Abstract
Background: “Theory of mind” (TOM) refers to the ability to attribute mental states (ie, beliefs and goals) to one's self and others and to recognize that behaviors are guided by these mental states. This capacity, critical for social competence, is impaired in schizophrenia. We undertook a study of TOM in a group of patients with schizophrenia and healthy controls. Method: We used positron emission tomography to identify the neural circuits recruited during a verbal task that required participants to attribute mental states to a character in a story of their creation. The comparison task consisted of reading aloud a neutral story, controlling for the speech component of the task. Results: Patients and controls generated the same percentage of TOM utterances. However, the two groups had markedly different patterns of brain activation. Compared with controls, patients had a lower blood flow in multiple regions in the left hemisphere including the frontal and visual association cortices, posterior hippocampus, and insula. The flow was also lower in contralateral areas in the lateral cerebellum and vermis, thalamus, and posterior insula. On the other hand, the flow was higher in the patients predominantly in the right hemisphere, including multiple frontal and parietal regions, insula, visual association cortex, and pulvinar. Discussion: The areas of lower flow are consistent with previous studies indicating impairment in recruiting cortical-cerebellar circuitry in schizophrenia. The areas of higher flow may reflect a need to draw on the right hemisphere to compensate for deficits in left hemisphere networks that include frontal cortex, anterior cingulate, cerebellum, and thalamus.
Keywords: schizophrenia, imaging, theory of mind, social cognition
Introduction
Although the majority of psychiatrists currently conceptualize schizophrenia primarily in terms of psychotic symptoms, the original classic description of the disorder was quite different. As formulated by the person who gave the illness the name that we currently use, disorganization of thinking (schiz = fragmented, phen = mind) was the central defining feature.1 In Dementia Praecox, or the Group of Schizophrenias, Eugen Bleuler described 4 features as primary or fundamental: associative loosening (fragmented thinking or “thought disorder”), autism, affective blunting, and ambivalence.1 These came to be known as the “4 As” and were considered to be the “diagnostic criteria” for schizophrenia up until the 1970s. (There were actually 2 more As in Bleuler's book, attentional impairment and avolition, but they for some reason were left out of the Central Dogma of psychiatry as taught in early and mid-20th century America.)
Having been “lost” or ignored for a number of years, several features of Bleuler's thinking are now reemerging. Some of his As are what we currently call negative symptoms. One of them is the emphasis of this theme issue: social cognition, or what he referred to as “autism.”1 Bleuler believed that the inability to relate empathically to others was one of the primary or fundamental symptoms of schizophrenia. He considered this symptom to be far more important than the delusions and hallucinations given so much emphasis in current diagnostic criteria. Bleuler described an impaired ability to appreciate the internal states of others as a fundamental characteristic of the illness. Instead of recognizing cues from the actions of others, patients with schizophrenia may be guided principally by their own personal representations of the world, which are sometimes idiosyncratic or even wrong. In other words, their behavior is “autistic.” This capacity, that appears diminished in schizophrenia, is variously referred to as the ability to mentalize or to have a “theory of mind (TOM).” TOM is defined as the ability to attribute mental states (such as beliefs, intentions, desires, goals, etc.) to self and others and to appreciate that behaviors are guided by these mental states.2–4
During recent years, there has been increased interest in TOM, principally in the area of child psychiatry and in philosophy of mind. As is often the case in the study of psychopathology, one major issue is identifying the best method for assessing it. A paradigm known as the false-belief test has been touted as the acid test for mentalizing abilities.5 In this paradigm, a research subject is asked to make inferences about another person's behavior when that person holds a false belief.2,3 Others have argued that it is too cognitively demanding for use in children or subjects who are psychiatrically ill.6–11 Its high level of specificity is undermined by a high false-negative rate. In fact, some individuals fail it and, yet, are clearly endowed with mentalizing faculties.7,9 Based on this rationale, alternative tasks have been designed. One promising approach has been to rate utterances for the occurrence of terms that usually denote mental states, the various types of those states, and whether they are attributed to self or others.12–15 This technique is now recognized as an alternative way to evaluate TOM in a naturalistic, sensitive, and reliable way.
Over the last decade, interest in social cognition in schizophrenia has increased, and some investigators have begun to examine TOM. In a study that compared patients with Asperger's syndrome or schizophrenia to healthy controls, Bowler used a problem-solving task (Peter thinks that Jane thinks that …), followed by asking them to explain their solutions.16 He found that both clinical groups performed as well as controls in the problem-solving task, but were deficient in their use of mental state terms when explaining the rationale behind their answers, thereby displaying a lack of intuitive knowledge of social behavior. On tasks requiring understanding of hints, conversational maxims, first- and second-order false-belief and deception tasks, and jokes, Frith and colleagues have found that negative, disorganized, and, to a lesser extent, paranoid symptoms were associated with TOM deficiencies.17–20 No such impairment was detected in patients with delusions of passivity or in those in remission. These findings, that have been partially replicated by others,21–23 lent support to a theoretical model that posits that delusions of persecution, delusions of reference, and third-person auditory hallucinations result from the patients’ inability to have a representation of their own mental activity and, consequently, attribute the content of their thoughts to others.24,25 In fact, the ability to hold metarepresentations (ie, higher order representations) of one's own mental states and those of others is believed to underlie TOM capacities.
These studies have relied solely on cognitive tests to measure TOM in schizophrenia. Ideally one would like to measure both behavior/cognition and its neural substrates, as is now possible with in vivo functional imaging techniques. In a functional magnetic resonance imaging (fMRI) study, Russell et al26 asked their subjects to choose between 2 words describing the mental state reflected in photographed eyes. Compared with healthy controls, patients with schizophrenia made more errors labeling the eyes and showed less blood oxygen level-dependent signal in the left middle/inferior frontal cortex bordering the insula (Brodmann area [BA] 9/44/45). In a positron emission tomography (PET) study, Brunet et al27 found that medicated patients with schizophrenia, unlike healthy controls, did not recruit the right prefrontal cortex during a nonverbal attribution-of-intentions task.
We have conducted a H2 15O-PET study of TOM in medication-free patients with schizophrenia, using a paradigm that permitted us to rate utterances for the occurrence of terms that usually denote mental states, the various types of those states, and whether they are attributed to self or others. In order to avoid the confounds of poor motivation and poor task performance, we used a task that would be relatively easy for patients to perform. We asked them to “make up a story,” using a situation that would induce them to attribute mental states to another person, and rated their utterances to assess their “mentalizing” capacities.9,13–15
Methods
Subjects
The subjects were 18 right-handed patients with a DSM-IV diagnosis of schizophrenia recruited through the mental health clinical research center at the University of Iowa. The diagnosis was based on an extensive evaluation using the Comprehensive Assessment of Symptoms and History (CASH).28 Their mean age was 32.5 (SD 11.0), and their mean educational achievement was 12.9 (SD 2.2). Fourteen were males and five females. On the day of the PET imaging, patients were either drug naïve (n = 8) or had been medication free for 3 weeks (n = 10). Clinical symptoms were rated on the day of the scan, using the Scale for the Assessment of Positive Symptoms and the Scale for the Assessment of Negative Symptoms.29 They were mildly to moderately symptomatic, rating 2.1 (SD 0.9) on the negative symptoms subscale, 2.8 (SD 1.4) on the psychotic one, and 1.8 (SD 1.3) on the disorganized one. A score of 2 is equivalent to a mild level of symptoms, while a score of 3 is moderate.
These were compared with 13 right-handed healthy control subjects recruited from the community. The controls were matched to the patient group on parents’ level of education. Six were males and seven females. They were screened to rule out a current or past history of psychiatric illness using a short version of the CASH. They were also evaluated to rule out any current or past history of neurological or general medical illness by history and physical examination. Their mean age was 26.5 years (SD 6.4) and their mean educational achievement 14.6 years (SD 0.9). The healthy controls data have been published elsewhere30 and are reported here for comparison.
All subjects gave written consent to a protocol approved by the University of Iowa Human Subjects Institutional Review Board.
Tasks
The experimental task examined the ability to attribute mental states to others by having the participants compose a story about the thoughts and internal experiences of another person. During this task, referred to as the “TOM Story”, the subject was told: “Imagine you sat next to a woman (for men; or next to a man for women) on a park bench and you realized she (he) was crying. Make up a story about what led up to her (his) crying.” These instructions were presented to the subjects on a video monitor positioned 12–13 inches from their eyes. Subjects were given 30 s to read the instructions and plan their narrative prior to beginning their “story.” They were allowed to speak for approximately 100 s, and PET data were acquired during this time interval. As described elsewhere,30 the narratives were scored to identify the number of mental state attributions (MSA), in order to determine the ability of subjects to attribute mental states to self or others.
The control/comparison task (“Read Story”) was to read aloud a neutral story that was presented on the video monitor. The subjects were required to read for 40 s. If they finished reading the story before the time was up, they had to restart from the beginning. The order of the tasks was counter balanced.
The experimental and control tasks both required the participants to read the instructions or other material and to speak aloud in continuous narrative speech, thereby isolating the TOM component in the experimental task. Subjects were audiotaped during the 2 conditions, and transcripts were prepared from the tapes. Both conditions were timed so that the subject began speaking 10 s prior to the arrival of the bolus of H2 15O in the brain.
Scoring
Scoring methods for MSA and their reliability have been previously described.30 Briefly, each transcript was rated independently and blindly by 2 raters for occurrences of MSA to self or others. Each utterance was scored as level 0 if no attributions were made, level I if it contained one level of attribution to self or others, and level II if at least 2 levels of attributions were made. The interrater agreement was 76%.
PET Imaging
In order to acquaint the participant with the imaging conditions and to ascertain stimulus timing, the time from injection to bolus arrival in the brain was individually measured by delivering a 15-mCi bolus during an initial scout injection. Quantitative PET blood flow data were acquired on a gradient echo (GE) 4096-plus whole-body scanner following the bolus injection of [15O]water. During the scout injection, the participant was asked to read a list of words presented on the monitor; this initial “sham” study served to familiarize the participants with the procedures and reduce anxiety once data collection began for the experimental and comparison tasks. All subsequent scans employed a 50 mCi [15O]water IV bolus dose. Imaging began at the time of injection (t = 0) and continued for 100 s in the form of twenty 5-s frames. Based on the time-activity curves over major cerebral arteries, the 8 frames reflecting the 40-s postbolus transit were summed and reconstructed into 2 mm voxels (128 × 128 matrix) using a Butterworth filter (order = 6, cut-off frequency = 0.35 Nyquist).31 Cerebral blood flow was calculated on a pixel-by-pixel basis using the autoradiographic method32 and normalized by dividing by global cerebral blood flow. In order to reduce anatomical variability, an 18-mm Hanning filter was applied. Imaging was repeated at approximately 15-min intervals.
Magnetic Resonance Imaging
Magnetic resonance (MR) scans, to be used for anatomic localization of functional activity, were obtained for each subject with a standard T1-weighted 3-dimensional spoiled grass sequence on a 1.5-T GE Signa scanner (time to echo = 5, repetition time = 24, flip angle = 40, number of excitations = 2, FOV = 26, matrix = 256 × 192, slice thickness = 1.5 mm).
Image Analysis
The normalized quantitative PET blood flow images and MR images were analyzed using the locally developed software package BRAINS.33–35 The outline of the brain was identified on the MR images by an artificial neural net.36 The anterior commissure-posterior commissure line was identified and used to realign the brains of all the subjects to a standard position to place each brain in standardized Talairach coordinate space.37 The PET image of each individual was then fit to that individual's MR scan using a surface-fit algorithm.38 Each injection was checked for head movement and individually refit as needed. The MR images of all the subjects were averaged, so that the data obtained with PET could be localized on a coregistered MR that represented the brains of all subjects in the study. The coregistered images were resampled and simultaneously visualized in all 3 orthogonal planes during the interpretation of the statistical analyses.
Statistical Analysis
Using nonparametric statistical techniques particularly appropriate for complex between-group comparisons in PET studies, we examined the differences in neural activation between patients and normal controls.39–41 Statistical techniques that rely on the general linear model for between-group comparisons make many assumptions about the data. Randomization analysis is a nonparametric statistical technique that makes no assumptions about variance and is not affected by between-group differences in variance. The randomization analysis was based on an initial subtraction of the 2 experimental conditions from one another. The randomization repeatedly sampled 2 groups of 18 and 13 without regard to diagnosis. After each resampling, voxel-wise t maps were generated. After 3000 randomizations, the distributions of the voxel-wise t values were stored to estimate the probabilities associated with the t. Areas where statistical analysis showed specific regional differences were identified as “peaks.” Consistent with our previous studies, we used an uncorrected P value of 0.005 as the minimum significance threshold for defining a peak. This threshold closely approximates the sizes of peaks defined by a t = 3.61 (uncorrected) using the Montreal method. This t threshold is consistently used in all our studies of healthy volunteers, and we have maintained an uncorrected P for all of our randomization analyses using patient vs control comparisons. Each significant peak is described in the tables by the number of adjacent pixels that comprise the peak and by Talairach atlas coordinates.
This randomization analysis relies on an across task and an across group comparison (TOM Story minus Read Story in patients compared with TOM Story minus Read Story in controls). The peaks identified represent statistical difference maps that indicate the brain regions where the 2 groups differ from one another during the experimental task, as compared with the control task. Interpretation of the direction of brain blood flow change in each group was determined in 2 ways. First, we examined and visually compared the within-group analyses of the 2 tasks generated by the Montreal method.42 Second, we examined the quantitative blood flow data for each peak identified as significantly different in the 2 groups by the randomization analysis. The voxels from the location with the highest significance level (as defined by the Talairach coordinates) were averaged for each condition within each group. The mean for the Read Story condition was then subtracted from the TOM Story condition. The difference produced by this subtraction is a direct indication of the regions in which cerebral flow is higher or lower during the TOM vs Read Story Tasks.
The tables indicate the brain regions where patients and controls differ significantly from one another, using region names based on inspection of the coregistered MR and PET images as well as the x, y, and z coordinates form the Talairach atlas. Areas containing at least 50 voxels with a t value greater that 3.61, the highest t value (t max), and the total number of voxels in the region are reported in the tables. Visual display of results is shown in 2 ways. One presentation shows only the peaks, as defined by the volume measurement, superimposed on the composite average MR brain. The other presentation, referred to as the “t map,” shows the color-coded t values for all voxels in the image. The peak map and the t map provide complementary information. The former identifies areas of activation by using a strict definition based on a relatively arbitrary cut-off point, while the latter provides a more descriptive picture of the geography of the circuitry involved.
A student's t test was used to analyze the differences in the behavioral performance across groups and conditions.
Results
Behavioral Data
Table 1 shows the performance of the participants during both the TOM and Read Story tasks. The patients and controls achieved a comparable percentage of TOM attributions: 56% vs 59%, respectively. This indicates that the patients were successful in attributing mental states to another person. Consistent with the lack of verbal fluency common in schizophrenia, the patients produced fewer words and utterances than the controls during the TOM Story (10.1 utterances [SD 4.1] and 156 words [SD 75]). However, they read the story at the same rate as the normal controls.
Table 1.
Behavioral Data: Demographics, Severity of Symptoms, Type and Number of TOM Utterances, and Rate of Speech during Both Tasks for Patients With Schizophrenia Spectrum and Healthy Controls
| Controls, n = 13 | Patients, n = 18 | ||
| Age | 26.5 (6.4) | 32.0 (11.4) | P = .11 |
| Full Scale Intelligence Quotient | 108 (9) | 87 (11) | P <.0001 |
| Parents’ Socioeconomic Status | 2.8 (0.4) | 3.3 (0.7) | P = .02 |
| Parents’ level of education | 12.0 (0.8) | 12.3 (2.4) | P = .7 |
| Negative symptoms | NA | 2.1 (0.9) | NA |
| Positive symptoms | NA | 2.8 (1.4) | NA |
| Disorganized symptoms | NA | 1.8 (1.3) | NA |
| Global rating | NA | 2.2 (0.9) | NA |
| Duration of illness (years) | NA | 8.96 (9.3) | NA |
| Level 0 utterances | 6.25 (3.7) | 4.1 (2.2) | P = .05 |
| Level I utterances | 8.25 (2.8) | 5.8 (3.4) | P = .04 |
| Level II utterances | 0.2 (0.5) | 0.2 (0.4) | P = .9 |
| Number of utterances | 14.7 (4.0) | 10.1 (4.3) | P = .005 |
| Rates of MSA/number of utterances | 0.59 (0.22) | 0.56 (0.23) | P = .7 |
| Rates of MSA × 100/number of words | 3.3 (0.9) | 3.9 (1.9) | P = .3 |
| Number of words in TOM Story | 257 (69) | 156 (75) | P = .0009 |
| Rate of words/min in TOM Story | 163 (26) | 95 (45) | P < .0001 |
| Number of words in Read Story | 114 (19) | 110 (20) | P = 0.6 |
| Rate of words/min in Read Story | 172 (29) | 163 (25) | P = 0.4 |
Note: MSA, mental state attribution; TOM, theory of mind; NA, not applicable. Statistically significant values appear in boldface.
Imaging Data
Tables 2 and 3 and figures 1 and 2 show the results of the randomization analysis that plots the “difference maps,” showing how the unmedicated patients differ from healthy volunteers in terms of having either lower or higher regional cerebral blood flow (rCBF) during the TOM task compared with the Read Story condition.
Table 2.
Cerebral Regions With Relatively Lower Regional Cerebral Blood Flow in Patients With Schizophrenia Spectrum Disorders
| Randomization (Significance of Peak)a |
Size of Significant Peak |
Difference in rCBF (ml/g/min) Between TOM and Read Story in Each Groupb |
Talairach Coordinates |
||||
| Brain region (Brodmann area) | P | Number of Voxels | Patients | Controls | x | y | z |
| Right inferior frontal gyrus | 0.001 | 91 | −6.61 | 1.92 | 27 | 40 | −20 |
| Left anterior frontal gyrus | 0.004 | 279 | −4.14 | 5.01 | −27 | 28 | −21 |
| Right anterior cingula | 0.0007 | 90 | −4.27 | 4.19 | 9 | −8 | 45 |
| Right posterior insula | 0.0012 | 87 | −4.99 | 3.13 | 44 | −8 | 0 |
| Right thalamus (dorsomedial) | 0.0005 | 287 | −3.47 | 5.47 | 5 | −19 | 2 |
| Left posterior hippocampus | 0.003 | 53 | −5.01 | 2.24 | −22 | −27 | −8 |
| Left lingual gyrus (BA 18) | 0.00001 | 6851 | −18.44 | -4.82 | −11 | −75 | 2 |
| Right cerebellum | 0.0001 | 1739 | −4.94 | 5.94 | 10 | −52 | −9 |
| Right cerebellum | 0.001 | 158 | 0.03 | 8.33 | 24 | −52 | −36 |
| Right cerebellum | 0.0018 | 162 | −2.07 | 5.68 | 11 | −69 | −34 |
| Vermis | 0.0027 | 74 | −5.69 | 1.84 | 1 | −71 | −18 |
The randomization analysis is a nonparametric statistical test that indicates the significance of the differences between patients with schizophrenia spectrum disorder and healthy volunteer subjects. It is based on an initial within-group subtraction of change in regional cerebral blood flow (rCBF) during the TOM task minus the Read Story condition, followed by a between-group comparison of the differences in change in rCBF during the 2 types of tasks. The P values indicate the magnitude of the significance level for each peak, indicating between-group differences in change. The overall size of the area of the peak is shown in the next column, which indicates the number of voxels that exceed the preset significance threshold. The differences in actual rCBF values for all significant randomization regions are shown for each group in the fourth and fifth columns of the table in order to indicate the differences in direction of change of rCBF. Positive values indicate that, overall, each group tends to have a higher rCBF in the specific area of the peak in the TOM condition compared with the Read Story one. Negative values indicate the opposite. The overall size of the area of the peak indicates the number of voxels that exceed the preset significance threshold (P < .005).
The difference in mean rCBF in each peak, based on the subtraction of TOM task from Read Story, is shown in order to indicate the differences in direction in each group. Negative values in patients and positive values in controls indicate that rCBF is lower in the patients in a given peak during the TOM task compared with Read Story condition.
Table 3.
Cerebral Regions With Relatively Higher Regional Cerebral Blood Flow in Patients With Schizophrenia Spectrum Disorders
| Randomization (Significance of Peak)a |
Size of Significant Peak |
Difference in rCBF (ml/g/min) Between TOM and Read Story in Each Groupb |
Talairach Coordinates |
||||
| Brain region (Brodmann area) | P | Number of Voxels | Patients | Controls | x | y | z |
| Right medial and lateral frontal gyri (BA 32/10/47/11) | 0.001 | 822 | 5.77 | −2.67 | 18 | 42 | −5 |
| Left middle frontal gyrus (BA 9/46) | 0.0002 | 399 | 9.32 | −0.84 | −18 | 39 | 13 |
| Left middle frontal gyrus (BA 8/9) | 0.0009 | 255 | 10.41 | 1.92 | −33 | 29 | 35 |
| Right inferior frontal gyrus (BA 45) | 0.0026 | 105 | 2.29 | −5.12 | 32 | 29 | 13 |
| Right middle frontal gyrus (BA 6) | 0.0018 | 90 | 5.36 | −2.44 | 19 | 21 | 47 |
| Right inferior frontal gyrus (BA 44/45) | 0.0005 | 259 | 0.35 | −8.79 | 41 | 17 | 17 |
| Right middle frontal gyrus | 0.0012 | 115 | 1.98 | −6.26 | 41 | 17 | 27 |
| Right anterior insula | 0.0011 | 119 | 2.34 | −5.95 | 39 | 6 | 8 |
| Postcentral gyrus (BA 43) | 0.0011 | 142 | 3.83 | −4.46 | −38 | −12 | 22 |
| Right thalamus (pulvinar) | 0.0004 | 276 | 5.95 | −3.18 | 13 | −26 | 13 |
| Right inferior parietal lobule (BA 40) | 0.0025 | 82 | 7.2 | −5.41 | 53 | −38 | 36 |
| Left middle temporal gyrus (BA 21) | 0.0025 | 86 | 0.83 | −6.63 | −38 | −48 | 36 |
| Right superior parietal lobule (BA 7) | 0.0002 | 972 | 11.45 | −4.49 | 39 | −64 | 36 |
| Right fusiform/inferior occipital gyrus (BA 18) | 0.0006 | 166 | 2.11 | −6.86 | 30 | −92 | −3 |
See table 2.
The difference in mean rCBF in each peak, based on the subtraction of TOM task from Read Story, is shown in order to indicate the differences in direction in each group. Positive values in patients with schizophrenia spectrum and negative values in healthy controls indicate that rCBF is higher in patients during the TOM task compared with Read Story condition.
Fig. 1.
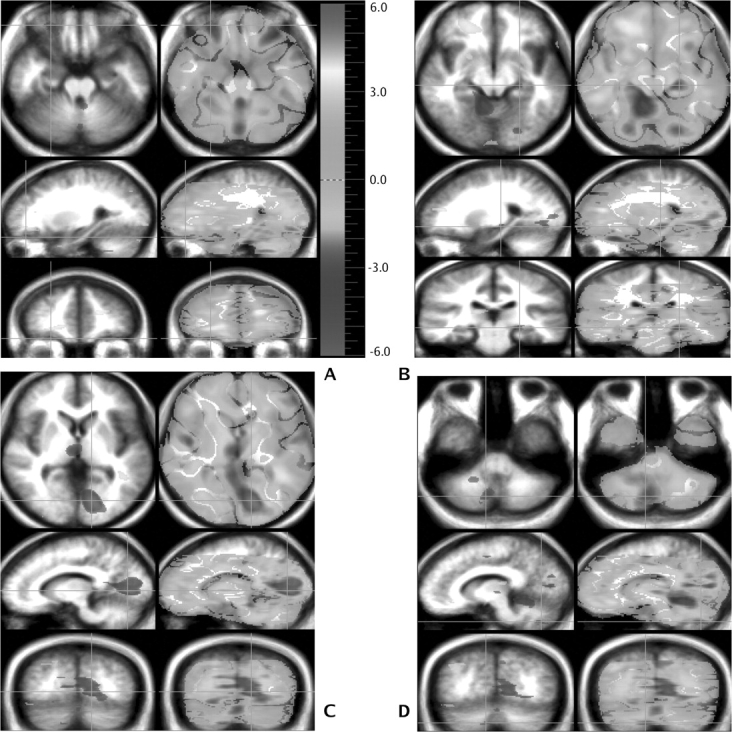
This figure shows brain regions recruited differently by the 2 study groups based on between-group randomization analysis comparing the theory of mind minus the Read Story subtraction in both groups (patients with schizophrenia – healthy individuals). Orientation is radiological convention. Four different sets of images have been selected in order to illustrate the location of peaks. Three orthogonal views are shown, with transaxial at the top, sagittal in the middle, and coronal on the bottom. Green crosshairs are used to show the location of the slice. Statistical maps of the PET data, showing regions that are differentially activated, are superimposed on a composite MR image derived by averaging the MR scans from the subjects. Two types of statistical maps are provided. The “peak map” (left side of image) shows the small areas where all contiguous voxels exceed the predefined threshold for statistical significance (P < .005). The “t map” (right side of image) shows the value of t for all voxels in the image and provides a general overview of the landscape of changes in regional cerebral blood flow (rCBF). Areas of higher rCBF appear in yellow, while those of lower rCBF are in blue. For each slice (axial, sagittal, and coronal planes), the green crosshairs show the location of the 2 other slices, and the intersection of the crosshairs indicates the pixel with the highest t value within the area marked by the crosshairs. In (A), an area of decreased flow in the left and right orbitofrontal area (BA 11) can be seen in the sagittal view. In (B), an area of lower rCBF in the left hippocampus is seen at the intersection of the crosshairs in all 3 planes. In (C), an area of lower rCBF in the left lingual gyrus (BA 18) shows all 3 planes; an area of decreased flow is also seen in the dorsomedial nucleus of the thalamus. In (D), in the transaxial view, a large area of lower rCBF is also seen in the cerebellum.
Fig. 2.
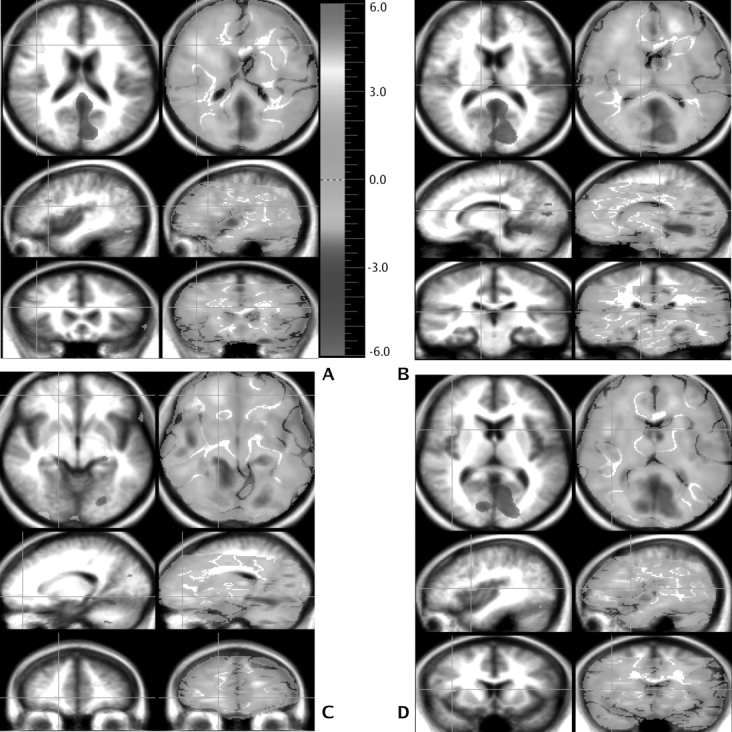
Several areas of higher regional cerebral blood flow (rCBF) (in yellow) in patients with schizophrenia are shown. In the axial plane, an area in the calcarine fissure (BA 17) and one in the right frontal area (BA 32/10/47/11) are seen. In addition, a left frontal area (BA 9/46) in the sagittal plane and another area in the thalamus in the frontal plane exhibit a higher rCBF in the patients. Finally, 3 regions of higher rCBF show in yellow: a right thalamic (Pulvinar) area in the sagittal view and a right cerebellar and a right superior parietal (BA 7) area in the frontal plane.
As shown in Table 2 and figure 1, the patients have lower rCBF in a widely distributed group of cortical and subcortical regions. The cortical areas include the left and right inferior frontal cortex, right anterior cingulate, right insular cortex, left posterior hippocampus, and the left visual association cortex (lingual gyrus). Also, the patients exhibited a lower rCBF in the right thalamus, probably the dorsomedial nucleus, and the cerebellum (primarily contralateral to the larger areas of lower rCBF, reflecting cross-hemispheric connections).
Table 3 and figure 2 show the regions where the patients, in comparison with the controls, displayed a higher rCBF while performing the TOM task vs the Read Story condition. These include multiple regions of the prefrontal cortex, including medial and dorsolateral frontal gyri, as well as the middle and inferior frontal gyri. Although some occur in the left hemisphere, the majority are on the right. In addition, a higher rCBF is observed in the right insular and parietal cortices, right visual association area, and the right pulvinar.
We have previously described the brain regions used by the healthy volunteers during the TOM task.30 This task activated an extensive neural network that included the medial frontal cortex, the superior frontal cortex, the anterior and retrosplenial cingulate, and the anterior temporal pole. Most of these activations were limited to the left hemisphere. In addition, the largest activation was in the contralateral right cerebellum, as well as the anterior vermis.
Relationship With Symptoms
Previous studies have identified a relationship between symptom profiles and performance on TOM tasks.18,19,43,20 Using a Pearson correlation analysis, we examined the correlations between symptom ratings and the patients’ performance on both tasks. We found no significant correlations, beyond chance level, between asociality, delusions, social adjustment (during the week and the 6 months prior to the study), positive, negative, and disorganized symptoms on the one hand and the various parameters of behavioral performance (listed in table 1) on the other hand.
Discussion
Behavioral Findings
This experiment was designed to evaluate the neural substrates of TOM in unmedicated patients suffering from schizophrenia. We began the study anticipating that the patients would show impaired performance on the behavioral task that we selected to assess TOM—a test that required them to imagine and describe the mental state of another person. To our surprise, their behavioral performance on this task was normal. Although they produced shorter “stories,” their density of TOM attributions was identical to that of the normal controls—59% in the controls vs 56% in the patients. Therefore, we must conclude that the patients demonstrated a normal capacity for mentalizing on this specific task, which requires a complex integration of language production with “mind reading.”
There are several likely reasons for this somewhat unexpected finding. Although a number of behavioral studies show impaired TOM in schizophrenia, not all do.44 In fact, the closer the TOM task is to “real life” in a particular study, the more likely the patients’ functioning will be normal.44 Furthermore, the role of IQ in performance on TOM tasks continues to be debated45; our patients had IQs in the normal range. Finally, this study only examined one facet of TOM. To assume that TOM is a single unitary entity is probably an oversimplification. As Frith points out, mentalizing during discourse is done “online” and may occur implicitly and even automatically in an effort to communicate.46 In an experimental TOM task such as the false-belief task, mentalizing is done “off-line” and must be executed by very explicit mental operations. Most of the behavioral studies reporting impaired TOM in schizophrenia have, in fact, relied largely on explicit “mentalizing.”
A possible limitation of the tasks is the fact that the experimental and baseline tasks do not control fully for the component of spontaneous speech generation. The reading task involves speech, but does not involve discourse planning. Therefore, the comparison of the baseline and experimental task could partially reflect the brain areas involved with speech generation in general and not completely isolate TOM activity.
Patterns of Brain Activation
Although they performed the TOM task at normal levels, the patients did so by using very different brain regions than the controls, as shown by the randomization analyses.
The profile of brain regions with lower rCBF in patients as compared with controls is relatively familiar and replicates much of our previous work. That is, the lower rCBF occurs in a widely distributed circuit that not only includes multiple cortical regions but also includes subcortical regions such as the thalamus and cerebellum. We have referred to this circuit as the cortico-cerebellar-thalamic-cortical circuit (CCTCC) and have noted in multiple studies that the cortical regions with decreased flow vary in ways that are task dependent, while the subcortical regions tend to have relatively consistent hypoperfusion that is task independent.47,48
In addition to the by-now-familiar decreases in rCBF in key components of this distributed circuit (ie, thalamus and cerebellum), we also find decreases in specific regions that are of particular interest for the TOM task.
For example, the patients show a decreased flow in the anterior cingulate gyrus. The more anterior and rostral part of the anterior cingula appears to be involved in emotional processing.49,50 Other imaging studies, in normal controls, have shown this area to be activated in TOM tasks. For example, in their fMRI study assessing TOM, Gallagher et al51 found a medial frontal activation closely associated with the anterior cingulate (BA 32) during the TOM tasks. In addition, the anterior cingulate has been incriminated both in monitoring mistakes during cognitive tasks such as the Stroop52,53 and in inhibiting prepotent responses.49,54 Furthermore, Carter et al55 have proposed that the anterior cingulate is involved in detecting high levels of response competition. The decreased flow in this region could reflect a greater demand either to monitor inappropriate statements or emotions (response competition) or to inhibit any affective or (Did you mean to say this way? Decreased flow reflecting greater demand) motor prepotent response that would have been irrelevant to the topic or inappropriate in such an emotional context.
In addition, the patients show decreased flow in right inferior and left anterior frontal gyrus. Based on single-cell recording studies in animals and on functional imaging and transcranial magnetic stimulation studies in humans, Rizzolatti et al56–59 have suggested that the inferior frontal gyrus (BA 44/45) is the site of a mirror system for gesture recognition which provides “a necessary bridge from doing to communicating.” Because “mirror neurons,” located there, are active both during observation and execution of a behavior, the authors proposed that they are part of a neural system that identifies and interprets the behavior of people based on one's experiences, making communication possible.60 Using the same data, Frith describes this area as part of a neural network subserving TOM.3 Thus the decreased flow in these regions in our patients may reflect a defect in the activity of mirror neurons in schizophrenia.
The insula is another area in which decreased flow is observed. The insula has been described to consist of 2 sections: the anteroventral part related to olfactory-gustatory-autonomic function and the posterodorsal insula specialized in auditory-somesthetic-skeletomotor function.61,62 In this study, we find a dissociation between the anterior and posterior insula in the patients with schizophrenia. They have a decrease in the posterior insula (table 2) and an increase in the anterior insula (table 3). The blood flow decrease in the posterior insula could reflect a more extensive mediation between the external environment and the internal milieu while completing the TOM task.61,62 In fact, because of its rich input from all 5 sensory modalities, Mesulam and Mufson62 proposed that the posterior insula might be in a unique position to interrelating events from the environment with internal motivational states and for associating sensory events with relevant emotional responses. Both of these processes are essential for a fully developed TOM. Reflecting their autism, the patients display an inability to access this region at normal levels, leading to a decrease in cerebral blood flow. The anterior insula is essentially a “limbic insula” that assists in connecting internal and external representations of emotional states, a capacity that is intimately related to social cognition and TOM. In order to perform the task as well as they do, the patients must “drive” this region harder than the normal controls.
Finally, the patients show a decreased flow in the left posterior hippocampus, another part of the limbic system known to be involved in encoding episodic memory.63 The process of attributing emotions to others requires accessing the episodic memories of past emotional experiences; an inefficiency in this process could explain yet another aspect of the autistic impairment in social cognition in schizophrenia.
Except for one,64 our previous studies comparing patients with schizophrenia and controls during a variety of tasks have found decreases in flow. In contrast, the mentalizing task in this study has elicited many areas of increased flow. There are numerous anterior, and mostly right-sided, regions that show a higher rCBF in patients with schizophrenia when they perform the TOM task. Whereas normal controls rely mainly, and almost exclusively, on their left cerebral hemisphere to perform this task, patients seem to draw more on the right one. Several lesion and functional imaging studies have highlighted the involvement of the right frontal lobe in TOM. It has been proposed, however, that this was not only due to the nonverbal nature of the task but also due to the design differences.65–67 That patients with schizophrenia recruit brain areas, possibly involved in nonverbal TOM tasks, while performing a highly verbal one, could be consistent with the proposed hypothesis that schizophrenia renders patients poorly efficient on cognitive tasks and forces them to rely on more brain resources to perform at the same levels as controls.68–76 Interestingly, as described above, the patients have decreased flow in their right inferior frontal gyrus, but they also have increases. The lower part of BA 6 and BA 44/45 are premotor areas believed to harbor “mirror” neurons that are activated by both movement observation and execution. This motor representation, on the left side, was postulated to be at the basis of the understanding of motor events and, therefore, makes communication and mind reading possible.57,58,60,77 However, to perform at a more satisfactory level, patients might have had to increase flow. As reported in table 3, patients with schizophrenia also show extensive activation of the left middle frontal gyrus (BA 8/9/46), site of verbal working memory.73,78
The extensive and stronger right-sided activation in patients while performing the TOM task, compared with controls, is also interesting for yet another reason. Because of the highly verbal nature of this task, the right-sided activation seen in patients with schizophrenia could hypothetically reflect the poor hemispheric lateralization typical of patients with schizophrenia according to Crow.79,80 Crow has repeatedly proposed that schizophrenia is “the price” Homo sapiens have to pay for their ability to verbally communicate.79–82 The fact that patients with schizophrenia show different patterns of brain activation when performing TOM tasks could be consistent with his proposal of impaired brain lateralization in this illness and explain our findings.
Another interesting increase in cerebral blood flow during the TOM task is in both right superior and inferior parietal lobules. Growing evidence from both animal and human studies supports the role of the parietal lobe as a sensorimotor interface.83,84 Parietofrontal circuits mediate the sensorimotor transformation for the control of specific actions. This has led researchers to postulate that space coding is performed in the context of the action to be executed.84 In addition, the parietal lobes bilaterally are sites of polymodal associations areas, and the right inferior parietal lobule is involved, to a larger extent, in spatial memory and attention,85–87 ie, evaluation of the external world, while the left lobe processing is directed mainly to the internally generated stimuli.88 We propose that patients with schizophrenia tend to rely more heavily on processing of external spatial cues, in addition of internal ones, for solving the TOM task.
Although most of our previous work has shown decreased flow in the thalamus in schizophrenia, this is the first study to show an increase, located in the pulvinar. This thalamic nucleus receives visual information from the superior colliculus and is involved in spatial processing.85,89
We have previously described a neural circuit that involves the paracingulate area, the anterior temporal and retrosplenial cingula, as well as the cerebellum in healthy controls conducting a TOM task.30 This study adds more evidence for an abnormal pattern of brain activation in schizophrenia, specifically in the CCTCC circuitry as shown in our previous studies.47,48 It reveals that patients tend to recruit their right hemisphere more extensively than controls on a highly verbal task, forcing the question of impaired brain lateralization in schizophrenia or poor efficiency of cognitive processes requiring excessive use of brain resources. On the other hand, it shows that patients might be using a different strategy in solving the task, failing to recruit the memory areas in the temporal lobe and relying, instead, more heavily on a neural network involved mainly in processing external cues and information and including the right frontal areas, the right parietal lobules, the anterior insula, and the pulvinar. Bowler16 argued that patients with mild pervasive developmental disorder and possibly those with schizophrenia rely on their relatively unimpaired cognitive skills to circumvent their lack of intuitive knowledge of social behavior, ie, the concept of logico-affective state. It is possible that the larger and bilateral neural circuit activated by patients with schizophrenia is a reflection of the more extensive “logical” processing rather than the “social” one. It might be that they need to rely more heavily on information from the outside world to solve such a mental task.
Funding
University of Iowa (MH40856, MH60990, MH19113, and MHCRC43271).
Acknowledgments
The present study was performed at the University of Iowa, Iowa City, IA, USA. Part of this work has been presented at the 2003 International Congress on Schizophrenia Research in Colorado Springs, CO, USA.
References
- 1.Bleuler E. Dementia Preacox or the Group of Schizophrenias. Madison, CT: International Universities Press, Inc; 1950. [Google Scholar]
- 2.Dennett DC. Beliefs about beliefs. Behav Brain Sci. 1978;1(4):568–569. [Google Scholar]
- 3.Frith CD, Frith U. Interacting minds—a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- 4.Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1(4):515–526. [Google Scholar]
- 5.Wimmer H, Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition. 1983;13(103–128) doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 6.Bloom P, German TP. Two reasons to abandon the false belief task as a test of theory of mind. Cognition. 2000;77(1):B25–B31. doi: 10.1016/s0010-0277(00)00096-2. [DOI] [PubMed] [Google Scholar]
- 7.Courtin C. The impact of sign language on the cognitive development of deaf children: the case of theory of mind. J Deaf Stud Deaf Educ. 2000;5:266–276. doi: 10.1093/deafed/5.3.266. [DOI] [PubMed] [Google Scholar]
- 8.Gray CD, Hosie JA. Deafness, story understanding, and theory of mind. J Deaf Stud Deaf Educ. 1996;1:217–233. doi: 10.1093/oxfordjournals.deafed.a014298. [DOI] [PubMed] [Google Scholar]
- 9.Marschark M, Green V, Hindmarsh G, Walker S. Understanding theory of mind in children who are deaf. J Child Psychol Psychiatry. 2000;41(8):1067–1073. [PubMed] [Google Scholar]
- 10.Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: the truth about false belief. Child Dev. 2001;72(3):655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- 11.Wellman HM, Cross D. Theory of mind and conceptual change. Child Dev. 2001a;72(3):702–707. doi: 10.1111/1467-8624.00309. [DOI] [PubMed] [Google Scholar]
- 12.Bartsch K, Wellman HM. Children Talk About the Mind. New York: Oxford University Press; 1995. [Google Scholar]
- 13.Moore C, Furrow D, Chiasson L, Patriquin M. Developmental relationships between production and comprehension of mental terms. First Lang. 1994;14:1–17. [Google Scholar]
- 14.Shatz M, Wellman HM, Silber S. The acquisition of mental verbs: a systematic investigation of the first reference to mental state. Cognition. 1983;14(3):301–321. doi: 10.1016/0010-0277(83)90008-2. [DOI] [PubMed] [Google Scholar]
- 15.Wellman HM. The Child's Theory of Mind. Cambridge: MIT Press; 1990. [Google Scholar]
- 16.Bowler DM. “Theory of mind” in Asperger's syndrome. J Child Psychol Psychiatry. 1992;33(5):877–893. doi: 10.1111/j.1469-7610.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 17.Corcoran R, Cahill C, Frith CD. The appreciation of visual jokes in people with schizophrenia: a study of ‘mentalizing’ ability. Schizophr Res. 1997;24(3):319–327. doi: 10.1016/s0920-9964(96)00117-x. [DOI] [PubMed] [Google Scholar]
- 18.Corcoran R, Mercer G, Frith CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res. 1995;17(1):5–13. doi: 10.1016/0920-9964(95)00024-g. [DOI] [PubMed] [Google Scholar]
- 19.Frith CD, Corcoran R. Exploring ‘theory of mind’ in people with schizophrenia. Psychol Med. 1996;26(3):521–530. doi: 10.1017/s0033291700035601. [DOI] [PubMed] [Google Scholar]
- 20.Pickup GJ, Frith CD. Theory of mind impairments in schizophrenia: symptomatology, severity and specificity. Psychol Med. 2001;31(2):207–220. doi: 10.1017/s0033291701003385. [DOI] [PubMed] [Google Scholar]
- 21.Doody GA, Gotz M, Johnstone EC, Frith CD, Owens DG. Theory of mind and psychoses. Psychol Med. 1998;28(2):397–405. doi: 10.1017/s003329179700648x. [DOI] [PubMed] [Google Scholar]
- 22.Drury VM, Robinson EJ, Birchwood M. ‘Theory of mind’ skills during an acute episode of psychosis and following recovery. Psychol Med. 1998;28(5):1101–1112. doi: 10.1017/s0033291798006850. [DOI] [PubMed] [Google Scholar]
- 23.Sarfati Y, Hardy-Bayle MC. How do people with schizophrenia explain the behaviour of others? A study of theory of mind and its relationship to thought and speech disorganization in schizophrenia. Psychol Med. 1999;29(3):613–620. doi: 10.1017/s0033291799008326. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg I, Guazzelli M. Schizophrenia—a disorder of the corollary discharge systems that integrate the motor systems of thought with the sensory systems of consciousness. Br J Psychiatry. 1999;174:196–204. doi: 10.1192/bjp.174.3.196. [DOI] [PubMed] [Google Scholar]
- 25.Frith CD. The Cognitive Neuropsychology of Schizophrenia. Erlbaum (UK): Taylor & Francis Publishers; 1992. [Google Scholar]
- 26.Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157(12):2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- 27.Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41(12):1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen NC, Flaum M, Arndt S. The Comprehensive Assessment of Symptoms and History (CASH). An instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry. 1992a;49(8):615–623. doi: 10.1001/archpsyc.1992.01820080023004. [DOI] [PubMed] [Google Scholar]
- 29.Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry. 1982;39(7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- 30.Calarge C, Andreasen NC, O'Leary DS. Visualizing how one brain understands another: a PET study of theory of mind. Am J Psychiatry. 2003;160(11):1954–1964. doi: 10.1176/appi.ajp.160.11.1954. [DOI] [PubMed] [Google Scholar]
- 31.Hurtig RR, Hichwa RD, O'Leary DS, et al. Effects of timing and duration of cognitive activation in [15O]water PET studies. J Cereb Blood Flow Metab. 1994;14(3):423–430. doi: 10.1038/jcbfm.1994.53. [DOI] [PubMed] [Google Scholar]
- 32.Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24(9):790–798. [PubMed] [Google Scholar]
- 33.Andreasen NC, Arndt S, Swayze V, II, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266(5183):294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 34.Andreasen NC, Cizadlo T, Harris G, et al. Voxel processing techniques for the antemortem study of neuroanatomy and neuropathology using magnetic resonance imaging. J Neuropsychiatry Clin Neurosci. 1993;5(2):121–130. doi: 10.1176/jnp.5.2.121. [DOI] [PubMed] [Google Scholar]
- 35.Andreasen NC, Cohen G, Harris G, et al. Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4(2):125–133. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 36.Magnotta VA, Heckel D, Andreasen NC, et al. Measurement of brain structures with artificial neural networks: two- and three-dimensional applications. Radiology. 1999;211(3):781–790. doi: 10.1148/radiology.211.3.r99ma07781. [DOI] [PubMed] [Google Scholar]
- 37.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 38.Cizadlo T. Image registration issues in the analysis of multiple-injection 150H20 PET studies: BRAINFIT. Proc SPIE—Int Soc Optical Engineer. 1994;2168:423–430. [Google Scholar]
- 39.Andreasen NC, Arndt S, Cizadlo T, et al. Sample size and statistical power in [15O]H2O studies of human cognition. J Cereb Blood Flow Metab. 1996;16(5):804–16. doi: 10.1097/00004647-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Arndt S, Cizadlo T, Andreasen NC, Heckel D, Gold S, O'Leary DS. Tests for comparing images based on randomization and permutation methods. J Cereb Blood Flow Metab. 1996;16(6):1271–1279. doi: 10.1097/00004647-199611000-00023. [DOI] [PubMed] [Google Scholar]
- 41.Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16(1):7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12(6):900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 43.Mazza M, De Risio A, Surian L, Roncone R, Casacchia M. Selective impairments of theory of mind in people with schizophrenia. Schizophr Res. 2001;47(2–3):299–308. doi: 10.1016/s0920-9964(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 44.McCabe R, Leudar I, Antaki C. Do people with schizophrenia display theory of mind deficits in clinical interactions? Psychol Med. 2004;34:401–412. doi: 10.1017/s0033291703001338. [DOI] [PubMed] [Google Scholar]
- 45.Brune M. Theory of mind and the role of IQ in chronic disorganized schizophrenia. Schizophr Res. 2003;60(1):57–64. doi: 10.1016/s0920-9964(02)00162-7. [DOI] [PubMed] [Google Scholar]
- 46.Frith CD. Schizophrenia and theory of mind. Psychol Med. 2004;34:385–389. doi: 10.1017/s0033291703001326. [DOI] [PubMed] [Google Scholar]
- 47.Andreasen NC. A unitary model of schizophrenia: Bleuler's “fragmented phrene” as schizencephaly. Arch Gen Psychiatry. 1999;56(9):781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 48.Andreasen NC, O'Leary DS, Cizadlo T, et al. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proc Natl Acad Sci USA. 1996;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–330. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 50.Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359(3):490–506. doi: 10.1002/cne.903590310. [DOI] [PubMed] [Google Scholar]
- 51.Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 52.Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154(12):1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- 53.Nordahl TE, Carter CS, Salo RE, et al. Anterior cingulate metabolism correlates with stroop errors in paranoid schizophrenia patients. Neuropsychopharmacology. 2001;25(1):139–148. doi: 10.1016/S0893-133X(00)00239-6. [DOI] [PubMed] [Google Scholar]
- 54.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 55.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 56.Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by positron emission tomography. 2. Observation compared with imagination. Exp Brain Res. 1996;112(1):103–111. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- 57.Rizzolatti G, Arbib MA. Language within our grasp. Trends Neurosci. 1998;21(5):188–194. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- 58.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 59.Rizzolatti G, Fadiga L, Matelli M, et al. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996;111(2):246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- 60.Gallese V, Goldman A. Mirror neurons and the simulation theory of mind-reading. Trends Cogn Sci. 1998;2(12):493–501. doi: 10.1016/s1364-6613(98)01262-5. [DOI] [PubMed] [Google Scholar]
- 61.Mesulam MM, Mufson EJ. Insula of the old world monkey. I. Architectonics in the insulo-orbito-temporal component of the paralimbic brain. J Comp Neurol. 1982;212(1):1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 62.Mesulam, MM, Mufson EJ Insula of the old world monkey. III: efferent cortical output and comments on function. J Comp Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 63.Fernandez G, Weyerts H, Schrader-Bolsche M, et al. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci. 1998;18(5):1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crespo-Facorro B, Paradiso S, Andreasen NC, et al. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- 65.Brunet E, Sarfati Y, Hardy-Bayle MC, Decety J. A PET investigation of the attribution of intentions with a nonverbal task. Neuroimage. 2000;11(2):157–166. doi: 10.1006/nimg.1999.0525. [DOI] [PubMed] [Google Scholar]
- 66.Stuss DT, Gallup GG, Jr., Alexander MP. The frontal lobes are necessary for “theory of mind”. Brain. 2001;124(pt 2):279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- 67.Winner E, Brownell H, Happe F, Blum A, Pincus D. Distinguishing lies from jokes: theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain Lang. 1998;62(1):89–106. doi: 10.1006/brln.1997.1889. [DOI] [PubMed] [Google Scholar]
- 68.Callicott JH, Bertolino A, Mattay VS, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- 69.Callicott JH, Mattay VS, Bertolino A, et al. Physiological characteristics of capacity constraints in working memory as revealed by functional MRI. Cereb Cortex. 1999;9(1):20–26. doi: 10.1093/cercor/9.1.20. [DOI] [PubMed] [Google Scholar]
- 70.Curtis VA, Bullmore ET, Morris RG, et al. Attenuated frontal activation in schizophrenia may be task dependent. Schizophr Res. 1999;37(1):35–44. doi: 10.1016/s0920-9964(98)00141-8. [DOI] [PubMed] [Google Scholar]
- 71.Goldberg TE, Berman KF, Fleming K, et al. Prefrontal cortical physiology: a PET rCBF study. Neuroimage. 1998;7(4):296–303. doi: 10.1006/nimg.1998.0338. [DOI] [PubMed] [Google Scholar]
- 72.Manoach DS, Gollub RL, Benson ES, et al. Schizophrenic subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry. 2000;48(2):99–109. doi: 10.1016/s0006-3223(00)00227-4. [DOI] [PubMed] [Google Scholar]
- 73.Manoach DS, Press DZ, Thangaraj V, et al. Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry. 1999;45(9):1128–1137. doi: 10.1016/s0006-3223(98)00318-7. [DOI] [PubMed] [Google Scholar]
- 74.Spence SA, Liddle PF, Stefan MD, et al. Functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. Focal dysfunction and distributed disconnectivity reappraised. Br J Psychiatry. 2000;176:52–60. doi: 10.1192/bjp.176.1.52. [DOI] [PubMed] [Google Scholar]
- 75.Stevens AA, Goldman-Rakic PS, Gore JC, Fulbright RK, Wexler BE. Cortical dysfunction in schizophrenia during auditory word and tone working memory demonstrated by functional magnetic resonance imaging. Arch Gen Psychiatry. 1998;55(12):1097–1103. doi: 10.1001/archpsyc.55.12.1097. [DOI] [PubMed] [Google Scholar]
- 76.Weinberger DR, Egan MF, Bertolino A, et al. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 77.Jeannerod M. Neural simulation of action: a unifying mechanism for motor cognition. Neuroimage. 2001;14(1 pt 2):S103–S9. doi: 10.1006/nimg.2001.0832. [DOI] [PubMed] [Google Scholar]
- 78.Jonides J, Smith EE. The architecture of working memory. In: Rugg MD, editor. Cognitive Neuroscience. Cambridge, MA: The MIT Press; 1997. pp. 243–276. [Google Scholar]
- 79.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20(8):339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 80.Crow TJ. Invited commentary on: functional anatomy of verbal fluency in people with schizophrenia and those at genetic risk. The genetics of asymmetry and psychosis. Br J Psychiatry. 2000;176:61–63. doi: 10.1192/bjp.176.1.61. [DOI] [PubMed] [Google Scholar]
- 81.Crow TJ. A Darwinian approach to the origins of psychosis. Br J Psychiatry. 1995;167(1):12–25. doi: 10.1192/bjp.167.1.12. [DOI] [PubMed] [Google Scholar]
- 82.Crow TJ. Schizophrenia as the price that homo sapiens pays for language: a resolution of the central paradox in the origin of the species. Brain Res Brain Res Rev. 2000;31(2–3):118–129. doi: 10.1016/s0165-0173(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 83.Freund HJ. The parietal lobe as a sensorimotor interface: a perspective from clinical and neuroimaging data. Neuroimage. 2001;14(1 pt 2):S142–S146. doi: 10.1006/nimg.2001.0863. [DOI] [PubMed] [Google Scholar]
- 84.Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14(1 pt 2):S27–S32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- 85.Chelazzi L, Corbetta M. Cortical mechanisms of visuospatial attention in the primate brain. In: Gazzaniga M, editor. The New Cognitive Neurosciences. Cambridge, MA: The MIT Press; 2000. pp. 667–686. [Google Scholar]
- 86.Luck SJ, Hillyard SA. The operation of selective attention at multiple stages of processing: evidence from human and monkey electrophysiology. In: Gazzaniga M, editor. The New Cognitive Neurosciences. Cambridge, MA: The MIT Press; 2000. pp. 687–700. [Google Scholar]
- 87.Vallar G. Extrapersonal visual unilateral spatial neglect and its neuroanatomy. Neuroimage. 2001;14(1 pt 2):S52–S58. doi: 10.1006/nimg.2001.0822. [DOI] [PubMed] [Google Scholar]
- 88.Sirigu A, Daprati E, Pradat-Diehl P, Franck N, Jeannerod M. Perception of self-generated movement following left parietal lesion. Brain. 1999;122(pt 10):1867–1874. doi: 10.1093/brain/122.10.1867. [DOI] [PubMed] [Google Scholar]
- 89.Rafal RD, Posner MI. Deficits in human visual spatial attention following thalamic lesions. Proc Natl Acad Sci USA. 1987;84(20):7349–7353. doi: 10.1073/pnas.84.20.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]


