 10.1177/107319119900600307. [DOI] [PubMed] [Google Scholar]
- 27.Paulsen JS, Stout JC, DeLaPena J, et al. Frontal behavioral syndromes in cortical and subcortical dementia. Assessment. 1996;3:327–337. [Google Scholar]
- 28.Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Res. 2002;113:227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- 29.Velligan DI, Lam YWF, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32:724–742. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the brief psychiatric rating scale: the drift busters. Int J Methods Psychiatr Res. 1993;3:221–224. [Google Scholar]
- 31.Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Manual for the expanded Brief Psychiatric Rating Scale. Int J Methods Psychiatr Res. 1993;3:227–244. [Google Scholar]
- 32.Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatr Res. 2000;97:129–135. doi: 10.1016/s0165-1781(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 33.Velligan DI, Prihoda TJ, Dennehy EB, et al. The Brief Psychiatric Rating Scale expanded version: how do new items affect factor structure? Psychiatr Res. 2005;135:217–228. doi: 10.1016/j.psychres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Barbato A, D'Avanzo B. Family interventions in schizophrenia and related disorders: a critical review of clinical trials. Acta Psychiatr Scand. 2000;102:81–97. doi: 10.1034/j.1600-0447.2000.102002081.x. [DOI] [PubMed] [Google Scholar]
- 35.Schooler NR. Relapse prevention and recovery in the treatment of schizophrenia. J Clin Psychiatry. 2006;67:19–23. [PubMed] [Google Scholar]
- 36.Nuechterlein KH, Dawson ME, Green MF. Information processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatr Scand. 1994;90:71–79. doi: 10.1111/j.1600-0447.1994.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 37.SAS/Secure (TS1M3): 9.1.6 for Windows5. Cary (NC): SAS Institute Inc; 200. [Google Scholar]
- 38.Kraemer HC, Blasey CM. Centering in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Cohen TJ. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 41.Velligan DI, Gonzalez JM. Rehabilitation and recovery in schizophrenia. Psychiatr Clin North Am. 2007;30:535–548. doi: 10.1016/j.psc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Velligan DI, Wang M, Diamond PM, et al. Relationships among measures of adherence to oral antipsychotic medications: can doctors identify adherent patients? Do patients accurately report their adherence? Psychiatr Serv. 2007;58:1187–1192. doi: 10.1176/ps.2007.58.9.1187. [DOI] [PubMed] [Google Scholar]
- 43.Turkington D, Kingdon D, Weiden PJ. Cognitive behavior therapy for schizophrenia. Am J Psychiatry. 2006;163:365–373. doi: 10.1176/appi.ajp.163.3.365. [DOI] [PubMed] [Google Scholar]
- 44.Miklowitz DJ, George EL, Richards JA, Simoneau TL, Suddath RL. A randomized study of family focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychatry. 2003;60:904–912. doi: 10.1001/archpsyc.60.9.904. [DOI] [PubMed] [Google Scholar]
- 45.Kemp R, Kirov G, Everitt B, Hayward P, David A. Randomized controlled trial of compliance therapy: 18-month follow-up. Br J Psychiatry. 1998;172:413–419. doi: 10.1192/bjp.172.5.413. [DOI] [PubMed] [Google Scholar]