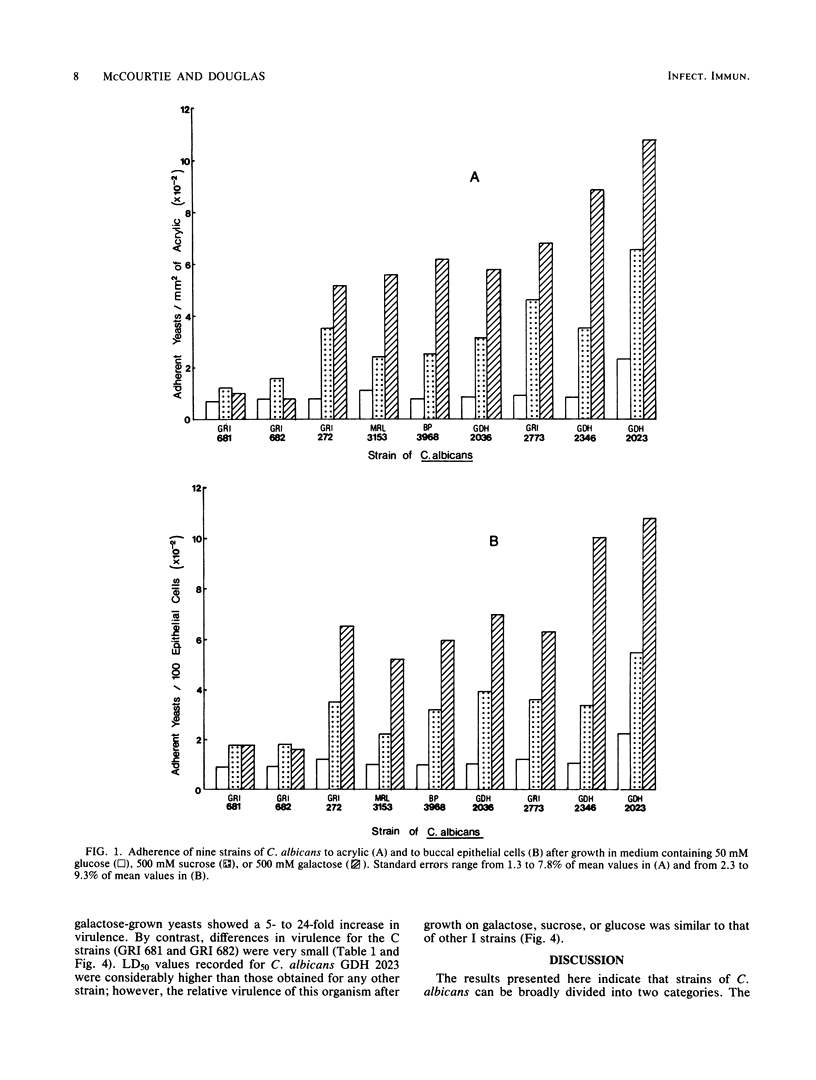
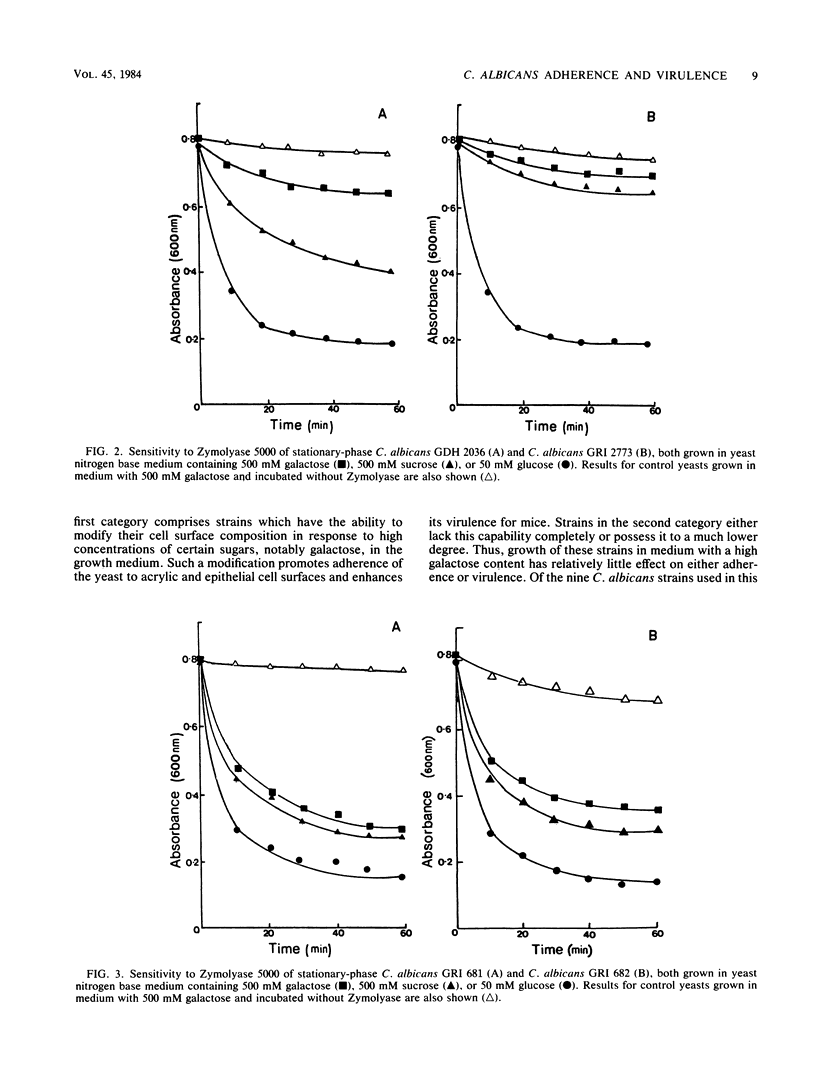
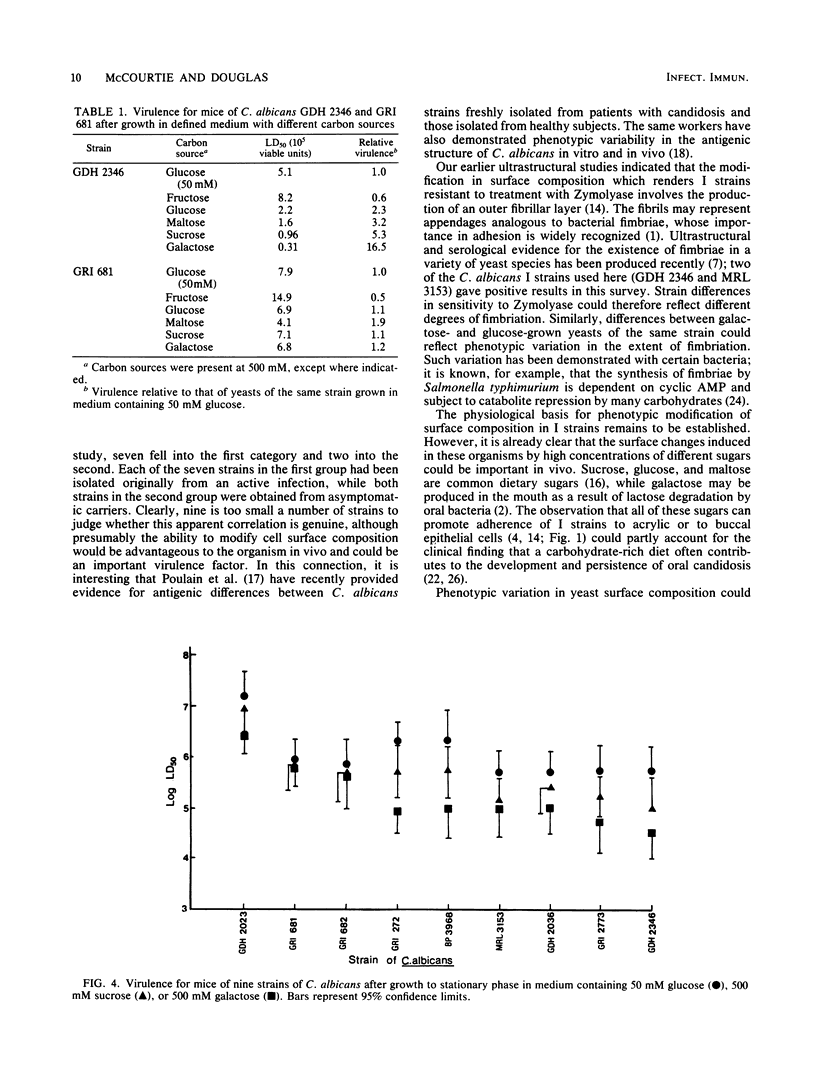
Abstract
A comparison was made of the adherence to acrylic and to human buccal epithelial cells of seven strains of Candida albicans isolated from active infections (I strains) and two strains obtained from asymptomatic carriers (C strains). After growth in defined medium containing a relatively low concentration (50 mM) of glucose as the carbon source, the adherence of I and C strains to either surface was similar and all strains were sensitive to spheroplast formation with Zymolyase 5000. Growth in medium containing a high concentration (500 mM) of sucrose or galactose enhanced the adherence of I strains up to 5- and 11-fold, respectively, and there were corresponding increases in resistance to spheroplast formation. Sucrose- or galactose-grown C strains showed only small increases in adherence and remained relatively sensitive to spheroplast formation. When inoculated intravenously into mice, I strains grown in 500 mM sucrose were up to five times more virulent than organisms grown in 50 mM glucose, while I strains grown in 500 mM galactose showed a 5- to 24-fold increase in virulence. Fifty percent lethal doses obtained for C strains were similar after growth on all three carbon sources. We conclude that I strains are able to modify their surface composition in response to high concentrations of certain sugars in the growth environment. Such modification can enhance both their ability to adhere to surfaces and their virulence. C strains lack this capability, or possess it to a lower degree, and may therefore have a lower pathogenic potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAUNCEY H. H., LIONETTI F., WINER R. A., LISANTI V. F. Enzymes of human saliva. I. The determination, distribution, and origin of whole saliva enzymes. J Dent Res. 1954 Jun;33(3):321–334. doi: 10.1177/00220345540330030501. [DOI] [PubMed] [Google Scholar]
- Hurley R. The pathogenic Candida species and diseases caused by candidas in man. Soc Appl Bacteriol Symp Ser. 1980;9:231–248. [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Adherence of Candida albicans to human buccal epithelial cells. Infect Immun. 1978 Jul;21(1):64–68. doi: 10.1128/iai.21.1.64-68.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., King R. D. Characterization of Candida albicans adherence to human vaginal epithelial cells in vitro. Infect Immun. 1983 Sep;41(3):1024–1030. doi: 10.1128/iai.41.3.1024-1030.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F., Odds F. C. Virulence for mice of a proteinase-secreting strain of Candida albicans and a proteinase-deficient mutant. J Gen Microbiol. 1983 Feb;129(2):431–438. doi: 10.1099/00221287-129-2-431. [DOI] [PubMed] [Google Scholar]
- McCourtie J., Douglas L. J. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun. 1981 Jun;32(3):1234–1241. doi: 10.1128/iai.32.3.1234-1241.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Lefebvre B., Husson M. O. Antigenic variability between Candida albicans blastospores isolated from healthy subjects and patients with Candida infection. Sabouraudia. 1982 Sep;20(3):173–177. [PubMed] [Google Scholar]
- Poulain D., Tronchin G., Vernes A., Popeye R., Biguet J. Antigenic variations of Candida albicans in vivo and in vitro--relationships between P antigens and serotypes. Sabouraudia. 1983 Jun;21(2):99–112. [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGOSA M., SHARPE M. E. Species differentiation of human vaginal lactobacilli. J Gen Microbiol. 1960 Aug;23:197–201. doi: 10.1099/00221287-23-1-197. [DOI] [PubMed] [Google Scholar]
- Richardson M. D., Smith H. Production of germ tubes by virulent and attenuated strains of Candida albicans. J Infect Dis. 1981 Dec;144(6):565–569. doi: 10.1093/infdis/144.6.565. [DOI] [PubMed] [Google Scholar]
- Richardson M. D., Smith H. Resistance of virulent and attenuated strains of Candida albicans to intracellular killing by human and mouse phagocytes. J Infect Dis. 1981 Dec;144(6):557–564. doi: 10.1093/infdis/144.6.557. [DOI] [PubMed] [Google Scholar]
- Ritchie G. M., Fletcher A. M., Main D. M., Prophet A. S. The etiology, exfoliative cytology, and treatment of denture stomatitis. J Prosthet Dent. 1969 Aug;22(2):185–200. doi: 10.1016/0022-3913(69)90246-7. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Jr, Schmidt M. R., Leibowitz M. Cyclic AMP-dependent synthesis of fimbriae in Salmonella typhimurium: effects of cya and pts mutations. J Bacteriol. 1978 Apr;134(1):356–358. doi: 10.1128/jb.134.1.356-358.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin R. L., Rogers A. L., Patterson R. J., Beneke E. S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982 Jan;35(1):79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Myers P. G., Kaye D., Levison M. E. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis. 1981 Jan;143(1):76–82. doi: 10.1093/infdis/143.1.76. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Bowie J. U., Gray G. R., Nelson R. D. Candidacidal activity of myeloperoxidase: mechanisms of inhibitory influence of soluble cell wall mannan. Infect Immun. 1983 Oct;42(1):76–80. doi: 10.1128/iai.42.1.76-80.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



