Abstract
The evolutionary origins of one of the most dramatic and seemingly deleterious behavioral phenotypes, the syndrome known as schizophrenia, are mysterious. Schizophrenia occurs in all cultures and is inherited. Although most phenotypes are said to be “selected for” based on adaptive qualities, it is difficult to understand how the genetic basis of schizophrenia could have operated under a similar framework. This has lead several theorists analyzing the proposed evolutionary origins of other disease states to that of schizophrenia. To date, several models have been applied. We have tried to conceptualize schizophrenia in a compensatory advantage framework whereby incomplete penetrance of the full disorder, or alternatively, the inheritance of risk alleles insufficient in number to manifest as the classic clinical syndrome, may manifest as a behavioral phenotype with adaptive advantages (eg, creative behavior or novel illuminating ideas). The idea that even full penetrance can also be advantageous has been offered as applied to religious experience and ancient social standing, but is unlikely. Can complex behavioral phenotypes such as schizophrenia, and particularly those that seem purely deleterious, be explained by mechanisms of Darwinian psychiatry? Can models from other disease classes be applied successfully to schizophrenia? Such ideas have generated intense speculation, but often in the absence of testable models. In this article, we will examine some of these proposed ideas and offer suggestions for future research.
Keywords: endophenotype, psychosis, risk genes, spandrel, exaptation, neurodevelopment
Introduction
Based on twin and adoption studies, “schizophrenia” is an undoubtedly genetic disorder1 that seems to exist in all cultures.2 It affects around 1% of people worldwide, although the universality of population prevalence statistics has been questioned recently.3,4 Based both on evidence from both epidemiologic studies and from discordant identical twin data, there are significant environmental risks for the disorder5 that may exert effects on early brain development (eg, maternal viral infections during pregnancy)6,7 or operate during adolescence (eg, cannabis smoking).8,9 Such environmental factors may well interact with genetic risk in ways that recent studies have begun to elucidate.10
Although it is possible that the disorder may represent an agglomeration of diseases caused by multiple, rare, individual mutations, schizophrenia is more likely a complex, multigene trait, with common risk alleles in the general population that may have relatively weak individual effects, be pleiotropic, and interact with each other multiplicatively. No single such allele is either necessary or sufficient for development of the full disorder.11 Several plausible candidate schizophrenia vulnerability genes have been identified; however, it is unclear how much of the attributable risk for schizophrenia is contributed by each, and to date, no candidate gene has been conclusively linked to schizophrenia (see recent reviews12–14).
Investigations have used linkage analysis and the quantitative trait locus (QTL) approaches to find nucleotide sequences that are related to phenotypes or are in linkage disequilibrium. Identified loci have been replicated in some studies; however, others have failed to replicate the association to schizophrenia or convey a more general risk for both schizophrenia and psychotic bipolar disorder. Promising chromosomal regions surviving scientific replication include 1q, 8p, 6p, 22q and 13q, including sequences that code for neuregulin 1 (NRG1), dysbindin (DTNBP1), catechol-O-methyltransferase (COMT), D-amino acid oxidase activator (DAOA), D-amino acid oxidase (DAO), translin-associated factor X/disrupted in schizophrenia 1 (TRAX/DISC1), V-AKT murine thymoma viral oncogene homolog 1 (AKT1), glutamate receptor metabotropic 3 (GRM3), and regulator of G-protein signaling 4 (RGS4), although emerging studies have identified additional possible loci that await further replication. It should be noted that even in the most compelling studies, the effect sizes are comparatively small, resulting in the current view that the total susceptibility effect arises from aggregation of small individual effects.
How Do the Genes Operate?
Because the basic physiological abnormalities that underlie clinical schizophrenia are not yet well understood, a properly integrated etiologic and pathophysiologic model does not yet exist. One possibility, however, is that schizophrenia risk alleles may operate analogously to genetic risk in amyloid cascade models of Alzheimer's disease, a disorder with a characteristic neuropathology, whose pathophysiology is better elucidated than that of schizophrenia. Despite the obvious difference that Alzheimer disease is neurodegenerative in nature and is typically first diagnosed in late life, whereas schizophrenia is primarily neurodevelopmental and most commonly first diagnosed in late adolescence, alterations in metabolic pathways in both disorders can presumably lead to pathological processes that begin early in life but do not necessarily manifest until considerably later. Using Alzheimer disease as an example, current hypotheses suggest that altered function at a series of several possible molecular bottlenecks can result in either amyloid overproduction or deficient amyloid removal, leading to a final common outcome of amyloid overaccumulation.15 This results in secondary neuronal damage, ultimately ushering in the emergence of the clinical disorder.
To extend the analogy further, for Alzheimer disease, there are several rare dominant mutations that could correspond to the DISC1 mutation associated with schizophrenia seen in the original Scottish pedigree16 and chromosomal-based partial deletions or replications, affecting the function of multiple genes, where increased risk of schizophrenia in velocardiofacial syndrome could be considered analogous to the increased risk of Alzheimer's disease in Down syndrome. However, in most cases of late-life–occurring “plain vanilla,” Alzheimer's disease remains cryptic with regard to susceptibility alleles. In our analogy, as is presumably occurring in the majority of cases of schizophrenia, this common type of Alzheimer's disease likely results from unfavorable combinations of individually low-risk gene variants influencing amyloid overaccumulation. In the case of schizophrenia, using knowledge of the molecular biology of identified risk genes to locate the relevant affected biological final common pathways in those cerebral cells exhibiting the greatest vulnerability to the collective mutational load would substantially advance our understanding of the pathogenesis of the disorder.
Current evidence suggests that in a general sense, schizophrenia risk genes likely act by impacting on neurodevelopmental mechanisms that play out subsequently as inefficient or disturbed neuronal communication. Such neurodevelopmental errors or normal single nucleotide polymorphisms (SNPs), copy number variants (CNVs), or insertions/deletions (indels) may disrupt or merely vary single or multiple metabolic or cellular processes; “different perturbations (eg, mutations) may ultimately have common outcomes.”17 Several major processes are implicated by schizophrenia risk genes identified to date. Some of these have been selected as examples in table 1.
Table 1.
Examples of Schizophrenia Risk Genes and Their Proposed Mechanism of Action in the Disorder
| Coding Gene | Biological Mechanism | Functional Significance | Pathophysiologic Mechanism |
| NRG1 (8p12) | ErbB4 receptor is a postsynaptic target of NRG NRG1/erbB4 signaling perturbation | May directly affect neuronal communication and have downstream neurodevelopmental consequences through signaling variations | Interfering with activity-dependent maturation and plasticity of excitatory synaptic structure and function at glutamatergic synapses, it can lead to loss of synaptic NMDA currents and thus to glutamatergic hypofunction18 may affect disrupted neural communication in schizophrenia directly through axon guidance and myelination19 |
| DTNBP1 (6p22.3) | Protein expressed in the human central nervous system pre- and postsynaptic vesicles, likely affecting glutamatergic transmission vesicles, and in microtubules where it binds snapin20; disrupted DA/NMDA signaling21,22 | Individuals with schizophrenia express less of the protein in dorsolateral prefrontal cortex23 and in hippocampus24 | Through altered glutamate neurotransmission or through structural changes that may affect the organization of cerebral dendrite fields25 |
| COMT (22q11) | Enzyme involved in catecholamine metabolism results in a 4-fold increase (high activity) in the enzymatic breakdown of DA effectively translating to lower DA levels in COMT valine (val) carriers compared with methionine (met) allele carriers (low activity). | Particularly important in the prefrontal cortex where there are fewer DA transporters26,27 | Schizophrenia patients with the val/val genotype perform especially poorly on working memory tasks associated with abnormal cortical connectivity28a |
| CHRNA-7 (15q13-q14) | Alpha-7 subunit of the nicotinic acetylcholine receptor, associated with decreased function and/or expression of the nicotine alpha receptor gene29 | Predominantly through nicotine acetylcholine receptors improves cognition, visual attention, and memory30–32 | Schizophrenia patients may be self-medicating by using tobacco, whose nicotine content ameliorates specific cognitive and physiologic abnormalities in the disorder |
Note: NRG1, neuregulin 1; NMDA, N-methyl-D-aspartic acid; DA, dopamine; CHRNA-7, nicotinic cholinergic receptor, alpha polypeptide 7; COMT, catechol-O-methyltransferase; DTNBP1, dysbindin;
More recent evidence reviewed in 33 has indicated that the relationship between COMT, brain function, and pathogenesis of schizophrenia is complex, but still worthy of continued study as a schizophrenia susceptibility gene. Bilder et al. 34 have suggested that this complex relationship may be better understood by a tonic-phasic dopamine explanation where the met allele is associated with increased tonic and decreased phasic dopamine subcortically with increased D1 in cortical regions resulting in increased stability with decreased flexibility of neural systems supported by these networks and in executive function.
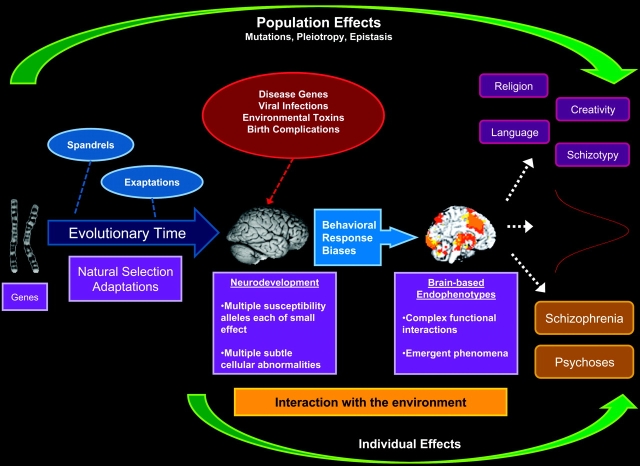
These examples of schizophrenia susceptibility genes found through familial aggregation and QTL studies are representative examples of the polygenic nature of schizophrenia transmission. The current view is that many of these genes, themselves of small individual effect, can aggregate by chance, assortative mating, or by other mechanisms to constitute increasing risk for schizophrenia.35 Thus, these genes may affect changes in attention, memory, language, or other cognitive functions through small effects on neurotransmitter function, cerebral structural organization, brain metabolism, or connectivity as they interact with nongenetic factors. The context under which we discuss the Darwinian mechanisms that propagate schizophrenia (figure 1) is not a Mendelian model but a more complicated polygenic emergent syndrome that may operate through Darwinian mechanisms of inheritance, variation, and selection.
Fig. 1.
Schizophrenia in an Evolutionary Framework. Schizophrenia is a disorder caused, in part, by multiple susceptibility alleles, each of small effect. The cumulative effect of evolutionary selection, mutations, and by-products that deposit liability on the population may result in observable traits, behaviors, and cognitive abilities that are distributed across and within individuals. Multiple individual genetic susceptibilities in the form of subtle cellular abnormalities interact with the environment through behavioral response biases, sometimes leading to positive and negative symptoms, cognitive distortions, or in combination, the overt clinical manifestation of the disorder.
What Kind of Inheritance Explains the Observed Patterns?
One possibility is that what is inherited is not schizophrenia as such but a highly unfavorable combination of singly occurring minor CNV- or SNP-driven “tweaks” or even minor variations in molecular efficiency within the normal range. These would not necessarily be sufficiently disadvantageous to be selected against individually; they may be effectively neutral or even confer minor benefits. Thus, susceptibility alleles may persist because they are “below the radar” of selection. These are not “dysfunctions” but components of normal variation and are retained presumably because they have utility under particular circumstances or in particular environments. However, if a sufficiently large number of such microlevel unfavorable variants in a particular individual all contribute unfavorably to a single molecular bottleneck process, then this may provide a mechanism for epistasis, as we argued earlier, similar to that hypothesized for the better understood, previously noted exemplar of Alzheimer disease. Random combinations of relatively common neutral or even beneficial genetically controlled traits may, only in epistatic combination, significantly increase disease risk.
In this way, the commonly accepted Diagnostic and Statistical Manual of Mental Disorders categorical disease perspective for schizophrenia collides with the continuous trait perspective, eg, schizophrenia vs schizotypal personality disorder. Schizotypy36,37 describes a constellation of traits that is both phenomenologically and genetically related to schizophrenia where increased schizotypal traits represent a latent liability for schizophrenia.38–41 Most factor analytic and clinical conceptualizations of this constellation of traits support a tridimensional model of schizotypy: traits that cluster along the positive, negative, and disorganized symptoms of schizophrenia.42 Conceptualizing psychiatric disorders or conditions of pathology as dimensional or as categorical can have important evolutionary implications. The dimensional approach applies a continuum model to traits that are seen on an individual level. The categorical model, in its strictest sense, describes traits or conditions as biological taxa. These ideas have been developed, eg, in the competing models expressed by Meehl and Eysenck. Meehl's categorical model describes a bimodal population distribution of 2 taxa, those with either schizophrenia or schizotypy and those without.37 Thus, those in the nonaffected taxon do not carry vulnerability for any degree of the disorder. Eysenck36 has proposed that these traits, or vulnerabilities, are distributed throughout the population such that each individual can be measured as having few or many of the traits, with the extreme tails of the distribution describing those without effect to those with an extreme phenotype (eg, schizophrenia). Those falling outside of the extreme case would be said to have few odd, or schizotypal traits, to many, but do not manifest the clinical disease state.
Expressed another way, susceptibility alleles for schizophrenia may be like individual cards in a randomly dealt hand; only the combination as a whole is extremely unfavorable. This can also be conceptualized as a “reverse slot machine” model, where a single allelic “cherry” contributes (along with other gene variants) to such ordinarily occult endophenotypic features as reduced P300 amplitude, poor oculomotor tracking, or (more speculatively) to positive putative endophenotypes such as enhanced creativity; 2 “cherries” contribute to schizotypy; and several to overt clinical schizophrenia. Schizophrenia susceptibility alleles may thus be individually associated with normal or increased fertility or be operating under positive selection, unlike the actual full-fledged clinical disorders. Additionally, carriers of small numbers of schizophrenia susceptibility genes are manyfold more numerous than cases of the disorder, and thus, their putative adaptive advantages may well overshadow the reproductive disadvantage of the latter. A commonly cited example is the above-average computational ability accompanied by reduced social skills in first-degree relatives of individuals with autism; the corresponding advantages selected for in first-degree relatives of schizophrenia patients are more elusive but have been suggested to include creativity.43
Campbell et al.44 conjecture that an individual allele may manifest as a sub-threshold abnormality, eg an endophenotype such as impaired immediate memory or decreased efficiency of dorsolateral prefrontal cortex26,45,46, which though harmful, is neither necessary nor sufficient for disorder. Expressed together, clusters of such endophenotypes, however, may cause complex conditions like schizophrenia. Particular endophenotypes might also be clinically useful in determining the choice of pharmacotherapy.47 This assumption of endophenotypes within the polygenetic mutation-selection balance model thus offers something additional that the traditional balancing selection account of schizophrenia48,49 would seem not to. Indeed, the correspondence of endophenotypes of a disorder to susceptibility alleles in subisolate populations could also offer clues to the endophenotypic structure of the disorder in more heterogeneous populations.
Adoption of such a model results in the reconceptualization of schizophrenia at several levels. First, a straightforward, binary disease/nondisease dichotomy yields to a more complex continuum with intermediate stages between disease and nondisease including schizoid and schizotypal personality disorder in addition to prodromal states, as argued recently.50 Supporting this hypothesis, recent observations from van Os et al51,52 of individuals without clinically diagnosable schizophrenia who nonetheless experience what appear to be auditory hallucinations unaccompanied by delusions, formal thought disorder, or deterioration in function may represent additional examples of such intermediate stages. Currently, schizoid and schizotypal behavior constellations are conceptualized as “personality disorders” in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition classification, rather than as less severe forms of schizophrenia, despite their genetic relationship,53 a point on which we expand below. Second, as summarized by Allen and Badcock,54 analysis at an evolutionary genetic level should focus less on overt clinical psychiatric disorders and more on genetic factors contributing to susceptibility to the disorders. Thus, what is most appropriately studied by researchers is those genes that result in meaningful variation in polygenetic traits and biologically how such variation may lead to individual differences in the functional efficiency of more complex psychobiological mechanisms such as novelty detection or sensorimotor gating or at an even more complex level to personality traits.
We argue that this changed research focus lends itself more readily to hypothesis testing. In order to test such a model more explicitly, one would have to estimate the whole-population distribution of particular putative susceptibility alleles, their relationship to various endophenotypes (where one such allele may be associated with several endophenotypes or several alleles with single endophenotypes) as well as the population distribution of the endophenotypes themselves. Even if defined statistically, 15% of the population manifests at least one schizophrenia-associated endophenotypic abnormality. Intermediate phenotypes certainly suggest themselves as footholds for exploring larger-scale dysfunctions such as schizophrenia, by being theoretically simpler in structure than the complex overt clinical disorder, closer to the gene, more individually quantifiable, and more readily lending themselves to an interpretation of their function, without which comprehending dysfunction is extremely hard55 (although for a counterargument, see Flint and Munafò56). Of the 40 or so endophenotypic abnormalities identified in individuals with schizophrenia and their first-degree relatives, some could be seen in this light. For example, the diminished P300 event-related potential could be a manifestation of altered responsiveness to environmentally salient alterations in the immediate environment.
Physiologically, while endophenotypes such as ventricular enlargement may be understood as representing aberrant brain development, others may represent relatively mild reductions in neural organization within the normal spectrum. For example, deviant eye tracking, reduced short-term memory processing, or less effective sensory-motor integration may be better understood as such relatively minor reductions in the efficiency of neural processing as argued by Cannon and Keller.57
Several observations tend to bear out this hypothesis. A literature review of schizophrenia endophenotype studies (G. D. Pearlson, unpublished data) reveals typical population prevalence for single abnormal endophenotypes in healthy volunteers of 15%–20%. If these figures are correct, then depending on the underlying genetic architecture, random matings will presumably result in some offspring displaying several endophenotypic abnormalities. Many research groups report anecdotally that subjects that they recruit and ascertain as having schizotypal personality disorder do not have first-degree relatives affected with schizophrenia, again emphasizing the higher prevalence of “fewer cherry”–related traits in the general population. The Epidemiological Catchment Area study also identified a high number of subthreshold cases of schizoid, paranoid, and schizotypal individuals.58,59 Results from this study can be interpreted as pointing to the arbitrary nature of using defined threshold points in defining cases of these individuals, particularly when only interpreting prevalence in terms of one side of a skewed distribution that does not take into account additive traits that span the threshold point.
Gene-Environment Interactions
Could schizophrenia have been the psychiatric complement of a “thrifty gene”60 or of a gene that was advantageous in a particular ancestral environment but that may be disadvantageous or confer disease risk in a different environment? This hypothesis has evolved as we begin to understand the genetic factors underlying forms of physical illness and disease. The models that we will extend as examples include hypertension, ischemic cardiac disease, diabetes, and familial hypercholesterolemia.
Through a “broken gene” model of the cytochrome P-450 3A5 (CYP3A5)*1/*3 polymorphism, the mechanism of action resulting in salt-sensitive hypertension for some ethnic groups is a complex interaction between dietary sodium intake and genes that regulate salt retention.61 Thus, individuals who have come from populations closer to hot equatorial regions have a properly working gene that allows ample sodium excretion. The “broken” version of the gene has become more abundant in the human genome overall and thus has been identified as carrying some “protective advantage.” Thus, there is evidence for relatively common genetic variance that was protective during evolutionary epochs of scarcity (salt retention when sodium was not readily available) but that with progressive abundance confers susceptibility for disease (overretention leading to hypertension). In the case of ischemic cardiac disease, variability in individual intrinsic inflammatory factors influences disease risk.62 Thus, there is an underlying internal biological environment (inflammation) that interacts with external environmental factors (stress, dietary choices, lifestyle, and exercise). The factors that underlie this susceptibility are polygenetic, and both the candidate gene and thrifty gene approaches have been theorized to underline disease risk for ischemic heart disease.
For diabetes, it is not the “damaged” genes that give susceptibility for disease but the properly expressed and working ones that during times of scarcity aided metabolic processes.63 Thus, when food supplies were scarce, hormonal changes could result in insulin resistance, thereby creating a more effective means of fat storage. However, those individuals with a thrifty gene in a calorie-abundant industrialized society are susceptible to unnecessary fat storage and obesity. James Neel first described the thrifty gene hypothesis in 1962 to describe his observation that Pima Indians have encountered enhanced rates of diabetes and obesity when introduced to modern diets and lifestyles that interacted deleteriously with their genetic propensity to conserve fat stores in traditional agrarian environments.60 When the natural cycle of food availability alternated between conditions of feast and famine, this genetic framework was adaptive in metabolic regulation, but in states of constant environmental “feast,” the genetic propensity to prepare for previous inevitable famines leads toward obesity and diabetes.
Another mechanism of disease that requires the interplay of genes, general environment, and to a large extent culture is the founder effect, which has been implicated in familial hypercholesterolemia.64 This effect occurs when a small group of individuals travels from their indigenous geographical environment to a distal one, and they subsequently become relatively isolated in their new environment. New progeny from this “founder population” come from a relatively select gene pool and have high rates of both dominant and recessive diseases. The founder effect has been proposed for the noted high rates of familial hypercholesterolemia in French Canadians living in Quebec65 through mutations disrupting the function of the low-density lipoprotein receptor.
Comparing these mechanisms with schizophrenia is challenging. Schizophrenia is most likely the result of complex polygenic inheritance and environmental susceptibility factors. Thus, a constellation of incremental traits interacting with environmental situations would need to confer a degree of adaptability if a similar model could be applied. Polimeni and Reiss66,67 have suggested that, through group selection, schizophrenia and related phenotypes may have provided a level of behavioral specialization that grew to be culturally essential. Comparing schizophrenia with the role of the nonreproducing task specialists in a honey bee colony, they suggest that the role conferred by schizophrenia and related traits is advantageous culturally even in the context of reduced fecundity and increased mortality. Additionally, the issue is complicated by evidence for positive selection for particular gene variants in recent evolutionary history that may be ongoing. Microcephalin regulates brain size in humans. Evans et al.68 have shown that the microcephalin D allele was introduced relatively recently in evolutionary terms (approximately 37 000 years ago) through a single progenitor copy and that it has spread to approximately 70% of modern humans. Thus, microcephalin may provide an example of genetic variation provided by Neanderthals that may have influenced the modern human brain. Thus, the modern human brain may not entirely be the result of slow evolutionary processes, but acute variation introduced by another closely related species may have introduced genes responsible for its modern aspects. Accordingly, there is evidence for a high rate of evolution in the human lineage based on analyses of genes involved in various brain functions.
Why Do Schizophrenia Risk Genes Persist in Evolution?
Schizophrenia that typically begins in early adult life or adolescence is chronic and generally nonfatal. The disorder is generally highly disabling, affects employability and productivity, and tends to lead to social isolation, what Keller and Miller69 refer to as “the very embodiment of maladaptive traits.” People with schizophrenia are less likely to marry, and when they do so are less likely to have children. Thus, it is unclear why genes that confer susceptibility to schizophrenia (and other common, harmful, inherited mental disorders that appear to reduce severely reproductive fitness) are still maintained by natural selection, given that selection mechanisms are generally extremely efficient at optimizing complex adaptations and eliminating genetic variants (susceptibility alleles) predisposing to such severe maladaptive conditions. Since the presentation of the idea, the “schizophrenia paradox”70 efforts to explain the stable existence of schizophrenia in spite of reduced fecundity71 and increased mortality72 have relied upon evolutionary theory to suppose a compensatory advantage for the genes associated with schizophrenia.67,73,74
One of the bedrock arguments cited in support of balancing selection series of schizophrenia is the supposedly invariant incidence of schizophrenia worldwide, eg75. However, in the last few years, several epidemiologists have produced strong evidence of substantial population variance in schizophrenia incidence. In addition, there are marked variations in schizophrenia risk within populations, groups at increased risk including first- and second-generation immigrants, and individuals born and raised in cities. These factors suggest environmental interactions with schizophrenia risk genes. A different type of environmental interaction has been shown for cannabis use with the COMT val/met allele.10 Whatever the true population prevalence of “case level,” ie, overt clinical schizophrenia and irrespective of whether this figure varies among different population groups, the illness is relatively common relative to serious Mendelian disorders. Because a very high percent of all human protein-coding genes are expressed in the brain, this organ may be especially affected by accumulations of mutational variation. In terms of natural selection, Gould76 has theorized that the inherent variability in human brain gene expression may have allowed unintended occurrences (such as language, aesthetics, etc.) that were by-products of the genes driven by natural selection for an intended purpose.
Crow77 has proposed that susceptibility genes for schizophrenia are inevitable trade-offs for adaptations related to key species–related innovations in humans. For example, Crow has theorized that schizophrenia is the price that humans pay for acquiring language. According to Crow, a change occurred in how homologous genes on the X and Y chromosomes became expressed. Because much of the Y chromosome does not recombine during meiosis, genetic drift can occur more frequently on the Y chromosome, and advantageous genes may be seen more frequently in the population. Crow has theorized that one of these genes was involved in both the speciation event that defined modern Homo sapiens and in language ability, the 2 being inextricably involved. Crow48,78 has asserted that the origins of schizophrenia and language are linked through cerebral asymmetry: hemispheric dominance for language increases the need for bilateral communication and increased plasticity and flexibility, but a collateral side effect of failed hemispheric lateralization may be psychosis. Thus, Crow has proposed that language disturbance and thought disorder, as seen in schizophrenia, may be the result of incomplete hemispheric specialization; there is some evidence to suggest that individuals with schizophrenia, their relatives, and those with schizotypal personality traits have anomalous cerebral lateralization. The benefit of incomplete hemispheric specialization, however, could result in increased interhemispheric communication, associative processing, and ideational fluency and flexibility.
These processes are similar to divergent thinking and the cognitive bases of creative thinking, which has been offered as a compensatory advantage for schizophrenia. Like schizophrenia, “creativity,” although difficult to operationalize, is heritable, particularly in monozygotic twins,79,80 but unlike schizophrenia, it does not appear at high levels within families.81 This has been explained by emergenesis and has received some empirical support. Similar to schizophrenia, as an emergent trait, creativity requires the culmination and integration of several other lower level traits (as opposed to a simple additive model), and it is unlikely that these phenotypes would exist simultaneously within individuals in families given the significant variation that exists among family members.82 Furthermore, emergenesis also stipulates that the unique gene combinations that result in the expression of emergenic traits are highly heritable.83
Considering that the concordance rate for schizophrenia is significantly greater in monozygotic twins than in dizygotic twins, Scandinavian studies have sought to elucidate the genetic relationship between creativity and mental illness using retrospective analyses of birth and medical records, finding that close relatives of schizophrenic patients were more successful in scholarly and academic professions,84 and they were more likely to become successful in professions that emphasized art and scholarship (published authors, honors graduates, doctorates, professors, and clergymen) rather than leadership (parliamentarians, lawyers, physicians, and engineers). The relatives studied in these samples represented 1/20 of the total population in Iceland, but 1/10 of the honor students in writing and poetry, and these qualitative differences appeared to be equally related to family history of either schizophrenia or manic-depressive psychosis.85 Although this relationship between psychotic relatives and academics and authors was confirmed 16 years later, it was also found to be true of mathematicians and of general school performance.86 In fact, excellent school performance was retrospectively linked to developing schizophrenia in a Finnish cohort,87 providing evidence that overall intellectual ability, including creativity, may be associated with schizophrenia.88
However, evidence from the whole-population UK National Childhood Development Study (NCDS89) disagrees with this observation. Children in NCDS subsequently diagnosed with schizophrenia as adults tended to have persistent childhood and adolescent reading impairment and lower than predicted (from parental intelligence measures) IQ scores. Recently, Nettle90 has examined the relationship between different modalities of creative production in relation to schizotypy, finding that schizophrenia may be related to enhancement in art, poetry, and divergent thinking, while other forms of psychopathology may be related to mathematical creativity.
Evolutionary explanations of schizophrenia must weigh whether the schizophrenia phenotype has arisen through adaptive natural selection or if it was the by-product of other traits that were themselves adaptive. These indirect mechanisms are the concepts of evolutionary spandrels91 and exaptations.92 Spandrels are useful but unintended side effects of the adaptive genetic design (phenotypes that occur not by purposeful selection [adaptive function] but simply because the confluence of other purposeful gene combinations have created them out of their “negative space”). Mental disorders have been conceptualized as maladaptive spandrels. Wakefield93 has asserted that psychopathology is the harmful failure of naturally selected functions. Failed spandrels are not automatically disorders because the malfunction was not in an intended function but in a failed by-product. These mechanisms would only produce disorders when the failure is in an inevitable by-product of adaptive functions. However, Murphy and Woolfolk94 have proposed that mental disorders, as spandrels, can exist in the absence of “dysfunction” where they may be processes related to mismatches in environmental design, in spite of proper gene expression. Panksepp and Moskal95 have speculated that social evolution may have created cultural spandrels, allowing a unique role for individuals with schizophrenia as objects of fascination or entertainment, similar to the argument of shamanism.96
Another nonadaptive mechanism for schizophrenia is through an exaptation, occurring when traits shaped by natural selection are co-opted for a new use (eg, bird feathers became useful for catching insects and for thermoregulation). Although environmental change may drive evolutionary change, environmental processes must exert their effects on a preexisting genetic framework or the availability of genetic variation that exist during the environmental change.43 The previously mentioned relationship between schizophrenia and language can also be placed in the context of an exaptation because nonlanguage brain regions were likely co-opted for language in humans.97 Are art, language, commerce, and schizophrenia spandrels of the human brain, or is schizophrenia a secondary consequence of creativity, religion, and originality? Others have proposed that schizophrenia is a side effect of sexual selection (the opposite of attractive sexually adaptive traits) that has evolved socially to allow for complex courtship rituals reliant on unique language attributes.98
The evidence that schizophrenia is polygenic, arising from complex pleiotropic and epistatic combinations, leaves room to consider schizophrenia as an unplanned by-product of other adaptive functions. However, others have asserted99 that before disorders are conceptualized in terms of spandrels, exaptations, and constraints, they must first fail adaptionist explanations. As such, schizophrenia would have to fail explanation as an adaptation of a particular function in order to be considered an indirect by-product of human evolution, a result that is particularly reliant upon measured data compared with possible theoretical mechanisms. Initial empirical evidence has been described for recent positive selection in some of the putative schizophrenia liability genes (DISC1, DTNBP1, and NRG1) compared with genes expressed in general brain function100 and that the frequent PPP1R1B haplotype coding for DARPP-32 was associated with increased frontostriatal connectivity and cognitive performance in addition to schizophrenia risk.101 Thus, there is emerging evidence that schizophrenia may be a maladaptive secondary effect of adaptive evolutionary mechanisms. At the very least, the discussion of evolutionary mechanisms that have allowed schizophrenia to develop and persist are contrary to eugenics. Barring its obvious ethical problems, eugenics has sought to oversimplify the role of diseases in human evolution to imply that mental disease must be totally maladaptive or at least that its risks outweigh its benefits. The evidence reviewed above has suggested that positive traits can be genetically and phenomenologically associated with schizophrenia and other mental disorders and that attempting to remove the genes for schizophrenia alone may also remove the genetic variation responsible for a myriad of other uniquely human abilities.
What Are the Possible Heterozygote Advantages?
As reviewed by Keller and Miller,69 evolutionary genetic theory offers 3 major hypotheses to explain persistent genetic variance in various behavioral traits under particular conditions: ancestral neutrality, balancing selection, and polygenic mutation-selection balance. Ancestral neutrality102 describes the condition where susceptibility alleles were not harmful among ancestors. It is unlikely that this mechanism can account for the persistence of susceptibility alleles because ancestral neutrality fails to explain low mental disorder frequencies and requires implausibly small selection coefficients against mental disorders given the data on the reproductive costs and impairment of mental disorders. On the other hand, the operational mechanism could be akin to balancing selection where susceptibility alleles sometimes increase fitness. Balancing selection103 (including spatiotemporal variation in selection, heterozygote advantage, antagonistic pleiotropy, and frequency-dependent selection) tends to favor environmentally contingent adaptations (which would show no heritability) or high-frequency alleles (which psychiatric genetics would have already found). Evolutionarily driven behavioral scientists have proposed that susceptibility alleles confer hidden adaptive benefits to patients with schizophrenia or alternatively to their first-degree relatives that might explain their persistence. Examples for schizophrenia include shamanism and in relatives increased intelligence104 or creativity.85,86 Certainly, for schizophrenia, an argument for increased fitness seems implausible, given that the serious maladaptive dysfunction associated with schizophrenia seems to be recognized as such by all cultures.
Earlier, we reviewed another variant of this argument that has been made for the selection of alleles that were formerly adaptive in ancestral environments but currently increase risk for various disorders in the example of thrifty genes in risk for type 2 diabetes. Analogous arguments might be made behavioral traits such as paranoia that might favor survival in dangerous ancestral environments where attacks from nonhuman predators or competing groups of humans were common and therefore highly salient.
Finally, through polygenic mutation-selection balance, mental disorders reflect the inevitable mutational load on the thousands of genes underlying human behavior. This theoretical explanation may be the most consistent with the data on mental disorder prevalence rates, fitness costs, the likely rarity of susceptibility alleles, and the increased risks of mental disorders with brain trauma, inbreeding, and paternal age. In modern environments, schizophrenia is associated with significantly diminished fertility, mediated by reduced survival, reduced attractiveness for mating, and lower marriage rates, as well as possibly via reduced fertility once married.
How Do We Test These Hypotheses?
Most of the hypotheses presented in this review would be best addressed by examining allele frequencies as they pertain to phenotypes over successive generations on a large scale. However, there are ways to approach identifying the evolutionary mechanisms involved in inheritance of schizophrenia that could be implemented realistically. For example, examining true prevalence rates of schizophrenia among different cultures is a unique way to hone gene-environment interactions and to apply characteristics of these cultures to what has been identified thus far in evolutionary history.
More specifically, using field survey methodology, eg, employing random digit dialing, a very large representative adult population sample of several thousand persons in size could be gathered. All individuals would be assessed on all available endophenotypic markers for psychotic illnesses across all domains. Similar ascertainment would take place in a large, representative sample of adults with schizophrenia, and all their available first-degree family members, as well as in a large representative sample of individuals with schizotypal personality disorder. The underlying architecture of endophenotypes could then be studied in the following manner. First, accurate population prevalence estimates for each endophenotype could be assessed. If the hypothesis is correct, the prevalence of single endophenotypic abnormalities in the healthy community sample will be high, in the range of 15%–20%. Second, cluster analysis will be informative as to which endophenotypes group together; endophenotypic clusters are likely to share common risk genes; this information would most pertinently be gathered in the schizophrenia and schizotypal groups. Finally, the gene clusters can themselves be interrogated to ascertain whether they converge on identified (or suggest novel) molecular bottlenecks.
We have presented a review of the current genetic evidence for susceptibility for schizophrenia and related behavioral phenotypes in the context of mechanisms that have been proposed to support the persistence of schizophrenia in human evolution. Through large population-based studies (QTLs) and family studies (linkage analysis), candidate susceptibility alleles have been and will continue to be identified. Associating allelic variance with specific endophenotypes seems to be a promising trajectory for ongoing research. It will be important for ongoing studies to recognize an evolutionary framework and to discuss findings in terms of possible evolutionary mechanisms that could be operating under the conditions of the resulting relationships.
Funding
National Institute of Mental Health (2RO1 MH43775 MERIT Award, 1RO1MH074797 and 1R01MH077945 to G.D.P. and 5T32MH18921 to B.S.F.); the National Alliance for Research on Schizophrenia and Depression (Distinguished Investigator Award to G.D.P.).
Acknowledgments
The authors would like to thank the anonymous reviewers for their helpful comments and suggestions in clarifying and expanding the manuscript.
References
- 1.Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. [PubMed] [Google Scholar]
- 2.Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020141. e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkbride JB, Fearon P, Morgan C, et al. Heterogeneity in incidence rates of schizophrenia and other psychotic syndromes: findings from the 3-center AeSOP study. Arch Gen Psychiatry. 2006;63:250–258. doi: 10.1001/archpsyc.63.3.250. [DOI] [PubMed] [Google Scholar]
- 4.McGrath JJ. Myths and plain truths about schizophrenia epidemiology–the NAPE lecture 2004. Acta Psychiatr Scand. 2005;111:4–11. doi: 10.1111/j.1600-0447.2004.00467.x. [DOI] [PubMed] [Google Scholar]
- 5.van Os J, Krabbendam L, Myin-Germeys I, Delespaul P. The schizophrenia envirome. Curr Opin Psychiatry. 2005;18:141–145. doi: 10.1097/00001504-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Wright P, Gill M, Murray RM. Schizophrenia: genetics and the maternal immune response to viral infection. Am J Med Genet. 1993;48:40–46. doi: 10.1002/ajmg.1320480110. [DOI] [PubMed] [Google Scholar]
- 7.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 8.Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 2002;325:1212–1213. doi: 10.1136/bmj.325.7374.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117. doi: 10.1192/bjp.184.2.110. [DOI] [PubMed] [Google Scholar]
- 10.Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-o-methyltransferase gene: longitudinal evidence of a gene X environment interaction. Biol Psychiatry. 2005;57:1117–1127. doi: 10.1016/j.biopsych.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 11.Botstein D, Risch N. Discovering genotypes underlying human phenotypes: past successes for Mendelian disease, future approaches for complex disease. Nat Genet. 2003;33(suppl):228–237. doi: 10.1038/ng1090. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy JL, Farrer LA, Andreasen NC, Mayeux R, St George-Hyslop P. The genetics of adult-onset neuropsychiatric disease: complexities and conundra? Science. 2003;302:822–826. doi: 10.1126/science.1092132. [DOI] [PubMed] [Google Scholar]
- 13.Riley B, Kendler KS. Molecular genetic studies of schizophrenia. Eur J Hum Genet. 2006;14:669–680. doi: 10.1038/sj.ejhg.5201571. [DOI] [PubMed] [Google Scholar]
- 14.Norton N, Williams HJ, Owen MJ. An update on the genetics of schizophrenia. Curr Opin Psychiatry. 2006;19:158–164. doi: 10.1097/01.yco.0000214341.52249.59. [DOI] [PubMed] [Google Scholar]
- 15.Chai CK. The genetics of Alzheimer's disease. Am J Alzheimers Dis Other Demen. 2007;22:37–41. doi: 10.1177/1533317506295655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 17.Gangestad SW, Yeo RA. Mutations, developmental instability, the red queen. Behav Brain Sci. 2006;29:412–413. [Google Scholar]
- 18.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbb4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntosh AM, Moorhead TW, Job D, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002103. doi:10.1038/sj.mp.4002103. [DOI] [PubMed] [Google Scholar]
- 20.Talbot K, Cho DS, Ong WY, et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054. doi: 10.1093/hmg/ddl246. [DOI] [PubMed] [Google Scholar]
- 21.Straub RE, Jiang Y, MacLean CJ, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang JX, Zhou J, Fan JB, et al. Family-based association study of DTNBP1 in 6p22.3 and schizophrenia. Mol Psychiatry. 2003;8:717–718. doi: 10.1038/sj.mp.4001287. [DOI] [PubMed] [Google Scholar]
- 23.Weickert CS, Straub RE, McClintock BW, et al. Human dysbindin (DTNBP1) gene expression in normal brain and in schizophrenic prefrontal cortex and midbrain. Arch Gen Psychiatry. 2004;61:544–555. doi: 10.1001/archpsyc.61.6.544. [DOI] [PubMed] [Google Scholar]
- 24.Talbot K, Eidem WL, Tinsley CL, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalus P, Bondzio J, Federspiel A, Muller TJ, Zuschratter W. Cell-type specific alterations of cortical interneurons in schizophrenic patients. Neuroreport. 2002;13:713–717. doi: 10.1097/00001756-200204160-00035. [DOI] [PubMed] [Google Scholar]
- 26.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egan MF, Goldberg TE, Gscheidle T, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001;50:98–107. doi: 10.1016/s0006-3223(01)01133-7. [DOI] [PubMed] [Google Scholar]
- 28.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends Neurosci. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Freedman R, Leonard S. Genetic linkage to schizophrenia at chromosome 15q14. Am J Med Genet. 2001;105:655–657. doi: 10.1002/ajmg.1548. [DOI] [PubMed] [Google Scholar]
- 30.Thiel CM, Zilles K, Fink GR. Nicotine modulates reorienting of visuospatial attention and neural activity in human parietal cortex. Neuropsychopharmacology. 2005;30:810–820. doi: 10.1038/sj.npp.1300633. [DOI] [PubMed] [Google Scholar]
- 31.Sacco KA, Termine A, Seyal A, et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- 33.Williams HJ, Owen MJ, O'Donovan MC. Is COMT a susceptibility gene for schizophrenia? Schizophr Bull. 2007;33:635–641. doi: 10.1093/schbul/sbm019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-o-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29:1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 35.Cannon TD. The inheritance of intermediate phenotypes for schizophrenia. Curr Opin Psychiatry. 2005;18:135–140. doi: 10.1097/00001504-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Eysenck HJ, Eysenck SBG. Psychoticism as a Dimension of Personality. London, UK: Hodder & Stoughton; 1976. [Google Scholar]
- 37.Meehl PE. Schizotaxia, schizotypy, schizophrenia. Am Psychol. 1962;17:827–838. [Google Scholar]
- 38.Chapman LJ, Chapman JP, Kwapil TR, Eckblad M, Zinser MC. Putatively psychosis-prone subjects 10 years later. J Abnorm Psychol. 1994;103:171–183. doi: 10.1037//0021-843x.103.2.171. [DOI] [PubMed] [Google Scholar]
- 39.Lenzenweger MF. Confirming schizotypic personality configurations in hypothetically psychosis-prone university students. Psychiatry Res. 1991;37:81–96. doi: 10.1016/0165-1781(91)90108-2. [DOI] [PubMed] [Google Scholar]
- 40.Tyrka AR, Cannon TD, Haslam N, et al. The latent structure of schizotypy: I. Premorbid indicators of a taxon of individuals at-risk for schizophrenia spectrum disorders. J Abnorm Psychol. 1995;104:173–183. doi: 10.1037//0021-843x.104.1.173. [DOI] [PubMed] [Google Scholar]
- 41.Tyrka AR, Haslam N, Cannon TD. Detection of a latent taxon of individuals at risk for schizophrenia-spectrum disorders. In: Raine A, Lencz T, Mednick SA, editors. Schizotypal Personality. Cambridge, UK: Cambridge University Press; 1995. pp. 168–191. [Google Scholar]
- 42.Claridge G, Beech T. Fully and quasi-dimensional constructions of schizotypy. In: Raine A, Lencz T, Mednick SA, editors. Schizotypal Personality. Cambridge, UK: Cambridge University Press; 1995. pp. 192–216. [Google Scholar]
- 43.Horrobin DF. The Madness of Adam and Eve: How Schizophrenia Shaped Humanity. London, UK: Bantam; 2001. [Google Scholar]
- 44.Campbell T, Osipova D, Kähkönenb S. Finland's galapagos: Founder effect, drift, and isolation in the inheritance of susceptibility alleles. Behav Brain Sci. 2006;29:409–410. [Google Scholar]
- 45.Bertolino A, Caforio G, Blasi G, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 46.Tuulio-Henriksson A, Arajarvi R, Partonen T, et al. Familial loading associates with impairment in visual span among healthy siblings of schizophrenia patients. Biol Psychiatry. 2003;54:623–628. doi: 10.1016/s0006-3223(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 47.Bertolino A, Caforio G, Blasi G, et al. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- 48.Crow TJ. A Darwinian approach to the origins of psychosis. Br J Psychiatry. 1995;167:12–25. doi: 10.1192/bjp.167.1.12. [DOI] [PubMed] [Google Scholar]
- 49.Haldane JBS. A mathematical theory of natural and artificial selection. Part V. Selection and mutation. Proc Camb Philol Soc. 1927;23:838–844. [Google Scholar]
- 50.Hyman SE. Can neuroscience be integrated into the DSM-V? Nat Rev Neurosci. 2007;8:725–732. doi: 10.1038/nrn2218. [DOI] [PubMed] [Google Scholar]
- 51.Rossler W, Riecher-Rossler A, Angst J, et al. Psychotic experiences in the general population: a twenty-year prospective community study. Schizophr Res. 2007;92:1–14. doi: 10.1016/j.schres.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Choong C, Hunter MD, Woodruff PW. Auditory hallucinations in those populations that do not suffer from schizophrenia. Curr Psychiatry Rep. 2007;9:206–212. doi: 10.1007/s11920-007-0020-z. [DOI] [PubMed] [Google Scholar]
- 53.Fanous A, Gardner C, Walsh D, Kendler KS. Relationship between positive and negative symptoms of schizophrenia and schizotypal symptoms in nonpsychotic relatives. Arch Gen Psychiatry. 2001;58:669–673. doi: 10.1001/archpsyc.58.7.669. [DOI] [PubMed] [Google Scholar]
- 54.Allen NB, Badcock PB. Darwinian models of depression: a review of evolutionary accounts of mood and mood disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:815–826. doi: 10.1016/j.pnpbp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 55.Troisi A, McGuire M. Darwinian psychiatry and the concept of mental disorder. Neuro Endocrinol Lett. 2002;23(suppl 4):31–38. [PubMed] [Google Scholar]
- 56.Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- 58.Nestadt G, Romanoski AJ, Samuels JF, Folstein MF, McHugh PR. The relationship between personality and DSM-III axis I disorders in the population: results from an epidemiological survey. Am J Psychiatry. 1992;149:1228–1233. doi: 10.1176/ajp.149.9.1228. [DOI] [PubMed] [Google Scholar]
- 59.Nestadt G, Eaton WW, Romanoski AJ, Garrison R, Folstein MF, McHugh PR. Assessment of DSM-III personality structure in a general-population survey. Compr Psychiatry. 1994;35:54–63. doi: 10.1016/0010-440x(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 60.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “Progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson EE, Kuttab-Boulos H, Witonsky D, Yang L, Roe BA, Di Rienzo A. CYP3A variation and the evolution of salt-sensitivity variants. Am J Hum Genet. 2004;75:1059–1069. doi: 10.1086/426406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andreotti F, Porto I, Crea F, Maseri A. Inflammatory gene polymorphisms and ischaemic heart disease: review of population association studies. Heart. 2002;87:107–112. doi: 10.1136/heart.87.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diamond J. The double puzzle of diabetes. Nature. 2003;423:599–602. doi: 10.1038/423599a. [DOI] [PubMed] [Google Scholar]
- 64.Betard C, Kessling AM, Roy M, Chamberland A, Lussier-Cacan S, Davignon J. Molecular genetic evidence for a founder effect in familial hypercholesterolemia among French Canadians. Hum Genet. 1992;88:529–536. doi: 10.1007/BF00219339. [DOI] [PubMed] [Google Scholar]
- 65.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1:445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 66.Polimeni J, Reiss JP. How shamanism and group selection may reveal the origins of schizophrenia. Med Hypotheses. 2002;58:244–248. doi: 10.1054/mehy.2001.1504. [DOI] [PubMed] [Google Scholar]
- 67.Polimeni J, Reiss JP. Evolutionary perspectives on schizophrenia. Can J Psychiatry. 2003;48:34–39. doi: 10.1177/070674370304800107. [DOI] [PubMed] [Google Scholar]
- 68.Evans PD, Gilbert SL, Mekel-Bobrov N, et al. Microcephalin, a gene regulating brain size, continues to evolve adaptively in humans. Science. 2005;309:1717–1720. doi: 10.1126/science.1113722. [DOI] [PubMed] [Google Scholar]
- 69.Keller MC, Miller G. Resolving the paradox of common, harmful, heritable mental disorders: which evolutionary genetic models work best? Behav Brain Sci. 2006;29:385–404. doi: 10.1017/S0140525X06009095. discussion. 405–352. [DOI] [PubMed] [Google Scholar]
- 70.Huxley J, Mayr E, Osmond H, Hoffer A. Schizophrenia as a genetic morphism. Nature. 1964;204:220–221. doi: 10.1038/204220a0. [DOI] [PubMed] [Google Scholar]
- 71.Larson CA, Nyman GE. Differential fertility in schizophrenia. Acta Psychiatr Scand. 1973;49:272–280. doi: 10.1111/j.1600-0447.1973.tb04421.x. [DOI] [PubMed] [Google Scholar]
- 72.Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry. 1997;171:502–508. doi: 10.1192/bjp.171.6.502. [DOI] [PubMed] [Google Scholar]
- 73.Brune M. Schizophrenia–an evolutionary enigma? Neurosci Biobehav Rev. 2004;28:41. doi: 10.1016/j.neubiorev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 74.Wilson DR. Evolutionary epidemiology. Integr Psychiatry. 1994;10:6–12. [Google Scholar]
- 75.McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gould SJ. Exaptation: a crucial tool for evolutionary psychology. J Soc Issues. 1991;47:43–65. [Google Scholar]
- 77.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 78.Crow TJ. A theory of the evolutionary origins of psychosis. Eur Neuropsychopharmacol. 1995;5(suppl):59–63. doi: 10.1016/0924-977x(95)00032-k. [DOI] [PubMed] [Google Scholar]
- 79.Grigorenko EL, LaBuda MC, Carter AS. Similarity in general cognitive ability, creativity, and cognitive style in a sample of adolescent Russian twins. Acta Genet Med Gemellol (Roma) 1992;41:65–72. doi: 10.1017/s000156600000252x. [DOI] [PubMed] [Google Scholar]
- 80.Nichols RC. Twin studies of ability, personality, and interests. Homo. 1978;29:158–173. [Google Scholar]
- 81.Rothenberg A, Wyshak G. Family background and genius. Can J Psychiatry. 2004;49:185–191. doi: 10.1177/070674370404900306. [DOI] [PubMed] [Google Scholar]
- 82.Waller NG, Bouchard TJ, Lykken DT, Tellegen A, Blacker DM. Creativity, heritability, familiality: which word does not belong? Psychol Inq. 1993;4:235–237. [Google Scholar]
- 83.Lykken DT. Research with twins: the concept of emergenesis. Soc Psychophys Res. 1981;19:361–372. doi: 10.1111/j.1469-8986.1982.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 84.Karlsson JL. Academic achievement of psychotic or alcoholic patients. Hereditas. 1983;99:69–72. doi: 10.1111/j.1601-5223.1983.tb00733.x. [DOI] [PubMed] [Google Scholar]
- 85.Karlsson JL. Creative intelligence in relatives of mental patients. Hereditas. 1984;100:83–86. doi: 10.1111/j.1601-5223.1984.tb00108.x. [DOI] [PubMed] [Google Scholar]
- 86.Karlsson JL. Mental abilities of male relatives of psychotic patients. Acta Psychiatr Scand. 2001;104:466–468. doi: 10.1034/j.1600-0447.2001.00515.x. [DOI] [PubMed] [Google Scholar]
- 87.Isohanni I, Jarvelin MR, Jones P, Jokelainen J, Isohanni M. Can excellent school performance be a precursor of schizophrenia? A 28-year follow-up in the northern Finland 1966 birth cohort. Acta Psychiatr Scand. 1999;100:17–26. doi: 10.1111/j.1600-0447.1999.tb10909.x. [DOI] [PubMed] [Google Scholar]
- 88.Karlsson JL. Inheritance of Creative Intelligence: A Study of Genetics in Relation to Giftedness and its Implications for Future Generations. Chicago, UK: Nelson-Hall; 1978. [Google Scholar]
- 89.Crow TJ, Done DJ, Sacker A. Childhood precursors of psychosis as clues to its evolutionary origins. Eur Arch Psychiatry Clin Neurosci. 1995;245:61–69. doi: 10.1007/BF02190732. [DOI] [PubMed] [Google Scholar]
- 90.Nettle D. Schizotypy and mental health amongst poets, visual artists, and mathematicians. J Res Pers. 2006;40:876–890. [Google Scholar]
- 91.Gould SJ, Lewontin RC. The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B Biol Sci. 1979;205:581–598. doi: 10.1098/rspb.1979.0086. [DOI] [PubMed] [Google Scholar]
- 92.Gould SJ, Vrba ES. Exaptation—a missing term in the science of form. Paleobiology. 1982;8:4–15. [Google Scholar]
- 93.Wakefield JC. Spandrels, vestigial organs, and such: reply to murphy and woolfolk's “The harmful dysfunction analysis of mental disorder”. Philos Psychiatr Psychol. 2000;7:253–269. [Google Scholar]
- 94.Murphy D, Woolfolk RL. The harmful dysfunction analysis of mental disorder. Philos Psychiatr Psychol. 2000;7:241–252. [Google Scholar]
- 95.Panksepp J, Moskal J. Schizophrenia: The elusive disease. Behav Brain Sci. 2004;27:863–864. [Google Scholar]
- 96.Polimeni J, Reiss JP. How shamanism and group selection may reveal the origins of schizophrenia. Med Hypotheses. 2002;58:244–248. doi: 10.1054/mehy.2001.1504. [DOI] [PubMed] [Google Scholar]
- 97.Haverkort M. The Evolution of Language: A Neurolinguistic Perspective on the Adaptation-Exaptation Debate. Wassenaar, The Netherlands: Netherlands Inst for Advanced Studies; 2003. [Google Scholar]
- 98.Shaner A, Miller G, Mintz J. Schizophrenia as one extreme of a sexually selected fitness indicator. Schizophr Res. 2004;70:101–109. doi: 10.1016/j.schres.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 99.Andrews PW, Gangestad SW, Matthews D. Adaptationism–how to carry out an exaptationist program. Behav Brain Sci. 2002;25:489–504. doi: 10.1017/s0140525x02000092. discussion 504–453. [DOI] [PubMed] [Google Scholar]
- 100.Crespi B, Summers K, Dorus S. Adaptive evolution of genes underlying schizophrenia. Proc R Soc Lond B Biol Sci. 2007;274:2801–2810. doi: 10.1098/rspb.2007.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Meyer-Lindenberg A, Straub RE, Lipska BK, et al. Genetic evidence implicating darpp-32 in human frontostriatal structure, function, and cognition. J Clin Invest. 2007;117:672–682. doi: 10.1172/JCI30413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tooby J, Cosmides L. On the universality of human nature and the uniqueness of the individual: the role of genetics and adaptation. J Pers. 1990;58:17–67. doi: 10.1111/j.1467-6494.1990.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 103.Mealey L. The sociobiology of sociopathy: an integrated evolutionary model. Behav Brain Sci. 1995;18:523–599. [Google Scholar]
- 104.Burdick KE, Lencz T, Funke B, et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568. doi: 10.1093/hmg/ddi481. [DOI] [PubMed] [Google Scholar]