Abstract
Endophenotypes represent intermediate phenotypes on the putative causal pathway from the genotype to the phenotype. They offer a potentially valuable strategy to examine the molecular etiopathology of complex behavioral phenotypes such as schizophrenia. Neurocognitive and neurophysiological impairments that suggest functional impairments associated with schizophrenia have been proposed as endophenotypes. However, few studies have examined the structural variations in the brain that might underlie the functional impairments as useful endophenotypes for schizophrenia. Over the past three decades, there has been an impressive body of literature supporting brain structural alterations in schizophrenia. We critically reviewed the extant literature on the neuroanatomical variations in schizophrenia in this paper to evaluate their candidacy as endophenotypes and how useful they are in furthering the understanding of etiology and pathophysiology of schizophrenia. Brain morphometric measures meet many of the criteria set by different investigators, such as being robustly associated with schizophrenia, heritable, quantifiable, and present in unaffected family members more frequently than in the general population. We conclude that the brain morphometric alterations appear largely to meet the criteria for endophenotypes in psychotic disorders. Some caveats for the utility of endophenotypes are discussed. A proposal to combine more than one endophenotype (“extended endophenotype”) is suggested. Further work is needed to examine how specific genes and their interactions with the environment may produce alterations in brain structure and function that accompany psychotic disorders.
Keywords: schizophrenia, endophenotypes, magnetic resonance, imaging, genetics, neuroanatomy, neuroimaging
Introduction
Research over the last century has led to major advances in the understanding of the neurobiology of schizophrenia. However, the neurobiological abnormalities are so varied that no single abnormality is observed across the entire diagnostic rubric subsumed under the Diagnostic and Statistical Manual of Mental Disorders, IV edition (DSM IV1)–defined schizophrenia. While one of the major reasons is poorly defined phenotypic boundaries, such inconsistent observations may be attributed to differing underlying genetic architecture of individuals with schizophrenia. The role of genetic factors in the etiology of schizophrenia has been supported by a high heritability for schizophrenia. This suggests that a large part of the variance for the etiology of schizophrenia is contributed by genetic factors. Having stated this, the precise nature of genetic basis of schizophrenia still remains unclear. It is still unclear regarding which gene or genes are responsible and variations in how many genes are required for the onset of the clinical syndrome. Current thinking is that the disease may be due to several genes of small effect that interact with environmental factors. Examining such interactions is a daunting task. In comparison to this, a larger challenge by several orders of magnitude is to precisely delineate the pathway(s) that connect genetic variations to the phenotype in question. Several factors contribute to this challenge. Besides the poorly defined phenotypic boundaries, genetic investigations have been hampered by small effect of the identified genetic risk loci, poorly understood pathophysiology, possible contribution of several genes, and complex interactions among the genes and with the contributing environmental factors. An alternative view is that schizophrenia is a common disease caused by multiple rare variants.2 Even with this possibility, the challenge is nevertheless not any smaller in identifying such variants and elucidating the pathophysiologic pathway.
Herein lies the potential utility of endophenotypes. Endophenotypes are quantitative traits in the putative pathophysiologic pathway from the genotype to the phenotype.3 They offer a valuable strategy to help address the above problems potentially more effectively. This is especially true in the current clinical scenario where the official psychiatric classification systems continue to be based on nonspecific symptoms and not on neurobiology.4 Further, syndromal definitions of the disorder are arbitrary and the disorders are considered categorically while ignoring the dimensional overlap of the symptoms. These factors make it difficult to map specific psychiatric illnesses as defined in the official classificatory systems to the genome.
There are many advantages of investing in closely examining the endophenotypes. They are often closer to the underlying genetic variations in the pathophysiologic pathway. These markers may be relatively simpler to examine compared with the clinical manifestations. The endophenotypic traits may be determined by fewer genes than the complex behavioral phenotypes; consequently, one could envision relatively fewer interacting factors that may portend fewer neuronal networks involved in its regulation. They may, therefore, offer a better “foothold” for the researcher to move closer to understanding the underlying causes and mechanisms of mental illnesses including the underlying genetic variations, relevant interacting environmental factors, their impact on the brain, and the associated clinical symptoms. However, these assumptions are overly simplistic. The “endophenotypic” markers may not be pathophysiologically or genetically simpler than the clinical phenotype. As would be clear from the discussion that follows, some endophenotypes may be common to more than one DSM IV–defined nosologic entity, while some are relatively more specific. Such critical observations could provide potential “branch points” to delineate the pathophysiologic pathways and biologically dissect the psychiatric syndromes. Another important advantage to examining the endophenotypes is that they are quantifiable as continuous measures, and therefore, a dimensional approach is tenable while employing endophenotypes as one of the outcome variables. Further, these markers may be utilized in the quantitative trait locus (QTL) approaches.
Definition of Endophenotypes and Its Scope
Gottesman and Gould5 offered a set of five defining criteria for endophenotypes. In order to be called an endophenotype, a biological marker should (a) be associated with the illness in the relevant population; (b) be state independent, ie, present both during periods of illness and wellness; (c) be heritable; (d) cosegregate with the illness within families; and (e) be present in unaffected family members more frequently than in the general population. The issue of state independence of a marker has been modified to include the epigenetic influence on a given marker. In the absence of a disease or before the onset of a disease, these markers should be able to be detected following a challenge, such as a cognitive challenge in Alzheimer's disease or schizophrenia or glucose challenge in diabetes mellitus.6
In addition to the above criteria, other investigators have suggested different but overlapping “criteria” sets.7–12 They are as follows: (1) Endophenotypes need to be related to the cause rather than the consequence of the illness (in other words, the marker should at least be involved in a biologically plausible mechanism of pathogenesis13,14), (2) they should have reliable psychometric properties or be reliably measured, (3) the markers should be stable over time, and (4) they should be related to the symptoms/features of the illness. In addition, some researchers propose that an endophenotype should be continuously quantifiable, should probabilistically predict the disease, and should be closer to the primary etiology than to the diagnostic category.12 Thus, the definition of endophenotype can vary in its stringency. At this stage, it is not clear how many of these criteria a marker should meet in order to consider it an endophenotype.
Certain caveats in the utility of endophenotypes merit discussion here. It is often questioned whether the endophenotypes are indeed internal (endo-) as opposed to being exophenotypes, eg, neurocognitive variations. For the latter, the term “intermediate phenotypes” is suggested, implying that these traits may be closer to the syndromal expression. The term “endophenotype” may be reserved for quantifiable, heritable traits that are not manifestly expressed, eg, the brain structural variations and brain oxygenation level–dependent (BOLD) responses measured using functional magnetic resonance imaging. These traits also may be localized within the pathophysiologic pathway whose impact may be externally manifested as an intermediate phenotype.
Several potential endophenotypic/intermediate markers have been proposed in schizophrenia, including neurocognitive impairments15–20 and electrophysiological abnormalities.21,22 Because the endophenotypes may be relatively closer to the genetic etiology of a disease, it is assumed that these traits may be associated with fewer interactions, determined by more discrete networks, and the effect size of genetic variations on the endophenotypes are presumed to be larger than on the clinical phenotype. This may not be applicable to all traits in the pathophysiologic pathway. For example, neurocognitive traits may be farther away from the genetic variations but closer to the clinical manifestation. Environmental influences and nongenetic factors, such as subject motivation, test administration, may influence the measurement process affecting the heritability estimates. Nevertheless, a recent genome-wide linkage study on a large sample of nuclear families with multiple affected members showed stronger association with neurocognitive endophenotypes than for the clinical diagnosis.23 For example, a locus on 4q13-25 within a 30cM region accounted for 33%, 33%, and 32% of the variation in delayed memory, semantic clustering, and verbal learning, respectively, using variance component analysis. When these investigators included the schizophrenia spectrum disorder as dichotomous variable, it greatly reduced the linkage signal. On the contrary, a large meta-analysis of published studies found that the effect size of genetic variations was not necessarily larger for the endophenotypes compared with the phenotype itself.24 These authors examined neurocognitive performance on Wisconsin card-sorting test (WCST) that measures executive function, N-back tasks for working memory, and the P300 event-related potential variables with one exonic single-nucleotide polymorphism (SNP) on catechol-O-methyl transferase gene (COMT; rs4680; Val/Met). All three examined measures showed small effect sizes; for perseverative errors on WCST and the N-back task performance, an estimated effect size was 0.5% of the phenotypic variance (VP), whereas it was 0.01% for the event-related potential P300. These small effects may be because, as authors point out, WCST and N-back structure may be complex. Further, performance on such tasks may be influenced by measurement and performance biases, testing conditions, and other variables not directly related to the pathology in question and may not be related to the genetic effects. In contrast to these measures, BOLD responses related to serotonin transporter gene polymorphism (5-HTT) and P50, an event-related potential related to 7-nicotinic acid receptor, were found to have larger effect sizes. These studies demonstrate that selection of endophenotypes needs closer examination of the trait using sufficiently powered samples, replicate studies, and meta-analyses.
While it remains unclear whether these putative endophenotypes help in linkage or association studies, the utility of these markers as quantitative traits in the QTL approach may provide better power to detect bigger effect sizes. In this approach, traits of interest are continuous variables compared with the dichotomous trait of diagnosis. Quantitative trait locus is a region of the chromosome that would be correlated with the quantitative trait. Such QTLs may be clustered together or scattered throughout the genome. Mouse genetic studies show that an overwhelming majority of loci contributed small proportion of variance to several quantitative traits, suggesting that even endophenotypes may pose a challenge in elucidating their genetic architecture.25 Another equally daunting challenge could be poor spatial localization of these markers in the brain. Therefore, more stable measures are recommended to be of greater utility as endophenotypes, such as brain structural measures. It must be noted that schizophrenia is a clinically and etiologically heterogeneous disorder. It is possible that some endophenotypes may be associated with certain distinct but not yet teased out biological subsyndromes, giving rise to a possibility of lower heritability or even lower effect sizes. For this reason, such observations need not deter one from examining the usefulness of endophenotypes.
One potential way forward is to improve the utility of endophenotypes by combining functionally related endophenotypes, eg, merging the brain structural changes with cognitive variations regulated by the same regions. We propose the term “extended endophenotypes” to a network of endophenotypes that are linked on the basis of putative or documented functional basis. Constructing such “extended endophenotypes” may improve our chances of delineating the pathway from the genetic variations to the behavioral phenotype and could help in deconstructing the schizophrenia phenotype into biologically more meaningful clinical phenotypes that may be amenable to developing rational pharmacotherapy. In addition, such a construct could help identify potential “branch points” for other related psychotic disorders.
Recent years have witnessed an expansion of neuroimaging research in psychiatric disorders, notably schizophrenia.26 Measurements of brain structure are highly reliable and, in some cases, correlate robustly with phenomenologic features of psychiatric disorders; this makes brain structural alterations potentially attractive endophenotypes. However, few studies have critically examined whether various brain structure abnormalities in schizophrenia meet the criteria for endophenotypes.
In this paper, we examine whether brain structural alterations as measured by magnetic resonance imaging (MRI) could be considered as endophenotypes. We searched the literature databases for meta-analyses and systematic reviews using terms “endophenotype,” “intermediate phenotype,” “neuroanatomy,” “brain structure,” “MRI,” “schizophrenia,” “genetics,” “psychosis,” “white matter,” “at-risk,” and “high-risk.” Additionally, we searched the literature for individual studies and included relevant studies. Wherever relevant, we also searched the literature including other disorders such as bipolar disorders. We briefly review the results from consistently replicated studies, systematic reviews, and meta-analyses of the relevant literature. We consider structural imaging studies in both patients with schizophrenia and the affective psychoses and studies on at-risk populations. Further, we discuss the value of bringing together more than one endophenotype to construct pathophysiologically meaningful “extended endophenotypes.” Such an approach enables integration of genetic and neuroimaging paradigms in our efforts to clarify whether MRI morphometric measures meet the criteria for endophenotypes of psychotic and related disorders.
Morphometric Measures and the Endophenotype Criteria
Structural MRI Variations Are Associated With the Illness
Over the past three decades, advances in in vivo neuroimaging techniques have led to the identification of a number of brain structural abnormalities in schizophrenia and have confirmed or extended earlier postmortem findings. Investigators have adopted either region of interest (ROI) or computational voxel-wise analytical approaches. The ROI approach entails precisely defining the anatomical boundaries of each hypothesized brain region and then reliable investigators manually tracing or parcellating each region for measurement. Automated ROI approaches are either fully automated programs or involve training a neural network program to trace the region and then manually editing them. Voxel-based morphometry (VBM) performs voxel-wise analysis for the intensity on images that are normalized onto a template image.
Systematic reviews and meta-analyses of structural MRI studies in schizophrenia indicate that the whole-brain volume is reduced, particularly in gray matter, while the ventricular volume is increased.27–30 These studies found the reductions in the temporal lobe, eg, two meta-analyses reported larger reductions in the hippocampus, amygdala, and superior temporal gyrus (STG).31,32 Meta-analysis of individual brain regions have also revealed consistent differences with modest effect sizes. For example, reductions in thalamic volume,33 corpus callosum area,34 hippocampal volume,35 and reduced leftward asymmetry of planum temporalè in schizophrenia patients27 have also been reported.
With advances in computational morphometric approaches, automated regional parcellation and VBM studies have validated results of earlier ROI-based approaches.35,36 Voxel-based morphometry studies consistently report gray matter density reductions in medial temporal lobes (MTLs) and the STG.36 High-dimensional nonlinear pattern classification techniques allow one to quantify the classification accuracy between schizophrenia and healthy controls. A recent study showed average discrimination accuracy of 82% for women and 85% for men for this method between schizophrenia and healthy controls.37
Relatively fewer studies have examined the white matter (WM) as an important endophenotype. Recent discoveries on the importance of WM beyond being a scaffolding tissue for the brain have renewed the interest of the researchers. Earlier studies on WM were somewhat equivocal in their findings. Some MRI studies using older methods of analyses did not find WM differences.38,39 Recent studies using diffusion tensor imaging (DTI) and magnetization transfer ratio (MTR) protocols have begun to shed more light on the WM deficits among schizophrenia patients. Magnetization transfer imaging is based on the signal loss due to an interaction of bound protons and free protons; a reduction in MTR reflects loss of myelin and axons. One study on first-episode schizophrenia patients found that the MTR was reduced in the medial prefrontal cortex, insula, and substantia uncinatus.40 Another study using T2 relaxation reported decreased myelin water fraction, specifically in the anterior genu of the corpus callosum.41 Studies using DTI42–44 have showed reductions in fractional anisotropy in the frontal regions, hippocampus, corpus callosum, left fronto-occipital fasciculus, and left inferior longitudinal fasciculus. Some of these observations have been made on the first-episode, never medicated schizophrenia patients, suggesting that such alterations exist at the onset of the disease.43 A study using statistical parametric mapping found frontotemporal WM deficits.45 Such WM abnormalities are expected to affect the anatomical connectivity and impact the function. This was examined in a study using concurrent DTI and electrophysiological methods. In a small sample of first-episode schizophrenia patients, reduced fractional anisotropy correlated with increased anterior alpha acivity.46 Such findings if replicated may emphasize WM abnormalities intuitively underlying schizophrenia as a “disconnection syndrome.” However, as can be noticed from the above review on the WM abnormalities among schizophrenia, some observations are replicated while others are not. With advances in technology to detect WM changes, the nature of abnormalities may become clearer.
Taken together, evidence so far points to the consistent association of brain structural alterations with schizophrenia. Majority of studies have suggested frontotemporal structural abnormalities to be relatively prominently present in schizophrenia patients.37,47 Therefore, MRI-observed cerebral structural variations may not represent diffuse changes in the brain. They may reflect localized pathology in specific neural circuitries critical to regulate brain function that underlies the disease.
Specificity to Schizophrenia
In order to evaluate the specificity of structural brain abnormalities in schizophrenia, we searched the published literature on psychotic disorder spectrum (bipolar disorders, major depressive disorders, and other psychotic disorders including delusional disorder). Structural neuroimaging literature in bipolar disorder is relatively scant; brain structure changes in affective disorder appear to be less marked but qualitatively similar to schizophrenia.48 Relatively few studies directly compare structural differences between schizophrenia and bipolar disorder patients. Furthermore, those studies that have been done so far have had relatively small samples. Additionally, few studies have examined the relationships between MRI findings and clinical or neurobehavioral features in affective disorders. In general, whole-brain volume reduction seen in schizophrenia is not evident in bipolar disorder.49–53 Bipolar patients may not show volume reductions in amygdala but may actually have volume increases.52 Meta-analyses show more frequent signal hyperintensities in affective disorder.50,54,55
A study on a cohort of schizophrenia, bipolar disorder patients and their unaffected relatives revealed reductions in WM density in the subgyral and anterior limb of the internal capsule among schizophrenia patients, whereas bipolar disorder patients showed reductions in the anterior limb of the internal capsule only.56 On the other hand, DTI studies on bipolar disorder patients suggests increased fractional anisotropy (FA) values in the midline genu57 and reduced FA values in the superior longitudinal fasciculus,58 superior frontal tracts,58,59 and cingulate–paracingulate WM.58 Among the euthymic bipolar patients, DTI tractography revealed increased number of reconstructed fibers between the left subgenual cingulate cortex and left amygdalo-hippocampal complex.60 Apparent diffusion coefficient, a measure of WM fiber integrity, was bilaterally increased in the orbitofrontal cortex.61 These data suggest that the WM anomalies are more widespread among bipolar disorder patients.
The reported similarities and differences in morphometric abnormalities in bipolar disorder and schizophrenia may be related to possible overlapping subgroups that may be associated with alterations in the same brain structures.62,63 One study that contrasted psychotic and nonpsychotic bipolar disorders observed that the former were associated with ventricular and left hippocampal abnormalities similar to those seen in schizophrenia.64 Likewise, Salokangas et al observed that patients with psychotic, but not nonpsychotic, depression have enlarged ventricles.65 Systematic neuroimaging studies on delusional disorder are even fewer. One imaging study on patients with late paraphrenia noted nonsignificantly reduced temporal lobe volumes but significant asymmetry of temporal lobes.66 Studies employing computerized tomographic scans have revealed similar ventricle brain ratio (∼13) for both late-onset paraphrenia and late-onset schizophrenia.67,68
In summary, brain structural alterations may cut across the traditional diagnostic boundaries between schizophrenia and affective disorders but may be unique to those with psychotic features and may discriminate between psychotic and nonpsychotic mood syndromes, suggesting some degree of specificity for psychotic illnesses.
MRI Abnormalities Are State Independent and Are Stable Over Time
This criterion is better applicable to functional changes than to the structural alterations. Structural brain abnormalities in schizophrenia could potentially be confounded by illness chronicity,69 medication,70 or substance abuse.71–73 There is now strong evidence that brain structural alterations in schizophrenia is present at illness onset, in first-episode schizophrenia patients who have not been treated with antipsychotics, and also in prodromal patients.74,75 A recent systematic review and meta-analysis of 68 studies compared brain volumes in patients with a first-episode of psychosis with the healthy control subjects.28 Pooled sample size from 52 studies was 1424 first-episode psychotic patients who were studied cross-sectionally. Sixteen studies had conducted longitudinal examination comprising 465 patients. Meta-analysis suggested that whole-brain and hippocampal volume were reduced (both P < 0.0001) and ventricular volume was increased (P < 0.0001) in these patients relative to healthy controls. Another meta-analysis of structural MRI studies in first-episode schizophrenia also revealed reduced whole-brain and hippocampus volumes.76 These observations suggest that the structural abnormalities are state independent and very likely predate the illness. However, one fact cannot be ruled out conclusively at present. The disease process is known to have its onset much before the clinical manifestation starts. Therefore, the structural changes that are observed may be related to the disease process but may be relatively stable after the onset of the disease.
Several studies have been conducted on clinically stable schizophrenia patients. One study reported deformations in the hippocampal shape, especially at the head of the hippocampus77 among clinically stable schizophrenia patients. The same authors observed similar deformations in the hippocampus among clinically ill schizophrenia patients.78 This evidence supports that some brain structural alterations are state independent. Deformations in this region of the hippocampus suggest that the neurons located in the head of the hippocampus that send projection fibers to the frontal cortex may be affected early in the course of the illness and later in the course.
Brain structural changes appear to persist during the course of the schizophrenic illness. Meta-analysis of longitudinal studies reported significant decrease of whole-brain volume after the diagnosis, with no changes noted in the WM and cerebellum.28 A longitudinal study found no difference in the rate of volume changes over time between patients with schizophrenia and healthy controls for any of the brain structures measured.79 However, other studies suggest ongoing changes in the brains of schizophrenic patients during the early years after diagnosis.80,81 Prescription medications and substance use confound the longitudinal studies. For example, treatment with typical antipsychotics was associated with increased basal ganglia volume and decreased cortical gray matter volume.70,82 This was detectable even after 12 weeks of treatment.83 In any case, such observed changes, if any, are likely to be short term, and the evidence for any lasting structural changes with long-term treatment is weak.84 Alcohol abuse may contribute to diffuse brain structure changes or show more prominent frontal or temporal lobe alterations73; however, the abnormalities in schizophrenia described earlier have been reported in patients with no history of alcohol abuse.85 At this time, it is hard to conclude whether the brain structural alterations remain stable or change over the time as the disease progresses. Further, with the available data, it is not definitive that the structural alterations are state independent.
One useful but inadequately examined dimension is the association of developmental trajectory of gray and WM with the structural alterations observed in schizophrenia. One study examined the ratio between the gray and WM volumes among schizophrenia patients and healthy subjects. Although not a longitudinal study, regression analyses suggested that the gray/white ratio in the frontal and temporal regions decreased linearly among the healthy subjects but remained unchanged among schizophrenia patients.86 However, there are no published studies to our knowledge as to whether the pattern of development itself could be an endophenotype. Such studies could provide insights into the neurodevelopmental trajectory and the precise developmental window to focus further research. Overall, brain structural alterations have been reported at every stage of the illness, although there is some debate about whether there is a progressive worsening of these changes with illness chronicity; these changes are unlikely to be explained by effects of medications or concomitant substance use.
Are Structural Abnormalities Related to the Cause or the Consequence of the Disorder? Do Structural Abnormalities Predict the Onset of Schizophrenia?
A review of current literature on structural brain alterations in first-episode, treatment-naïve patients suggests that these are unlikely to be the consequence of the illness. Foregoing discussion of longitudinal studies does not conclusively prove that the structural changes are indeed consequences of the disorder either. However, it is hard to directly establish the causality of structural brain abnormalities with the disease using the available evidence. Schizophrenia has been considered a neurodevelopmental disorder. The etiological factors have been proposed to impact the brain development either early in life (“early-hit” theories) or during the adolescence (“late-hit” theories). Therefore, many structural alterations observed before the onset of the illness may be considered to be related to the cause rather than the consequence of the disorder. More specifically, structural changes that predate the onset and predict the clinical manifestation may be considered to be related to the cause of the disorder. An important caveat is that in a disorder that is intertwined with the development, it is hard to discriminate the structural changes due to the causes or those that develop as a consequence of abnormal development. Brain structural changes observed before the onset of the illness may be a consequence of the preclinical or subclinical disease process. Some indirect evidence to that effect is provided by characterizing structural brain alterations before the onset of the illness among prodromal schizophrenia patients who later develop the illness.87 These investigators found abnormalities in the temporal (medial and lateral) and frontal (inferior frontal and orbitofrontal) regions in those who developed the disorder compared with those who did not. This suggests that the frontotemporal abnormalities in schizophrenia may be the earliest to appear. Another study on at-risk individuals reported increased prefrontal gyrification in those who developed the illness compared with those who did not.88 Gray matter changes in the prefrontal cortex have been proposed as predictive of later onset of psychosis in high-risk subjects.89,90 For this reason, it can be concluded that some of the brain changes probably lie in the pathophysiological pathway that leads to the development of clinical manifestation and also probabilistically increase the risk for the future development of the disorder. The latter have been proposed by some to be important characteristics of endophenotypes.12
Brain Structural Measures Are Heritable
Brain structure varies between individuals, and such variability may be determined by genetic (ie, single or multiple genes) or environmental factors (eg, nutritional factors) or their interactions between these two factors. Heritability is the proportion of VP attributable to genetic variance (VG). Heritability is a complex construct. As stated earlier, the total VP is the sum of environmental variance (VE) and VG. Genetic variance may be broken down into additive genetic effects and dominance effects. Broad-sense heritability (H2) takes all VGs into account, whereas narrow-sense heritability (h2) accounts for the additive VGs only. Similarly, environmental variance may be accounted for by additive effects of multiple environmental factors. Estimation of heritability for human traits is further made complex by the fact that simple partitioning of genetic and environmental components cannot be applied.91 This is because the genetic and environmental factors may be shared. Therefore, heritability estimates that assume VG and VE are independent may be inaccurate. If VP is accounted for by multiplicative interactions between VG and VE, then the heritability estimates may need to be suitably adjusted. For a multifactorial polygenic complex trait, such as schizophrenia, as the number of variances increase, the explanatory power of the model would decrease. Additionally, heritability estimates are affected by the power of the sample (eg, smaller samples may inflate heritability estimates), type of sample studied (eg, closer relatives such as twins provide better estimates of heritability compared with distant relatives), and the environmental differences between the samples. While reviewing the heritability estimates, it is important to note that a particular endophenotype may account for a portion of the VP. Therefore, lower heritability endophenotypes may still be useful in examining the genotype-phenotype relationship. However, the jury is still out regarding how low a heritability estimate is acceptable for a given endophenotype.
Investigation of the genetic and environmental contributions to phenotypic variability is made possible by twin studies. While monozygotic (MZ) twins share 100% of the genes, dizygotic (DZ) twins share on average, 50% of the genes. Assuming that the twins grow up in the same environment, the variations in a measured trait may be attributable to genetic factors. This allows one to dissect the common genetic vs the common environmental contributions to variations in brain structure.
Heritability estimates from twin studies for schizophrenia range from 0.6 to 0.9.92 Evidence for a high degree of heritability for human brain morphometric measures has been marshaled in recent neuroimaging studies. Heritability estimates were 81% for the intracranial volume, 79% for the midline cross-sectional area of the corpus callosum, and 79% for the lateral ventricular volume93 in a study comparing the MZ twins with the DZ twins. Estimated heritability for the total brain volume was 94% in another study employing a smaller subset of younger MZ and DZ twins.94 Low heritability was especially seen for the temporal horn of the lateral ventricles.95 Wright et al examined the heritability of regional gray matter using path analysis and structural equation modeling.96 There was significant evidence of a familial effect in several brain regions (14 of the 46 regions had large heritability estimates (>50%) (see table 1). Those regions indicated the cingulate, precentral gyrus, hippocampus and parahippocampal gyrus, superior and transverse temporal gyri, retrosplenial and supramarginal gyri, corpus striatum, and cerebellum. Asymmetries between the two hemispheres were not heritable. In addition, environmental factors appear to strongly influence ventricular volume99,101 (See table 1). Bartley et al also found that cortical gyral patterns were heritable, though to a lesser extent than cerebral volumes, but were more strongly influenced by environmental factors.94 Carmelli et al reported heritability of 65% for the ventricular volumes in 85 elderly twin pairs.104 There was a substantial genetic contribution to corpus callosum size and shape.93,105 Studies of elderly twins suggest that hippocampal volume may have relatively low heritability (40%).95 It is possible that the environmental factors exert greater influences on more malleable structures such as the hippocampus than other regions that may be under larger genetic control. Such a finding may be brought in greater relief in studies of elderly twins whose longevity has allowed greater exposure to environmental factors.
Table 1.
Studies Reporting Heritability of Global and Regional Cerebral Volumes
| References | Population | Heritability and Environmental Effects |
| Bartley et al94 | 10 pairs of MZ and 9 pairs of same-sex DZ twins | Heritability TBV 94% |
| Pennington et al97 | 25 MZ and 23 DZ pairs in which at least 1 member of each pair had reading disability (RD) and 9 MZ and 9 DZ pairs in which neither member had RD | Heritability: 2 brain morphometric measures (“cortical” and “subcortical”) accounted for 64% of the variance |
| Pfefferbaum et al93 | 44 MZ and 40 DZ twin pairs (elderly) | Heritability ICV 81% cc 79%, LV 79% CC 79%; environmental effects LV 58% |
| Posthuma et al98 | 111 twin pairs and 34 additional siblings | Heritability cerebellum 88%; ICV 65% |
| Baare et al99 | 54 MZ and 58 DZ twin pairs | Heritability TBV 90% LV 0%; Environmental effects LV 41% |
| Sullivan et al95 | 44 MZ and 40 DZ twin pairs (elderly) | Heritability HC 40% |
| Wright et al96 | 10 pairs of healthy MZ and 10 pairs of same-sex MZ twins | Heritability TBV 66%, high (>50%) heritability for right sup parietal, R frontal, bilateral post cingulate, bilateral anterior temporal, right supramarginal, right inferior postcentral, bilateral HC, R corpus striatum, R putamen, R insula and bilateral cerebellum |
| Scamvougeras et al100 | 14 MZ and 12 DZ twin pairs | Heritability CC 94% |
| Rijsdijk et al101 | 14 MZ twin pairs concordant for schizophrenia, 10 MZ discordant pairs, 17 MZ control pairs, 22 discordant sibling pairs, three concordant sibling pairs, and 114 healthy control subjects. | Heritability LV 67% TBV 88%; environmental effects LV 67% |
| Wallace et al102 | Pediatric sample of 90 MZ twin pairs, 38 same-sex DZ twin pairs, and 158 unrelated typically developing singletons | Heritability TGM 82%, TWM (85%), frontal cortex (77%), parietal cortex (86%), temporal cortex (88%), caudate (80%), LV %; CC 85% |
| Hulshoff Pol et al103 | 54 MZ and 58 DZ twin pairs and 34 of their siblings | Heritability: left, right CC (82%, 80%), medial frontal cortex (78%, 83%), superior frontal cortex (76%, 80%), superior temporal cortex (80%, 77%), left posterior cingulate cortex (83%), right parahippocampal cortex (69%), and amygdala (80%, 55%). |
CC, corpus callosum; DZ, dizygotic; ICV, intracranial volume; LV, lateral ventricle; MZ, monozygotic; TBV, total brain volume; TGM, total gray matter volume; TWM, total white matter volume; Hc, hippocampal complex.
The estimates of heritability for regional volumes among schizophrenia patients are relatively scanty. In a study on twins, high correlations between MZ twin sets were seen suggesting a high degree of heritability of brain structure volumes.106 In a recent study of schizophrenia patients, unaffected siblings, and controls, heritability estimates for volumetric differences between patients and their siblings were small107; however, for volumetric reduction in the cortical volumes and hippocampal volumes, the heritability estimates were high (Risch’s λ, hippocampus = 3.1, left cerebral cortex = 4.9, and right cerebral cortex = 8.5).108 In the latter case, it is assumed that the volumetric reduction is a disease marker.
On the other hand, heritability estimates for WM are quite variable. While one study finds no heritability for the WM volumes,108 other studies have reported higher heritability estimates. One study on healthy twins found a significant age by heritability interaction in that the heritability for the gray matter decreased with age, whereas the heritability for the WM increased as an individual got older.102 In this study, the broad-sense heritability (H2) for the gray matter varied from 0.80 to 0.89 among MZ twins compared with 0.29 to 0.53 among the DZ twins. For the WM, however, the heritability estimates ranged from 0.90 to 0.91 for MZ twins and 0.46 to 0.65. In addition, among the WM tracts that showed heritability was the superior occipitofrontal fasciculus.109
Childhood-onset schizophrenia could be unique in that the genetic factors may play a larger role in the etiology and manifest more severe symptoms with poorer prognosis. Current evidence, although inconsistent, suggests that the age of onset has genetic influence.110,111 A higher number of cytogenetic abnormalities has also been reported among the childhood-onset schizophrenia (reviewed in references 112,113). Some notable changes in MRI-measured brain morphology showed absence of prefrontal and MTL abnormalities with more prominent lateral ventricular enlargement and total gray matter volume reduction among childhood-onset schizophrenia compared with adult-onset patients.114–117 However, this specific group has not been studied adequately for endophenotype characterization.
Overall, morphometric measures appear to be as highly heritable as the disease phenotype and can therefore serve as possible endophenotypes in our efforts to study gene-behavior relationships. Global cerebral measures might have higher heritability; the relative contributions of gene and environmental factors may differ across the different brain structures. In general, the larger the samples, the more valid the heritability estimates are likely to be. Statistical power is also likely to be enhanced by the use of extended twin designs, such as using siblings of twins.99 More studies are needed among schizophrenia patients and their relatives to clearly estimate the range of heritability estimates.
Brain Structural Abnormalities Cosegregate With the Illness
A review of extant literature clearly suggests that the brain structural variations cosegregate with the illness. The volume of the dorsolateral prefrontal cortex dose dependently correlates with the degree of genetic loading for schizophrenia in a sample of twins.118,119 Additionally, hippocampal volume differences became more prominent in a step-wise fashion, with each increase in the genetic load for schizophrenia.120 Indeed, a meta-analysis of structural imaging studies on relatives of schizophrenia patients found that the largest difference was found in the volume of the hippocampus (d = 0.31, 95% confidence interval [CI] 0.13–0.49).121 However, in twin samples, among MZ twins discordant for schizophrenia, affected twins had more hippocampal volume reduction than the healthy twins, suggesting that nongenetic and disease-related effects may be involved as well.118,119,122 Structural MRI abnormalities have also been observed to incrementally increase with the proximity to the affected relative.123 Comparison of the hypothalamic volumes in multiplex families with the singleplex, first-degree relatives of schizophrenia patients and healthy relatives found that the volumes were increased in patients and relatives compared with healthy controls. The volume increase was linear from singleplex to multiplex families.124 Overall, the available data suggest that brain structural abnormalities cosegregate in a dose-dependent manner, with the risk for schizophrenia.
MRI Abnormalities Are More Frequently Observed in Unaffected Relatives
Structural imaging data have been reported in offspring, siblings, parents, as well as the unaffected cotwins of schizophrenia patients. Early ROI studies of young relatives at risk (offspring and siblings) showed the amygdala and hippocampus volume reductions in relatives compared with controls, but most relatives did not have volume reductions to pathological levels.125,126 A recent systematic review and meta-analysis of studies of relatives found hippocampal reductions in relatives, with an effect size of about 0.3, and additional differences between relatives and patients.121 Voxel-based morphometry studies have found reduced gray matter in the prefrontal cortex in relatives at high risk for schizophrenia.127,128 Reductions in gray matter density have been observed in prefrontal regions and thalamus in schizophrenia as opposed to no reductions in gray matter in these regions in bipolar disorder.56,129 Reductions in the thalamus have been reported as a measure of genetic liability to psychosis in general.129,130 Decreased volume and surface area in the right cingulate gyrus, a bilateral decrease in cingulate thickness, and decreased surface area in the superior temporal lobe have been reported in a series of healthy relatives of schizophrenia patients.131 Cortical thickness and surface area are proposed as indicators of cortical cytoarchitectural integrity. Using high-dimensional pattern classification approach, unaffected family members had highly overlapping structural variations. Increased WM and decreased gray matter in the orbitofrontal cortex contributed significantly to the classification.132
Twin studies have played an important role to dissect genetic and environmental factors in the brain abnormalities found in schizophrenia.119,133,134 Comparison of affected and unaffected MZ twins sheds light on possible shared environmental factors, while the differences between unaffected twins and healthy twin pairs reflects genetic contributions. In general, several studies have suggested that genetic contributions to brain structure are seen for decreased volumes of the intracranium, whole brain, cerebrum, hippocampus, and thalamus.103 Baare et al compared MZ and DZ twin pairs discordant for schizophrenia, with matched control twin pairs; they observed reduced whole-brain volumes and increased ventricular volumes in the unaffected members of discordant twin pairs, with a further reduction of whole-brain volume and increase in ventricular volume in the affected probands.99
White matter volume was found to be increased among first-degree relatives of schizophrenia/schizoaffective disorder compared with patients and healthy controls.135 In a study examining the relative contribution of genetic and environmental factors on a twin sample, global WM decreases was suggested to be due to the genetic factors, whereas gray matter was found to be associated with disease-related factors, possibly nongenetic.136
Taken together, the majority of studies point to the presence of brain structural alterations in unaffected relatives and cotwins, although quantitatively less severe than patients with already manifest illness. This strongly supports the view that the genetic risk for schizophrenia may underlie brain structural alterations.
Are Brain Structural Alterations Associated With Symptoms of Schizophrenia?
Several structural abnormalities have been associated with the symptom clusters and individual symptoms in schizophrenia. STG volumes correlate with positive symptoms, while MTL reductions correlate with memory impairment.26,137,138 Entorhinal cortex139 and parahippocampal gyrus140 have been associated with severity of delusions. Similarly orbitofrontal cortex has been associated with negative symptoms.141 In general, the association of each symptom to individual regions of interest is understandably relatively weak. General consensus is that the symptoms are the result of a network of different brain regions. This is further complicated by poorly defined phenomenological boundaries and inherent subjective biases in eliciting and documenting the findings. Despite these drawbacks, many investigators have reported correlations between the severity of symptoms and the volumetric changes.
Value of Studying “Extended Endophenotypes”
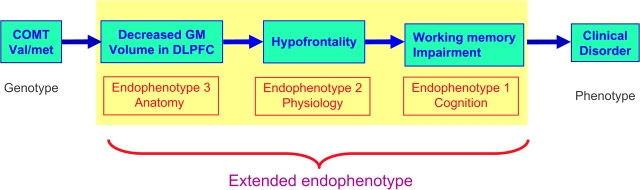
In complex psychiatric disorders where genetic, environmental, and epigenetic factors are at play, endophenotypes are likely to vary quantitatively in relatives at risk, irrespective of whether the disease is expressed using conventional phenotypic definitions (such as diagnosis). For this reason, use of multiple intermediate phenotypes that are functionally associated with each other may be more useful in understanding the pathophysiologic pathways (fig 1). We propose the term “extended endophenotypes” for such a functionally linked set of endophenotypes. For example, neurocognitive deficits and personality traits that cosegregate with the illness along with correlated brain structural and functional measures may be considered “extended endophenotypes.” Neurocognitive variations have been found to be heritable in multiplex, multigenerational families with schizophrenia.142 There is strong evidence for cosegregation of personality traits such as schizotypy families with schizophrenia probands.143 Brain structural alterations have been associated with these measures. Therefore, combining the structural variations along with cognition and/or behavioral traits such as schizotypy in the same cohort may identify the markers that may be pathophysiologically more valuable. Some suggestions for such “extended endophenotypes” follow.
Fig. 1.
A conceptual diagram of “extended endophenotype.”
First, there is strong evidence that abnormalities in prefrontally mediated cognitive functions such as attention, working memory and reasoning, and related alterations in prefrontal structure and functions may reflect the inherited vulnerability to schizophrenia.144 Neurocognitive functions discriminated the schizophrenia probands from their relatives and healthy controls in a sample of multiplex, multigenerational families with schizophrenia.142 Consistent with this, the dorsolateral prefrontal structural abnormalities are dose dependently correlated with the degree of genetic loading for schizophrenia in a sample of twins.119 Second, impairments in declarative memory processes represent another neurocognitive domain consistently abnormal in high-risk samples and nonpsychotic relatives of schizophrenia patients.145,146 These memory processes are tightly linked to the integrity of hippocampal structure and function, suggesting that they may reflect common inherited variations that tend to occur together. Consistent with this, a significant relationship has been described between hippocampal volume and declarative memory in relatives of schizophrenia patients.120,147 Additionally, hippocampal volume differences became more prominent in a step-wise fashion with each increase in the genetic load for schizophrenia.120 Indeed, a meta-analysis of structural imaging studies on relatives of schizophrenia patients found that the largest difference was found in the volume of the hippocampus (d = 0.31, 95% CI 0.13–0.49).121 However, in twin samples, among MZ twins discordant for schizophrenia, affected twins had more hippocampal volume reduction than the healthy twins, suggesting that nongenetic and disease-related effects may be involved as well.119,122
Elevated scores on schizotypy, a measure of inherited diathesis for schizophrenia, have been consistently observed in relatives at risk for schizophrenia.143 MRI studies of schizotypal personality disorder have revealed gray matter reductions in frontal, thalamus, and parietal gyri similar to schizophrenia.148 In high-risk adolescents, those with elevations in schizotypy have more prominent gray matter losses compared with those without.127
These observations point to the value of examination of cosegregation between MRI measures and neurocognitive and/or behavioral traits, thereby examining the “extended endophenotypes” in this illness. Examination of co-occurrence of a given cognitive variation and abnormalities in the structure and function of a consistently replicated brain region associated with such a trait could shed more light. For example, one could combine individuals with a reduction in prefrontal cortical volume and poor performance on executive function and working memory tasks. Both the prefrontal cortical volume and impaired prefrontally regulated cognitive functions have been associated with poor prognosis. Specific allelic variations on some of the schizophrenia susceptibility genes have been associated with prefrontal cortical structure and function (see table 2). Therefore, examining individuals with specific allelic variant associated with prefrontal cortical structure and related cognitive functions in those with poor prognosis could be a potentially useful strategy. Following criteria may be useful to construct such “extended endophenotypes”: (1) Each trait included within the extended endophenotype should meet criteria for the endophenotype, (2) the extended endophenotype should involve two or more candidate biomarkers at distinct levels of analyses, eg, cognitive and physiological, neurochemical and physiological, and structural and physiological, (3) those endophenotypes should be correlated within the individuals and should co-occur within the families of affected individuals, and (4) the traits included within the extended endophenotype should be mechanistically related, eg, declarative memory deficits and hippocampal functional abnormalities, prefrontal BOLD response abnormalities, and γ-band oscillatory changes.
Table 2.
Schizophrenia Susceptibility Genes and MR Structural Variations
| Gene | Sample Studied | Findings | References |
| RGS4 | First-episode, antipsychotic naïve schizophrenia and healthy subjects | Prefrontal cortex volume reduction in patients | Prasad et al149 |
| Healthy subjects | Prefrontal cortical gray and WM reduction; frontoparietal and frontotemporal network activity and network coupling while performing working memory tasks | Buckholtz et al150 | |
| COMT | Schizophrenia patients | Increased gray matter density in the STG associated with rs2097603 (a P2 promoter SNP) and increased WM density in the left inferior frontal pole associated with rs4680 (Val allele) | Zinkstok et al (2007)151 |
| Chronic schizophrenia patients | Decreased volume of left anterior cingulate cortex, thalamus, amygdala uncus and left middle temporal gyrus associated with Val allele homozygosity | Ohnishi et al152 | |
| First-episode, antipsychotic naïve schizophrenia and healthy subjects | Val/Val patients showed decreased gray matter in the dorsolateral prefrontal cortex and increased gray matter in the substantia nigra and ventral tegmental area compared with Met/Met patients | Prasad et al153 | |
| First-episode nonaffective psychoses | Increased volume of lateral ventricles associated with Met/Met patients | Crespo-Faccoro et al154 | |
| Individuals genetically at risk for schizophrenia | Subjects carrying Val allele had decreased anterior cingulate cortex | McIntosh et al155 | |
| Healthy Subjects | Subjects homozygous for Val allele showed decreased temporal lobe and hippocampal volumes | Taylor et al156 | |
| PRODH | Schizophrenia patients | Bilateral frontal WM density reductions (rs2008720), decreased GM in the cerebellum, inferior frontal gyrus, and cuneus (rs450046), increased GM in STG, parahippocampal gyrus and inferior parietal lobe (rs372055) | Zinkstok et al151 |
| DISC1 | Normal control subjects | Reduced hippocampal gray matter volume associated with Ser allele of SNP10 | Callicott et al157 |
| Monozygotic twin samples (with healthy and schizophrenia probands) | One haplotype block near the translocation break point (HEP1) associated with focal reductions in superior and inferior frontal gray matter; rare AATG haplotype of the combined HEP2/HEP3 segments associated with focal reductions in gray matter in the superior and middle frontal gyri and superior temporal and parietal cortexes | Cannon et al158 | |
| Healthy subjects | Reduced volume of anterior and posterior cingulate gyri and increased volume of the lateral ventricle, interhemispheric fissure and the Sylvian fissure associated with Cys carrier status | Hashimoto et al159 |
GM, gray matter; RGS4, regulator of G-protein signaling, subtype 4; SNP, single-nucleotide polymorphism; STG, superior temporal gyrus; WM, white matter; HEP1, haplotype block; HEP2 & HEP3: haplotype blocks.
Conclusions
Our review supports the view that MRI morphometric measures meet many but not all of the criteria in order to be considered endophenotypes. The brain structural alterations (1) are robustly associated with schizophrenia subjects, (2) are relatively specific to schizophrenia, (3) are present in unaffected relatives, (4) cosegregate with the disorder, (5) appear to covary with the presence of broader spectrum psychopathology related to schizophrenia, (6) are moderately to highly heritable, (7) are reliably quantifiable, and (8) have some regional variations that probabilistically predict the disease. Existing data do not definitively support the view that brain structural alterations are state independent, there is relative stability across the course of the disorder, and their association with the cause rather than the consequence of the disorder exists. Furthermore, such alterations have also been shown to be associated with specific allelic variations of schizophrenia susceptibility genes (discussed later). In this sense, they appear to largely meet several criteria set forth by different investigators.5,7–12 A number of caveats need to be considered in future studies involving neuromorphometric measures as intermediate phenotypes for future genetic studies.
First, recent conceptualizations of endophenotypes have pointed to the need for the assessment leading to candidate markers to be easily and rapidly administered with minimal participant demands and also to have strong test-retest and cross-site reliability. While MRI studies are still somewhat expensive ($200–600 per scan), a standard structural imaging sequence can be carried out at most medical centers in less than half an hour and is noninvasive. Additionally, unlike many laboratory-based neurocognitive and electrophysiological measures, structural MRI studies pose minimal risk and are less likely to be affected by motivational demands.
Second, a key expectation of an ideal endophenotypic marker is that it should have a well understood and discrete neurobiologic mechanism tied to the proposed pathophysiology of the disorder in question as well as to the set of genes linked to the disorder. This raises the question, to what extent do brain structural measures relate to the specific genes recently implicated in schizophrenia? Table 2 shows the results of such studies that have reported associations between brain structural alterations and variations in schizophrenia susceptibility genes. Table 2 includes only those genetic variations that have been replicated in different populations. Currently replicated genetic associations in schizophrenia seem to point to a set of genes that may be pathophysiologically linked.160 Genes likely lead to abnormalities in brain structure and function by impacting neurodevelopmental processes.
Undoubtedly, such effects reflect the combined result of several susceptibility genes and their interactions with environmental factors such as perinatal complications,161–164 exposure to infectious agents,165 and drug abuse.166,167 Nevertheless, promising, but preliminary, clues have begun to emerge in regard to the nature of such susceptibility genes. For example, there is an association between reduced prefrontal gray matter volume and the polymorphisms of the gene encoding the regulator of G-protein signaling, subtype 4 (RGS4).149,150 Polymorphisms in the brain-derived neurotrophic factor (BDNF) gene have been suggested to be associated with schizophrenia.168 Alterations in volumes of frontal169 as well as MTL structures170,171 have been associated with this gene. Hippocampal structure is associated with DISC1 polymorphisms.157,172 COMT variants have been associated with increased gray matter density in the STG associated with rs2097603 (a P2 promoter SNP) and increased WM density in the left inferior frontal pole associated with rs4680 (Val allele).151 Among chronic schizophrenia patients, decreased volume of left anterior cingulate cortex, thalamus, amygdala uncus, and left middle temporal gyrus associated with Val allele homozygosity was reported.152 Other genes that have shown associations with various brain regions are pericentriolar material 1 (PCM 1) with orbitofrontal gyrus,173 and interleukin-1 receptor antagonist with reduced dorsolateral prefrontal cortex, lateral ventricles, and hippocampal volumes.174 In addition, in general, however, these relationships have been revealed in small samples of patients and healthy controls; direct understanding of the functional meaning of such relationships with genetic variation remain unclear and are beginning to be explored using functional MRI.175 It is very likely that such multiple genes contribute interactively to regional brain structure and function.
Third, it remains unclear whether any of the MRI endophenotypes associated with schizophrenia are unique to this illness or are shared by other forms of psychosis such as bipolar disorder. Family and twin studies point to significant coaggregation of schizophrenia and bipolar disorder in families; several genes recently linked to schizophrenia such as DISC1, G72, and NRG1 have also been linked to bipolar disorder, increasingly blurring the long-held Kraepelinian divide.26,62,176 A case study on the triplets discordant for schizophrenia further supports the weakness in such dichotomy.177 The similarity between these two disorders in regard to brain structural parameters reviewed above suggests that brain structural endophenotypes may cut across traditional diagnostic boundaries. This may be a blessing in disguise because charting the markers across the ill-defined diagnostic boundaries may help the field to redraw more valid neurobiologic boundaries that possess better construct and predictive validity than those currently available.
The use of brain structural measures as endophenotypic biomarkers will likely enhance the power of molecular genetic studies to clarify the pathophysiology of major psychiatric disorders such as schizophrenia. Such information should help us to eventually identify the specific genetic variations responsible for psychiatric disorders. Identifying specific genes associated with complex traits such as those seen in schizophrenia will remain challenging, given the many genes of small effect and their interactions with the environment. Nevertheless, such an effort will ultimately help in better characterizing psychiatric diseases and develop more specific, etiologically relevant treatments or preventative strategies.
Funding
NIMH (MH72995) and NARSAD Young Investigator Award to K.M.P. and NIMH (MH64023, MH45156, and MH01180) and a NARSAD Independent Investigator award to M.S.K.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.McClellan JM, Susser E, King MC. Schizophrenia: a common disease caused by multiple rare alleles. Br J Psychiatry. 2007;190:194–199. doi: 10.1192/bjp.bp.106.025585. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122(566):15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Craddock N, Jones I, Kirov G, Jones L. The Bipolar Affective Disorder Dimension Scale (BADDS)—a dimensional scale for rating lifetime psychopathology in bipolar spectrum disorders. BMC Psychiatry. 2004;4:19. doi: 10.1186/1471-244X-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 6.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006;60(2):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet. 2006;22(6):306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Berrettini WH. Genetic bases for endophenotypes in psychiatric disorders. Dialogues Clin Neurosci. 2005;7(2):95–101. doi: 10.31887/DCNS.2005.7.2/wberrettini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waldman ID. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57(11):1347–1356. doi: 10.1016/j.biopsych.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Doyle AE, Faraone SV, Seidman LJ, et al. Are endophenotypes based on measures of executive functions useful for molecular genetic studies of ADHD? J Child Psychol Psychiatry. 2005;46(7):774–803. doi: 10.1111/j.1469-7610.2005.01476.x. [DOI] [PubMed] [Google Scholar]
- 11.Skuse DH. Endophenotypes and child psychiatry. Br J Psychiatry. 2001;178:395–396. doi: 10.1192/bjp.178.5.395. [DOI] [PubMed] [Google Scholar]
- 12.Almasy L, Blangero J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am J Med Genet. 2001;105(1):42–44. [PubMed] [Google Scholar]
- 13.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nat Rev Neurosci. 2002;3(8):617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 14.Tsuang MT. Genotypes, phenotypes, the brain. A search for connections in schizophrenia. Br J Psychiatry. 1993;163:299–307. doi: 10.1192/bjp.163.3.299. [DOI] [PubMed] [Google Scholar]
- 15.Cannon TD, Huttunen MO, Lonnqvist J, et al. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am J Hum Genet. 2000;67(2):369–382. doi: 10.1086/303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faraone SV, Matise T, Svrakic D, et al. Genome scan of European-American schizophrenia pedigrees: results of the NIMH genetics initiative and millennium consortium. Am J Med Genet. 1998;81(4):290–295. [PubMed] [Google Scholar]
- 18.Glahn DC, Therman S, Manninen M, et al. Spatial working memory as an endophenotype for schizophrenia. Biol Psychiatry. 2003;53(7):624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- 19.Hoff AL, Svetina C, Maurizio AM, Crown TJ, Spokes K, Delisi LE. Familial cognitive deficits in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2005;133(1):43–49. doi: 10.1002/ajmg.b.30120. [DOI] [PubMed] [Google Scholar]
- 20.Myles-Worsley M, Park S. Spatial working memory deficits in schizophrenia patients and their first degree relatives from Palau, Micronesia. Am J Med Genet. 2002;114(6):609–615. doi: 10.1002/ajmg.10644. [DOI] [PubMed] [Google Scholar]
- 21.Bramon E, McDonald C, Croft RJ, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Myles-Worsley M. P50 sensory gating in multiplex schizophrenia families from a Pacific island isolate. Am J Psychiatry. 2002;159(12):2007–2012. doi: 10.1176/appi.ajp.159.12.2007. [DOI] [PubMed] [Google Scholar]
- 23.Paunio T, Tuulio-Henriksson A, Hiekkalinna T, et al. Search for cognitive trait components of schizophrenia reveals a locus for verbal learning and memory on 4q and for visual working memory on 2q. Hum Mol Genet. 2004;13(16):1693–1702. doi: 10.1093/hmg/ddh184. [DOI] [PubMed] [Google Scholar]
- 24.Flint J, Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37(2):163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdar W, Solberg LC, Gauguier D, et al. Genome-wide genetic association of complex traits in heterogeneous stock mice. Nat Genet. 2006;38(8):879–887. doi: 10.1038/ng1840. [DOI] [PubMed] [Google Scholar]
- 26.Pearlson GD, Calhoun V. Structural and functional magnetic resonance imaging in psychiatric disorders. Can J Psychiatry. 2007;52(3):158–166. doi: 10.1177/070674370705200304. [DOI] [PubMed] [Google Scholar]
- 27.Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29(1):26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- 28.Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- 29.Ward KE, Friedman L, Wise A, Schulz SC. Meta-analysis of brain and cranial size in schizophrenia. Schizophr Res. 1996;22(3):197–213. doi: 10.1016/s0920-9964(96)00076-x. [DOI] [PubMed] [Google Scholar]
- 30.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157(1):16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 31.Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. doi: 10.1192/bjp.172.2.110. [DOI] [PubMed] [Google Scholar]
- 32.Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry. 1998;55(5):433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- 33.Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry. 2001;49(1):28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- 34.Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58(4):457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giuliani NR, Calhoun VD, Pearlson GD, Francis A, Buchanan RW. Voxel-based morphometry versus region of interest: a comparison of two methods for analyzing gray matter differences in schizophrenia. Schizophr Res. 2005;74(2–3):135–147. doi: 10.1016/j.schres.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 37.Davatzikos C, Shen D, Gur RC, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62(11):1218–1227. doi: 10.1001/archpsyc.62.11.1218. [DOI] [PubMed] [Google Scholar]
- 38.Moreno D, Burdalo M, Reig S, et al. Structural neuroimaging in adolescents with a first psychotic episode. J Am Acad Child Adolesc Psychiatry. 2005;44(11):1151–1157. doi: 10.1097/01.chi.0000179055.46795.3f. [DOI] [PubMed] [Google Scholar]
- 39.Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49(3):195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]
- 40.Bagary MS, Symms MR, Barker GJ, Mutsatsa SH, Joyce EM, Ron MA. Gray and white matter brain abnormalities in first-episode schizophrenia inferred from magnetization transfer imaging. Arch Gen Psychiatry. 2003;60(8):779–788. doi: 10.1001/archpsyc.60.8.779. [DOI] [PubMed] [Google Scholar]
- 41.Flynn SW, Lang DJ, Mackay AL, et al. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8(9):811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- 42.Price G, Cercignani M, Parker GJ, et al. Abnormal brain connectivity in first-episode psychosis: a diffusion MRI tractography study of the corpus callosum. Neuroimage. 2007;35(2):458–466. doi: 10.1016/j.neuroimage.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheung V, Cheung C, McAlonan GM, et al. A diffusion tensor imaging study of structural disconnectivity in never-medicated, first-episode schizophrenia. Psychol Med. 2007;Oct 22:1–9. doi: 10.1017/S0033291707001808. [DOI] [PubMed] [Google Scholar]
- 44.Hao Y, Liu Z, Jiang T, et al. White matter integrity of the whole brain is disrupted in first-episode schizophrenia. Neuroreport. 2006;17(1):23–26. doi: 10.1097/01.wnr.0000195664.15090.46. [DOI] [PubMed] [Google Scholar]
- 45.Whitford TJ, Grieve SM, Farrow TF, et al. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. Am J Psychiatry. 2007;164(7):1082–1089. doi: 10.1176/ajp.2007.164.7.1082. [DOI] [PubMed] [Google Scholar]
- 46.Begre S, Federspiel A, Kiefer C, et al. Reduced hippocampal anisotropy related to anteriorization of alpha EEG in schizophrenia. Neuroreport. 2003;14(5):739–742. doi: 10.1097/00001756-200304150-00016. [DOI] [PubMed] [Google Scholar]
- 47.Pukrop R, Schultze-Lutter F, Ruhrmann S, et al. Neurocognitive functioning in subjects at risk for a first episode of psychosis compared with first- and multiple-episode schizophrenia. J Clin Exp Neuropsychol. 2006;28(8):1388–1407. doi: 10.1080/13803390500434425. [DOI] [PubMed] [Google Scholar]
- 48.Pearlson GD, Barta PE, Powers RE, et al. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biol Psychiatry. 1997;41(1):1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- 49.McDonald C, Bullmore ET, Sham PC, et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Arch Gen Psychiatry. 2004;61(10):974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 50.Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3(3):106–150. doi: 10.1034/j.1399-5618.2001.030302.x. [DOI] [PubMed] [Google Scholar]
- 51.Hoge EA, Friedman L, Schulz SC. Meta-analysis of brain size in bipolar disorder. Schizophr Res. 1999;37(2):177–181. doi: 10.1016/s0920-9964(98)00149-2. [DOI] [PubMed] [Google Scholar]
- 52.McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56(6):411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Strakowski SM, DelBello MP, Adler C, Cecil DM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disord. 2000;2(3 Pt 1):148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- 54.Videbech P. MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatr Scand. 1997;96(3):157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- 55.Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. Am J Psychiatry. 1995;152(8):1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- 56.McIntosh AM, Job DE, Moorhead TW, Harrison LK, Lawrle SM, Johnstone EC. White matter density in patients with schizophrenia, bipolar disorder and their unaffected relatives. Biol Psychiatry. 2005;58(3):254–257. doi: 10.1016/j.biopsych.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 57.Yurgelun-Todd DA, Silveri MM, Gruber SA, Rohan ML, Pimentel PJ. White matter abnormalities observed in bipolar disorder: a diffusion tensor imaging study. Bipolar Disord. 2007;9(5):504–512. doi: 10.1111/j.1399-5618.2007.00395.x. [DOI] [PubMed] [Google Scholar]
- 58.Frazier JA, Breeze JL, Papadimitriou G, et al. White matter abnormalities in children with and at risk for bipolar disorder. Bipolar Disord. 2007;9(8):799–809. doi: 10.1111/j.1399-5618.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 59.Adler CM, Adams J, DelBello MP, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry. 2006;163(2):322–324. doi: 10.1176/appi.ajp.163.2.322. [DOI] [PubMed] [Google Scholar]
- 60.Houenou J, Wessa M, Douaud G, et al. Increased white matter connectivity in euthymic bipolar patients: diffusion tensor tractography between the subgenual cingulate and the amygdalo-hippocampal complex. Mol Psychiatry. 2007;12(11):1001–1010. doi: 10.1038/sj.mp.4002010. [DOI] [PubMed] [Google Scholar]
- 61.Beyer JL, Taylor WD, MacFall JR, et al. Cortical white matter microstructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30(12):2225–2229. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 62.Potash JB. Carving chaos: genetics and the classification of mood and psychotic syndromes. Harv Rev Psychiatry. 2006;14(2):47–63. doi: 10.1080/10673220600655780. [DOI] [PubMed] [Google Scholar]
- 63.Potash JB, Willour VL, Chiu YF, et al. The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry. 2001;158(8):1258–1264. doi: 10.1176/appi.ajp.158.8.1258. [DOI] [PubMed] [Google Scholar]
- 64.Strasser HC, Lilyestrom J, Ashby ER, et al. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry. 2005;57(6):633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 65.Salokangas RK, Cannon T, Van Erp T, et al. Structural magnetic resonance imaging in patients with first-episode schizophrenia, psychotic and severe non-psychotic depression and healthy controls. Results of the schizophrenia and affective psychoses (SAP) project. Br J Psychiatry. 2002;43(suppl):s58–s65. doi: 10.1192/bjp.181.43.s58. [DOI] [PubMed] [Google Scholar]
- 66.Howard R, Mellers J, Petty R, et al. Magnetic resonance imaging volumetric measurements of the superior temporal gyrus, hippocampus, parahippocampal gyrus, frontal and temporal lobes in late paraphrenia. Psychol Med. 1995;25(3):495–503. doi: 10.1017/s0033291700033419. [DOI] [PubMed] [Google Scholar]
- 67.Naguib M, Lavy R. Late paraphrenia: neuropsychological impairment and structural brain abnormalities on computed tomography. Int J Geriatr Psychiatry. 1987;2(2):83–90. [Google Scholar]
- 68.Rabins P, Pearlson GD, Jayaram G, Steele C, Tune LE. Increased ventricle-to-brain ratio in late-onset schizophrenia. Am J Psychiatry. 1987;144(9):1216–1218. doi: 10.1176/ajp.144.9.1216. [DOI] [PubMed] [Google Scholar]
- 69.Schwarzkopf SB, Olson SC, Coffman JA, Nasrallah HA. Third and lateral ventricular volumes in schizophrenia: support for progressive enlargement of both structures. Psychopharmacol Bull. 1990;26(3):385–391. [PubMed] [Google Scholar]
- 70.Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344(8934):1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 71.Borne J, Riascos R, Cuellar H, Vargas D, Rojas R. Neuroimaging in drug and substance abuse part II: opioids and solvents. Top Magn Reson Imaging. 2005;16(3):239–245. doi: 10.1097/01.rmr.0000192154.34563.6b. [DOI] [PubMed] [Google Scholar]
- 72.Rojas R, Riascos R, Vargas D, Cuellar H, Borne J. Neuroimaging in drug and substance abuse part I: cocaine, cannabis, and ecstasy. Top Magn Reson Imaging. 2005;16(3):231–238. doi: 10.1097/01.rmr.0000192156.46492.24. [DOI] [PubMed] [Google Scholar]
- 73.Spampinato MV, Castillo M, Rojas R, Palacios E, Frascheri L, Descrates F. Magnetic resonance imaging findings in substance abuse: alcohol and alcoholism and syndromes associated with alcohol abuse. Top Magn Reson Imaging. 2005;16(3):223–230. doi: 10.1097/01.rmr.0000192175.26243.a7. [DOI] [PubMed] [Google Scholar]
- 74.Keshavan MS, Berger G, Zipursky RB, Wood SJ, Pantelis C. Neurobiology of early psychosis. Br J Psychiatry. 2005;48(suppl):s8–18. doi: 10.1192/bjp.187.48.s8. [DOI] [PubMed] [Google Scholar]
- 75.Kuroki N, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry. 2006;163(12):2103–2110. doi: 10.1176/appi.ajp.163.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res. 2006;82(1):75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Csernansky JG, Joshi S, Wang L, et al. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proc Natl Acad Sci U S A. 1998;95(19):11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Csernansky JG, Wang L, Jones D, et al. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry. 2002;159(12):2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- 79.Whitworth AB, Kemmler G, Honeder M, et al. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Res. 2005;140(3):225–237. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 80.DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Res. 2004;130(1):57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60(6):585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- 82.Chakos MH, Lieberman JA, Bilder RM, et al. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151(10):1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- 83.Scherk H, Falkai P. Effects of antipsychotics on brain structure. Curr Opin Psychiatry. 2006;19(2):145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- 84.McClure RK, Phillips I, Jazayerli R, Barnett A, Coppola R, Weinberger DR. Regional change in brain morphometry in schizophrenia associated with antipsychotic treatment. Psychiatry Res. 2006;148(2–3):121–132. doi: 10.1016/j.pscychresns.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 85.Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV. Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry. 2003;60(3):245–252. doi: 10.1001/archpsyc.60.3.245. [DOI] [PubMed] [Google Scholar]
- 86.Bartzokis G, Nuechterlein KH, Lu PH, Gitlin M, Rogers S, Mintz J. Dysregulated brain development in adult men with schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 2003;53(5):412–421. doi: 10.1016/s0006-3223(02)01835-8. [DOI] [PubMed] [Google Scholar]
- 87.Pantelis C, Velakoulis D, McGorry PD, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361(9354):281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 88.Harris JM, Moorehead TWJ, Miller P, et al. increased prefrontal gyrification in a large high-risk cohort characterizes those who develop schizophrenia and reflects abnormal prefrontal development. Biol Psychiatry. 2007;62(7):722–799. doi: 10.1016/j.biopsych.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 89.Job DE, Whalley H, Johnstone EC, Lawrence E. Grey matter changes over time in high risk subjects developing schizophrenia. Neuroimage. 2005;25(4):1023–1030. doi: 10.1016/j.neuroimage.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Job DE, Whalley HC, McIntosh AM, Owens DG, Johnstone EC, Lawrie SM. Grey matter changes can improve the prediction of schizophrenia in subjects at high risk. BMC Med. 2006;4:29. doi: 10.1186/1741-7015-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Strachan T, Reed AP. Human Molecular Genetics. 3rd ed. New York: Garland Science; 2004. [Google Scholar]
- 92.Kendler KS. Schizophrenia: genetics. In: Sadock BJ, Sadock VA, editors. Comprehensive Textbook of Psychiatry. Vol 1. 7th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. pp. 1147–1159. [Google Scholar]
- 93.Pfefferbaum A, Sullivan EV, Swan GE, Carmelli D. Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiol Aging. 2000;21(1):63–74. doi: 10.1016/s0197-4580(00)00086-5. [DOI] [PubMed] [Google Scholar]
- 94.Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120(Pt 2):257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- 95.Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: equivalent influence from genes and environment. Hippocampus. 2001;11(6):754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- 96.Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. Genetic contributions to regional variability in human brain structure: methods and preliminary results. Neuroimage. 2002;17(1):256–271. doi: 10.1006/nimg.2002.1163. [DOI] [PubMed] [Google Scholar]
- 97.Pennington BF, Filipek PA, Lefly D, et al. A twin MRI study of size variations in human brain. J Cogn Neurosci. 2000;12(1):223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- 98.Posthuma D, de Geus EJ, Neale MC, et al. Multivariate genetic analysis of brain structure in an extended twin design. Behav Genetics. 2000;30(4):311–319. doi: 10.1023/a:1026501501434. [DOI] [PubMed] [Google Scholar]
- 99.Baare WF, Hulshoff Pol HE, Boomsma DI, et al. Quantitative genetic modeling of variation in human brain morphology. Cereb Cortex. 2001;11(9):816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- 100.Scamvougeras A, Kigar DL, Jones D, Weinberger DR, Witelson SF. Size of the human corpus callosum is genetically determined: an MRI study in mono and dizygotic twins. Neurosci Lett. 2003;338(2):91–94. doi: 10.1016/s0304-3940(02)01333-2. [DOI] [PubMed] [Google Scholar]
- 101.Rijsdijk FV, van Haren NE, Picchioni MM, et al. Brain MRI abnormalities in schizophrenia: same genes or same environment? Psychol Med. 2005;35(10):1399–1409. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- 102.Wallace GL, Eric Schmitt J, Lenroot R, et al. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47(10):987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- 103.Hulshoff Pol HE, Schnack HG, Mandl RC, et al. Gray and white matter density changes in monozygotic and same-sex dizygotic twins discordant for schizophrenia using voxel-based morphometry. Neuroimage. 2006;31(2):482–488. doi: 10.1016/j.neuroimage.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 104.Carmelli D, Reed T, DeCarli C. A bivariate genetic analysis of cerebral white matter hyperintensities and cognitive performance in elderly male twins. Neurobiol Aging. 2002;23(3):413–420. doi: 10.1016/s0197-4580(01)00336-0. [DOI] [PubMed] [Google Scholar]
- 105.Oppenheim JS, Skerry JE, Tramo MJ, Gazzaniga MS. Magnetic resonance imaging morphology of the corpus callosum in monozygotic twins. Ann Neurol. 1989;26(1):100–104. doi: 10.1002/ana.410260117. [DOI] [PubMed] [Google Scholar]
- 106.Thompson PM, Cannon TD, Narr KL, et al. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- 107.Honea RA, Meyer-Lindenberg A, Hobbs KB, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;31(2–3):147–156. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldman AL, Pezawas L, Mattay VS, et al. Heritability of brain morphology related to schizophrenia: a large-scale automated magnetic resonance imaging segmentation study. Biol Psychiatry. 2008;63(5):475–483. doi: 10.1016/j.biopsych.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 109.Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Hulshoff Pol HE. Genetic influences on human brain structure: a review of brain imaging studies in twins. Hum Brain Mapp. 2007;28(6):464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kallmann FJ, Roth B. Genetic aspects of preadolescent schizophrenia. Am J Psychiatry. 1956;112(8):599–606. doi: 10.1176/ajp.112.8.599. [DOI] [PubMed] [Google Scholar]
- 111.Kendler KS, Tsuang MT, Hays P. Age at onset in schizophrenia. A familial perspective. Arch Gen Psychiatry. 1987;44(10):881–890. doi: 10.1001/archpsyc.1987.01800220047008. [DOI] [PubMed] [Google Scholar]
- 112.Nicolson R, Lenane M, Hamburger SD, Fernandez T, Bedwell J, Rapoport JL. Lessons from childhood-onset schizophrenia. Brain Res Brain Res Rev. 2000;31(2–3):147–156. doi: 10.1016/s0165-0173(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 113.Kumra S, Shaw M, Merka P, Nakayama E, Augustin R. Childhood-onset schizophrenia: research update. Can J Psychiatry. 2001;46(10):923–930. doi: 10.1177/070674370104601004. [DOI] [PubMed] [Google Scholar]
- 114.Frazier JA, Giedd JN, Hamburger SD, et al. Brain anatomic magnetic resonance imaging in childhood-onset schizophrenia. Arch Gen Psychiatry. 1996;53(7):617–624. doi: 10.1001/archpsyc.1996.01830070065010. [DOI] [PubMed] [Google Scholar]
- 115.Jacobsen LK, Giedd JN, Berquin PC, et al. Quantitative morphology of the cerebellum and fourth ventricle in childhood-onset schizophrenia. Am J Psychiatry. 1997;154(12):1663–1669. doi: 10.1176/ajp.154.12.1663. [DOI] [PubMed] [Google Scholar]
- 116.Jacobsen LK, Giedd JN, Vaituzis AC, et al. Temporal lobe morphology in childhood-onset schizophrenia. Am J Psychiatry. 1996;153(3):355–361. doi: 10.1176/ajp.153.3.355. [DOI] [PubMed] [Google Scholar]
- 117.Jacobsen LK, Giedd JN, Tanrikut C, et al. Three-dimensional cortical morphometry of the planum temporale in childhood-onset schizophrenia. Am J Psychiatry. 1997;154(5):685–687. doi: 10.1176/ajp.154.5.685. [DOI] [PubMed] [Google Scholar]
- 118.Cannon TD, Thompson PM, van Erp TG, et al. Mapping heritability and molecular genetic associations with cortical features using probabilistic brain atlases: methods and applications to schizophrenia. Neuroinformatics. 2006;4(1):5–19. doi: 10.1385/NI:4:1:5. [DOI] [PubMed] [Google Scholar]
- 119.Cannon TD, Thompson PM, van Erp TG, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci U S A. 2002;99(5):3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Seidman LJ, Faraone SV, Goldstein JM, et al. Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry. 2002;59(9):839–849. doi: 10.1001/archpsyc.59.9.839. [DOI] [PubMed] [Google Scholar]
- 121.Boos HBM, Aleman A, Cahn W, Pol HH, Kahn RS. Brain volumes in relatives of patients with schizophrenia: a meta-analysis. Arch Gen Psychiatry. 2007;64(3):297–304. doi: 10.1001/archpsyc.64.3.297. [DOI] [PubMed] [Google Scholar]
- 122.van Erp TG, Saleh PA, Huttunen M, et al. Hippocampal volumes in schizophrenic twins. Arch Gen Psychiatry. 2004;61(4):346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- 123.Sharma T, Du Boulay G, Lewis S, Sigmundsson T, Gurling H, Murray RM. The Maudsley family study I: structural brain changes on magnetic resonance imaging in familial schizophrenia. Prog Neuro-psychopharmacol Biol Psychiatry. 1997;21(8):1297–1315. doi: 10.1016/s0278-5846(97)00165-6. [DOI] [PubMed] [Google Scholar]
- 124.Goldstein JM, Seidman LJ, Makris N, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry. 2007;61(8):935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 125.Keshavan MS, Montrose DM, Pierri JN, et al. Magnetic resonance imaging and spectroscopy in offspring at risk for schizophrenia: preliminary studies. Prog Neuro-psychopharmacol Biol Psychiatry. 1997;21(8):1285–1295. doi: 10.1016/s0278-5846(97)00164-4. [DOI] [PubMed] [Google Scholar]
- 126.Lawrie SM, Whalley H, Kestelman JN, et al. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet. 1999;353(9146):30–33. doi: 10.1016/S0140-6736(98)06244-8. [DOI] [PubMed] [Google Scholar]
- 127.Diwadkar VA, Montrose DM, Dworakowski D, Sweeney JA, Keshavan MS. Genetically predisposed offspring with schizotypal features: an ultra high-risk group for schizophrenia? Prog Neuro-psychopharmacol Biol Psychiatry. 2006;30(2):230–238. doi: 10.1016/j.pnpbp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 128.Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrle SM. Voxel-based morphometry of grey matter densities in subjects at high risk of schizophrenia. Schizophr Res. 2003;64(1):1–13. doi: 10.1016/s0920-9964(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 129.McDonald C, Bullmore E, Sham P, et al. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. Br J Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- 130.McIntosh AM, Job DE, Moorhead WJ, et al. Genetic liability to schizophrenia or bipolar disorder and its relationship to brain structure. Am J Med Genet B Neuropsychiatr Genet. 2006;141(1):76–83. doi: 10.1002/ajmg.b.30254. [DOI] [PubMed] [Google Scholar]
- 131.Goghari VM, Rehm K, Carter CS, MacDonald AW., 3rd Regionally specific cortical thinning and gray matter abnormalities in the healthy relatives of schizophrenia patients. Cereb Cortex. 2007;17(2):415–424. doi: 10.1093/cercor/bhj158. [DOI] [PubMed] [Google Scholar]
- 132.Fan Y, Gur RE, Gur RC, et al. Unaffected family members and schizophrenia patients share brain structure patterns: a high-dimensional pattern classification study. Biol Psychiatry. 2008;63(1):118–124. doi: 10.1016/j.biopsych.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noga JT, Bartley AJ, Jones DW, Torrey EF, Weinberger DR. Cortical gyral anatomy and gross brain dimensions in monozygotic twins discordant for schizophrenia. Schizophr Res. 1996;22(1):27–40. doi: 10.1016/0920-9964(96)00046-1. [DOI] [PubMed] [Google Scholar]
- 134.Reveley AM, Reveley MA, Clifford CA, Murray RM. Cerebral ventricular size in twins discordant for schizophrenia. Lancet. 1982;1(8271):540–541. doi: 10.1016/s0140-6736(82)92047-5. [DOI] [PubMed] [Google Scholar]
- 135.Marcelis M, Suckling J, Woodruff P, Hofman P, Bullmore E, van Os J. Searching for a structural endophenotype in psychosis using computational morphometry. Psychiatry Res. 2003;122(3):153–167. doi: 10.1016/s0925-4927(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 136.Hulshoff Pol HE, Brans RG, van Haren NE, et al. Gray and white matter volume abnormalities in monozygotic and same-gender dizygotic twins discordant for schizophrenia. Biol Psychiatry. 2004;55(2):126–130. doi: 10.1016/s0006-3223(03)00728-5. [DOI] [PubMed] [Google Scholar]
- 137.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70(2–3):117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 138.Lawrie SM, Johnstone EC, Weinberger DR. Schizophrenia: From Neuroimaging to Neuroscience. Oxford: Oxford University Press; 2005. [Google Scholar]
- 139.Prasad KMR, Patel AR, Sweeney JA, Keshavan MS. Entorhinal cortex in first episode psychotic disorders: a structural magnetic resonance study. Am J Psychiatry. 2004;161(9):1612–1619. doi: 10.1176/appi.ajp.161.9.1612. [DOI] [PubMed] [Google Scholar]
- 140.Prasad KMR, Rohm B, Keshavan MS. Parahippocampal gyrus in first episode psychotic disorders: a structural magnetic resonance imaging study. Prog Neuro-psychopharmacol Biol Psychiatry. 2004;28:651–658. doi: 10.1016/j.pnpbp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 141.Lacerda AT, Hardan AB, Yorbik O, Vemulapalli M, Prasad KMR, Keshavan MS. Orbitofrontal cortex in first episode schizophrenia: relationship with negative symptomatology. Prog Neuro-psychopharmacol Biol Psychiatry. 2007;31(2):510–516. doi: 10.1016/j.pnpbp.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 142.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocogntive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164(5):813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 143.Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19(2):261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 144.Snitz BE, MacDonald AW, III, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Byrne M, Hodges A, Grant E, Owens DC, Johnstone EC. Neuropsychological assessment of young people at high genetic risk for developing schizophrenia compared with controls: preliminary findings of the Edinburgh High Risk Study (EHRS) Psychol Med. 1999;29(5):1161–1173. doi: 10.1017/s0033291799001002. [DOI] [PubMed] [Google Scholar]
- 146.Seidman LJ, Yurgelun-Todd D, Kremen WS, et al. Relationship of prefrontal and temporal lobe MRI measures to neuropsychological performance in chronic schizophrenia. Biol Psychiatry. 1994;35(4):235–246. doi: 10.1016/0006-3223(94)91254-8. [DOI] [PubMed] [Google Scholar]
- 147.O'Driscoll GA, Florencio PS, Gagnon D, et al. Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Res. 2001;107(2):75–85. doi: 10.1016/s0925-4927(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 148.Koo MS, Dickey CC, Park HJ, et al. Smaller neocortical gray matter and larger sulcal cerebrospinal fluid volumes in neuroleptic-naive women with schizotypal personality disorder. Arch Gen Psychiatry. 2006;63(10):1090–1100. doi: 10.1001/archpsyc.63.10.1090. [DOI] [PubMed] [Google Scholar]
- 149.Prasad KMR, Chowdari KV, Nimgaonkar VL, Talkowski ME, Lewis DA, Keshavan MS. Polymorphism of the RGS4 gene and the DLPFC morphometry in first episode schizophrenia: a ROI study using the structural magnetic resonance imaging. Mol Psychiatry. 2005;10:213–219. doi: 10.1038/sj.mp.4001562. [DOI] [PubMed] [Google Scholar]
- 150.Buckholtz JW, Meyer-Lindenberg A, Honea RA, et al. Allelic variation in RGS4 impacts functional and structural connectivity in the human brain. J Neurosci. 2007;27(7):1584–1593. doi: 10.1523/JNEUROSCI.5112-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zinkstok J, Schmitz N, van Amelsvoort T. Genetic variation in COMT and PRODH is associated with brain anatomy in patients with schizophrenia. Genes, Brain Behav. 2008;7(1):61–69. doi: 10.1111/j.1601-183X.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 152.Ohnishi T, Hashimoto R, Mori T, et al. The association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and morphological abnormalities of the brain in chronic schizophrenia. Brain. 2006;129(Pt 2):399–410. doi: 10.1093/brain/awh702. [DOI] [PubMed] [Google Scholar]
- 153.Prasad KMR, Chowdari KV, Talkowski ME, et al. Cerebral morphometry and COMT Val/Met polymorphism in first-episode schizophrenia. Neuropsychopharmacology. 2004;29(suppl 1):S222. [Google Scholar]
- 154.Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, et al. Low-activity allele of catechol-O-methyltransferase (COMTL) is associated with increased lateral ventricles in patients with first episode non-affective psychosis. Prog Neuro-psychopharmacol Biol Psychiatry. 2007;31(7):1514–1518. doi: 10.1016/j.pnpbp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 155.McIntosh AM, Baig BJ, Hall J, et al. Relationship of catechol-O-methyltransferase variants to brain structure and function in a population at high risk of psychosis. Biol Psychiatry. 2007;61(10):1127–1134. doi: 10.1016/j.biopsych.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 156.Taylor WD, Zuchner S, Payne ME, et al. The COMT Val158Met polymorphism and temporal lobe morphometry in healthy adults. Psychiatry Res. 2007;155(2):173–177. doi: 10.1016/j.pscychresns.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Callicott JH, Straub RE, Pezawas L, et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc Natl Acad Sci U S A. 2005;102(24):8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cannon TD, Hennah W, van Erp TG, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62(11):1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 159.Hashimoto R, Numakawa T, Ohnishi T, et al. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15(20):3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- 160.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 161.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159(7):1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 162.Geddes JR, Verdoux H, Takei N, et al. Schizophrenia and complications of pregnancy and labor: an individual patient data meta-analysis. Schizophr Bull. 1999;25(3):413–423. doi: 10.1093/oxfordjournals.schbul.a033389. [DOI] [PubMed] [Google Scholar]
- 163.Gilbert AR, Montrose DM, Sahni SD, Diwadkar VA, Keshavan MS. Obstetric complications correlate with neurobehavioral and brain structural alterations in young relatives at risk for schizophrenia. Ann N Y Acad Sci. 2003;1008:269–275. doi: 10.1196/annals.1301.030. [DOI] [PubMed] [Google Scholar]
- 164.Stefanis N, Frangou S, Yakeley J, et al. Hippocampal volume reduction in schizophrenia: effects of genetic risk and pregnancy and birth complications. Biol Psychiatry. 1999;46(5):697–702. doi: 10.1016/s0006-3223(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 165.Prasad KMR, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first episode schizophrenia. Mol Psychiatry. 2007;12(1):105–113. doi: 10.1038/sj.mp.4001915. [DOI] [PubMed] [Google Scholar]
- 166.Bangalore S, Prasad KM, Montrose DM, Goradia D, Diwadkar VA, Keshavan MS. Cannabis use and brain structural alterations in first episode schizophrenia—a region of interest, voxel-based morphometric study. Schizophr Res. 2008;99(1–3):1–6. doi: 10.1016/j.schres.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 167.Szeszko PR, Robinson DG, Sevy S, et al. Anterior cingulate grey-matter deficits and cannabis use in first-episode schizophrenia. Br J Psychiatry. 2007;190:230–236. doi: 10.1192/bjp.bp.106.024521. [DOI] [PubMed] [Google Scholar]
- 168.Jonsson EG, Edman-Ahlbom B, Sillen A, et al. Brain-derived neurotrophic factor gene (BDNF) variants and schizophrenia: an association study. Prog Neuro-psychopharmacol Biol Psychiatry. 2006;30(5):924–933. doi: 10.1016/j.pnpbp.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 169.Agartz I, Sedvall GC, Terenius L, et al. BDNF gene variants and brain morphology in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141(5):513–523. doi: 10.1002/ajmg.b.30338. [DOI] [PubMed] [Google Scholar]
- 170.Szeszko PR, Lipsky R, Mentschel C, et al. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Mol Psychiatry. 2005;10(7):631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- 171.Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24(45):10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roberts RC. Schizophrenia in translation: disrupted in schizophrenia (DISC1): integrating clinical and basic findings. Schizophr Bull. 2007;33(1):11–15. doi: 10.1093/schbul/sbl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gurling HM, Critchley H, Datta SR, et al. Genetic association and brain morphology studies and the chromosome 8p22 pericentriolar material 1 (PCM1) gene in susceptibility to schizophrenia. Arch Gen Psychiatry. 2006;63(8):844–854. doi: 10.1001/archpsyc.63.8.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Papiol S, Molina V, Desco M, et al. Ventricular enlargement in schizophrenia is associated with a genetic polymorphism at the interleukin-1 receptor antagonist gene. Neuroimage. 2005;27(4):1002–1006. doi: 10.1016/j.neuroimage.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 175.Hariri AR, Weinberger DR. Imaging genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- 176.Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. Br J Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- 177.McGuffin P, Reveley A, Holland A. Identical triplets: non-identical psychosis? Br J Psychiatry. 1982;140:1–6. doi: 10.1192/bjp.140.1.1. [DOI] [PubMed] [Google Scholar]