Abstract
It has been proposed that the loose associations characteristic of thought disorder in schizophrenia result from an abnormal increase in the automatic spread of activation through semantic memory. We tested this hypothesis by examining the time course of neural semantic priming using event-related potentials (ERPs). ERPs were recorded to target words that were directly related, indirectly related, and unrelated to their preceding primes, while thought-disordered (TD) and non-TD schizophrenia patients and healthy controls performed an implicit semantic categorization task under experimental conditions that encouraged automatic processing. By 300–400 milliseconds after target word onset, TD patients showed increased indirect semantic priming relative to non-TD patients and healthy controls, while the degree of direct semantic priming was increased in only the most severely TD patients. By 400–500 milliseconds after target word onset, both direct and indirect semantic priming were generally equivalent across the 3 groups. These findings demonstrate for the first time at a neural level that, under automatic conditions, activation across the semantic network spreads further within a shorter period of time in specific association with positive thought disorder in schizophrenia.
Keywords: schizophrenia, N400, semantic priming
Introduction
Language and thought disturbances have long been considered core features of schizophrenia,1 at their extreme manifesting as “positive thought disorder.”2 Because positive thought disorder is often characterized by the intrusion of inappropriate semantic associations between words, some have posited that it results from an abnormally heightened spread of activation within semantic memory.3 This study used event-related potentials (ERPs) to demonstrate a faster rate in the spread of activation across semantic memory in thought-disordered (TD) schizophrenia patients at the neural level by examining early and late N400 semantic priming effects.
Semantic memory is often conceptualized as a network of interconnected nodes organized by semantic relationship.4,5 The semantic priming effect describes the faster response to targets preceded by semantically related, relative to unrelated, primes.6 This facilitated response to the target is thought to arise, in part, from its partial preactivation due to an automatic spread of activation from the activated prime. Indirect semantic priming provides the most compelling evidence for spreading activation7,8 because it occurs when the prime is not directly related to the target but when it is associated with an unseen mediator that is, in turn, associated with the target (eg, lion stripes via tiger). Strategic processes can also contribute to semantic priming; however, a short interval (<400 milliseconds) between the onset of the prime and the target (the stimulus-onset asynchrony, SOA) and a low proportion of related words in the stimulus set reduce the influence of such controlled processes.9
Under automatic conditions, schizophrenia patients with positive thought disorder show increased direct10–12 and indirect3,13–15 semantic priming relative to both non-TD patients and healthy controls. Other investigators, however, have reported normal16 and sometimes even reduced17 direct semantic priming in schizophrenia at short SOAs (although most of these latter studies have not accounted for thought disorder). (Under controlled processing conditions in which strategic processes can operate, semantic priming is generally reduced in schizophrenia [see Minzenberg et al18 and Kuperberg et al19 for reviews of this literature]; a recent neuroimaging study of controlled semantic priming in schizophrenia indicates that thought disorder is associated with inappropriate increases in hemodynamic activity to directly and indirectly related words, relative to unrelated words.20)
While reaction time (RT) has been the most common measure of semantic priming in schizophrenia, it is potentially confounded both by the overall slower RTs typically exhibited by patients21 and by the inherent necessity of requiring a behavioral response to each trial of interest. Consequently, even RT data collected under automatic conditions may partially reflect decision-making processes in addition to automatic spreading activation at the neural level.
ERPs are scalp-recorded fluctuations in voltage that are an online measure of brain activity and that can be passively recorded in the absence of behavioral responses. The N400 component, a negative-going waveform peaking approximately 400 milliseconds after target onset, reflects the difficulty of semantically integrating a word into its preceding context.22 Words preceded by semantically unrelated words elicit a larger, more negative N400 than words preceded by semantically related words,23,24 a modulation known as the N400 effect.25 We have recently demonstrated a significant N400 effect to indirectly primed (relative to unrelated) targets under automatic conditions. This effect was smaller than that evoked to directly primed (relative to unrelated) targets under these conditions, suggesting that the N400 is sensitive to automatic spreading activation.7
There have been 2 ERP studies of automatic semantic processing in schizophrenia. In a lexical decision task with a 350-millisecond SOA, Condray et al26 found an abnormally reduced semantic priming N400 effect to directly related (relative to unrelated) targets in schizophrenia patients but the relationship between thought disorder and N400 modulation was not reported. Mathalon et al27 used a picture-word–matching task with a SOA of 325 milliseconds to demonstrate that when target words were preceded by pictures representing the same word, no differences in N400 amplitude were found between schizophrenia patients and healthy controls. When target words were preceded by moderately related pictures belonging to the same superordinate categories, schizophrenia patients exhibited smaller N400 amplitudes than healthy controls, although positive thought disorder failed to predict N400 amplitude. The smaller N400 amplitudes to these moderately related targets provides intriguing evidence for hyperactivity of the semantic network in schizophrenia. However, because there were no completely unrelated picture-word pairs, the degree of semantic priming per se (the N400 effect to related relative to unrelated targets) was not assessed.
Thus, to date, the finding of increased automatic semantic priming in schizophrenia has not been replicated at the neural level. One reason for this may be that ERP studies in schizophrenia have only examined automatic priming using directly related word pairs. Because directly associated words are presumably automatically activated by both patients and controls, indirect semantic priming may be a more sensitive measure of any heightened activation (or reduced inhibition) in the semantic memory network.3 In addition, both the ERP studies reviewed above26,27 required participants to make a decision on each trial and thus may be somewhat confounded by the same decision-making processes as RT studies.
The present study utilized ERPs to index automatic spreading activation in schizophrenia patients and healthy controls. Both directly and indirectly related word pairs were examined using a short SOA. Additionally, because the results from both the behavioral and ERP literature have been mixed, possibly, in part, because of strategic confounds, we used an implicit semantic categorization task that required no behavioral response on trials of interest.28 If positive thought disorder is indeed associated with a faster and further spread of activation, this would predict both increased direct and indirect neurophysiological priming—an increased N400 effect to both directly and indirectly related (each relative to unrelated) targets—in TD patients relative to non-TD patients or healthy controls.
METHODS
Participants
Eighteen outpatients meeting Diagnostic and Statistical manual of Mental Disorders, Fourth Edition, Text Revision, criteria for schizophrenia were recruited from the Lindemann Mental Health Center, Boston, MA, and 18 demographically matched healthy volunteers, screened to exclude histories of psychiatric disorders29 and any current medication affecting the central nervous system, were recruited by advertisement. All participants were right-handed30,31 native English speakers and had normal/corrected-to-normal vision, no history of traumatic head injury, and no substance abuse within the past 6 months or any history of substance dependence. Written informed consent was obtained from all participants according to Massachusetts General Hospital and Tufts Human Subjects Research Committees guidelines. All but one unmedicated patient were receiving stable doses of antipsychotic medication; no patients were taking anticholinergic medication. Patients’ symptomatology was rated using the Scales for the Assessment of Negative Symptoms (SANS)32 and the Positive and Negative Syndrome Scale (PANSS).33 Thought disorder was assessed in depth using the Scale for the Assessment of Thought Language and Communication (TLC); TLC ratings were performed by the first author on the basis of a clinical interview on the day of ERP testing.34,35 A language disorganization or positive thought disorder score was calculated by summing the following TLC items: circumstantiality, tangentiality, loss of goal, derailment, illogicality, and incoherence.36,37 A median split on this score was used to subdivide patients into positively TD and non-TD groups. The 3 resulting groups were well matched on demographic characteristics such as gender and race distribution, education, parental socio economic status,38 and premorbid IQ39 (all Ps > .27, see table 2), except that the TD patients were older than either the non-TD patients [t15 = 3.555, P < .01] or the healthy controls [t24 = 2.254, P < .05]. The TD and non-TD groups were matched on clinical characteristics including medication dosage, overall psychopathology, hallucinations, delusions, and negative symptoms (all Ps > .13), except that the mean length of illness was greater in the TD than the non-TD group [t14 = 2.505, P < .05]. In all analyses, we covaried for both years of illness and age whenever significant effects emerged between groups.
Table 2.
Demographic and Psychopathological Data of Healthy Controls, Non-TD, and TD Patients With Schizophrenia
| Parameter | Control | Non-TD | TD |
| Gender (M/F) | 12/6 | 7/2 | 5/3 |
| Race (C/AA) | 16/2 | 9/0 | 6/2 |
| Age (y) | 42 (8) | 37 (8) | 49 (6) |
| Education (y) | 15 (2) | 13 (3) | 13 (2) |
| Hollingshead index | 3 (1) | 3 (2) | 3 (1) |
| Premorbid IQ | 113 (9) | 109 (14) | 109 (12) |
| CPZ equivalent | — | 393 (365) | 456 (542) |
| Duration of illness (y) | — | 11 (8) | 23 (12) |
| PANSS total | — | 53.9 (13.0) | 65.0 (16.0) |
| PANSS hallucination | — | 2.4 (1.8) | 3.7 (2.3) |
| PANSS delusion | — | 2.1 (1.4) | 3.4 (2.3) |
| SANS total | — | 30.9 (18.0) | 39.8 (11.0) |
| TLC total | — | 1.4 (1.7) | 6.8 (3.7) |
Note: Means are shown with SD in brackets. Hollingshed Index was used as a measure of parental socioeconomic status.35 American version of the National Adult Reading Test was used as a measure of premorbid IQ.36 TD, thought-disordered; M, Male; F, Female; C, Caucasian; AA, African American; CPZ, chlorpromazine equivalent; PANSS, Positive and Negative Syndrome Scale; SANS, Scales for the Assessment of Negative Symptoms; TLC, Thought Language and Communication.
Design and Stimulus Materials
The development of the stimuli is described in detail by Kreher et al.7 Word pairs were designed such that, across all participants, exactly the same target was seen in each of the 3 relatedness conditions but that no individual saw the same prime or target more than once (avoiding repetition priming effects). Two hundred one triplets were developed in which target words (eg, stripes) were paired with directly related primes (eg, tiger), indirectly related primes (eg, lion), or unrelated primes (eg, truck, see table 1). Targets were counterbalanced across 3 lists in a Latin square design (67 pairs per condition). Each participant saw only one list.
Table 1.
Example of Word Pairs Counterbalanced Across Conditions, Derived From the Triplet “Lion-Tiger Stripes”
| Relatedness Condition | Example | Frequency | Word Length |
| Directly related | Tiger stripes | Prime: 92.16 (139.16) | Prime: 5 (2) |
| Target: 96.37 (478.96)a | Target: 5 (2)a | ||
| Indirectly related | Lion stripes | Prime: 70.55 (159.01) | Prime: 6 (2) |
| Target: 96.37 (478.96)a | Target: 5 (2)a | ||
| Unrelated | Truck stripes | Prime: 70 (133) | Prime: 5 (1) |
| Target: 96.37 (478.96)a | Target: 5 (2)a |
Note: Means are shown with SD in brackets.
The frequency and word length of the targets across the 3 conditions are identical because the exactly same words were counterbalanced, across participants, across the 3 conditions. There were also no significant differences in the frequency43 of prime words across the 3 conditions (direct vs unrelated t200 = .745, P > .1; indirect vs unrelated t200 = −.826, P > .1; direct vs indirect t200 = 1.845, P > .05).
ERP Procedure
Participants performed an implicit semantic categorization task28 in which they monitored all words (primes and targets) for occasional exemplars of probe (food) words that were introduced as filler items and pressed a button when probes were detected. No response was required to other (nonfood) words. This task ensured that participants attempted to process all words at a deep semantic level. There were no food words in the prime-target pairs of interest.
Each trial began with a central fixation point (500 milliseconds), followed by a 500-millisecond blank screen. Prime and target words were then each presented for 250 milliseconds (inter-stimulus intervals [ISI] 100 milliseconds). Following a 900-millisecond ISI (to avoid component overlap), a second word pair trial appeared. In between every 2 trials, the 900-millisecond ISI was followed by a cue indicating that participants could blink and rest their eyes that remained on the screen for 3000 milliseconds. Participants were given 18 practice trials prior to the experiment.
Electrophysiological Recording
Twenty-nine active tin electrodes were held in place on the scalp by an elastic cap (Electro-Cap International, Inc, Eaton, OH), see figure 1 for montage. Electrodes were placed below the left eye and at the outer canthus of the right eye to monitor vertical and horizontal eye movements and also over the left mastoid (reference) and right mastoid (recorded actively to monitor for differential mastoid activity). Electroencphalogram (EEG) electrode impedances were maintained below 5 kΩ.
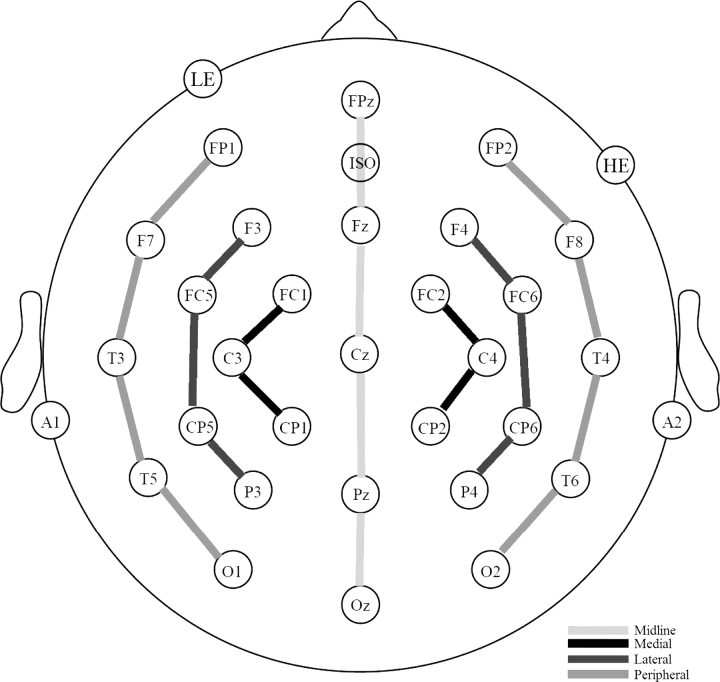
Fig. 1.
Electrode Montage. Electrodes placed in the standard International 10–20 System locations included 5 sites along the midline (FPz, Fz, Cz, Pz, and Oz) and 8 lateral sites, 4 over each hemisphere (F3/F4, C3/C4, T3/T4, and P3/P4). Eight additional 10–20 sites were altered to form a circle around the perimeter of the scalp. These altered sites included FP1′/FP2′ (33% of the distance along the circle between T3/T4), F7′/F8′ (67% of the distance between FPz and T3/T4), T5′/T6′ (33% of the distance between T3/T4 and Oz), and O1′/O2′ (67% of the distance between T3/T4 and Oz). In addition, 8 extended 10–20 system sites were also used (FC1/FC2, FC5/FC6, CP1/CP2, and CP5/CP6). The dotted lines represent the 4 columns used in analyses (ie, midline, medial, lateral, and peripheral).
The EEG signal was amplified by an Isolated Bioelectric Amplifier System Model HandW-32/BA (SA Instrumentation Co, San Diego, CA) with a bandpass of 0.01–40 Hz and was continuously sampled at 200 Hz by an analogue-to-digital converter. The stimuli and participants’ behavioral responses were simultaneously monitored by a digitizing computer.
ERP Data Analysis
Averaged ERPs, time locked to target words, were formed off line from trials free of ocular and muscular artifacts and were quantified by calculating the mean amplitude (relative to a 100-millisecond prestimulus baseline) and peak latency values in time windows of interest. All sites were included in a systematic, comprehensive columnar “pattern of analyses” applied in prior studies in healthy individuals7 and in patients with schizophrenia.37 This approach yielded statistical information about differences in the distribution of effects along the anterior-posterior (AP) axis of the scalp and across the 2 hemispheres at columns covering the whole scalp (see figure 1).
At each column, a series of planned mixed model repeated measures analyses of variance (ANOVAs) were performed. We were interested, a priori, in examining differences between groups in direct N400 priming effects (directly related vs unrelated targets) and indirect N400 priming effects (indirectly related vs unrelated targets). Thus, our initial ANOVAs contained 3 levels of the between-subjects factor group (control, TD, or non-TD) and 2 levels of the within-subjects factor relatedness (either directly related vs unrelated or indirectly related vs unrelated). Any difference in direct or indirect N400 priming between groups would therefore be manifest by group by relatedness interactions. Significant interactions between group and relatedness were then followed up by conducting 2 × 2 ANOVAs, in order to examine differences between 2 groups (control vs TD, TD vs non-TD, and control vs non-TD) in N400 priming effects. Any group by relatedness interactions in these 2 × 2 ANOVAs were then followed up in 2 ways: first by conducting within-group ANOVAs to determine whether N400 effects were significant within each group separately and second by conducting between-group ANOVAs to determine whether the amplitudes of the N400 to each type of target was significantly different between groups.
In all these ANOVAs, in addition to relatedness, within-subject factors included AP distribution (number of levels depending on the number of electrode sites in each column, figure 1) and, at the 3 lateral electrode columns, hemisphere (2 levels: left, right). We used the Geisser-Greenhouse correction to protect against type 1 error resulting from violations of sphericity40 and a significance level of alpha = .05 because, in all cases, we were testing a priori hypotheses.
To obtain a fine-grained analysis of the time course of the N400 effect, we subdivided the N400 time window into two 100-millisecond time windows: 300–400 millisecond (capturing the upslope and onset of the effect) and 400–500 millisecond (capturing the downslope and offset of the effect) and conducted statistical analyses within each of these early and late subcomponents. (In order to ensure that we were capturing the true onset and offset of the N400, we performed an additional individualistic analysis: each individual's N400 peak was located, and then mean amplitudes were measured 100 millisecond before and after their peak. Thus, our early and late N400 effects did not reflect a single time window but each individual's true upslope and downslope of the N400 component. The results obtained through these analyses were identical to those reported here, indicating that we did capture the onset and offset of the N400 effect for our participants in the 2 selected time windows.) There were no main effects of group except at the medial column for both early and late direct and indirect N400 priming effects (all Fs > 3.4, all Ps < .05) due to larger absolute N400 amplitudes in TD patients. Below, we focus on main effects and interactions involving relatedness that were of most theoretical interest. Any interactions between relatedness, hemisphere, and/or AP distribution not noted below or in table 3 were nonsignificant (all Ps > .07).
Table 3.
Mixed Model ANOVAs at all Electrode Columns
| Main Effect of Relatedness |
Group (Control, TD, and Non-TD) × Relatedness |
Group (TD and Non-TD) × Relatedness |
Group (TD and Control) × Relatedness |
|||||
| df | F Value | df | F Value | df | F Value | df | F Value | |
| A. Directly related vs unrelated | ||||||||
| (i) N400: Early | ||||||||
| Midline | 1,32 | 2.99∼ | 2,32 | 1.23 | — | — | — | — |
| Medial | 1,32 | 3.48∼ | 2,32 | 1.34 | — | — | — | — |
| Lateral | 1,32 | 1.50 | 2,32 | 1.19 | — | — | — | — |
| Peripheral | 1,32 | <1 | 2,32 | 1.76 | — | — | — | — |
| (ii) N400: Late | ||||||||
| Midline | 1,32 | 14.21** | 2,32 | <1 | — | — | — | — |
| Medial | 1,32 | 14.81** | 2,32 | 1.28 | — | — | — | — |
| Lateral | 1,32 | 11.13** | 2,32 | <1 | — | — | — | — |
| Peripheral | 1,32 | 7.90** | 2,32 | <1 | — | — | — | — |
| B. Indirectly related vs unrelated | ||||||||
| (i) N400: Early | ||||||||
| Midline | 1,32 | <1 | 2,32 | 2.99∼ | 1,15 | 9.14** | 1,24 | 3.06∼ |
| Medial | 1,32 | 1.34 | 2,32 | 3.11∼ | 1,15 | 8.07* | 1,24 | 2.79 |
| Laterala | 1,32 | <1 | 2,32 | 3.43* | 1,15 | 10.22** | 1,24 | 3.05∼ |
| Peripheral | 1,32 | <1 | 2,32 | 4.32* | 1,15 | 18.22** | 1,24 | 3.64∼ |
| (ii) N400: Late | ||||||||
| Midline | 1,32 | 4.09∼ | 2,32 | <1 | — | — | — | — |
| Medial | 1,32 | 6.13* | 2,32 | 1.45 | — | — | — | — |
| Lateralb | 1,32 | 4.27* | 2,32 | 1.73 | — | — | — | — |
| Peripheral | 1,32 | 3.53∼ | 2,32 | 1.51 | — | — | — | — |
Note:Dashes in empty cells indicate that no follow-ups were conducted because of the absence of higher order effects. ANOVA, analysis of variance. ∼P < .10, *P < .05, **P < .01.
There was also a 4-way interaction between group, relatedness, anterior-posterior (AP) distribution, and hemisphere, F6,96 = 2.77, P < .05. This arose due to the tendency for TD patients to show increased indirect N400 priming, particularly at right hemisphere and posterior sites compared with healthy controls (group by relatedness by hemisphere interaction, F1,24 = 4.06, P = .055) and non-TD patients (group by relatedness by AP distribution by hemisphere, F3,45 = 4.74, P < .05; group by relatedness by hemisphere, F1,15 = 4.11, P = .06).
There was also a trend toward a 4-way interaction between group, relatedness, AP distribution, and hemisphere, F6,96 = 2.354, P = .06. Follow-up simple effects ANOVAs comparing the TD and non-TD patients at this electrode column demonstrated a significant group by relatedness by AP distribution by hemisphere interaction (F3,45 = 3.31, P = .05) and a trend toward a group by relatedness interaction (F1,15 = 3.57, P = .08); these interactions arose because TD patients showed increased indirect N400 priming, particularly at right hemisphere and more anterior sites (F4: t15 = 2.22, P < .05; FC6: t15 = 2.50, P < .05; all other Ps > .1). No interactions involving group and relatedness were observed in comparing TD patients and healthy controls or non-TD patients and healthy controls (all Fs < 1.7, all Ps > .2).
Results
Although both patients and controls correctly identified food words more than 90% of the time, patients were significantly less accurate than controls [t34 = 3.409, P < .01]. However, there was no significant difference in accuracy between TD and non-TD groups [t15 = 1.131, P = .3]. One patient with a 40% error rate was excluded from all ERP analyses. Thirteen percent of experimental trials were rejected for artifact. These did not differ significantly between conditions or between the 3 groups (all Ps > .4).
Early ERP Components
There were no significant main effects of relatedness (all Fs < 1) or group by relatedness interactions (all Fs < 3, all Ps > .07) on the amplitude of ERPs during the first 200 millisecond.
N400 Peak Latency
ANOVAs examining N400 peak latency, measured between 300–500 millisecond across the 3 relatedness conditions, failed to reveal main effects of relatedness or group by relatedness interactions (all Fs < 2.3, all Ps > .08). There was a main effect of group only at the midline column [F2,32 = 3.399, P < .05) that arose because TD patients had slightly later (on average 30 millisecond) N400 peak latencies than the non-TD patients (all Fs > 4.8, all Ps < .05); there were no differences in N400 peak latency between TD patients and healthy controls (all Fs < 1).
N400 Amplitude
Direct N400 Priming Effect
300–400 Milliseconds
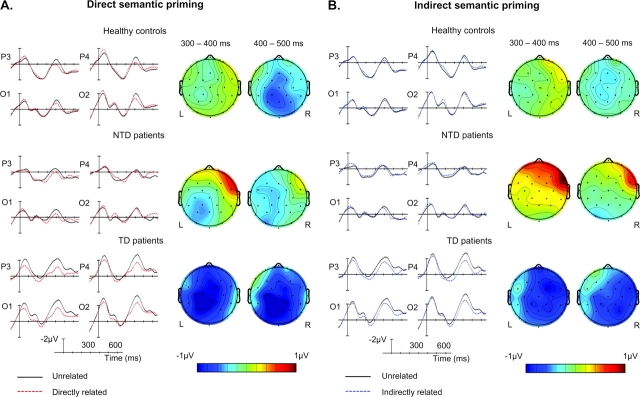
Comparisons between directly related and unrelated targets revealed a significant early N400 effect across all participants (figure 2A), particularly over the right hemisphere, as evidenced by significant relatedness by hemisphere interactions at all columns (medial: F1,32 = 7.033, P < .05; lateral: F1,32 = 5.022, P < .05; peripheral: F1,32 = 5.313, P < .05). No interactions involving group and relatedness were observed (table 3Ai), indicating that the degree of direct semantic priming did not differ across the 3 groups.
Fig. 2.
(A) Event-Related Potentials (ERPs) to Target Words at 4 Parietal-Occipital Sites. Linearly interpolated voltage maps showing the scalp distribution of differences in ERPs elicited by target words produced using EEGLAB v4.512 for MatLab software: scalp distributions of comparisons between unrelated-directly related in controls, TD patients and non-TD patients in early and late N400 windows.(B)ERPs to target words at 4 parietal-occipital sites; linearly interpolated voltage maps showing the scalp distribution of differences in ERPs elicited by target words produced using EEGLAB v4.512 for MatLab software: scalp distributions of comparisons between unrelated-indirectly related in controls, TD patients, and non-TD patients in early and late N400 windows; note that ERPs to unrelated targets are identical to those of figure 2A.
400–500 Milliseconds
A significant semantic priming late N400 effect to directly related (relative to unrelated) targets was again observed across all participants (figure 2A), reflected by main effects of relatedness at all columns (table 3Aii). There were no interactions involving group and relatedness (table 3Aii), again indicating that the degree of direct priming was similar across groups.
Indirect N400 Priming Effect
300–400 Milliseconds
In comparing indirectly related with unrelated target words, group by relatedness interactions reached or approached significance at all columns (table 3Bi). Planned 2 × 2 follow-ups revealed significantly greater early N400 effects to indirectly related (relative to unrelated) in the TD than the non-TD patients (figure 2B) as indicated by highly significant group by relatedness interactions at all columns (table 3Bi). The TD patients also appeared to show greater indirect N400 priming effects in this time window than the healthy controls (figure 2B) as reflected by trends toward group by relatedness interactions (table 3Bi) and toward group by relatedness by hemisphere interactions at the medial (F1,24 = 3.333, P = .08) and lateral (F1,24 = 4.057, P = .06) columns. Indirect N400 priming effects did not, however, differ between non-TD patients and healthy controls (no group by relatedness interactions: all Fs < 1.5, all Ps > .23).
To follow up the group by relatedness interactions in the 2 × 2 ANOVAs, both within-group and between-group ANOVAs were carried out. Within-group ANOVAs examined N400 priming effects to indirectly related (relative to unrelated) targets within each of the 3 groups separately. Significant early N400 priming effects to indirectly related (relative to unrelated) targets were observed in the TD patients (midline: F1,7 = 5.807, P < .05; medial: F1,7 = 4.533, P = .07; lateral: F1,7 = 5.557, P = .05; peripheral: F1,7 = 11.035, P < .05). In the non-TD patients, there was a slight reversed early N400 priming effect to indirectly related (relative to unrelated) targets that reached significance only at the peripheral column (F1,8 = 6.824, P < .05); in the non-TD patients, indirectly related targets elicited more negative N400 amplitudes than unrelated targets at some electrode sites. Finally, no significant early N400 priming effects to indirectly related (relative to unrelated) targets were seen in the healthy controls (all Fs < 1). Between-group ANOVAs examined absolute N400 amplitudes elicited by target words in each relatedness condition separately. There were no differences in the amplitude of the N400 to the indirectly related targets between any of the 3 groups (all Fs < 2.5, all Ps > .1). The unrelated targets evoked a more negative absolute N400 amplitudes in TD patients relative to healthy controls (midline: F1,24 = 6.186, P < .05; medial: F1,24 = 8.269, P < .01; lateral: F1,24 = 5.778, P < .05; peripheral: F1,24 = 3.494, P = .07). However, no differences in the amplitude of the N400 to the unrelated targets were found between TD and non-TD patients (all Fs < 3.18, all Ps > .1) or between controls and non-TD patients (all Fs < 2.33, all Ps > .14).
400–500 Milliseconds
A late N400 effect to indirectly related (relative to unrelated) targets was present across all participants (figure 2B), indicated by main effect of relatedness that reached or approached significance at all columns (table 3Bii). There were no group by relatedness interactions (table 3Bii).
Potential Confounds and Effects of Clinical Variables
ANOVAs were repeated after covarying for factors that differed significantly between groups to determine whether they confounded findings. (To determine whether any of the factors found to differ significantly between groups [age, length of illness, and accuracy] exerted differential effects on N400 effects between groups, we tested for interactions between these variables and group. These variables were cross multiplied with our group variable and the cross product terms were entered into analyses of covariance (ANCOVAs); in each model, the main effects of group and either age, length of illness, or accuracy were retained. These interaction terms were neither significant in any ANCOVA [all Fs < 1.7, all Ps > .21] nor did these factors interact significantly with priming effects [all Fs < 2.3, all Ps > .14 ]. We therefore removed these interaction terms from our ANCOVA analyses.) First, after covarying for age, at lateral and peripheral columns, particularly over the right hemisphere, TD patients still showed a larger early N400 effect to indirectly related (relative to unrelated) targets than non-TD patients; they also showed a significantly larger early N400 effect to indirectly related (relative to unrelated) targets than healthy controls (group by relatedness by hemisphere interactions, Fs > 4.8, Ps < .05). Second, after covarying for length of illness, the early N400 effect to indirectly related (relative to unrelated) targets remained larger in the TD than the non-TD patients (Fs > 4.8, Ps < .05 at all columns). Finally, because the TD patients performed slightly less accurately than controls on probe trials, we repeated analyses covarying for behavioral performance. In these models, TD patients showed significantly larger early N400 effects to indirectly related (relative to unrelated) targets than controls, particularly over the right hemisphere (group by relatedness by hemisphere interactions at lateral and peripheral columns, Fs > 4.5, Ps < .05).
The specificity of these findings to positive thought disorder was further examined through analyses covarying for negative symptoms (SANS) and overall psychopathology excluding thought disorder (total PANSS excluding conceptual disorganization score). Differences in early indirect N400 priming between the TD and non-TD patients remained significant (Fs > 6.0, Ps < .05 at all columns).
Finally, we carried out Spearman rank correlations within the patient group to explore relationships between direct and indirect N400 priming effects in both time windows (calculated by subtracting mean amplitudes of primed from unprimed target words at the CP2 electrode site, where N400 effects were maximal in all groups) and various clinical measures. In the early N400 time window, language disorganization on the TLC correlated with the magnitude of both the direct priming effect, ie, the difference in the N400 amplitude between directly related and unrelated targets (Spearman r = −.52, P < .05), and the indirect priming effect, ie, the difference in the N400 amplitude between indirectly related and unrelated targets (Spearman r = −.55, P < .05) (figure 3A and B). In the later N400 time window, there was a trend toward a correlation between language disorganization and the direct priming effect (Spearman r = −.44, P = .07) but not the indirect priming effect (P > .1). These effects were specific: there were no significant correlations between direct or indirect N400 priming effects in the early or late time window and negative symptoms, hallucinations, delusions, and overall psychopathology excluding conceptual disorganization, chlorpromazine equivalents, or length of illness (all Ps > .09).
Fig. 3.
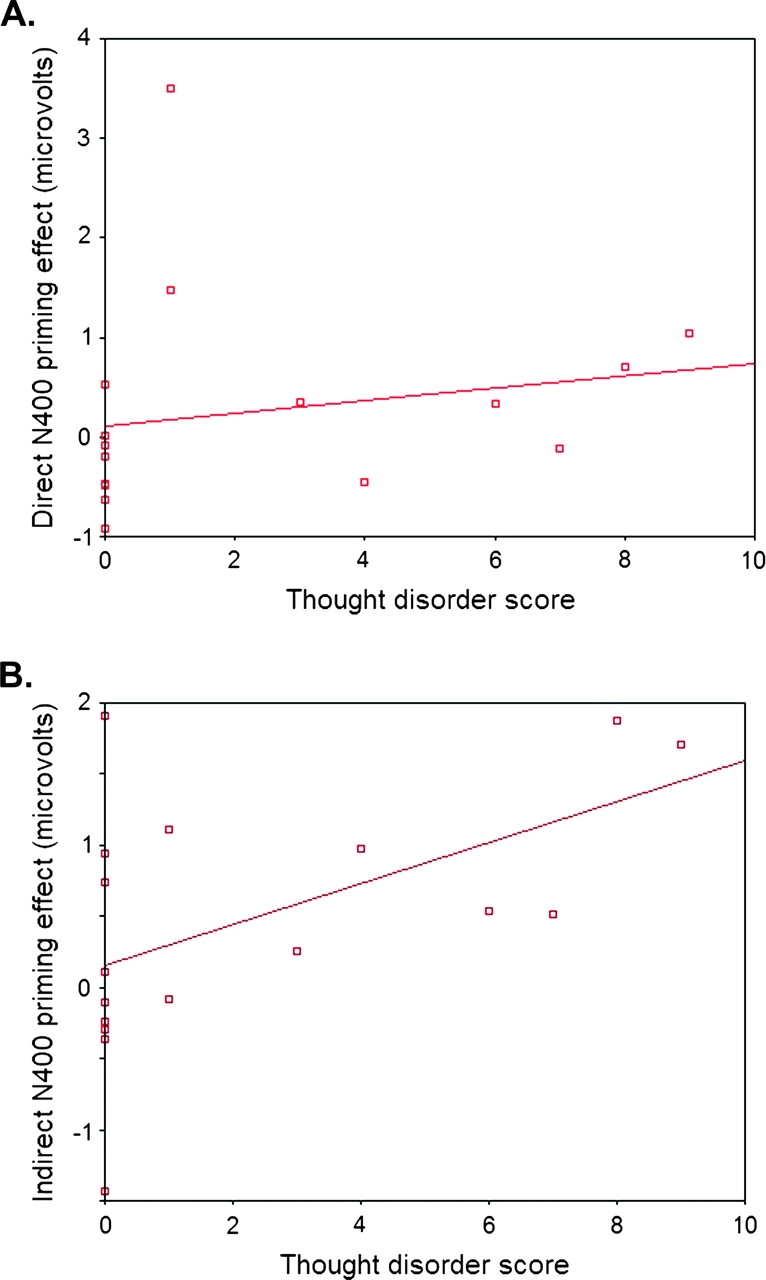
(A) Correlation Between Early Direct N400 Priming and Thought Disorder at Electrode CP2. Absolute N400 values are multiplied by −1 to yield positive values for larger difference scores, ie, larger N400 effects. (B) Correlation between early indirect N400 priming and thought disorder at electrode CP2. Absolute N400 values are multiplied by −1 to yield positive values for larger difference scores, ie, larger N400 effects.
Discussion
Under automatic experimental conditions, and using an implicit task that had the advantage of requiring no behavioral response on trials of interest, TD patients, unlike healthy controls, exhibited significant and fairly widespread indirect neural priming 300–400 milliseconds after target word onset, while non-TD patients exhibited reversed indirect neural priming at some electrode sites. Moreover, within the patient group, severity of thought disorder predicted the degree of both direct and indirect neural priming between 300 and 400 milliseconds after target onset. These relationships were highly specific. Effects remained significant even after covarying for other psychopathological variables, and, within the patient group, no other clinical symptom predicted either early or late N400 priming effects to directly related or indirectly related (each relative to unrelated) targets. Although TD patients were older and therefore had longer durations of illness than the non-TD patients, these factors are unlikely to have accounted for these findings because the differences in indirect priming between groups remained significant when covarying for years of illness and age. Moreover, in healthy individuals, the N400 effect decreases with age,41 and, in schizophrenia patients, behavioral semantic priming effects decrease with length of illness.42
The increased N400 priming effect to indirectly related (relative to unrelated) targets in TD patients is broadly consistent with previous behavioral findings under automatic experimental conditions.3,13 However, our results extend these findings in 2 important ways. First, they are the first demonstration of increased indirect priming at the neural level in specific association with positive thought disorder. Neither of the 2 previous ERP studies examining automatic semantic priming in schizophrenia26,27 specifically examined neurophysiological activity to indirectly related word pairs, and neither demonstrated a specific association between neural priming and positive thought disorder. Second, the excellent temporal resolution of ERPs provided novel information about the time course of semantic priming in schizophrenia. Although the crucial variable allowing us to detect the effects of an automatic spread of activation from prime to target was our short SOA, by subdividing the N400 into early and late subcomponents, we gained additional insights into how the speed of this spread of activity impacted the processing of the target. Group differences across these 2 time windows were unlikely to reflect an artifact of capturing different stages of processing in different individuals because when we adopted an individualistic analysis, locating each individual's N400 peak and measuring the true onset and offset of each individual's N400, our results were unchanged.
By 300–400 milliseconds after target word presentation, indirect neural priming was observed only in the TD patient group. We interpret this as suggesting a further spread of activation within a shorter period of time in schizophrenic thought disorder: more remote associates to prime words were activated relatively more rapidly in TD patients. (TD patients exhibited significantly more negative N400 amplitudes to unrelated targets than healthy controls. This may have reflected an increased rigidity of semantic boundaries within this patient group. Such a general increase in rigidity of semantic boundaries in TD patients, however, is unlikely to account for the entire pattern of findings. First, it would predict a more robust N400 effect to directly related [relative to unrelated] targets than to indirectly related [relative to unrelated] targets. The opposite pattern was, in fact, observed. Second, the amplitude of the N400 to unrelated targets in the TD patients was the same as in the non-TD patients, suggesting that any such increased difficulty in integrating unrelated targets was not specific to TD schizophrenia patients. It is, nonetheless, possible that the increased indirect priming effect in the TD patients [relative to the healthy controls] partially resulted from difficulty in integrating unrelated targets in these patients.)
Although there was no significant difference in direct N400 priming between the TD and non-TD groups as a whole, the correlation between severity of thought disorder and direct N400 priming reached significance in the early N400 time window and approached significance within the late N400 time window. These findings suggest that, in the most severely TD patients, activation spread more rapidly even to directly related targets.
By 400–500 milliseconds after target presentation, N400 priming effects to directly related and indirectly related (relative to unrelated) targets were not significantly different at most electrode sites between the 3 groups.
Taken together, these findings are consistent with inappropriate activity within the semantic networks of TD schizophrenia patients: TD patients, relative to non-TD patients and healthy controls, exhibited an increased neural spread of activation such that activation spread further (to more remote associates) within a shorter period of time. By the later N400 time window, healthy controls appeared to have “caught up” with TD patients’ more accelerated spread of activation.
It is important to note that our patients were outpatients with chronic schizophrenia. Although there were no significant correlations between antipsychotic medication and N400 measures in the present study, it will be important to replicate these findings with first-episode and medication-naive patients.
In sum, we have shown increased early indirect semantic priming at a neural level in patients with positive thought disorder, relative to both non-TD patients and healthy controls. These findings bolster the theory that clinical language disturbances in schizophrenia are a consequence of semantic memory dysfunction3,20 and provide a potential neural correlate of the “loose associations” long considered central to the disorder of schizophrenia.1
Funding
National Institutes of Health (R01 MH071635 to G.R.K., R01 HD25889, R01 HD043251 to P.J.H.); National Alliance for Research on Schizophrenia and Depression (with the Sidney Baer Trust) to G.R.K.; Massachusetts General Hospital (Claflin Distinguished Scholars Award to G.R.K.).
Acknowledgments
We are grateful to Sarah Choi, Katherine Hunter, Kalli Feldman, and Anjuli Singh for their assistance in data collection; Daphne Holt for providing helpful comments and assisting with patient recruitment; and Tatiana Sitnikova and Courtney Brown for assistance with figures.
References
- 1.Bleuler E. In: Dementia Praecox or the Group of Schizophrenias. Zinkin J, translator. New York, NY: International Universities Press; 1950. [Google Scholar]
- 2.DSM-IV:Diagnostic and Statistical Manual of Mental Disorders. 4th ed, revised. Washington, DC: American Psychiatric Press; 1990. American Psychiatric Association. [Google Scholar]
- 3.Spitzer M, Braun U, Hermle L, Maier S. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry. 1993;34:864–877. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- 4.Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82:407–428. [Google Scholar]
- 5.Anderson JR. The Architecture of Cognition. Cambridge, Mass: Harvard University Press; 1983. [Google Scholar]
- 6.Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: evidence of a dependence between retrieval operations. J Exp Psychol. 1971;90:227–234. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- 7.Kreher DA, Holcomb PJ, Kuperberg GR. An electrophysiological investigation of indirect semantic priming. Psychophysiology. 2006;43:550–563. doi: 10.1111/j.1469-8986.2006.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neely JH. Semantic priming effects in visual word recognition: a selective review of current findings and theories. In: Besner D, Humphreys GW, editors. Basic Processes in Reading: Visual Word Recognition. Hillsdale, NJ: Lawrence Earlbaum Associates; 1991. pp. 264–336. [Google Scholar]
- 9.Neely JH. Semantic priming and retrieval from lexical memory: roles of inhibitionless spreading activation and limited capacity attention. J Exp Psychol. 1977;106:226–254. [Google Scholar]
- 10.Manschreck TC, Maher BA, Milavetz JJ, Ames D, Weisstein CC, Schneyer ML. Semantic priming in thought-disordered schizophrenic patients. Schizophr Res. 1988;1:61–66. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer M, Weisker I, Winter M, Maier S, Hermle L, Maher BA. Semantic and phonological priming in schizophrenia. J Abnorm Psychol. 1994;103:485–494. doi: 10.1037//0021-843x.103.3.485. [DOI] [PubMed] [Google Scholar]
- 12.Moritz S, Mersmann K, Kloss M, et al. Enhanced semantic priming in thought-disordered schizophrenic patients using a word pronunciation task. Schizophr Res. 2001;48:301–305. doi: 10.1016/s0920-9964(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 13.Weisbrod M, Maier S, Harig S, Himmelsbach U, Spitzer M. Lateralised semantic and indirect semantic priming effects in people with schizophrenia. Br J Psychiatry. 1998;172:142–146. doi: 10.1192/bjp.172.2.142. [DOI] [PubMed] [Google Scholar]
- 14.Moritz S, Mersmann K, Kloss M, et al. “Hyper-priming” in thought-disordered schizophrenic patients. Psychol Med. 2001;31:221–229. doi: 10.1017/s0033291701003105. [DOI] [PubMed] [Google Scholar]
- 15.Moritz S, Woodward TS, Kuppers D, Lausen A, Schickel M. Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophr Res. 2002;59:181–186. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 16.Barch DM, Cohen JD, Servan-Schreiber D, Steinberger S, Steinhauer SS, van Kammen DP. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- 17.Ober BA, Vinogradov S, Shenaut GK. Automatic versus controlled semantic priming in schizophrenia. Neuropsychology. 1997;11:506–513. doi: 10.1037//0894-4105.11.4.506. [DOI] [PubMed] [Google Scholar]
- 18.Minzenberg MJ, Ober BA, Vinogradov S. Semantic priming and schizophrenia: a review and synthesis. J Int Neuropsychol Soc. 2002;8:699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- 19.Kuperberg GR, Ditman T, Kreher DA, Goldberg TE. Approaches to understanding language dysfunction in neuropsychiatric disorders: insights from the study of schizophrenia. In: Wood S, Allen N, Pantelis C, editors. Handbook of Neuropsychology of Mental Illness. Cambridge University Press; [Google Scholar]
- 20.Kuperberg GR, Deckersbach T, Holt DJ, Goff D, West WC. Increased temporal and prefrontal activity in response to semantic associations in schizophrenia. Arch Gen Psychiatry. 2007;64:138–151. doi: 10.1001/archpsyc.64.2.138. [DOI] [PubMed] [Google Scholar]
- 21.Chapman LJ, Chapman JP. Problems in the measurement of cognitive deficit. Psychol Bull. 1973;79:380–385. doi: 10.1037/h0034541. [DOI] [PubMed] [Google Scholar]
- 22.Holcomb PJ. Semantic priming and stimulus degradation: implications for the role of the N400 in language processing. Psychophysiology. 1993;30:47–61. doi: 10.1111/j.1469-8986.1993.tb03204.x. [DOI] [PubMed] [Google Scholar]
- 23.Bentin S, McCarthy G, Wood CC. Event-related potentials, lexical decision and semantic priming. Clin Neurophysiol. 1985;6:343–355. doi: 10.1016/0013-4694(85)90008-2. [DOI] [PubMed] [Google Scholar]
- 24.Rugg MD. The effects of semantic priming and word repetition on event-related potentials. Psychophysiology. 1985;22:642–647. doi: 10.1111/j.1469-8986.1985.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 25.Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- 26.Condray R, Siegle GJ, Cohen JD, van Kammen DP, Steinhauer SR. Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biol Psychiatry. 2003;5:1134–1148. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 27.Mathalon DH, Faustman WO, Ford JM. N400 and automatic semantic processing abnormalities in patients with schizophrenia. Arch Gen Psychiatry. 2002;59:641–648. doi: 10.1001/archpsyc.59.7.641. [DOI] [PubMed] [Google Scholar]
- 28.Misra M, Holcomb PJ. Event-related potential indices of masked repetition priming. Psychophysiology. 2003;40:115–130. doi: 10.1111/1469-8986.00012. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID) I: history, rationale and description. Arch Gen Psychiatry. 1992;49:642–649. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 30.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 31.White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- 32.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Br J Psychiatry. 1989;155:53–58. [PubMed] [Google Scholar]
- 33.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 34.Andreasen NC. Thought, language and communication disorders. I. Clinical assessment, definition of terms, and evaluation of their reliability. Arch Gen Psychiatry. 1979;36:1315–1321. doi: 10.1001/archpsyc.1979.01780120045006. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen NC. Thought, language and communication disorders. II. Diagnostic significance. Arch Gen Psychiatry. 1979;36:1325–1330. doi: 10.1001/archpsyc.1979.01780120055007. [DOI] [PubMed] [Google Scholar]
- 36.Kuperberg GR, McGuire PK, David A. Reduced sensitivity to linguistic context in schizophrenic thought disorder: evidence from online monitoring for words in linguistically anomalous sentences. J Abnorm Psychol. 1998;107:423–434. doi: 10.1037//0021-843x.107.3.423. [DOI] [PubMed] [Google Scholar]
- 37.Kuperberg GR, Sitnikova T, Goff D, Holcomb PJ. Making sense of sentences in schizophrenia: abnormal interactions between semantic and syntactic processes. J Abnorm Psychol. 2006;115:243–256. doi: 10.1037/0021-843X.115.2.251. [DOI] [PubMed] [Google Scholar]
- 38.Hollingshead AB. Two Factor Index of Social Position. New Haven, Conn: Yale University Press; 1965. [Google Scholar]
- 39.Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- 40.Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- 41.Kutas M, Iragui V. The N400 in a semantic categorization task across 6 decades. Electroencephalogr Clin Neurophysiol. 1998;108:456–471. doi: 10.1016/s0168-5597(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 42.Maher BA, Manschreck TC, Redmond D, Beaudette S. Length of illness and the gradient from positive to negative semantic priming in schizophrenia. Schizophr Res. 1996;22:127–132. doi: 10.1016/s0920-9964(96)00066-7. [DOI] [PubMed] [Google Scholar]
- 43.Kučera H, Francis WN. Computational Analysis of Present-Day American English. Providence, RI: Brown University Press; 1967. [Google Scholar]