Abstract
The social significance of imitation is that it provides internal tools for understanding the actions of others by simulating or forming internal representations of these actions. Imitation plays a central role in human social behavior by mediating diverse forms of social learning. However, imitation and simulation ability in schizophrenia has not been adequately addressed. The major aim of the present study was to investigate imitation ability in schizophrenia patients and healthy individuals by examining simple motor imitation that involved the replication of meaningless manual and oral gestures, and the imitation of emotional facial expressions, which has implications for mentalizing. A secondary aim of the present study was to investigate the relationships among imitation ability, social functioning, and working memory. Subjects were asked to mimic hand gestures, mouth movements, and facial expressions of others, online. Clinical symptoms, social competence, and working memory were also assessed. Patients with schizophrenia were significantly impaired on all imitation tasks. Imitation errors were significantly correlated with reduced social competence and increased negative symptoms. However, imitation ability was only weakly associated with working memory. To summarize, the present study examined the ability of patients with schizophrenia to imitate the behaviors demonstrated by others. The results indicate a fundamental impairment in imitation ability in schizophrenia and implicate a possible difficulty in simulation. Further research to determine the neural and developmental origins of this difficulty could be extremely helpful in elucidating the role of simulation in schizophrenia and to establish the complex relationships among mental representation, imitation, and social cognition.
Keywords: imitation, simulation, mirror neuron, social cognition, schizophrenia, theory of mind, working memory, mentalizing, mind reading
Introduction
The human is indissolubly linked with imitation: a human being only becomes human at all by imitating other human beings.
Theodor Adorno1
Social impairment is a cardinal feature of schizophrenia, present throughout the course of illness starting at the prodromal stage.2–5 Social functioning is instrumental in determining quality of life and functional outcome, but difficulties in conceptualizing and assessing social functions have impeded progress towards successful targeted treatment and intervention strategies.6–8 One way to tackle these challenges is to segregate anomalous social behavior into theoretically constrained components that can then be systematically examined in the laboratory. Access to such “building blocks” that cascade into social impairments could lead to a better understanding of the cognitive and neural origins of these deficits.
In this context, we consider social cognition research in schizophrenia, which has been dominated by the Theory-of-Mind (ToM) paradigm. ToM refers to the ability to attribute mental states, such as thoughts, feelings, intentions, and beliefs to self and others in order to explain and predict behavior.9 Frith,10 who proposed that several key symptoms of schizophrenia could be explained by ToM impairment, sparked early interest in ToM in schizophrenia. Accumulating evidence for the presence of ToM deficit in schizophrenia supports Frith's position.11,12 However, many tasks that are used to assess ToM (the hinting task, false belief task, story comprehension, etc.) are not specific to tapping mental state attributions and instead, recruit an assortment of cognitive functions, ranging from working memory and selective attention to semantic memory and pragmatics. Thus, it is unclear how and why deficits in ToM arise in patients with schizophrenia.
There are 2 broad theories regarding the cognitive processes that underpin ToM; the theory theory (or meta-representation) and the simulation theory.13 The theory theory postulates that normal mentalizing or mind reading depends on the acquisition, over the first few years of life, of a stored body of knowledge about mental states and rules of inference concerning how mental states relate to behavior. These rules are maintained online in order to reason about the actions of others.14,15 Thus, according to the theory theory, how we infer about the mental states of others is similar to how scientists go about testing their hypotheses.
In contrast, the simulation theory proposes that normal mentalizing or “mind reading” involves internally modeling the observed behaviors of others. It is a process of mentally “stepping into the shoes” of another person.13,16 By generating an internal simulation, one can model the thoughts, emotions, and intentions behind the behaviors of others. In its strongest form, the simulation account implies that understanding others' minds requires no meta-representation or theory formulation.
The theory theory and simulation theory of mentalizing do not have to be mutually exclusive. It is possible that both are employed, depending on the nature of the problem to be solved. Imagine being shipwrecked on a tropical island with no previous exposure to the language and culture of the indigenous people. How does one attempt to understand the actions and behaviors of the hosts? According to the theory theory, one could apply previously acquired knowledge about one's own culture, and because there are many universals among our species, some of these attempts will yield fruitful mind reading results. However, such application of top-down strategy may not be as successful for culturally unique repertoires of behavior. When one encounters a completely novel behavior or action, which does not fit into any previously acquired database of knowledge, then simulation may provide an internal model, which then can be used as an input for a theory about that behavior. Thus, the nature and the context of the social interaction would influence the interaction between the top-down strategy exemplified by the theory-theory account and the more bottom-up strategy of simulation.
In the case of schizophrenia, neither the theory theory nor the simulation theory has been fully explored to link them to the behaviors of schizophrenia patients. Does the theory theory account explain experimental findings and clinical observations in schizophrenia with respect to social cognition? Schizophrenia patients do not necessarily lack the conceptual knowledge about mental states, and many patients acknowledge that beliefs can be false. This suggests that individuals with schizophrenia are able to use meta-representation.17
Furthermore, individuals with schizophrenia who have difficulties in appreciating the belief of other people also find it difficult to simulate other visual perspectives.17 The most parsimonious explanation for these co-occurring deficits is that people with schizophrenia find it difficult to simulate another person's subjective experience, whether the content of that experience is cognitive, perceptual, or affective.17 With respect to the simulation theory of mentalizing, it has been largely overlooked in schizophrenia research yet the construct of simulation overlaps with a fundamental cognitive function, working memory, which may be a core feature of schizophrenia. Individuals with schizophrenia have deficits in the maintenance and manipulation of internal representations in working memory and an associated difficulty in using these representations to guide behavior.18–20 Mental simulation may be described as a process by which internal representations of external events such as movements and actions of others are generated and maintained. Viewed in this way, working memory and simulation share similar constructs. They both depend on the generation, maintenance, and manipulation of internal representation. Given the widespread deficits in working memory in schizophrenia, one would expect simulation to be also affected. Simulation provides one possible mechanism by which we model behaviors of others, and deficits in mental simulation could lead to misunderstanding of actions of others. However, simulation ability in schizophrenia has not been adequately studied to date.
A simple and practical way of studying simulation experimentally is to utilize imitation. The simulation theory of ToM simply argues that humans understand the actions of others by replicating or mimicking other individuals. In other words, simulation of others' mental states requires the generation of internal representations, and thus, it is a form of mental imitation.21
In its most generic sense of the word, imitation is the copying of the actions of someone else. As Thorndike22 stated succinctly, imitation is “learning to do an act from seeing it done.” It follows that there is a causal relationship between observing the movement or behavior of a model and reproducing that specific movement by imitation.23
Imitation plays a central role in human social behavior13 by mediating diverse forms of social learning.24,25 Whether learning to tango in Buenos Aires or to bow fittingly to elderly ladies in Seoul, replicating actions demonstrated by others lies at the heart of skill acquisition. Analogous to imitation of movements, mental imitation or simulation of behaviors of others is suggested to be a core component of mind reading.21 Overall, a substantive body of literature indicates the importance of imitation in social learning and in the development of the ToM.13 Thus, it is not surprising to find autism-specific deficits in a broad range of imitation tasks, including imitation of meaningless gestures and emotional expressions, as well as symbolic movements, which are not caused by problems in visual memory or motor control.26–28
The biological and social significance of imitation is that it provides internal tools for understanding the actions of others by simulating or forming internal representations of these actions. Evidence suggests that there is a strong link between the simulation function required for imitation and ToM.29 Further support for the relationship between the process of simulation and imitation comes from investigations into the neural correlates of these functions. The discovery of mirror neurons30–32 in the premotor cortex (F5) of nonhuman primates and their role in action understanding provide insight into the neural mechanism supporting mental simulation in imitation. The term mirror neurons was coined to reflect their functional property; they are active both during the execution of one's own goal-directed action and during the observation of the same action performed by others31,32. Analogous to the mirror neurons in the monkey premotor cortex that support action understanding, the human mirror mechanism33 is mediated by a direct mapping of the observed action and its motor representation in the neural circuit that includes the inferior frontal gyrus (IFG) within Broca's area (human homolog of F5) and the right posterior parietal cortex.34 Evidence suggests that the human mirror mechanism is also involved in simulating the thoughts, intentions, and emotions of others. A recent fMRI experiment that investigated the neural correlates of empathy found increased activation of the neural circuits that include the IFG and right PP during observation and imitation of facial emotional expressions, which suggests that the mirror mechanism is involved in empathy.35
Given the deficits in ToM ability in schizophrenia and the close relationship between ToM and imitation ability, it would seem reasonable to hypothesize that individuals with schizophrenia also show imitation deficits. However, very little research has been conducted on this topic. There is some evidence from emotion processing studies which suggests that individuals with schizophrenia have impairments in their ability to pose emotional expressions,36 and one recent study37 has explicitly investigated emotional facial imitation ability in schizophrenia and found reduced accuracy in imitation in the absence of deficits in the identification of all emotional facial expressions. Although these findings suggest that patients with schizophrenia are impaired in imitating emotional expressions of others, they did not address the question of whether imitation ability itself is problematic. In other words, do patients with schizophrenia have deficits in imitation even if the action or movement generated by the model is simple and meaningless?
The present study was conceived to examine 3 modes of imitation in schizophrenia patients and healthy individuals: the imitation of hand gestures, mouth movements, and emotional facial expressions. These imitation tasks belong to 2 categories. Complex imitation, such as emotional face imitation is related to mental state attributions and has implications for mentalizing. Simple imitation, such as hand and mouth imitation that involve the replication of meaningless and disembodied manual and oral gestures, do not require the observer to engage in mentalizing.
Based on the results of Schwartz and colleagues37, impairment in facial expression imitation in schizophrenia may be predicted. However, the investigation of simple imitation of meaningless movements is necessary to determine whether the basic cognitive ability to imitate and simulate is intact in schizophrenia. If there exists a fundamental impairment in the process of simulation in schizophrenia, then deficits should be observed in schizophrenia for all forms of imitation.
A secondary aim of the present study was to investigate the relationship between imitation ability and social functioning to explore if impaired imitation might be associated with difficulties in social behavior. Lastly, the relationship between simulation ability as probed by imitation tasks and the ability to generate and manipulate mental representations in working memory was investigated, motivated by the notion that both simulation and working memory functions require generation and maintenance of mental representations and are supported by overlapping neural circuits. It has been hypothesized that the core deficit in schizophrenia is an impairment of working memory or guidance of behavior by internal representation.18–20 It is possible that social deficits such as impairments in imitation and cognitive deficits such as working memory abnormalities share common roots in problems in generating and simulating the external world.
Method
Participants
Twenty schizophrenia outpatients (7 women) were recruited from 2 outpatient psychiatric facilities in Nashville, TN. Diagnoses were made according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria38 using structured clinical interviews (SCID-IV). All patients were taking atypical antipsychotic medications. The mean chlorpromazine (CPZ) equivalent dose was 297.3 mg (SD = 128.1). Sixteen healthy control subjects (9 women) without a history of DSM-IV Axis 1 disorders in themselves or their families were recruited from the same urban area. Schizophrenia patients (SZ) and controls (CO) were matched for age, education, intelligence, and handedness (see table 1). Exclusion criteria were substance use, neurological disorders, and history of head injury. All subjects had normal or corrected-to-normal vision. All subjects gave written informed consent approved by the Vanderbilt University Institutional Review Board and were paid.
Table 1.
Demographic and Clinical Variables
| Schizophrenia Patients | Healthy Controls | |
| Age | 38.6 (10.3) | 37.9 (8.6) |
| Year of education | 14.2 (2.8) | 14.3 (2.5) |
| Edinburgh handedness | 60.2 (42.0) | 73.7 (45.2) |
| Full scale IQ | 99.1 (17.2) | 96.2 (11.7) |
| Zigler social competence | 3.45 (1.2) | 5.94 (1.5) |
| BPRS | 18.0 (9.9) | — |
| SAPS | 15.8 (16.5) | — |
| SANS | 15.2 (12.0) | — |
Note: BPRS, Brief Psychiatric Rating Scale; SAPS, Scale for the Assessment of Positive Symptoms; SANS, Scale for the Assessment of Negative Symptoms.
Design and Procedure
Clinical and Demographic Measures
Subjects were asked to complete questionnaires and participate in behavioral tasks. Symptoms ratings for SZ were obtained on the same day using the Brief Psychiatric Rating Scale39 (BPRS), the Scale for the Assessment of Positive Symptoms40 (SAPS), and the Scale for the Assessment of Negative Symptoms41 (SANS). For all subjects, social competence was estimated by the Zigler Social Competence Scale,42 which is based on demographic factors such as age, education, employment, and marital status. The Zigler score can range from 0 to 8 with higher scores indicating greater social competence. Intelligence was assessed using the Wechsler Abbreviated Scale of Intelligence.43 Handedness was assessed using the Modified Edinburgh Handedness Inventory44 (see table 1).
Working Memory
Verbal working memory (VWM) span was measured using the Letter Number Sequencing task.45 Subjects were verbally presented a series of letters and numbers and asked to report back the numbers in numerical order, followed by the letters in alphabetical order.
Simple Imitation
Subjects participated in 2 tasks of simple imitation, one involving hand gestures (see figure 1a) and the other mouth movements (see figure 1b). All participants were given explicit instructions to copy and imitate the movements of the “model” as they were watching the stimulus presentation online. They were asked to be as accurate as possible and were given a block of practice trials before starting each imitation task to ensure that they understood the task.
Fig. 1.
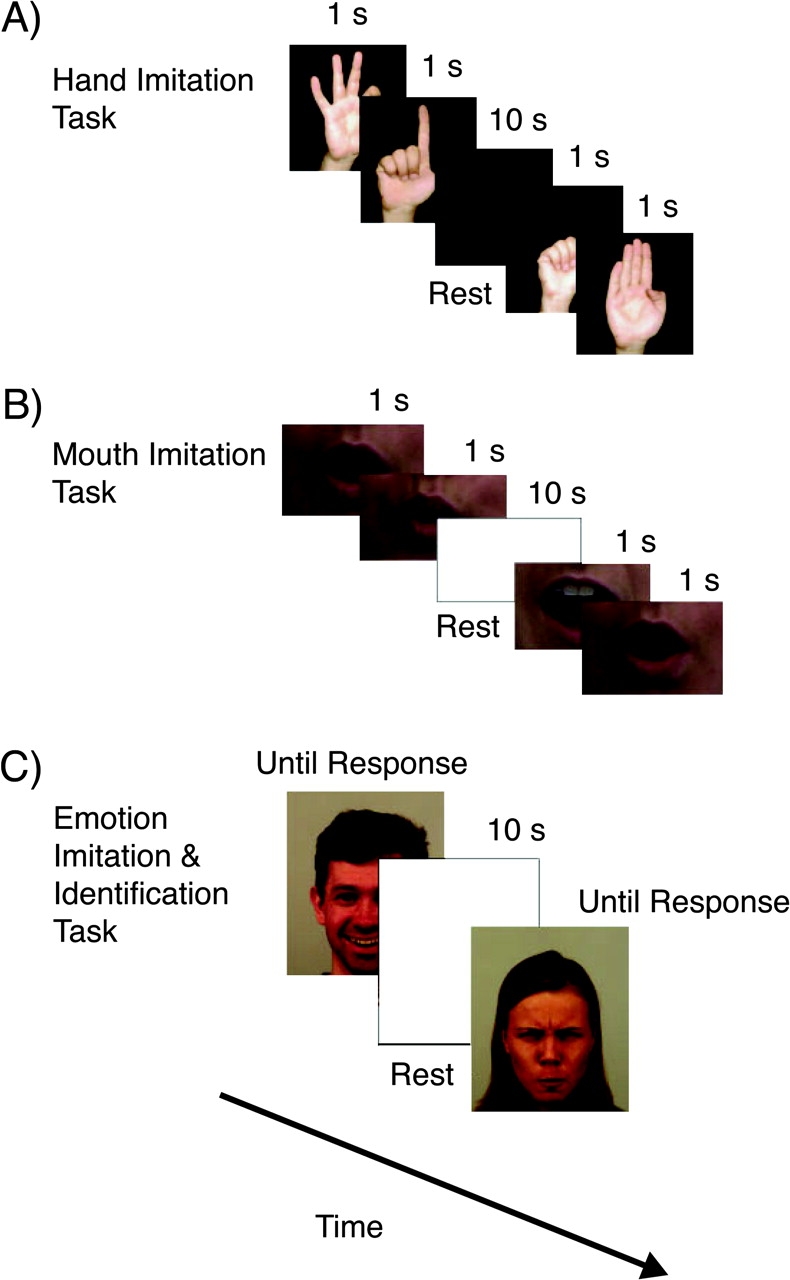
The Procedure for the 3 Imitation Tasks. (a) Hand movement imitation task. (b) Mouth movement imitation task. (c) Emotional face imitation and identification task.
In the hand imitation task, subjects were shown images of a hand forming letters of the alphabet from the American Sign Language (ASL). No participant had prior knowledge of the ASL. Each trial began with a blank screen. When subjects were ready to begin a trial, they pressed the spacebar. Then 2 gestures (ie, 2 ASL letters) appeared on the screen sequentially for 1 s each. Subjects were instructed to imitate each gesture online while they were observing the stimuli. This was followed by a rest period of 10 s. During imitation, each response was compared with the presented stimulus and scored as correct (scoring 1) or incorrect (scoring zero) by a trained experimenter who sat next to the subject with a preprinted scoring form. The imitated gesture was required to be a precise copy of the stimulus gesture (ie, a correct ASL sign); all 5 fingers had to be correctly positioned to receive the correct score for a sign. As each trial consisted of 2 ASL signs, the maximum score for a trial was 2 and the minimum score was 0. There were 2 blocks of 30 trials, one for the left and one for the right hand. The reliability of scoring was established in a separate session with 3 independent raters judging the task performance by 2 individuals (1 male and 1 female), who were not subjects in this study and who imitated the entire stimulus set. The intraclass correlation coefficient was 0.92.
For the mouth imitation task, stimuli were created from video clips of a person producing binary combinations of the English vowels, A, E, I, O, and U (eg, AE, OI, UA, IA). Only the mouth region of the face was presented to the subjects to ensure that they were focusing on that part of the face. During practice trials, subjects watched the video with the sound on and were asked to imitate the mouth to produce the same sounds. The vowel sounds are correctly produced only if the mouth moves appropriately. After practice trials, the sound was switched off. Subjects were instructed to move their mouths as they watched the silent video clip and to produce the corresponding vowel sounds. Each trial began with a blank screen. When subjects were ready to begin a trial, they pressed the spacebar. As shown in figure 1b, a video clip was presented of a person moving her mouth to form 2 vowel sounds one after the other. Each mouth movement corresponding to a single vowel sound was presented for 1 s. Therefore each trial lasted 2 s. Subjects moved their mouths to pronounce the 2 vowel sounds as they were watching the video clip. Then there was a 10-s rest period. There were 30 trials. The responses were scored as correct (score of 1) if subjects accurately replicated the mouth movements, accompanied by the correct vowel sound, and incorrect (score of 0) if the mouth and the vowel sound did not match the stimulus. As each trial consisted of 2 mouth movements, the maximum score for a trial was 2 and the minimum score was 0. An experimenter sat next to the subject during the task and scored the response as correct or incorrect on a preprinted scoring form. The reliability of scoring was established in a separate session with 3 independent raters judging the task performance by 2 individuals (1 male and 1 female) who were not subjects in this study and who imitated the entire stimulus set of mouth movements for vowel pronunciations. The intraclass correlation coefficient was 0.64.
Complex Imitation
Subjects were asked to mimic facial expressions (see figure 1c) and to identify the emotion expressed by the stimulus faces. A block of practice trials was given before the experiment began. For the facial emotion imitation and emotion identification tasks, stimuli were photographs of faces chosen from the Karolinska Directed Emotional Faces.46 Seven emotions (angry, afraid, happy, sad, surprise, disgust, and neutral) were represented by each face. There were 8 male and 8 female stimulus faces. Each trial began with a blank screen. When subjects were ready, they pressed the spacebar. As shown in figure 1c, a stimulus face was presented and subjects were instructed to accurately mimic the facial expression as they were viewing the stimulus face. The stimulus remained on the screen until subjects finished imitating the facial expression. Immediately following imitation, subjects were asked to identify the emotion expressed by the stimulus face by choosing from 7 emotions: anger, fear, happiness, sadness, surprise, disgust, and no emotion (neutral). This was followed by a 10-s resting period. A trained experimenter sat with the subject and scored each face imitation as correct (2 points), partially correct (1 point), or incorrect (0 point) on a preprinted scoring sheet for each trial. The reliability of scoring was established in a separate session with 3 independent raters judging the task performance by 2 participants (1 male and 1 female) who were not subjects in this study and who imitated the entire set of face stimuli. The intraclass correlation coefficient was 0.83. There were 112 trials.
The order of presentation of the hand, mouth, and face tasks was counterbalanced across subjects.
Results
For each task, the accuracy (% correct) was computed. Imitation accuracy on the hand, mouth, and emotional face imitation tasks and the identification accuracy of emotional expression were compared between SZ and CO with analyses of variance (ANOVAs). In addition, correlations were computed to examine the relationships among the imitation ability, clinical symptoms, social competence, and VWM. All significance tests were 2 tailed unless otherwise stated.
Simple Imitation
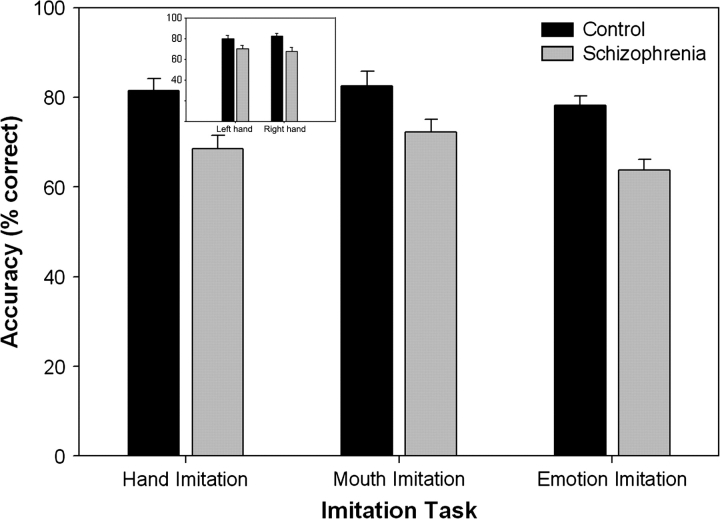
The accuracy of performance of SZ on the hand imitation task was compared with CO using a mixed model ANOVA with hand (left, right) as the within-subjects factor and group (SZ, CO) as the between-subjects factor. This analysis revealed a significant main effect of group, with CO (M = 84.1, SD = 8.4) performing more accurately than SZ (M = 66.4, SD = 12.4), (F(1, 34) = 23.68, P < .001) (see figure 2). There was no significant main effect of hand (F (1, 34) = 0.085, P = .77) and no significant hand-by-group interactions (F (1, 34) = 0.93, P = .34), with CO performing more accurately than SZ for both left- and right-hand gestures. As shown in figure 2, CO (M = 85.1, SD = 9.3) also performed more accurately on the mouth imitation task compared with SZ (M = 70.3, SD = 12.8) (F (1, 34) = 14.9, P = .005).
Fig. 2.
Accuracy (% Correct) of Schizophrenia Patients and Healthy Control Participants on the 3 Imitation Tasks.
Complex (Emotional Face) Imitation and Identification
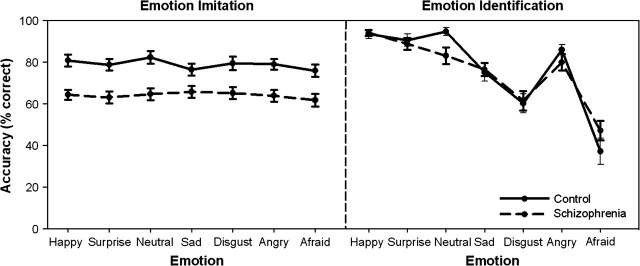
The accuracy of performance of SZ on the emotional face imitation task was compared with CO using a mixed model ANOVA with emotion type (angry, happy, sad, surprised, afraid, disgust, and neutral) as the within-subjects factor and group (schizophrenia, controls) as the between-subjects factor. This analysis revealed a significant main effect of group, with CO (M = 79.4, SD = 7.9) more accurate than SZ (M = 62.9, SD = 9.3) (F (1, 34) = 32.0, P < .001) (see figure 2). There were no significant main effects of emotion type (F (6, 204) = 0.94, P = .46) and no significant emotion-by-group interaction (F (6, 204) = 0.51, P = .77). Performance of SZ and CO on the emotional identification task was also compared using a mixed model ANOVA with emotion type (angry, happy, sad, surprised, afraid, disgust, and neutral) as the within-subjects factor and group (schizophrenia, controls) as the between-subjects factor. There were no significant differences between SZ (M = 75.9, SD = 7.9) and CO (M = 76.9, SD = 6.7) for identification of emotion (F (1, 34) = 0.12, P = .73; see figure 2 and figure 3) and no significant emotion-by-group interaction (F (6, 204) = 1.73, P = .14). There was however a significant main effect of emotional valence (F (6, 204) = 53.25, P < .001) with identification performance for sad, disgust, and afraid emotions significantly lower than that for neutral, happy, surprise, and angry emotions. A correlation was performed between the scores on the emotional identification and emotional imitation tasks to investigate the relationship between emotion identification and imitation ability. The correlation between emotional face identification and emotional face imitation across the 2 groups was not statistically significant but there appears to be a trend (r(36) = 0.30, P = .08). Within SZ, the correlation between emotional face imitation and identification was not statistically significant but there was a trend (r(20) = 0.421, P = .07). Within CO, there was no correlation between the imitation accuracy and the performance on the identification of emotional faces (r(16) = 0.21, P = .43).
Fig. 3.
Accuracy (% Correct) of Schizophrenia Patients and Healthy Control Participants on the Emotional Face Imitation and Identification Tasks.
Relation to Working Memory
Although there was no statistically significant difference in VWM span (F(1, 34) = 2.92, P = .09) between SZ (M = 5.3, SD = 1.2) and CO (M = 5.9, SD = 0.8), there appears to be a trend toward SZ displaying reduced VWM span compared with CO. Correlations between VWM span and accuracy on the 3 imitation tasks and that between VWM span and performance on the emotional identification task were examined across the 2 groups and within each group (see table 2 for a summary of the correlations). Correlations were performed on the total hand imitation score (collapsed across hands) and on the total emotional face imitation, and emotional face identification scores (collapsed across the 7 types of emotions). Across the 2 groups, although the correlation was not statistically significant, there was a trend toward an association between hand imitation accuracy and VWM. In addition, there was a significant correlation between emotion identification accuracy and VWM across the 2 groups; increased accuracy was associated with greater WM span. Within the SZ group, there were no significant correlations among VWM and imitation or identification accuracy. Within CO, there were no significant correlations among VWM and imitation accuracy, but there was a trend toward significant correlation between VWM and emotion identification accuracy, with increased identification accuracy associated with increased WM span (see table 2).
Table 2.
Correlations Among the Performance on Imitation and Emotional Identification Tasks and VWM and Zigler Scores
| VWM Span |
Zigler Social Competence |
|||||
| All Subjects | CO | SZ | All Subjects | CO | SZ | |
| Hand imitation | 0.32+ | 0.13 | 0.21 | 0.42* | −0.19 | 0.08 |
| Mouth imitation | 0.20 | −0.11 | 0.12 | 0.49** | 0.15 | 0.23 |
| Emotional face imitation | 0.16 | −0.36 | 0.093 | 0.55** | 0.07 | 0.21 |
| Emotional face identification | 0.37* | 0.45+ | 0.31 | 0.57* | 0.41 | 0.46* |
Note: Correlations are presented both collapsed across groups and for the within CO and SZ groups separately. VWM, verbal working memory; CO, control; SZ, schizophrenia patients.
*P < .05, **P < .01, and + trend level P <. 09.
Relation to Social Competence Measure
Accuracy on the imitation tasks and the emotion identification task were correlated with the Zigler Social Competence Scores across the 2 groups and within the CO and SZ groups separately. Across the 2 groups, social competence was significantly correlated with hand imitation, mouth imitation, emotional face imitation, and emotional face identification, with higher social competence associated with great imitation and emotion identification accuracy. There were no correlations between the Zigler score and the performance on the imitation or identification tasks in the CO group; however, a significant correlation between social competence and emotional identification was observed in SZ. See table 2 for a summary of the correlations.
Relation to Clinical Symptoms
Correlations among clinical symptoms ratings, and imitation accuracy, identification accuracy, and VWM span are presented in table 3. Overall psychiatric symptoms (BPRS) and negative symptoms (SANS) were significantly associated with imitation performance in SZ, such that higher scores on the BPRS and SANS were associated with poor imitation performance. There was no relationship between positive symptoms (SAPS) and imitation accuracy. There was also no association between clinical symptoms and VWM span.
Table 3.
Correlations Among Clinical Symptoms, and Imitation Accuracy, Emotional Identification Accuracy, and VWM Span in Schizophrenia Patients
| BPRS | SAPS | SANS | |
| Hand imitation | −.50* | −.31 | −.54* |
| Mouth imitation | −.53* | −.24 | −.45* |
| Emotional face imitation | −.45* | −.01 | −.63** |
| Emotional face identification | −.65** | −.39 | −.41 |
| VWM span | −0.24 | −0.26 | −0.32 |
*P < .05 and **P < .01.
Discussion
The major aim of this study was to examine imitation ability in schizophrenia in relation to social functioning, clinical symptoms, and working memory. Several findings emerge from this study. Individuals with schizophrenia were less accurate than controls in all imitation tasks, regardless of the modality and complexity of the task. Patients with schizophrenia had difficulty in imitating meaningless manual and oral gestures, as well as facial emotional expressions. This suggests a basic deficit in the imitation ability, which may reflect a difficulty in simulation.
Impaired facial expression imitation in schizophrenia reported in the present study replicates previous findings.36,37 It is important to note that the emotional face imitation impairment was not accompanied by a deficit in the identification of facial emotional expressions, and the correlation between imitation and identification scores was not significant. Therefore, it seems unlikely that the face imitation deficits were caused by a general impairment in emotion processing. It is possible that because emotion identification was always preceded by emotional face imitation, identification may have been facilitated. Then those emotions with the highest imitation accuracy ratings should be associated with the highest identification scores. Conversely, those emotions with the lowest imitation accuracy ratings should be associated with the lowest identification scores. But the imitation accuracy score is almost invariant whereas the range of identification scores is about 50% for both groups across all 7 emotions. Thus, accurate imitation does not necessarily lead to accurate identification.
Taken together, these results suggest that individuals with schizophrenia have a fundamental impairment in imitation ability regardless of the motor output system (eg, hand, mouth, face). Given the important role of imitation in all aspects of learning and skill acquisition, such impairment may have profound consequences for social behavior. Social impairment is one of the most disabling clinical features of schizophrenia,7,8 and it is possible that imitation impairments may be precursors to these social deficits. In support of this hypothesis, it was found that those who were low on social competence scores were also likely to be impaired on all imitation tasks across the 2 groups. Interestingly, the relationship between the imitation accuracy and social competence was strongest for the emotional face imitation task. There are a number of reasons why this may be the case. First, emotional face imitation ability and empathy may be associated.35 Because emotional states are linked to certain facial expressions, observation of a facial expression might result in the recruitment of the mirrored mechanism in the observer leading to a corresponding emotional state even if the observer is not able to verbally identify the emotion. Such a process might help to explain the phenomenon of emotional contagion in which people automatically mirror facial expressions and moods of others.47–49 This automatic facial mimicry response has been proposed to facilitate empathy through a process of internal simulation of the corresponding facial expressions,50,51 and there is evidence to support that this process is achieved through the mirror mechanism.35 This seems plausible in view of the close connections between the superior temporal sulcus and the amygdala, which have been identified as subserving the neural circuitry that implements the ToM.52 If the same system underlying simulation is a critical component in empathy, then impairments in the mirror mechanism could lead to reduced sensitivity to the emotional states of others, eventually cascading into social dysfunction. One would then expect those individuals who exhibit impairments in imitation to show deficits in empathy.
In the present study, poor imitation ability was significantly associated with the presence of negative symptoms across all imitation tasks, especially for the emotional face imitation condition. This finding highlights the importance of negative symptoms for emotion processing and social cognition.53 Moreover, abnormal frontal cortical function54 and structure55 may be responsible for both negative symptoms and anomalous human mirror mechanism.33,34,56 Moreover, the role of the prefrontal cortex in working memory is well established. It was hypothesized that an impairment in the ability to generate and manipulate internal representations, as observed in working memory tasks,18,19 would be related to the process of simulation in which internal representations of the actions of others are generated. In the present study, a weak correlation between hand gesture imitation and verbal working memory was observed at trend level. Compared with the much more robust correlations among social functioning, negative symptoms, and imitation ability, this association between imitation ability and working memory is fragile. It is important to note that this group of patients with schizophrenia performed rather well on the VWM task, and therefore, the range of the VWM span was very much restricted. The reduced range of VWM scores may have contributed to the weak association between working memory and imitation scores. Langdon and her colleagues17 have suggested that individuals with schizophrenia have a particular impairment in the ability to simulate another person's first–person viewpoint, whether the content of that experience is cognitive, perceptual, or affective. In the present study, all tasks of imitation required this kind of perspective taking because the stimuli consisted of other people's hands, mouths, and faces. In contrast, the letter-number task of verbal working memory is a nonsocial task that requires sequencing over time. Thus, it is possible that a relationship exists between imitation ability and working memory, but the letter-number task was not sensitive enough to detect it.
There are several limitations to the present study. First, it is unknown whether the imitation deficit we observed is a permanent feature of schizophrenia or is state dependent. The patients with schizophrenia in this study were outpatients taking atypical antipsychotic medications and whose clinical symptoms were stable. Thus, it is not possible to determine whether the imitation deficit fluctuates over time or if it is a trait marker. Impairments in mentalizing may be a trait marker,12 but the existing evidence is mixed and some studies have reported intact ToM in remitted patients with schizophrenia.57,58 Future studies should specify the relationship between clinical symptoms and mental simulation as probed by imitation tasks. With respect to potential antipsychotic drug effects, medication does not seem to cause ToM deficits,59 but their potential effects on imitation ability are not known. Second, although the simulation account of ToM motivated the context for this study, ToM was not directly investigated. The role of imitation and simulation should be directly addressed in a future study in which different components of mental state attribution are examined concurrently. Third, the rating system used in the present study should be further refined where possible. It was not possible for the raters to be blind to the diagnosis of participants because upon interacting with the participant it becomes apparent who has psychosis and who does not, at least to clinically trained researchers. One possibility is using sensors on a “data glove” to detect accurately the hand movements made by the subject.60 By transducing the movements of the fingers, the velocity profile of each digit's flexion or extension can be used to segment movements made during an imitation and compared with the corresponding gestures made by the model. Therefore, a multivariate quantitative description of each imitation, including its temporal characteristics, as well as spatial errors can be obtained. Utilizing such methods can bypass the potential problems in rater bias and reliability. A quantitative imitation study using the data glove technology is currently in progress in the authors' laboratory.
To summarize, the present study examined the ability of patients with schizophrenia to imitate the behaviors demonstrated by others. The results indicate a fundamental impairment in imitation ability in schizophrenia and implicate a possible difficulty in simulation. The present study did not directly address the potential relationship between imitation and ToM, but instead it sought to examine whether individuals with schizophrenia are able to engage in mental simulation of the world outside. The fact that patients with schizophrenia show any deficits in imitating simple manual and oral gestures is astonishing. Given that children are prolific “imitation machines”61 beginning in infancy,62,63 it is puzzling why human adults would have a difficulty replicating manual gestures. Further research to determine the neural and developmental origins of this difficulty could be extremely helpful in elucidating the role of simulation in schizophrenia and to establish the complex relationships among internal representation, imitation, and social cognition.
Funding
NARSAD Independent Investigator Award to Park.
References
- 1.Adorno TW. Minima Moralia. Reflections From Damaged Life. London: NLB; 1974. (First published in German in 1951) [Google Scholar]
- 2.Bellack AS, Morrison RL, Wixted JT, Mueser KT. An analysis of social competence in schizophrenia. Br J Psychiatry. 1990;156:809–818. doi: 10.1192/bjp.156.6.809. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin RH, Lewis JA, Cornblatt BA, Erlenmeyer-Kimling L. Social competence deficits in adolescents at risk for schizophrenia. J Nerv Ment Dis. 1994;182(2):103–108. doi: 10.1097/00005053-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Hans SL, Auerbach JG, Asarnow JR, Styr B, Marcus J. Social adjustment of adolescents at risk for schizophrenia: the Jerusalem Infant Development Study. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1406–1414. doi: 10.1097/00004583-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33(3):688–702. doi: 10.1093/schbul/sbm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2007;32:S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green MF, Penn D, Bentall R, et al. Social Cognition in Schizophrenia: an NIMH Workshop on Definitions, Assessment, and Research Opportunities. Schizophr Bull. 2008 doi: 10.1093/schbul/sbm145. doi:10.1093/schbul/sbm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith TE, Hull JW, Huppert JD, Silverstein SM. Recovery from psychosis in schizophrenia and schizoaffective disorder: symptoms and neurocognitive rate-limiters for the development of social behavior skills. Schizophr Res. 2002;55(3):229–237. doi: 10.1016/s0920-9964(01)00231-6. [DOI] [PubMed] [Google Scholar]
- 9.Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:515–526. [Google Scholar]
- 10.Frith CD. The Cognitive Neuropsychology of Schizophrenia. Hove, UK: Lawrence Erlbaum; 1992. [Google Scholar]
- 11.Brüne M. Theory of mind in schizophrenia: a review of the literature. Schizophr Bull. 2005;31:21–42. doi: 10.1093/schbul/sbi002. [DOI] [PubMed] [Google Scholar]
- 12.Sprong M, Schothorst P, et al. Theory of mind in schizophrenia: meta-analysis. Br J Psychiatry. 2007;191:5–13. doi: 10.1192/bjp.bp.107.035899. [DOI] [PubMed] [Google Scholar]
- 13.Hurley S, Charter N. Introduction: the importance of imitation. In: Hurley S, Charter N, editors. Perspectives on Imitation. Vol 2. Cambridge, MA: MIT press; 2005. pp. 1–52. [Google Scholar]
- 14.Gopnik A, Wellman HM. The theory theory. In: Hirschfeld LA, Gelman SA, editors. Mapping the Mind: Domain Specificity in Cognition and Culture. New York, NY: Cambridge University Press; 1994. pp. 257–293. [Google Scholar]
- 15.Langdon R, Coltheart M. Mentalising, schizotypy, and schizophrenia. Cognition. 1999;71:43–71. doi: 10.1016/s0010-0277(99)00018-9. [DOI] [PubMed] [Google Scholar]
- 16.Heal J. Replication and functionalism. In: Butterfield J, editor. Language, Mind and Logic. Cambridge, UK: Cambridge University Press; 1986. pp. 135–150. [Google Scholar]
- 17.Langdon R, Coltheart M, Ward P, Catts S. Visual and cognitive perspective-taking impairments in schizophrenia: a failure of allocentric simulation? Cognit Neuropsychiatry. 2001;6(4):241–269. [Google Scholar]
- 18.Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry. 1994;6:348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Park S. Working memory impairments in schizophrenia: a meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 21.Goldman AI. Imitation, mind reading, and simulation. In: Hurley S, Charter N, editors. Perspectives on Imitation. Vol 2. Cambridge, MA: MIT press; 2005. pp. 79–93. [Google Scholar]
- 22.Thorndike EL. Animal intelligence: experimental studies. Second edition. Edison, NJ: Transaction Publishers; 1999. [Google Scholar]
- 23.Heyes CM. Causes and consequences of imitation. Trends Cogn Sci. 2001;5:253–261. doi: 10.1016/s1364-6613(00)01661-2. [DOI] [PubMed] [Google Scholar]
- 24.Dijksterhuis A. Why we are social animals: the high road to imitation. In: Hurley S, Charter N, editors. Perspectives on Imitation. Vol 2. Cambridge, MA: MIT press; 2005. pp. 207–220. [Google Scholar]
- 25.Bandura A. Social Learning Theory. New York, NY: General Learning Press; 1977. [Google Scholar]
- 26.Rogers S. An examination of the imitation deficit in autism. In: Nadel J, Butterworth G, editors. Imitation in Infancy. Cambridge, UK: Cambridge University Press; 1999. pp. 254–283. [Google Scholar]
- 27.Rogers S, Pennington B. A theoretical approach to the deficits in infantile autism. Dev Psychopathol. 1991;3:137–162. [Google Scholar]
- 28.Williams JHG, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 29.Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2:493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- 30.Gallese V, Fadiga L, et al. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- 31.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 32.Rizzolatti G, Craighero L. The mirror neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 33.Iacoboni M. Neural mechanisms of imitation. Curr Opin Neurobiol. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Iacoboni M, Woods RP, et al. Cortical mechanisms of human imitation. Science. 1999;24:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 35.Carr L, Iacoboni M, et al. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100:497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putnam KM, Kring AM. Accuracy and intensity of posed emotional expressions in unmedicated schizophrenia patients: vocal and facial channels. Psychiatry Res. 2007;151(1–2):67–76. doi: 10.1016/j.psychres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz BL, Mastropaolo J, et al. Imitation of facial expressions in schizophrenia. Psychiatry Res. 2006;145:87–94. doi: 10.1016/j.psychres.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Arlington, VA: APPI; 1997. [Google Scholar]
- 39.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 40.Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) Iowa City, IA: The University of Iowa; 1984. [Google Scholar]
- 41.Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- 42.Zigler E, Levine J. Premorbid competence in schizophrenia: what is being measured? J Consult Clin Psychol. 1981;49:96–105. doi: 10.1037//0022-006x.49.1.96. [DOI] [PubMed] [Google Scholar]
- 43.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 44.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 45.Gold JM, Carpenter C, Randolf C, Goldberg TE, Weinberger DR. Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry. 1997;54:159–165. doi: 10.1001/archpsyc.1997.01830140071013. [DOI] [PubMed] [Google Scholar]
- 46.Lundquist D, Flykt A, Ohman A. The Karolinska Directed Emotional Faces [CD-ROM] Stockholm, Sweden: The Karolinska Institute; 1998. [Google Scholar]
- 47.Dimberg U. Facial reactions to facial expression. Psychophysiology. 1982;19:643–647. doi: 10.1111/j.1469-8986.1982.tb02516.x. [DOI] [PubMed] [Google Scholar]
- 48.Dimberg U, Lundquist O. Facial reactions to facial expressions: sex differences. Psychophysiology. 1988;25:442–443. [Google Scholar]
- 49.Bush LK, Barr CL, et al. The effects of facial control and facial mimicry on subjective reactions to comedy routines. Motiv Emot. 1989;13:31–52. [Google Scholar]
- 50.Wallbott HG. Recognition of emotion from facial expressions via imitation? Some indirect evidence for an old theory. Br J Soc Psychol. 1991;30:207–219. doi: 10.1111/j.2044-8309.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 51.Niedenthal PM, Brauer M, et al. When did her smile drop? Facial mimicry and the influences of the emotional state on the detection of change in emotional expression. Cogn Emot. 2001;15:853–864. [Google Scholar]
- 52.Baron-Cohen S. Mindblindness. An Essay on Autism and Theory of Mind. Cambridge, UK: MIT Press; 1995. [Google Scholar]
- 53.Gur RE, Kohler CG, Ragland JD, et al. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophr Bull. 2006;32(2):279–287. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolkin A, Sanfilipo M, Wolf AP, Angrist B, Brodie JD, Rotrosen J. Negative symptoms and hypofrontality in chronic schizophrenia. Archives of General Psychiatry. 1992;49(12):959–965. doi: 10.1001/archpsyc.1992.01820120047007. [DOI] [PubMed] [Google Scholar]
- 55.Wolkin A, Choi SJ, Szilagyi S, Sanfilipo M, Rotrosen JP, Lim KO. Inferior frontal white matter anisotropy and negative symptoms of schizophrenia: a diffusion tensor imaging study. Am J Psychiatry. 2003;160(3):572–574. doi: 10.1176/appi.ajp.160.3.572. [DOI] [PubMed] [Google Scholar]
- 56.Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3:e79. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pousa E, Duñó R, Brébion G, David AS, Ruiz AI, Obiols JE. Theory of mind deficits in chronic schizophrenia: evidence for state dependence. Psychiatry Res. 2008;158(1):1–10. doi: 10.1016/j.psychres.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Pousa E, Ruiz AD, David AS. Mentalising impairment as a trait marker of schizophrenia? Br J Psychiatry. 2008;192:312. doi: 10.1192/bjp.192.4.312. [DOI] [PubMed] [Google Scholar]
- 59.Mizrahi R, Korostil M, Starkstein SE, Zipursky RB, Kapur S. The effect of antipsychotic treatment on Theory of Mind. Psychol Med. 2007;37(4):595–601. doi: 10.1017/S0033291706009342. [DOI] [PubMed] [Google Scholar]
- 60.Gold BJ, Pomplun M, Rice NJ, Sekuler R. A new way to quantify the fidelity of imitation: preliminary results with gesture sequences. Exp Brain Res. 2008;187(1):139–152. doi: 10.1007/s00221-008-1291-2. [DOI] [PubMed] [Google Scholar]
- 61.Tomasello M. The Cultural Origins of Human Cognition. Cambridge, MA: Harvard University Press; 1999. [Google Scholar]
- 62.Meltzoff AN, Moore MK. Interpreting ‘imitative’ responses in early infancy. Science. 1979;205:217–219. doi: 10.1126/science.451596. [DOI] [PubMed] [Google Scholar]
- 63.Meltzoff AN, Moore MK. Early imitation within a functional framework: the importance of person identity, movement, and development. Behav Dev. 1992;15:479–550. doi: 10.1016/0163-6383(92)80015-M. [DOI] [PMC free article] [PubMed] [Google Scholar]