Abstract
Many previous observers have reported some qualitative similarities between the normal mental state of dreaming and the abnormal mental state of psychosis. Recent psychological, tomographic, electrophysiological, and neurochemical data appear to confirm the functional similarities between these 2 states. In this study, the hypothesis of the dreaming brain as a neurobiological model for psychosis was tested by focusing on cognitive bizarreness, a distinctive property of the dreaming mental state defined by discontinuities and incongruities in the dream plot, thoughts, and feelings. Cognitive bizarreness was measured in written reports of dreams and in verbal reports of waking fantasies in 30 schizophrenics and 30 normal controls. Seven pictures of the Thematic Apperception Test (TAT) were administered as a stimulus to elicit waking fantasies, and all participating subjects were asked to record their dreams upon awakening. A total of 420 waking fantasies plus 244 dream reports were collected to quantify the bizarreness features in the dream and waking state of both subject groups.
Two-way analysis of covariance for repeated measures showed that cognitive bizarreness was significantly lower in the TAT stories of normal subjects than in those of schizophrenics and in the dream reports of both groups.
The differences between the 2 groups indicated that, under experimental conditions, the waking cognition of schizophrenic subjects shares a common degree of formal cognitive bizarreness with the dream reports of both normal controls and schizophrenics. Though very preliminary, these results support the hypothesis that the dreaming brain could be a useful experimental model for psychosis.
Keywords: dreaming, schizophrenia, REM sleep
Introduction
The classical psychiatric and psychoanalytic literature has commented upon qualitative similarities between the normal state of dreaming and the abnormal state of psychosis, such as the loosening of associations, the incongruity and bizarreness of personal experience, and the distortion of time and space parameters.
In 1907, Carl Gustav Jung wrote, “If we could imagine a dreamer walking around and acting his own dream as if he were awake, we would see the clinical picture of dementia praecox.”1 In his Psychoanalytic Notes on an Autobiographical Account of a case of Paranoia, Sigmund Freud stated that acute psychotic episodes and dreams sometimes share common features.2 In his classical book Dementia praecox or the group of Schizophrenias, Eugene Bleuler also proposed that “The modalities of thinking of schizophrenic subjects are very similar to dreaming” and hypothesized that the dreaming state “has its own rules, and that most of the characteristics of schizophrenic thinking (particularly delusional thinking) are explained by the differences between the dreaming and the wakefulness way of thinking.”3 In Bleuler's clinical perspective, one of the most striking psychopathological traits of schizophrenia is that people who are affected with the disorder are detached from reality, living in a private world with their own desires, which suggests a strong similarity with the dream experience. In 1927, Eugène Minkowski, a French psychopathologist fellow of Bleuler, wrote “The dreaming man sleeps and it is the sleeping condition which allows the desires to reveal themselves as bizarre and incomprehensible dreams, by temporarily stopping the connections with the real world …. A patient suffering from schizophrenia doesn't sleep but sometimes his inner life shows the same characteristics.”4
In the second half of the last century, the dreaming mental state was also assimilated to the fantastic mental activity of waking and a direct relationship between the severity of schizophrenia and the incongruity of fantastic activity was reported.5 Consistent with this finding, a direct relationship between the severity of psychopathology and the bizarreness of sleep fantasy was also reported in schizophrenic subjects.6 After the discovery of the rapid eye movement (REM)-non rapid eye movement (NREM) cycle, many experimental data from independent lines of research seem to reasonably substantiate this clinical point of view: the mental activity of normal waking subjects appears to be more bizarre when it is elicited immediately after awakening from REM sleep, rather than after awakening from NREM sleep7; over 65% of normal subjects’ dreams show auditory hallucinations8; the progression from waking to NREM and REM sleep in normal subjects is correlated with a progressive modification in mental state from thinking to hallucinosis.9
Since the early days of sleep research, it has been observed that acutely ill schizophrenic patients fail to develop REM pressure when deprived of sleep,10 suggesting—in some way—a dysfunction in the neurochemical mechanisms underpinning the REM-NREM cycle. Furthermore, the presence of a similar disperceptive psychopathology (visual and auditory hallucinations) raised the hypothesis of a common genetic substrate underlying a REM-NREM cycle disorder like narcolepsy or schizophrenia.11 Data emerging from the field of research and theory that link REM sleep to memory processes appear to be of relevant interest for the topic. REM sleep plays an important role in promoting 2 special forms of memory called context memory, a composite memory formed by merging together many elements of experience in a particular context,12 and relational memory, the flexible ability to generalize across existing stores of information.13 It seems interesting to note that both context analysis and the ability to make inferences are typically defectual in schizophrenic subjects.14
Furthermore, a model has been proposed in which REM sleep is considered a functional state of the brain necessary to restructure the complexity of cerebral networks modulated by the day's experiences; serious mental disturbances such as obsessions, hallucinatory associations, and delusions might be the result of a failure in the underpinned neurofunctional mechanisms.15 More specifically, the psychotic symptoms of schizophrenia have been related to a defect in the physiological inhibition of the memory of dream events.16
A certain degree of convergence between the dreaming state, schizophrenia, and the hallucinogenic drug state have also been reported in the literature, and the hallucinogenic drug-induced mental state has been considered a model of psychosis.17 Lysergic acid diethylamide, one of the most powerful hallucinogens known to date, antagonizes serotonin in peripheral systems and depresses the firing rate of raphe units, increasing the frequency of ponto-geniculo-occipital spikes in the waking cat. This has been postulated as a possible explanation for visual hallucinations.18
The reciprocal and mutual relationships between serotonergic and cholinergic systems in the brain also reveal some intriguing similarities in dreaming and psychosis: both are characterized by a fall in serotonergic and noradrenergic firing and a hyperactivation of the cholinergic system.19–21
Studies on anatomofunctional segregation in the brain also seem to lead in the same direction, with functional neuroimaging studies in normal subjects having consistently associated REM sleep with a hyperactivation of limbic structures and a reciprocal hypoactivity of the frontal cortex, especially in its dorsolateral prefrontal regions22; it is striking that a similar neurofunctional pattern of cerebral organization appears to underpin the psychotic mental state in schizophrenia.23
An interesting similarity also seems to emerge from studies on the electrophysiological model of prepulse inhibition, where the negative component of the evoked potential is reduced—in normal subjects—in the second response to 2 sensory stimulations delivered at short intervals: a lack of these central inhibitory processes is observed in the normal subject during REM sleep and in the schizophrenic subject during waking and REM sleep.21
This convergence of phenomenological and biological aspects of the mental organization of both dreaming and psychosis leads to the recent hypothesis of the dreaming brain as a biological model of psychosis, with dreaming considered as hallucinatory and thoughtless (or delusional) as so-called mental illness.24 However, what kind of psychosis dreaming most closely mimics or to what degree the cognitive similarities between these mental states overlap has not yet been rigorously investigated. With this study, we addressed the second question by focusing on formal cognitive bizarreness in dream reports and verbal productions elicited by the Thematic Apperception Test (TAT) of schizophrenic patients and normal controls. For this purpose, we defined cognitive bizarreness as a distinctive formal property of dreams, which is specifically characterized by discontinuities and incongruities of dream perception and cognition. Dream bizarreness has been quite consistently related to REM sleep, having been considered a phenomenological correlate of REM sleep neurophysiology.25,26 More specifically, it has been suggested that the atypical patterns of brain activation during REM sleep may account for the bizarreness of dream imagery.27 It has also been suggested that bizarre phenomena in dreams may be assimilated to specific neuropsychological symptoms and/or syndromes—such as prosopoagnosia and Capgras delusion—which are typically defined by aspects of reality distortion similar to those found in the positive symptomatology of schizophrenic patients.28
Furthermore, in order to evaluate whether the formal bizarreness of schizophrenic subjects may somehow be contaminated by their psychopathological features, we evaluated the relationship between psychopathology—expressed as the Brief Psychiatric Rating Scale (BPRS) total score—and bizarreness in these subjects.
METHODS
Subjects
Dream reports and TAT story responses were collected from 30 Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision actively psychotic, schizophrenic patients and 30 normal controls (table 1).
Table 1.
Demographic and Clinical Characteristics of Subjects
| Psychotic Subjects | Normal Controls | |
| Demographic characteristics | ||
| Age—y | 39.4 ± 11.3 | 40.2 ± 15.3 |
| Sex—n (%) | ||
| Male | 19 (63) | 12 (40) |
| Female | 11 (37) | 18 (60) |
| Education—ya | 11.9 ± 3 | 14 ± 4 |
| Marital status—n (%) | ||
| Married | 3 (10) | 14 (47) |
| Previously married | 3 (10) | 2 (6) |
| Never married | 24 (80) | 14 (47) |
| Psychiatric assessment | ||
| Age of onset | 25.8 ± 7.8 | — |
| Diagnosis—n (%) | ||
| Paranoid schizophrenia | 22 (73) | — |
| Disorganized schizophrenia | 5 (17) | — |
| Undifferentiated schizophrenia | 3 (10) | — |
| Medication—n (%) | ||
| Typical antipsychotic | 6 (20) | — |
| Atypical antipsychotic | 24 (80) | — |
| BPRS | ||
| BPRS total score | 49.6 ± 10.1 | — |
| BPRS hallucinations item score | 3.26 ± 2.35 | — |
Note: Plus or minus values are means ± SD.
One-way analysis of variance: F1,57 = 5.38, P = 0.024.
The level of education was the only significantly different demographic variable between the 2 groups (one-way analysis of variance: F1,57 = 5.38, P = 0.024). Table 1 also shows that the male-to-female ratio of our sample is inverted in the 2 groups (2:3 in the schizophrenia group vs 3:2 in the control group), but the difference is not statistically significant (χ2 [1] 3.27, P = 0.07).
We are aware of the fact that sex influences the cognitive style in humans,29 and many studies over the years have specifically revealed sex-related differences in dream content, such as greater cognitive activity and dream intensity in women and greater auditory activity and presence of strangers in men.30,31 Nevertheless, because of the statistically nonsignificant differences between the 2 groups and in order to avoid any confounding bias in the evaluation of our results, we decided—at this point of our work—not to include this variable in the statistical analysis.
All patients were on therapy with antipsychotic and occasionally hypnotic drugs. Exclusion criteria for both groups were a present or past history of a serious medical or neurological disease, sleep disorders, mental retardation, alcohol and psychoactive substance abuse, perinatal injury, or cranial trauma. An additional exclusion criterion for the patient group was a past or present history of any mental disorder other than schizophrenia; a present or past history of any mental disorder was an additional exclusion criterion for the control group.
All participants were unpaid volunteers, and informed consent was obtained from all subjects after full explanation of the study purpose and design.
Data Collection—TAT and Dream Reports
Seven of the 20 TAT pictures were used to elicit the subjects’ projections (1, 3GF/3BM, 6GF/6BM, 9GF/9BM, 12F/12M, 15, 18GF/18BM); we used a shortened version of the test because the cards were employed as an experimental stimulus to produce stories and not as a standardized projective test. An additional consideration was that several specific neuropsychological traits of psychotic patients, such as deficits in sustained attention and working memory,32 could have limited the administration of the complete TAT. Furthermore, shortened versions of this test have been used in similar experimental studies.7 The test was administered in the morning, and the instructions conformed to the standardized TAT procedure33; the stories recounted in response to the stimulus were tape-recorded and then transcribed. During the same week in which the TAT was administered, the subjects were instructed to write down each morning a report of the dreams they had had the night before. The data set comprised 121 dream reports and 210 TAT stories from the schizophrenic subjects and 123 dream reports and 210 TAT stories from the normal controls.
Scoring Bizarreness
The standardized criteria for measuring bizarreness are reported in table 2.26 Dream reports and TAT stories were scored by 2 judges blind to diagnosis who practiced the procedure until they had reached a sufficient degree of agreement.
Table 2.
Two-Stage Scoring System for Dream Bizarreness
| Stage I identifies items as bizarre if they are physically impossible or improbable* aspects of |
| A. The plot, characters, objects, or action |
| B. The thoughts of the dreamer or dream characters |
| C. The feeling state of the dreamer or dream characters |
| Stage II then characterizes the item as exhibiting |
| 1. Discontinuity (change of identity, time, place, or features thereof) |
| 2. Incongruity (mismatching features) |
| 3. Uncertainty (explicit vagueness) |
Probability of occurrence <0.05.
They then independently scored the same dream and TAT reports of a sample of 10 subjects (5 cases and 5 controls). A total of 70 TAT (17% of the total) and 46 dream reports (19% of the total) were scored for interrater reliability in conformance with the method adopted in the development of the bizarreness scale.26 The reliability coefficient was 0.92 for the TAT reports and 0.89 for the dream reports (table 3). Each judge then randomly selected one-half of the reports and independently scored 210 TAT reports and 122 dream reports.
Table 3.
Reliability Analysis—Scale (Alpha)
| Intraclass Correlation Coefficients |
|||
| Reports (no.) | Single Measure (Lower/Upper) | Average Measure (Lower/Upper) | Alpha |
| TAT (70) | 0.8608 (0.5371/0.9635) | 0.9252 (0.6989/0.9814) | 0.9252 |
| DREAMS (46) | 0.8158 (0.4200/0.9508) | 0.8986 (0.5916/0.9748) | 0.8986 |
Note: Reliability coefficients are no. of subjects = 10; no. of raters = 2.
Both judges were blind to diagnosis at all stages of the procedure. In the following examples, the reader is shown how the bizarreness scale is applied to written reports: whenever a bizarre element is identified, it is italicized and scored with an alphabetical letter indicating the narrative domain to which it belongs and an Arabic numeral indicating the specific subtype of bizarreness.
Examples
TAT Picture 15 (38-Year-Old Male Patient)
An extraterrestrial or a devil or an evil man (A3) … or he seems evil (B3), no he doesn't seem evil, he's … an orchestra conductor (A1) who's between all the seats in his concert … how do you call it? I can't recall the words … an orchestra conductor who's looking at the seats in his theatre.
Dream (23-Year-Old Female Control)
I was in my hometown (though it actually had nothing to do with the place I know) (A2) and I had to marry. I knew (B2) the person I had to marry had already married me, but now it was my turn to marry him (A2). So I thought I had to ask him whether or not the fact of having married me had a meaning for both of us. There were many church pews and many guests. I was in a white dress at the altar, but he didn't arrive (A2). Angry, I sat amongst the guests, and suddenly groups of guests dressed up like soldiers or like a marching band passed in front of me (A1 + A2). At the end of the parade was a small open train with him on top, in front of a table full of different food dishes (amongst which I remember some meat). He stopped in front of me and ate some of the meat (A2), I looked at him with surprise, and he said he was proud I had waited for him and had watched him eat (C2).
The following indices were calculated for each dream and TAT response:
1. Bizarreness Intensity (BI) determined as the number of bizarre events in the domains of plot, cognition, and affect and
2. Bizarreness Density (BD) determined by dividing BI by the report's word count. This was done to normalize our data because of the remarkable lack of uniformity in the Number of Words (NW) of the TAT and dream reports that emerged in the present investigation (NW in TAT reports: normal subjects = 52.2 ± 28.8, schizophrenic subjects = 51.7 ± 38.4; NW in dreams reports: normal subjects = 167 ± 102.3, schizophrenic subjects = 65.8 ± 53.2).
The following indices were then calculated for each subject:
1. Bizarreness Density Index (BDI) for dreams determined as the mean of the dream BD indices and
2. BDI for TAT tables determined as the mean of the TAT table BD indices.
The square root of the BDI was then calculated in order to normalize the data distribution.
Statistical Analysis
Two-way analysis of covariance for repeated measures34—with the education level as a covariate—was the statistical tool we used to assess the influence of diagnosis, experimental conditions (TAT stories and dream reports), and education on bizarreness; diagnosis was the independent variable, and the experimental conditions were the within-subjects’ factors. With this type of analysis, we were able to independently estimate the effects of diagnosis and test conditions and the interaction between them.
In the schizophrenic subjects group, Pearson's correlation coefficient r34 was used to measure the strength of the linear relationship between the BDI and the BPRS total score and, separately, between the BDI of dream reports and TAT reports.
Results
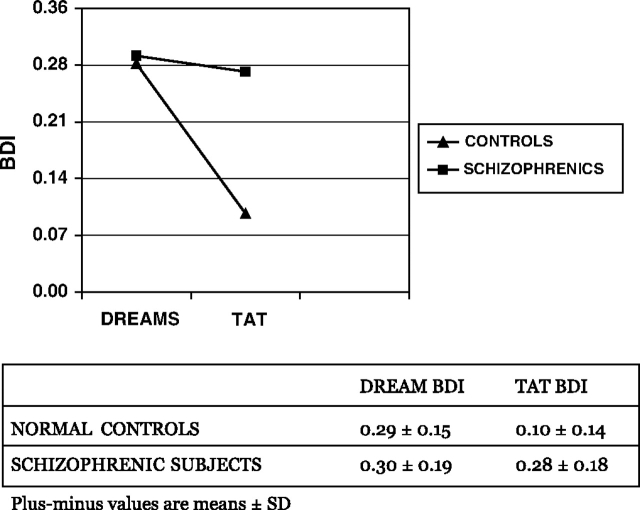
Figure 1 shows the dream and TAT BDI in schizophrenic and normal subjects. Analysis of the data (table 4) reveals that
Fig. 1.
BDI Values in the Dream Reports and TAT Stories of Normal Controls and Schizophrenic Subjects. The BDI values reported are means of the square roots.
Table 4.
Two-Way Analysis of Covariance Summary Table
| Main Effects | F | P |
| Condition | F1,57 < 1 | P = NS |
| Diagnosis | F1,57 = 14.2 | P < 0.001 |
| Education | F1,57 = 3.5 | P = 0.07 |
| Interactions | ||
| Condition by diagnosis | F1,57 = 6.3 | P = 0.01 |
| Condition by education | F1,57 < 1 | P = NS |
1. The effect of diagnosis was significant: BDI was higher in schizophrenic subjects than in normal controls F1,57 = 14.2, P < 0.001. The interaction diagnosis by experimental conditions was also significant F1,57 = 6.3, P = 0.001. Controls and schizophrenic subjects showed roughly the same level of cognitive bizarreness in their dreams (0.29 ± 0.15 and 0.30 ± 0.19, respectively), whereas the level of cognitive bizarreness was significantly higher—and similar to that of dreams—in the TAT stories of schizophrenic subjects compared with normal controls (0.28 ± 0.18 vs 0.10 ± 0.14).
2. The effect of education was not significant.
3. The interaction experimental conditions by education was not significant.
4. There is almost no linear correlation at all between BDI of dream reports and BPRS total score (r = −0.039, NS) and between BDI of TAT reports and BPRS total score (r = −0.006, NS).
Discussion
To the best of our knowledge, this is the first study that tries to experimentally evaluate the formal properties of dreaming and waking mentation of normal subjects compared with schizophrenic patients; therefore, our results should be cautiously evaluated and need to be replicated.
The quantitative difference between the BDIs of the schizophrenic subjects and the normal controls seems to indicate that, under experimental conditions, awake psychotic subjects show a degree of formal cognitive bizarreness in verbal reports elicited by TAT tables comparable to that found in written reports of dreams of both normal and schizophrenic subjects. This finding seems to indicate that formal bizarreness, which is a peculiar cognitive pattern of the dreaming mental state, is still present as a formal distinctive property of the waking cognitive organization in schizophrenia. Though still very preliminary, these results appear to be consistent with the hypothesis of the dreaming brain as a possible experimental model for psychosis.24
We are fully aware that the issue of dreams and psychosis is still open to debate and that it needs to be addressed with rigor; moreover, it must be stressed that the absence of phenomenologic difference—in terms of cognitive bizarreness—between the dreaming and waking state of psychotic subjects does not necessarily mean that these conditions are pathophysiologically similar.
A relevant consideration must be made about the relationship between cognitive bizarreness and the psychopathological peculiarities of schizophrenic patients. If the intrinsic illogical and outlandish nature of these subjects’ positive symptoms were to influence cognitive bizarreness in waking and dreaming reports through a straightforward inclusion of delusional thoughts in the narratives, a direct correlation between the severity of psychopathology and the BDI score could be expected. The data of the present investigation show no correlation between these variables so we can consider cognitive bizarreness an inherent characteristic of mental organization independent of the psychopathological traits of schizophrenia. In our opinion, the fact that our data deny this hypothesis confirms the validity of an approach that centered the experimental design on the formal aspects of brain activation rather than on contents related to the experience of mental illness.
Furthermore, viewing dream mentation as a model of psychosis, one could hypothesize a correlation between BDI in dreams and waking fantasies in schizophrenic patients; however, this hypothesis is not supported by our results (r = 0.062, NS), and the data show that whereas the mean of BDI is similar among the variables, each case appears to be higher in dream score or in TAT score or sometimes equal in both. Not easily interpretable, this result could depend on the methodology the study used: TAT stories are verbally reported and dreams are written, so the style of reporting could randomly influence the score of single subjects depending, eg, on their own writing or verbal capacities.
Indeed, it is well known that studies focused on the neuropsychological features of schizophrenia consistently report impairments in different aspects of language and writing abilities in these subjects.35
Our sample is still quite small; even so, it may be possible to correlate BDI to specific neuropsychological traits related to writing and language once it has been broadened.
A functional consequence of the similarities between the psychotic mental state and dreaming in the domain of brain organization and neurochemical demodulation could be a decrease in executive ego functions (including thinking) and an increase in emotion-driven cognition. This could account for both the delusional and uncritical thinking, consistent with the hypothesis that the delusionality of schizophrenia and dreaming arise when information integration and processing is deregulated by an excess of emotion.36
Furthermore, the changes in neuromodulatory balance could play a critical role in the defects already attributed to regional activation differences as specified above and to the hallucinosis and the bizarre mentation that we have begun to study here.
Comparison of New Results With Previous Research
In the early years of dream research, dream and TAT reports compared with test psychoanalytic hypotheses unveiled considerable similarities between the psychotic and the dreaming state6,37; however, these similarities have never been quantified or compared with normal dreaming. Studies such as Gordon's investigated normal subjects but did not compare the results with a patient group nor did they adopt the formal approach to mental contents taken here. Cartwright's study tested the characteristics of REM and NREM mental activity in normal and schizophrenic subjects but did not include comparable samples from the waking state. The only study using neuroimaging techniques to directly investigate the similarities between schizophrenia and REM sleep showed no similarity between these 2 mental states.38 Many relevant flaws in the design of the study and the fact it has never since been replicated clearly warrant very cautious interpretation of those data.
Methodological Issues
Though very promising, this preliminary study does have some noteworthy drawbacks. First of all, the psychophysiological heterogeneity of psychotic mental states somehow limits our possibility to generalize. We began studying schizophrenia because our patients were relatively numerous and cooperative, whereas other psychotic conditions such as acute mania and acute organic psychosis are less easily accessible in an experimental setting. Moreover, the schizophrenic subjects were taking psychoactive drugs at the time of data collection, which might have interfered with both dream activity and dream recall.
It is also well known that verbal reports are secondary to individual factors such as intellectual and verbal skills and level of motivation39 and that low motivation and impairment in verbal abilities can be present in schizophrenic subjects.
As far as the different stages of sleep are concerned, we did not monitor the kind of sleep from which the reports derived. As a result, no distinction was made between the reports that fulfilled all the formal characteristics of hallucinoid dreams and those simply describing thought-like activity.40
Another important issue is the evaluation of the subjective experience: if we consider the written reports of a strongly subjective experience such as dreams much less objective than the verbal construction of a fantastic story in response to an external stimulus, our data could be read in the light of first person vs third person perspective. The sleeping brain can, in fact, either generate its own perceptions or it can think about them, but it cannot do both at the same time. Put another way, we could speculate that written reports of dreams and verbal reports of TAT projections could be respectively assimilated to first and third person perspectives. Two of the authors have previously demonstrated that the shift from first to third person perspective can help to modify the insight of delusional patients about their own delusional experience.41
Another relevant point concerns the scientific validity of comparing mental states as apparently different as dreaming and waking. Evidences collected from independent lines of research indeed support a continuum hypothesis between the brain organization of these 2 states, suggesting the appropriateness of their experimental comparison. From a neurophysiological point of view, it has recently been reported that the anatomofunctional pattern of sensorial analyses is preserved during REM sleep28 and according to some authors, REM sleep and wakefulness can be considered fundamentally equivalent brain states, probably subserved by an intrinsic thalamocortical loop, with the main difference lying in the influence of sensory inputs on cognition.42 Furthermore, specific elements of presleep time, such as working life events and stressors, have been shown to influence dream content,43,44 and psychological daytime well-being has been reported to be inversely correlated with the level of aggressiveness and hostility in dreams.45 Moreover, experimental data emerging from neurocognitive research seem to indicate that the dreaming brain generates phenomenological consciousness events (ie, subjective and first person experiences) that are overall similar to those experienced during wakefulness.27
Another point to be emphasized is the remarkable difference in the NWs in the dream reports of the 2 subject groups. No consistent data have been reported in the literature with respect to this matter,31 so our finding needs cautious evaluation. The lack of motivation inherent to the psychopathological conditions of the patients could in part explain the relative shortness of their dream reports, and it could be consistent with the functional deactivation of the dorsolateral prefrontal cortex, which is closely related to the well-known impairment in cognitive abilities such as verbal fluency.35
Conclusive Remarks
In summary, despite the specified methodological problems and the need for replicating the study with larger samples, our data appear to be promising in view of clarifying the mutual relationships between the cognitive characteristics of dreaming and psychotic mental states. Our experimental approach appears to be useful in the light of the convergent evidence from independent lines of research that validate the hypothesis that anatomofunctional modifications of dreaming and psychosis are underpinned by concomitant neurophysiological and common neurochemical patterns of brain activity. In this perspective, bizarreness patterns could be further tested in relation to hypomonoaminergic and hypercholinergic systems linked to the demodulation of cortical activity and to the hyperactivation of subcortical regions such as the limbic system.
This field of research could prove a valuable tool for an experimental evaluation of brain-mind isomorphism in order to shed light on the complex relationship between phenomenologically measurable cognitive traits and underlying neurophysiological activity.
Acknowledgments
We thank K. Britsch for his assistance during the revision of the manuscript.
References
- 1.Jung CG. The psychology of dementia praecox. In: Jung CG, editor. Collected Works Vol. 3—The Psychogenesis of Mental Disease. New York, NY: Nervous and Mental Disease Publ. Co.; 1936. [Google Scholar]
- 2.Freud S. Psychoanalytic notes on an autobiographical account of a case of paranoia. In: Strachey J, editor. Standard Edition of the Complete Psychological Works of Sigmund Freud Vol. 12. London: Hogarth Press; 1958. [Google Scholar]
- 3.Bleuler E. Dementia Praecox or the Group of Schizophrenias. New York, NY: International Universities Press; 1966. [Google Scholar]
- 4.Minkowski E. La schizophrénie. Paris, France: Editions Payot & Rivages; 1997. [Google Scholar]
- 5.Cappon D. Morphology and other parameters of phantasy in the schizophrenias. Arch Gen Psychiatry. 1959;1:17–34. doi: 10.1001/archpsyc.1959.03590010033005. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright RD. Sleep fantasy in normal and schizophrenic persons. J Abnorm Psychol. 1972;80:275–279. doi: 10.1037/h0033738. [DOI] [PubMed] [Google Scholar]
- 7.Fiss H, Klein GS, Bokert E. Waking fantasies following interruption of two types of sleep. Arch Gen Psychiatry. 1966;14:543–551. doi: 10.1001/archpsyc.1966.01730110095015. [DOI] [PubMed] [Google Scholar]
- 8.McCarley RW. Dreams: disguise of forbidden wishes or transparent reflections of a distinct brain state? In: Bilder RM, LeFefer FF, editors. Neuroscience of the Mind on the Centennial of Freud's Project for a Scientific Psychology. New York, NY: The New York Academy of Sciences; 1998. pp. 116–133. [DOI] [PubMed] [Google Scholar]
- 9.Fosse R, Stickgold R, Hobson JA. Thinking and hallucinating: reciprocal changes in sleep. Psychophysiology. 2004;41:298–305. doi: 10.1111/j.1469-8986.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 10.Gillin JC, Buchsbaum MS, Jacobs LS, et al. Partial REM sleep deprivation, schizophrenia and field articulation. Arch Gen Psychiatry. 1974;30:653–662. doi: 10.1001/archpsyc.1974.01760110073009. [DOI] [PubMed] [Google Scholar]
- 11.Saucerman SA. Dissociation of W/REM/NREM states may cause psychotic symptoms. Schizophr Res. 1997;25:261–263. doi: 10.1016/s0920-9964(97)00030-3. [DOI] [PubMed] [Google Scholar]
- 12.Johnson JD. REM sleep and the development of context memory. Med Hypotheses. 2005;64:499–504. doi: 10.1016/j.mehy.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Ellenbogen JM. Human relational memory requires time and sleep. Proc Natl Acad Sci USA. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brüne M. “Theory of Mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 15.Crick F, Mitchison G. The function of REM sleep. Nature. 1983;304:111–114. doi: 10.1038/304111a0. [DOI] [PubMed] [Google Scholar]
- 16.Kelly PH. Defective inhibition of dream event memory formation: a hypothesized mechanism in the onset and progression of symptoms of schizophrenia. Brain Res Bull. 1998;46:189–197. doi: 10.1016/s0361-9230(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 17.Fischman LG. Dreams, hallucinogenic drug states, and schizophrenia: a psychological and biological comparison. Schizophr Bull. 1983;9:73–94. doi: 10.1093/schbul/9.1.73. [DOI] [PubMed] [Google Scholar]
- 18.Morgane PJ, Stern WC. Relationship of sleep to neuroanatomical circuits, biochemistry and behaviour. Ann N Y Acad Sci. 1972;193:95–111. doi: 10.1111/j.1749-6632.1972.tb27827.x. [DOI] [PubMed] [Google Scholar]
- 19.Benson KL, Zarcone VP. Schizophrenia. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders Company; 2000. pp. 1159–1167. [Google Scholar]
- 20.Bymaster FP, McKinzie DL, Felder CC. New evidence for the involvement of muscarinic cholinergic receptors in psychoses. In: Silman I, Soreq H, Anglister L, Michaelson D, Fisher A, editors. Cholinergic Mechanisms: Function and Dysfunction. London: Taylor & Francis; 2004. pp. 331–343. [Google Scholar]
- 21.Gottesmann C. The dreaming sleep stage: a new neurobiological model of schizophrenia? Neuroscience. 2006;140:1105–1115. doi: 10.1016/j.neuroscience.2006.02.082. [DOI] [PubMed] [Google Scholar]
- 22.Drummond SP, Smith MT, Orff HJ, Chengazi V, Perlis ML. Functional imaging of the sleeping brain: review of findings and implications for the study of insomnia. Sleep Med Rev. 2004;8:243–247. doi: 10.1016/j.smrv.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Weinberger DR, McClure RK. Neurotoxicity, neuroplasticity, and magnetic resonance imaging morphometry: what is happening in the schizophrenic brain? Arch Gen Psychiatry. 2002;59:553–558. doi: 10.1001/archpsyc.59.6.553. [DOI] [PubMed] [Google Scholar]
- 24.Hobson A. A model for madness? Nature. 2004;430:21. doi: 10.1038/430021a. [DOI] [PubMed] [Google Scholar]
- 25.Williams J, Merritt J, Rittenhouse C, Hobson JA. Bizarreness in dreams and fantasies: implications for the activation-synthesis hypothesis. Conscious Cogn. 1992;1:172–185. [Google Scholar]
- 26.Hobson JA, Hoffman SA, Helfand R, Kostner D. Dream bizarreness and the activation-synthesis hypothesis. Hum Neurobiol. 1987;6:157–164. [PubMed] [Google Scholar]
- 27.Revonsuo A. Revonsuo A, ed. Inner Presence: Consciousness as a Biological Phenomenon. Cambridge, Mass: MIT Press; 2006. Binding in dreams; pp. 239–253. [Google Scholar]
- 28.Schwartz S, Maquet P. Sleep imaging and the neuropsychological assessment of dreams. Trends Cogn Sci. 2002;6:23–30. doi: 10.1016/s1364-6613(00)01818-0. [DOI] [PubMed] [Google Scholar]
- 29.Kocel KM. Cognitive abilities, handedness, familial sinistrality and sex. Ann N Y Acad Sci. 1977;299:233–243. doi: 10.1111/j.1749-6632.1977.tb41910.x. [DOI] [PubMed] [Google Scholar]
- 30.Hall CS, Van De Castle RL. The Content Analysis of Dreams. New York, NY: Meredith Publishing Company; 1966. [Google Scholar]
- 31.Kramer M. Manifest dream content in psychopathologic states. In: Kramer M, editor. Dream Psychology and the New Biology of Dreaming. Springfield, Ill: Charles C. Thomas Publisher; 1969. pp. 377–396. [Google Scholar]
- 32.Braff DL. Psychophysiological and information processing approaches in schizophrenia. In: Charney DS, Nestler E, Bunney BS, editors. Neurobiological Foundations of Mental Illness. New York, NY: Oxford University Press; 1999. pp. 258–271. [Google Scholar]
- 33.Murray HA. Thematic Apperception Test Manual. Cambridge, MA: Harvard University Press; 1938. [Google Scholar]
- 34.SPSS 11.0. Chicago, IL: SPSS Inc; 2001. [Google Scholar]
- 35.Williamson P. Williamson P, ed. Mind, Brain, and Schizophrenia. New York, NY: Oxford University Press; 2006. Neuropsychological studies. [Google Scholar]
- 36.Mujica-Parodi LR, Malaspina D, Sackheim HA. Logical processing, affect and delusional thought in schizophrenia. Harv Rev Psychiatry. 2000;8:73–83. [PubMed] [Google Scholar]
- 37.Gordon HL. A comparative study of dreams and responses to the thematic apperception test: I. A need-press analysis. J Pers. 1953;22:234–253. doi: 10.1111/j.1467-6494.1953.tb01808.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiler MA, Buchsbaum MS, Gillin JC, Tafalla R, Bunney WE., Jr Explorations in the relationship of dream sleep to schizophrenia using positron emission tomography. Neuropsychobiology. 1990;23:109–118. doi: 10.1159/000119435. [DOI] [PubMed] [Google Scholar]
- 39.Schredl M. Dream research: integration of physiological and psychological models. Behav Brain Sci. 2000;23:1001–1003. [Google Scholar]
- 40.Casagrande M, Violani C, Lucidi F, Buttinelli E, Bertini M. Variations in sleep mentation as a function of time of night. Int J Neurosci. 1996;85:19–30. doi: 10.3109/00207459608986348. [DOI] [PubMed] [Google Scholar]
- 41.Gambini O, Barbieri V, Scarone S. Theory of Mind in schizophrenia: first person vs third person perspective. Conscious Cogn. 2004;13:39–46. doi: 10.1016/S1053-8100(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 42.Llinàs RR, Paré D. Of dreaming and wakefulness. Neuroscience. 1991;44:521–535. doi: 10.1016/0306-4522(91)90075-y. [DOI] [PubMed] [Google Scholar]
- 43.Schredl M, Hofmann F. Continuity between waking activities and dream activities. Conscious Cogn. 2003;12:298–308. doi: 10.1016/s1053-8100(02)00072-7. [DOI] [PubMed] [Google Scholar]
- 44.Grenier J, Cappeliez P, St-Onge M, et al. Temporal references in dreams and autobiographical memory. Mem Cognit. 2005;33:280–288. doi: 10.3758/bf03195317. [DOI] [PubMed] [Google Scholar]
- 45.Pesant N, Zadra A. Dream content and psychological well-being: a longitudinal study of the continuity hypothesis. J Clin Psychol. 2006;62:111–121. doi: 10.1002/jclp.20212. [DOI] [PubMed] [Google Scholar]