Abstract
Background: Face recognition has important implications for patients with schizophrenia, who exhibit poor interpersonal and social skills. Previous reports have suggested that patients with schizophrenia have deficits in their ability to recognize faces, and because face recognition relies heavily on information about the configuration of faces, we hypothesized that patients with schizophrenia would have specific problems in processing configural information. Methods: We measured the performance of 20 patients with schizophrenia and 20 normal subjects in a face-discrimination task, using upright and inverted pairs of face photographs that differed in featural or configural information. Results: The patients with schizophrenia showed disproportionately poorer performance in discriminating configural compared with featural face sets. Conclusion: The result suggests that the face-recognition deficit in schizophrenic patients is due to specific impairments in configural processing of faces.
Keywords: face, featural, recognition, schizophrenic
Poor social functioning constitutes the core symptomatology of schizophrenia, and effective social interactions depend greatly on the ability to recognize identity and emotional responses from faces and facial expressions.1 Although numerous studies have found that persons with schizophrenia have a deficit in their ability to evaluate facial expressions,2–5 fewer studies have examined face recognition in patients with schizophrenia. Faces differ from other kinds of visual objects in that recognition relies heavily on the configuration of their parts.6 Disproportionate impairment in recognition when faces, compared with other visual objects, are presented upside down has been interpreted as evidence of configural processing for face recognition because inversion leads to much greater disruption of configural information than of featural information.7–10 We hypothesized that if patients with schizophrenia have a specific deficit in face recognition, they will have a higher degree of deficit in the processing of configural information than of the featural information of faces. Patients with schizophrenia were expected to show lower performance in face-discrimination tasks relying on configural information compared with tasks relying on featural information; we also expected this difference to be enhanced with face inversion.
METHODS
Subjects
Twenty patients (mean age = 26.8 years, SD = 4.8; 11 men and 9 women), all of whom fulfilled the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for schizophrenia, were diagnosed using the Structured Clinical Interview for DSM-IV (SCID-I). They were recruited from the inpatient unit and outpatient clinic of the Department of Psychiatry, Seoul National University Hospital. None of the patients had any history of traumatic brain injury, epilepsy, alcohol or substance abuse, or any other neurological issues. All the patients were right handed. Table 1 provides details of the demographic and clinical characteristics of the patients with schizophrenia and of the normal healthy subjects. As shown in table 1, no significant group differences were observed in the mean years of education among the subjects or in the mean socioeconomic status of the subjects or their parents.11 The mean IQ in the patient group was lower than that of the normal control group (94.50 ± 11.52 vs 114.40 ± 9.88, t = 5.86, P < .001). In the schizophrenia group, the average age of onset was 22.5 years (SD = 5.7), the mean illness duration was 4.9 years (SD = 3.7), and the mean Positive and Negative Syndrome Scale score12 was 52.8 (SD = 13.3). All the patients had received atypical antipsychotics. Eight of the patients were currently taking risperidone at a daily mean dose of 3.93 mg (SD = 2.29), another 8 were on clozapine at a mean daily dose of 212.5 mg (SD = 121.74), 3 took olanzapine at a mean daily dose of 9.16 mg (SD = 5.20), 2 took aripiprazole at a dosage of 10 mg, and 1 took quetiapine at a dosage of 250 mg. Twenty age- and sex-matched healthy controls (mean age = 25.6 years, SD = 2.4; 11 men and 9 women), all of whom had been recruited from the community via newspaper advertisements and screened by the Structured Clinical Interview for DSM-IV, Non-Patient Version (SCID-NP), were included in the study. After they had been completely apprised of the study protocols, we obtained written informed consent from all the subjects. This study was conducted in accordance with the guidelines provided by the institutional review board at Seoul National University Hospital.
Table 1.
Demographic and Clinical Characteristics of the Patients With Schizophrenia and the Normal Control Subjects
| Schizophrenia (n = 20) |
Controls (n = 20) |
|||
| Variables | Mean | SD | Mean | SD |
| Age (y) | 26.81 | 4.777 | 25.55 | 2.460 |
| Education (y) | 14.62 | 1.987 | 15.40 | 1.635 |
| IQ* | 94.50 | 11.52 | 114.40 | 9.88 |
| Subjects' SES | 3.17 | 0.576 | 2.90 | 0.447 |
| Parents' SES | 2.96 | 0.475 | 3.00 | 0.649 |
| PANSS total score | 52.77 | 13.32 | — | — |
| Positive score | 14.23 | 4.780 | — | — |
| Negative score | 12.91 | 3.650 | — | — |
| General score | 27.09 | 5.748 | — | — |
| Duration (y) | 4.88 | 3.72 | — | — |
| Onset (y) | 22.48 | 5.66 | — | — |
Note: SES, socioeconomic state; PANSS, Positive and Negative Syndrome Scale.
P < .001.
Stimuli
We made 2 sets of pairs of faces: a configural set composed of faces that differed in the spacing among features within the face (between the eyes and between the nose and mouth) and a featural set composed of faces that differed in the shapes of individual features.9,13,14 Gray-scaled, emotionally neutral face photographs of 2 Korean males and 2 Korean females, 350 × 350 pixels in size, were modified to create the configural and featural sets of face stimuli for the face-discrimination task. For the faces in the configuration set, eyes were moved 5 pixels in and out horizontally and mouths were moved 5 pixels up and down vertically, relative to the original image. For the faces in the feature set, eyes in the photographs were changed by pasting in eyes from 3 other faces of people of the same gender, while maintaining the whole configuration. For a control task, chair-discrimination stimuli were prepared. A pair of photographs of chairs that were similar to each other in general contour but different in some features such as the armrest, back, and legs was extracted from the Hemera Photo-Objects image database (Figure 1).
Fig. 1.

Samples of Task Stimuli: a) Configural Face Set, b) Featural Face Set, and c) Featural Chair Set.
Procedure
Using the 2 sets of pairs of faces, we asked the subjects to recognize the faces by preferentially relying on configural or featural information. As shown in Figure 1, a pair of 2 photographs in a set, one of which had been resized to 300 × 300 pixels from 350 × 350 pixels to prevent a mechanical discrimination strategy such as comparing the length of any landmarks within a face, was presented for 1000 milliseconds after a 500-millisecond presentation of a cross as the prompt. The subjects were seated approximately 80 cm away from a 15-inch monitor having 1024 × 768 pixel resolution and were required to decide whether the 2 photographs were the same or different as quickly and as accurately as possible. They were asked to press the left button of the mouse if the 2 photographs were the same and the right button if they were different. For each presentation, chance performance was 50% accuracy, given that the number of same or different photographs was equal in the stimuli. Face blocks comprised 50 configural and 50 featural trials in each set of upright and inverted blocks; thus, for the 4 types of blocks, a total of 200 trials were conducted. The chair block contained 50 trials in each set of upright and inverted blocks; thus, the 2 types of blocks included 100 trials in total. The order of presenting the blocks and the side-of-face location were counterbalanced. The stimuli were presented in random order in blocks. A stimulus was shown for 1 second after the 500-millisecond “+” as a prompt, and the next trial appeared only after the subject responded. The accuracy and reaction times were measured automatically, using Presentation 9.0 software (Neurobehavioral Systems, Albany, CA). All participants finished the task within 15 minutes.
Statistical analysis
Discrimination accuracy was calculated from the number of correct trials in each type of block for the 20 patients with schizophrenia and the 20 normal controls. Analysis of variance tests (ANOVAs) with configural vs featural faces and upright vs inverted faces as within-subjects factors and patient vs control subjects as a between-subjects factor were carried out for the accuracy and the response times (RTs) for correct trials. Additional ANOVA with featural face vs featural chair and upright vs inverted stimuli as within-subjects factors and patient vs control subjects as a between-subjects factor were carried out to compare the featural chair and featural face sets. The RTs for correct trials were compared between the patients with schizophrenia and the control subjects using t tests.
Results
A 2 (group) × 2 (stimulus type) × 2 (orientation) ANOVA for face-discrimination accuracy revealed a significant main effect of group (F1,38 = 34.49, P < .001). As seen in figure 2, the patients with schizophrenia showed lower accuracy in discriminating the upright configuration set faces (t = −7.316, df = 38, P < .001) and upright feature set faces (t = −2.151, df = 38, P < .05), as well as the inverted configuration set faces (t = −2.932, df = 38, P < .01), but not the inverted featural set faces (t = −1.338, df = 38, P > .1), compared with the control subjects.
Fig. 2.
Performances on Face and Chair Discrimination Task in Patients With Schizophrenia and Healthy Controls. A 2 (group) × 2 (stimulus type) × 2 (orientation) analysis of variance (ANOVA) for face discriminating accuracy show a main effect of group (F1,38 = 34.49, P < .001), stimulus type (F1,38 = 67.97, P < .005), in addition to interactions of group by stimulus type (F1,38 = 12.97, P < .005), group by orientation (F1,38 = 26.99, P < .001), and group by stimulus type by orientation (F1,38 = 18.93, P < .001). A 2 (group) × 2 (stimulus: chair vs face) × 2 (orientation) ANOVA between featural chair and featural face sets revealed main effect of group (F1,38 = 4.58, P < .05), face vs chair (F1,38 = 20.50, P < .001), and orientation (F1,38 = 20.50, P < .001).Error bar represents SD.*P < .05, **P < .01, ***P < .001.
Configural Vs featural face processing
A main effect of stimulus type (F1,38 = 67.97, P < .005) was detected, suggesting that the accuracy of face discrimination was lower for the configural face set than for the featural face set. Both control and patient groups showed lower accuracy for the configural face set compared with the featural face set (67.55 ± 14.50% vs 76.60 ± 11.88%, t = −5.015, P < .001 and 46.15 ± 15.07% vs 70.70 ± 12.11%, t = −7.598, P < .001, respectively) (table 2). Significant interactions were found of group × stimulus type (F1,38 = 12.97, P < .005), indicating that lower accuracy for the configural face set was more prominent in patients with schizophrenia than in control subjects.
Table 2.
The Face Discrimination Performance in the Patients With Schizophrenia and the Normal Control Subjects
| Upright condition (%) | Inverted condition (%) | |
| Schizophrenia | ||
| Configural set | 45.3 ± 19.6 | 47.0 ± 9.0 |
| Featural set | 75.6 ± 11.1 | 65.8 ± 11.2 |
| Control | ||
| Configural set | 79.1 ± 6.6 | 56.0 ± 10.4 |
| Featural set | 82.9 ± 10.3 | 70.3 ± 10.0 |
Inversion effect
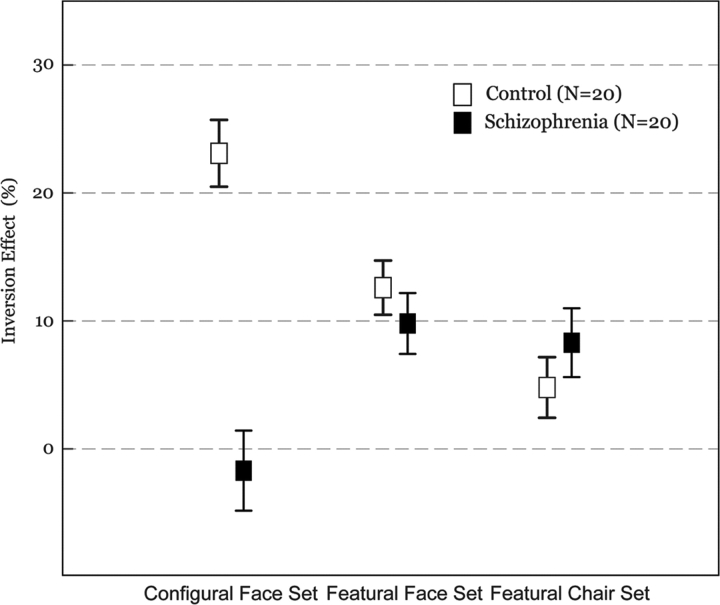
A significant interaction of group × orientation (F1,38 = 26.99, P < .001) was observed, indicating that face discrimination was disrupted by inversion in the control group (81.00 ± 8.74% vs 63.15 ± 12.39%, t = 9.584, P < .001) but not in the patients with schizophrenia (60.45 ± 21.97% vs 56.40 ± 13.84%, t = 1.880, P > .06). Although there was no interaction of stimulus type × orientation (F1,38 = 0.04, P > .80), a significant interaction of group × stimulus type × orientation (F1,38 = 18.93, P < .001) was detected. As shown in Figure 3, the inversion effect was observed in the featural face set (t = 4.104, df = 19, P < 0.005) but not in the configural face set in patients with schizophrenia (t = −0.541, df = 19, P > 0.59). However, in the control group, the inversion effect was observed regardless of stimulus type, ie, both in the featural face set (t = 5.949, df = 19, P < 0.001) and in the configural face set (t = 8.825, df = 19, P < 0.001). A 2 (group) × 2 (orientation) analysis of featural chair sets revealed a main effect of orientation (F1,38 = 13.31, P < .01) with no interaction of group × orientation (F1,38 = 0.95, P > .33).
Fig. 3.
Inversion Effect Between Patients With Schizophrenia and Normal Subjects. Inversion effect was measured by the performance difference: performance for the upright stimulus minus performance for the inverted stimulus. Error bar represents standard error.
Chair Vs face
When analysis was confined to featural chair and featural face sets, a 2 (group) × 2 (stimulus: chair vs face) × 2 (orientation) analysis revealed a main effect of group (F1,38 = 4.58, P < .05), indicating that the control group showed higher performance compared with the patients with schizophrenia (79.35 ± 10.62% vs 74.63 ± 12.06%, t = 2.63, P < .01); a main effect of face vs chair (F1,38 = 20.50, P < .001), indicating higher performance for featural chairs than for featural faces (80.33 ± 9.80% vs 73.65 ± 12.28%, t = 3.80, P < .001); and a main effect of orientation (F1,38 = 20.50, P < .001), indicating higher performance for the upright stimuli compared with the inverted stimuli (81.43 ± 9.60% vs 72.55 ± 11.72%, t = 5.24, P < 0.001). However, no interactions occurred for group vs orientation (F1,38 = 0.64, P > .43), group vs stimulus (F1,38 = 0.01, P > .89), or group vs stimulus vs orientation (F1,38 = 2.28, P > .14).
RT for correct trials
A 2 (group) × 2 (stimulus type) × 2 (orientation) ANOVA for the RTs of correct face-discrimination trials revealed no significant main effect of group (F1,38 = 0.09, P > .77) and no main effect of stimulus type (F1,38 = 3.59, P > .06). However, a main effect of orientation (F1,38 = 4.76, P < .05) was detected, with the trend of shorter RTs for the upright stimuli than for the inverted stimuli (1341.13 ± 325.85 vs 1361.68 ± 341.96 milliseconds, t = −1.74, P = .08). Interactions were found for group × orientation (F1,38 = 4.37, P < .05) and group × stimulus type × orientation (F1,38 = 5.58, P < .05).
The following 2 (stimulus type) × 2 (orientation) ANOVA for each group showed that while a main effect of orientation (F1,19 = 7.21, P < .05) was found in the control group, indicating that RTs for the upright stimuli were shorter than for the inverted stimuli (1344.61 ± 325.15 vs 1385.92 ± 347.30 milliseconds, t = −2.72, P = .01), no main effect of orientation (F1,19 = 0.01, P > .94) was observed in the patient group, which showed no difference in RTs between the upright and inverted stimuli (1336.60 ± 330.62 vs 1337.44 ± 339.18 milliseconds, t = −0.05, P > .96). In the control group, no main effect for stimulus type (F1,19 = 1.59, P > .22) or for interaction of stimulus type × orientation (F1,19 = 0.44, P > .51) was found. In the patient group, a main effect of stimulus type (F1,19 = 4.43, P < .05) was detected, which indicates that RTs for discriminating the configural face set were longer than for the featural face set (1363.94 ± 348.97 vs 1310.09 ± 317.94 milliseconds, t = 2.24, P < .05). In the patient group, an interaction of stimulus type × orientation (F1,19 = 5.99, P < .05) was seen, which indicates that RTs were shorter for upright than for inverted stimuli in the featural face set (1284.99 ± 292.94 vs 1335.20 ± 346.92 milliseconds, t = −2.17, P < .05), while a trend existed toward longer RTs for upright compared with inverted stimuli for the configural face set (1388.20 ± 364.63 vs 1339.68 ± 340.27 milliseconds, t = 2.09, P = .05).
Discussion
Patients with schizophrenia showed disproportionately poor performance and long RTs in discriminating faces that differed in configural information compared with faces that differed in featural information. This disproportionately poor performance in configural processing both for upright and inverted faces led to the face-inversion effect not being shown in patients with schizophrenia, in contrast to the normal inversion effect shown by control subjects. These results pose strong evidence against the view that the face-recognition deficit in persons with schizophrenia merely mirrors a general impairment of memory and the executive function of schizophrenia.15,16 Although little behavioral research has focused on face recognition in people with schizophrenia, recent neuroimaging studies have consistently implied a dysfunction in face recognition in these persons. Reduced N170 amplitudes in schizophrenic compared with control subjects17,18; a reduced volume of the fusiform gyrus, which is known to be involved in face perception19,20; and failure of the fusiform gyrus to activate during face perception in patients with schizophrenia21 suggest dysfunction in face recognition in persons with schizophrenia.
Despite abundant research on configural face processing in normal subjects and its strong relevance to social functioning, few studies have focused on face recognition per se in persons with schizophrenia. Two previous studies regarding face discrimination among neutral faces yielded conflicting findings. Although those studies involved the same task (a Benton facial recognition test [BFRT]), one revealed significantly lower performance in patients with schizophrenia compared with normal subjects,22 while the other found no difference between the patients and normal subjects.23 However, the BFRT requires the subject to select a face with the same identity as a reference face and is not adequate for specific evaluation of face recognition. Patients with prosopagnosia have been reported to score in the normal range on the BFRT; those participants reported that they relied heavily on featural information rather than configural information in discriminating faces.24
Schwartz et al25 tackled the issue of configural face processing by subjects with schizophrenia using face inversion. They reported that subjects with schizophrenia, similar to control subjects, showed the inversion effect in a face-memory task and concluded that those with schizophrenia had no deficit in configural face processing. In contrast, our results show that patients with schizophrenia had a specific deficit in configural processing. Two reasons may explain the differences in results. First, Schwartz et al25 did not differentiate between configural and featural face processing, which might have lessened their ability to detect differences in the face inversion effect between patients and control subjects. We found the inversion effect for the featural face set in patients with schizophrenia, similar to the normal subjects, although the effect size was much smaller than that for the configural face set. Differences in features inevitably lead to small differences in relational information, which is consistent with several previous reports.26,27 Therefore, by not differentiating between configural and featural processing, it is possible that those researchers may have missed differences between the subject groups with regard to the face inversion effect. Second, unlike the memory task of Schwartz et al25 in which subjects were instructed to memorize the identity of previous faces, our task paradigm did not adopt a memory task; instead, we focused on discriminating perception in face recognition. The rigor of our task paradigm may also have differed from that of Schwartz et al.25 In our study, mean performance was around 80% across all 3 types of upright stimuli, which is in contrast to the greater than 90% performance in the task of Schwartz et al25; the performance value in our study may have enhanced our ability to detect differences between patients and normal subjects.
A limitation of our study was that all the subjects with schizophrenia were taking antipsychotic medications. The generally lower performance in recognition across all types of stimulus sets in the patient group could reflect medication use. However, medication cannot explain the unequal cognitive impairment between configural and featural face processing. Furthermore, we found no difference in the RTs for correct trials between the patient and control groups, which also lessens the probability of a medication effect as an explanation for the results. Notably, evidence exists that the face recognition deficit in schizophrenia could be an endophenotype not associated with variables of chronic illness including medications.28 Calkins et al28 reported that the first-degree relatives of persons with schizophrenia had impaired face recognition but not object recognition.
To our knowledge, this is the first study to show a deficit specific to configural face processing in persons with schizophrenia. A specific deficit in configural face processing at the early stage of face discrimination, which is less confounded by higher cognition such as memory or executive function, suggests how perceptual deficit could result in higher social dysfunction in patients with schizophrenia.
Funding
Cognitive Neuroscience Program of the Korean Ministry of Science and Technology (M10644020003-06N4402-00310).
References
- 1.Posamentier MT, Abdi H. Processing faces and facial expressions. Neuropsychol Rev. 2003;13:113–143. doi: 10.1023/a:1025519712569. [DOI] [PubMed] [Google Scholar]
- 2.Shannon AM. Facial expression of emotion: recognition patterns in schizophrenics and depressives. Nurs Res Conf. 1971;7:131–146. [PubMed] [Google Scholar]
- 3.Berndl K, von Cranach M, Grusser OJ. Impairment of perception and recognition of faces, mimic expression and gestures in schizophrenic patients. Eur Arch Psychiatry Neurol Sci. 1986;235:282–291. doi: 10.1007/BF00515915. [DOI] [PubMed] [Google Scholar]
- 4.Archer J, Hay DC, Young AW. Movement, face processing and schizophrenia: evidence of a differential deficit in expression analysis. Br J Clin Psychol. 1994;33(Pt 4):517–528. doi: 10.1111/j.2044-8260.1994.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson EK, Jones CA, Johnston RA, Meaden A, Wink B. Facial emotion recognition from moving and static point-light images in schizophrenia. Schizophr Res. 2006;85:96–105. doi: 10.1016/j.schres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? J Exp Psychol Hum Percept Perform. 1995 Jun;21:628–634. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- 7.Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol Rev. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka JW, Sengco JA. Features and their configuration in face recognition. Mem Cognit. 1997;25:583–592. doi: 10.3758/bf03211301. [DOI] [PubMed] [Google Scholar]
- 9.Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: direct evidence. Perception. 2000;29:159–170. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- 10.Valentine T. Upside-down faces: a review of the effect of inversion upon face recognition. Br J Psychol. 1988;79(Pt 4):471–491. doi: 10.1111/j.2044-8295.1988.tb02747.x. [DOI] [PubMed] [Google Scholar]
- 11.Hollingshead AB, Redlich FL. Social Class and Mental Illness. New York, NY: Wiley; 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 13.Mondloch CJ, Le Grand R, Maurer D. Configural face processing develops more slowly than featural face processing. Perception. 2002;31:553–566. doi: 10.1068/p3339. [DOI] [PubMed] [Google Scholar]
- 14.Le Grand R, Mondloch CJ, Maurer D, Brent HP. Neuroperception. Early visual experience and face processing. Nature. 2001;19:410–890. doi: 10.1038/35073749. [DOI] [PubMed] [Google Scholar]
- 15.Hellewell JS, Connell J, Deakin JF. Affect judgement and Fusiform gyrus volume reduction and facial recognition memory in schizophrenia. Psychopathology. 1994;27:255–261. doi: 10.1159/000284879. [DOI] [PubMed] [Google Scholar]
- 16.Whittaker JF, Deakin JF, Tomenson B. Face processing in schizophrenia: defining the deficit. Psychol Med. 2001;31:499–507. doi: 10.1017/s0033291701003701. [DOI] [PubMed] [Google Scholar]
- 17.Onitsuka T, Niznikiewicz MA, Spencer KM, et al. Functional and structural deficits in brain regions subserving face perception in schizophrenia. Am J Psychiatry. 2006;163:455–462. doi: 10.1176/appi.ajp.163.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. Am J Psychiatry. 2004;161:915–917. doi: 10.1176/appi.ajp.161.5.915. [DOI] [PubMed] [Google Scholar]
- 19.Lee CU, Shenton ME, Salisbury DF, et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- 20.Onitsuka T, Shenton ME, Kasai K, et al. Fusiform gyrus volume reduction and facial recognition in chronic schizophrenia. Arch Gen Psychiatry. 2003;60:349–355. doi: 10.1001/archpsyc.60.4.349. [DOI] [PubMed] [Google Scholar]
- 21.Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry. 2003;15(53):1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- 22.Kubota Y, Querel C, Pelion F, et al. Facial affect recognition in pre-lingually deaf people with schizophrenia. Schizophr Res. 2003;61:265–270. doi: 10.1016/s0920-9964(02)00298-0. [DOI] [PubMed] [Google Scholar]
- 23.Hall J, Harris JM, Sprengelmeyer R, et al. Social cognition and face processing in schizophrenia. Br J Psychiatry. 2004;185:169–170. doi: 10.1192/bjp.185.2.169. [DOI] [PubMed] [Google Scholar]
- 24.Duchaine BC, Nakayama K. Developmental prosopagnosia and the Benton Facial Recognition Test. Neurology. 2004;62:1219–1220. doi: 10.1212/01.wnl.0000118297.03161.b3. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz BL, Marvel CL, Drapalski A, Rosse RB, Deutsch SI. Configural processing in face recognition in schizophrenia. Cognit Neuropsychiatry. 2002;7:15–39. doi: 10.1080/13546800143000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leder H, Carbon C-C. Part-to-whole effects and configural processing in faces. Psychol Sci. 2004;46:531–543. [Google Scholar]
- 27.Rhodes G, Brake S, Atkinson AP. What's lost in inverted faces? Cognition. 1993;47:25–57. doi: 10.1016/0010-0277(93)90061-y. [DOI] [PubMed] [Google Scholar]
- 28.Calkins ME, Gur RC, Ragland JD, Gur RE. Face recognition memory deficits and visual object memory performance in patients with schizophrenia and their relatives. Am J Psychiatry. 2005;162:1963–1966. doi: 10.1176/appi.ajp.162.10.1963. [DOI] [PubMed] [Google Scholar]