Classifying Psychotic Disorders
For the last several decades, diagnosis in psychiatry has been rule based, related to phenomenology and standardized. It has given psychiatry an unearned advantage in communicating about its illnesses, unearned because the molecular basis for this standardization has remained elusive. However, it has provided a language for successful communication about psychiatric syndromes and supported practical functions for which categorization is helpful; functions as disparate as insurance reimbursement and drug development have been enabled with this language. Moreover, this standardization has had additional practical advantages beyond communication and labeling, specifically in terms of public familiarity.
Further, these standardized categories have been postulated without any real knowledge about the biological nature of the underlying brain disturbances or their mechanisms. Imagine categorizing diabetes by phenomenology before 1922 or infectious disease before the microscope and antibiotics. It is hard to intuit how one might successfully use nonspecific illness descriptors of phenomenology to sort affected individuals into homogeneous enough categories to discover molecular disease mechanisms, whether the diseases involve disorders of the pancreas, heart, or brain.
In psychiatry, despite the practical importance of the Diagnostic and Statistical Manual of Mental Disorder (DSM) nomenclature, the diagnostic system remains a hypothesis of disease categories, awaiting a refinement of categorization based on mechanisms and molecules. Not that we should be persuaded to discard this current system, until another one, more biologically based, is in place. But, because this current system may not provide the final correct illness categories, it may be time to experiment with other systems, within research indications. In this context, scientists and clinicians alike have developed an informed skepticism, whose goal is to promote mechanism-oriented research into the major psychoses with the goal of defining the mechanistic basis of the brain diseases with cognitive and affective expression.
There is consistent evidence that genes contribute to the etiology of psychosis. Recent findings from genetic studies provide evidence for an overlap in genetic susceptibility across the traditional psychosis categories. Candidate genes show strong associations with component symptom complexes, such as psychosis, that are not projected directly onto Kraepelinian disease entities. Genetic studies suggest that psychosis may be conceptualized as a clinical phenotype with specific genetic etiologies. Hypothetically genes or sets of genes, interacting with environmental factors, may predetermine vulnerability to psychosis. Depending on additional syndrome-specific genetic influence and environmental interactions, psychosis may coexist with other clinical phenotypes, eg, mood symptoms or cognitive dysfunction, composing categorical diagnoses. This conceptualization of psychosis is well illustrated by epidemiological and molecular genetic studies. In this chapter, we will review the phenomenology and genetics of psychosis, across different diagnoses. Other aspects of the psychosis overlap will be presented in other articles in this volume.
The Bipolar Disorder-Schizophrenia Distinction
Kraepelin divided insanity into a bipolar type and a schizophrenia type in the 1890s, distinguished by symptom profile and by overall outcome. Since that time, clinical scientists have discussed whether this is a useful division or a false dichotomy. Whether this categorization conforms to a biological distinction between these 2 syndromes likely to be molecularly based, remains a question. Formulating answers to this question highlights the current controversy of whether it more advantageous to utilize traditional diagnostic categories or to pursue dimensional constructs. To advance this controversy, we explore here the dimension of “psychosis” from a practical point of view, as a representative dimensional construct, using phenomenologic and genetic data as they address this question. Other perspectives are presented elsewhere in this issue.
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), distinguishes between schizophrenia and psychotic mood disorders, mainly based upon psychosis being the core-defining feature of the schizophrenia diagnosis, whereas in mood disorders it is considered a secondary clinical phenomenon. In fact, there is no DSM-IV diagnostic category for psychotic bipolar disorder, although psychosis is included as a specifier for severe mood episodes. While in the current diagnostic system psychosis in mood disorders is treated as a secondary feature, recent research has suggested that there is a subpopulation of patients with bipolar disorder, in which psychosis appears to be a consistent syndrome which may have a genetic basis.1
Therefore, a dimensional approach to categorization has emerged, driven by clinical observations and research need. It has been recently directed toward facilitating novel drug development in schizophrenia for symptoms domains without treatments, specifically for cognition. It is applicable more broadly for research in pathophysiology and etiology. The idea is clinically based and practical, taking “component symptom complexes” and targeting these for evaluation, disease hypotheses, and drug development.2 Component symptom complexes (or clinical domains) are groups of symptoms which associate in an illness and appear to have a common pharmacology, neural basis, and putative pathophysiology. Purportedly, eg, the symptom construct psychosis could be supported by a common disease mechanism across different psychiatric diagnoses, a concept which is important for clinical prediction, mechanistic research, and drug development. We will discuss the similarities and distinctions in phenomenology across the 2 psychotic illnesses, schizophrenia and psychotic bipolar 1 disorder (BD-1) as illustrative of developing this formulation further.
The Phenomenology of Psychosis as a Component Symptom Complex
Psychosis receives a range of definitions in the DSM-IV. The narrowest definition is restricted to “delusions and prominent hallucinations, with the hallucinations occurring in the absence of insight into their pathological nature.” In a less restrictive formulation, it includes “prominent hallucinations that the individual realizes are hallucinatory experiences.” This definition uses insight into the phenomenon to distinguish the symptoms. Another broader psychosis definition also includes “other positive symptoms of schizophrenia (ie, disorganized thought process, grossly disorganized or catatonic behavior).” Finally, the most generalized, conceptual definition of psychosis is “a loss of ego boundaries or a gross impairment of reality testing.”
Schizophrenic psychosis is distinguished more by its course than by the nature of the psychotic symptoms. Usually the course of psychosis is chronic with either continuous or episodic psychotic symptoms, mostly without full interepisode recovery. Although courses of schizophrenia with a good prognosis are described in DSM-IV (eg, a single episode with partial or full remission), they are uncommon. In contrast, the course of psychosis in other psychotic illnesses can be fleeting, with relatively short episodes of hallucinations and delusions manifest at the peak of severe depression or mania, decompensated medical conditions, or episode of illicit substance use. Over the full course of schizophrenia, psychotic symptoms tend to become less severe with time, whereas cognitive function worsens and negative symptoms become more apparent. A further deterioration in the patient's baseline functioning can follow each relapse of schizophrenia psychosis. This failure to return to baseline functioning after each relapse is commonly taken as the major distinction between schizophrenia and psychotic mood disorders. However, recent studies report that 20%–25% of patients with affective illnesses show considerable long-term impairment in daily functioning.3–5 Without treatment, psychosis in schizophrenia is incapacitating and contributes, along with negative symptoms and cognitive dysfunction, to an overall poor prognosis.
Comparative studies across diagnoses, contrasting predictions of clinical course based on dimensional composites compared with traditional diagnostic categories, have shown that the use of component symptom complexes have an advantage; moreover, demand for psychiatric care, treatment outcome, social adaptation, and global prognosis for the illness seem also to be facilitated by dimensional categorization.6,7 Recently, the use of component symptom complexes has been applied in research, as well as clinical, paradigms to inform genetic and intermediate phenotype studies, and as novel symptom targets for drug development. Dimensional composites of schizophrenia are, at least partially, pharmacologically independent and may have independent etiologies and mechanisms. Psychotic symptoms are satisfactorily treated with first- and second-generation antipsychotic drugs; however, no effective treatments exist either for cognitive deficits or for negative symptoms.8 Clinical experience and research data show that psychosis in different diagnostic categories shows a similar pharmacological response to antipsychotic drug treatment, including psychosis in schizophrenia, bipolar disorder, depression, substance-induced psychoses, and organic psychoses.9 One of the current goals of drug development for schizophrenia is treatment for cognitive dysfunction, with hypothetical treatment targets focused on glutamatergic, cholinergic, and serotoninergic neurotransmitter systems.8–16
The course of schizophrenia varies across individuals but is generally described as starting with premorbid signs and symptoms followed by a prodromal phase of illness, as the illness evolves. However, in practice, it is not always possible to distinguish these 2 periods precisely; several reasons account for this failure, including the presence of schizophrenia spectrum personality disorders, traits, or the presence of minor psychotic symptoms appearing long before the onset of the diagnosis. In the typical, although not invariable premorbid picture of schizophrenia, affected probands have schizoid or schizotypal personality features and are characterized as quiet, introverted, emotionally aloof, and seclusive. They prefer solitary activities, seem content by themselves, and are unattached to family members. When schizotypal features are predominant, thinking, perception, and behavior may be odd. Various psychotic-like symptoms, such as magical thinking, peculiar beliefs, ideas of reference, perceptual illusions, odd fantasies, and derealization-depersonalization symptoms, are commonly present. Especially during the adolescent period, they may have poor communication skills and distorted social judgment. This often leads to feelings of isolation, “not fitting in,” and withdrawal from social interactions. Although different types of personalities (including paranoid, avoidant, obsessive-compulsive, and borderline) are also seen in the premorbid period in individuals with schizophrenia, schizoid and schizotypal personality traits are the most common. Still, the presence of these symptoms does not inevitably develop into schizophrenia.
The presence of prodromal symptoms for BD-1 with psychosis is unclear. Although clinical observations suggest that symptoms like mood lability, impulsivity, destructibility, and physical hyperactivity are often present long before the development of affective psychosis, the phenomenology of the BD prodrome is not well characterized. A recent study suggested that there are unique prodromal characteristics distinguishing psychotic and nonpsychotic mania. This study was conducted with early onset BD-1 individuals.17 Specifically, attenuated late psychotic symptoms during the prodrome accompanied by increased energy and goal-directed activity were more common in individuals with eventual psychotic mania. The phenomenology of prodromal symptoms in schizophrenia and affective psychosis revealed considerable overlap, including such symptoms as suspiciousness, hallucinatory experiences, anxiety, and insomnia. On the other hand, depressed mood, suicidality, mood lability, difficulty communicating clearly, lack of energy, obsessions, and physical agitation were more prevalent in the mania prodrome.17
At present, the prodromal syndrome is a hypothetical construct; its actual existence can only be confirmed after the diagnosis of schizophrenia or BD-1 has been made. During the prodromal phase, patients characteristically lack insight about their developing symptoms, although some individuals experience a sense of change. In clinical situations, information about the prodrome is developed from the history of events proximal to illness onset; often this information is more reliable from relatives than from the patient. In the area of research, studies are focused on large samples of individuals who are at increased risk for developing schizophrenia (eg, offspring of ill parents or members of high density families, especially, those with mild psychotic symptoms). These populations are being studied for the rate of conversion of these individuals to a diagnosis of schizophrenia (approximately 30%–35%) and predictive factors for illness onset.18,19
Family studies of schizophrenia and affective psychoses show that psychosis aggregates in families.1,20–23 Having a relative with schizophrenia or bipolar disorder is a single most powerful risk factor for developing psychosis. A familial liability to psychosis is not disorder specific, in that many pedigrees show familial aggregation of various functional psychoses, including schizophrenia, schizoaffective disorder, psychotic bipolar and major depressive disorder, substance-induced psychoses, delusional disorder, and other psychoses.24–26 Certain clinical phenotypes or psychopathological dimensions seem to predict familial risk of psychosis across the DSM-IV categorical diagnoses.27–29 Overall, studies suggest that familial liability for psychosis crosses DSM-IV categories of schizophrenia and mood disorders. From a dimensional perspective, psychosis may represent a shared phenotype with unique genetic etiologies, running through family generations.
Psychosis Genetics
Family Studies
Family studies of schizophrenia and affective psychoses show that psychosis aggregates in families.1,20–23 The lifetime risk for developing schizophrenia increases approximately 8- to 12-folds in first-degree biological relatives of schizophrenic probands. Although the results of genetic studies in affective psychoses are less consistent, the familial aggregation of bipolar disorder and major depressive disorder has been observed. First-degree relatives of individuals with BD-1 have elevated rates of BD-1 (4%–24%), bipolar II disorder (1%–5%), and major depressive disorder (4%–24%). A familial liability to psychosis is not disorder specific, in that many pedigrees show familial aggregation of various functional psychoses, including schizophrenia, schizoaffective disorder, psychotic bipolar and major depressive disorder, substance-induced psychoses, delusional disorder, and other psychoses. Although there are a few studies that note coaggregation of bipolar disorder and schizophrenia in families of bipolar disorder or schizophrenia probands,24,25 the vast majority of studies carried out in large epidemiological samples show that the familial risks for schizophrenia and bipolar disorders are mostly independent of each other.30,31 On the other hand, bipolar disorder has been associated with increased risk of schizophrenia in relatives. In one family study in Spain, it was reported that relatives of women with early onset of bipolar disorder had the highest morbid risks for both bipolar illness and schizophrenia.26 In this study, the presence of more than one patient with bipolar disorder in a family increased the risk for schizophrenia nearly 4-fold. In a different study, it was shown that affective disorders are more frequently inherited from the same parental side of the family as schizophrenia psychosis,25 consistent with the hypothesis that in some cases the same genes could contribute to susceptibility to both schizophrenia and affective psychoses. Schizoaffective disorder occurs at similarly increased rates both in families of probands with schizophrenia and bipolar disorder. Both schizophrenia and bipolar disorder have been shown to occur at increased rates in relatives of probands with schizoaffective disorder.32
Certain clinical phenotypes or psychopathological dimensions seem to predict familial risk of psychosis across the DSM-IV categorical diagnoses.27–29 Consistently between the studies, presence of negative symptoms and insidious early onset of illness in probands are predictive of schizophrenia in their first-degree relatives, whereas familial morbid risk of affective psychosis is specifically predicted by history of mania in probands.28,29 The syndrome characterized by bizarre behavior, inappropriate affect, catatonia, and poor rapport was reported to be predictive of psychosis independent of DSM-IV categories in biological relatives of psychotic probands.29 Overall, studies suggest that familial liability for psychosis crosses DSM-IV categories of schizophrenia and mood disorders. From a dimensional perspective, psychosis may represent a shared phenotype with unique genetic etiologies, running through family generations.
Twin Studies
Twin studies show that the concordance rate for schizophrenia is higher in monozygotic twins (47%–56%) than in dizygotic twins (12%–16%), suggesting a strong heritability component for the illness. Some studies reported the concordance rates for monozygotic twins over 80% in cases of severe schizophrenia with typical core symptoms.33 Further, twin studies suggest that a schizophrenia diagnosis in one twin increases risk for both schizophrenia and affective psychosis in the cotwin.34,35 An overlap in genetic risk for schizophrenia, schizoaffective, and manic syndrome is also suggested by a report, based on the Maudsley twin series: the maximum monozygotic/dizygotic concordance ratio was produced by a combination of schizophrenia, affective disorder with mood-incongruent psychotic features, schizotypal personality disorder, and atypical psychosis.
Genetic Linkage Studies
In the past decade, numerous genetic studies have implicated chromosomal loci and candidate risk genes associated with schizophrenia.36–38 Several large meta-analyses have found strong evidence of numerous genetic linkages of which 6p24-22, 1q21-22, and 13q32-43 are the best supported.38,39 Highly suggestive linkages have been identified in 8p21-22, 6p22, 6q21-25, 22q11-12, 5q21-33, 10p15-11, and 1q42.36,38–41 Genome-wide scans of bipolar disorder have produced inconsistent evidence for specific linkage, despite interesting leads in earlier studies (eg, chromosomes 2,42 11,43 18,44 and “X.”45 Several meta-analyses of bipolar disorder data sets indicated no significant linkages by a priori criteria, but the most promising linkages were to 18q22, 21q21, 4p16, and 12q2440,41 and 13q and 22q.46
Recent large meta-analyses of linkage studies based on the clinical phenotype have identified several loci that overlap between schizophrenia and bipolar disorder including 1q32, 10p11-15, 13q32, 18p11.2, and 22q11-13.36,40,46–48 Interestingly, Park et al 49 identified several putative loci associated with psychosis in bipolar disorders pedigrees (with significant linkage to 9q31 and 8p21 and suggestive linkage to 5q33, 6q21, 8p12, 8q24, 13q32, 15q26, 17p12, 18q21, and 20q13). This study supports that psychosis is a potentially useful phenotype informative for future exploring of schizophrenia- and bipolar disorder–shared genetics markers. A recent genome-wide linkage scan in schizoaffective disorder confirmed the existence of loci that influence susceptibility across the functional psychosis spectrum.50 This study demonstrated genome-wide significant linkage at chromosome 1q42 and suggestive linkages at 22q11 and 19p13. Noteworthy, 2 candidate genes, Disrupted in Schizophrenia 1 (DISC1) and catechol-O-methyltransferase (COMT), which have been consistently implicated in schizophrenia and, more recently, in bipolar disorder, map to 1q42 and 22q11, respectively.
Studies of Individual Genes
Association studies have identified several putative candidate genes involved in etiology of schizophrenia. Some of these risk genes include DISC1 on 1q42,51–53 COMT on 22q11,54–56 dystrobrevin-binding protein 1 (dysbindin) on 6p22.3,57–62 neuregulin 1 (NRG1) on 8p12,63–67 d-amino acid oxidase activator (G72)/G30) (DAOA (G72)/G30) on 13q33,64,68–70 brain-derived neurotrophic factor (BDNF),71–73 and regulator of G protein signaling (RGS4) on 1q23,74–77 although the reports vary considerably. Recent functional candidate gene studies have specified that several candidate genes for schizophrenia may also be associated with bipolar disorder, including DAOA (G72)/G30,69,78–80 BDNF,48,81,82 DISC1,83 and NRG1.84 Of these, association with G72 may be most robust; however, G72 haplotypes and polymorphisms associated with bipolar disorder are not consistent. In the recent comprehensive review in genetics of bipolar disorder,85 additional associations between bipolar disorder and TRPM2 (21q22.3),86 GPR50 (Xq28),87 Citron (12q24),88 CHMP1.5 (18p11.2),89 GCHI (14q22-24),90 MLC1 (22q13),91 GABRA5 (15q11-q13),92 BCR (22q11),93 CUX2, FLJ32356 (12q23-q24),94 and NAPG (18p11)95 have been suggested, although future replicating studies are warranted. From gene expression analysis, PDLIM5, somatostatin, and the mtDNA 3243 mutation were found to be related to bipolar disorder.85 Additionally, recent reports have suggested BmaL1,96,97 TIMELESS,96 PERIOD3,97 and CLOCK98–101 as candidate loci associated with the circadian rhythm phenotype in bipolar disorder, although not all reports are consistent.97,102,103
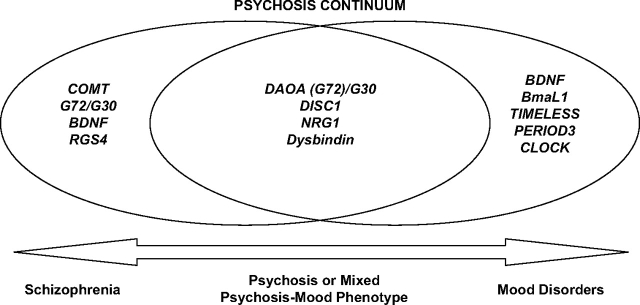
Several recent reports on candidate genes implicate variation at the same loci influencing susceptibility to both schizophrenia and bipolar disorder104; most notably these are association findings at DAOA (G72)/G30,64,68–70,74,78,79 DISC1,51–53,83,105 NRG1,66,84 and dysbindin106 (figure 1). For instance, a recent study found the association of NRG1 with a clinical phenotype of bipolar disorder with mood-incongruent psychotic symptoms, as well as with schizophrenia with lifetime manic episodes.84 This suggests that NRG1 may confer susceptibility to a specific clinical phenotype with combined features of psychosis and mania. A number of independent genetic linkage and association studies in diverse populations support the formulation that variation in DISC1 gene influences susceptibility to disorders of psychosis spectrum, including schizophrenia, schizoaffective disorder, and bipolar disorder.107 Although dysbindin has been extensively implicated in schizophrenia, a recent preliminary report has also suggested an association between polymorphism in this gene and a clinical subtype of bipolar disorder with recurrent psychotic episodes.106 Overall, these findings implicate variations in NRG1, DISC1, and dysbindin in the susceptibility to psychosis or mixed phenotype with features of both psychosis and mood symptoms rather than to the DSM-IV schizophrenia phenotype. A large recent study from the United Kingdom implicated a genetic variation in G72 (DAOA)/G30 in susceptibility for major mood episodes across the traditional bipolar disorder and schizophrenia categories.108 This report suggests that even though this locus was originally described as a schizophrenia risk gene, it appears to be more strongly associated with mood symptoms domain than with psychosis; future replication studies are warranted.
Fig. 1.
Candidate Genes in Schizophrenia—Bipolar Disorder Boundary.
Conclusions
Epidemiological and genetic studies support the hypothesis that psychosis is a clinical phenotype with multiple etiologies and a genetic component. Psychosis strongly aggregates in families. Twin studies have suggested high heritability estimates for psychosis and a complex mode of transmission. Whole-genome linkages studies have identified chromosomal loci that influence susceptibility to psychosis, independent of diagnostic categories. Detailed studies of linked genomic regions have identified several putative candidate genes (NRG 1, dysbindin, DISC 1, COMT, G72/G30, BDNF, RGS 4), which appear to be involved in schizophrenia and affective psychoses. Understanding the biological effect of risk genes is complex. Even though several such genes have been implicated, it is difficult to determine the disease mechanism of each risk gene. Interactions between risk genes add to the complexity of the picture. In addition, environmental factors, interacting with risk genes, contribute to psychosis susceptibility.
While originally the candidate risk genes were implicated in schizophrenia, recent findings provide evidence that many show strong associations with symptom dimensions, such as psychosis (NRG1, DISC1, and dysbindin) or mood symptoms (G72/G30, BDNF), across the schizophrenia-mood disorder continuum. A growing number of reports suggest that psychosis may be a clinical phenotype with a unique genetic background from categorical diagnoses. Although intriguing, these observations are preliminary in establishing evidence for genetic associations in complex, polygenetic illnesses. Future genetic studies, focusing on the symptom dimensions across the functional psychosis continuum, are urgently needed. Dimensional approach may provide more direct clues to understanding the mechanisms of psychotic illnesses.
References
- 1.Potash JB, Willour VL, Chiu YF, et al. The familial aggregation of psychotic symptoms in bipolar disorder pedigrees. Am J Psychiatry. 2001;158(8):1258–1264. doi: 10.1176/appi.ajp.158.8.1258. [DOI] [PubMed] [Google Scholar]
- 2.Hyman SE, Fenton WS. MEDICINE: what are the right targets for psychopharmacology? Science. 2003;299(5605):350–351. doi: 10.1126/science.1077141. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg JF, Harrow M. Consistency of remission and outcome in bipolar and unipolar mood disorders: a 10-year prospective follow-up. J Affect Disord. 2004;81(2):123–131. doi: 10.1016/S0165-0327(03)00161-7. [DOI] [PubMed] [Google Scholar]
- 4.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62(12):1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy N, Foy K, Sherazi R, McDonough M, McKeon P. Long-term social functioning after depression treated by psychiatrists: a review. Bipolar Disord. 2007;9(1–2):25–37. doi: 10.1111/j.1399-5618.2007.00326.x. [DOI] [PubMed] [Google Scholar]
- 6.Allardyce J, Gaebel W, Zielasek J, van Os J. Deconstructing psychosis conference February 2006: the validity of schizophrenia and alternative approaches to the classification of psychosis. Schizophr Bull. 2007;33(4):863–867. doi: 10.1093/schbul/sbm051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenman S, Korten A, Medway J, Evans M. Dimensional vs. categorical diagnosis in psychosis. Acta Psychiatr Scand. 2003;107(5):378–384. doi: 10.1034/j.1600-0447.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 8.Gray JA, Roth BL. Molecular targets for treating cognitive dysfunction in schizophrenia. Schizophr Bull. 2007;33(5):1100–1119. doi: 10.1093/schbul/sbm074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamminga CA, Davis JM. The neuropharmacology of psychosis. Schizophr Bull. 2007;33(4):937–946. doi: 10.1093/schbul/sbm063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158(9):1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan RW, Freedman R, Javitt DC, Abi-Dargham A, Lieberman JA. Recent advances in the development of novel pharmacological agents for the treatment of cognitive impairments in schizophrenia. Schizophr Bull. 2007;33(5):1120–1130. doi: 10.1093/schbul/sbm083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heresco-Levy U, Javitt DC, Ebstein R, et al. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biol Psychiatry. 2005;57(6):577–585. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 13.McGurk SR, Green MF, Wirshing WC, et al. Antipsychotic and anticholinergic effects on two types of spatial memory in schizophrenia. Schizophr Res. 2004;68(2–3):225–233. doi: 10.1016/S0920-9964(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 14.O'Grada C, Dinan T. Executive function in schizophrenia: what impact do antipsychotics have? Hum Psychopharmacol. 2007;22(6):397–406. doi: 10.1002/hup.861. [DOI] [PubMed] [Google Scholar]
- 15.Tsai G, Lane HY, Yang P, Chong MY, Lange N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol Psychiatry. 2004;55(5):452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Tamminga CA. The neurobiology of cognition in schizophrenia. J Clin Psychiatry. 2006;67(9):e11. [PubMed] [Google Scholar]
- 17.Correll CU, Penzner JB, Frederickson AM, et al. Differentiation in the preonset phases of schizophrenia and mood disorders: evidence in support of a bipolar mania prodrome. Schizophr Bull. 2007;33(3):703–714. doi: 10.1093/schbul/sbm028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67(2–3):131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 19.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65(1):28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray RM, Sham P, Van Os J, Zanelli J, Cannon M, McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophr Res. 2004;71(2–3):405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Asarnow RF, Nuechterlein KH, Fogelson D, et al. Schizophrenia and schizophrenia-spectrum personality disorders in the first-degree relatives of children with schizophrenia: the UCLA family study. Arch Gen Psychiatry. 2001;58(6):581–588. doi: 10.1001/archpsyc.58.6.581. [DOI] [PubMed] [Google Scholar]
- 22.Goes FS, Zandi PP, Miao K, et al. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21-33. Am J Psychiatry. 2007;164(2):236–247. doi: 10.1176/ajp.2007.164.2.236. [DOI] [PubMed] [Google Scholar]
- 23.Schurhoff F, Szoke A, Meary A, et al. Familial aggregation of delusional proneness in schizophrenia and bipolar pedigrees. Am J Psychiatry. 2003;160(7):1313–1319. doi: 10.1176/appi.ajp.160.7.1313. [DOI] [PubMed] [Google Scholar]
- 24.Arajarvi R, Ukkola J, Haukka J, et al. Psychosis among “healthy” siblings of schizophrenia patients. BMC Psychiatry. 2006;6:6. doi: 10.1186/1471-244X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henn S, Bass N, Shields G, Crow TJ, DeLisi LE. Affective illness and schizophrenia in families with multiple schizophrenic members: independent illnesses or variant gene(s)? Eur Neuropsychopharmacol. 1995;5(suppl):31–36. doi: 10.1016/0924-977x(95)00026-l. [DOI] [PubMed] [Google Scholar]
- 26.Valles V, van Os J, Guillamat R, et al. Increased morbid risk for schizophrenia in families of in-patients with bipolar illness. Schizophr Res. 2000;42(2):83–90. doi: 10.1016/s0920-9964(99)00117-6. [DOI] [PubMed] [Google Scholar]
- 27.Dikeos DG, Wickham H, McDonald C, et al. Distribution of symptom dimensions across Kraepelinian divisions. Br J Psychiatry. 2006;189:346–353. doi: 10.1192/bjp.bp.105.017251. [DOI] [PubMed] [Google Scholar]
- 28.Peralta V, Cuesta MJ. The relationship between syndromes of the psychotic illness and familial liability to schizophrenia and major mood disorders. Schizophr Res. 2007;91(1–3):200–209. doi: 10.1016/j.schres.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 29.van Os J, Marcelis M, Sham P, Jones P, Gilvarry K, Murray R. Psychopathological syndromes and familial morbid risk of psychosis. Br J Psychiatry. 1997;170:241–246. doi: 10.1192/bjp.170.3.241. [DOI] [PubMed] [Google Scholar]
- 30.Kendler KS, Gardner CO. The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychol Med. 1997;27(2):411–419. doi: 10.1017/s003329179600445x. [DOI] [PubMed] [Google Scholar]
- 31.Laursen TM, Labouriau R, Licht RW, Bertelsen A, Munk-Olsen T, Mortensen PB. Family history of psychiatric illness as a risk factor for schizoaffective disorder: a Danish register-based cohort study. Arch Gen Psychiatry. 2005;62(8):841–848. doi: 10.1001/archpsyc.62.8.841. [DOI] [PubMed] [Google Scholar]
- 32.Maier W, Lichtermann D, Minges J, et al. Continuity and discontinuity of affective disorders and schizophrenia. Results of a controlled family study. Arch Gen Psychiatry. 1993;50(11):871–883. doi: 10.1001/archpsyc.1993.01820230041004. [DOI] [PubMed] [Google Scholar]
- 33.Franzek E, Beckmann H. [Genetic heterogeneity of schizophrenia. Results of a systematic twin study] Nervenarzt. 1996;67(7):583–594. [PubMed] [Google Scholar]
- 34.Cardno AG, Rijsdijk FV, Sham PC, Murray RM, McGuffin P. A twin study of genetic relationships between psychotic symptoms. Am J Psychiatry. 2002;159(4):539–545. doi: 10.1176/appi.ajp.159.4.539. [DOI] [PubMed] [Google Scholar]
- 35.Farmer AE, McGuffin P, Gottesman II. Twin concordance for DSM-III schizophrenia. Scrutinizing the validity of the definition. Arch Gen Psychiatry. 1987;44(7):634–641. doi: 10.1001/archpsyc.1987.01800190054009. [DOI] [PubMed] [Google Scholar]
- 36.Baron M. Genetics of schizophrenia and the new millennium: progress and pitfalls. Am J Hum Genet. 2001;68(2):299–312. doi: 10.1086/318212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 38.Owen M, Williams N, O'Donovan M. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2003;9:14–17. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- 39.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am J Hum Genet. 2003;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berrettini WH. Are schizophrenic and bipolar disorders related? A review of family and molecular studies. Biol Psychiatry. 2000;48(6):531–538. doi: 10.1016/s0006-3223(00)00883-0. [DOI] [PubMed] [Google Scholar]
- 41.Segurado R, Detera-Wadleigh SD, Levinson DF, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part III: bipolar disorder. Am J Hum Genet. 2003;73(1):49–62. doi: 10.1086/376547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu J, Juo SH, Dewan A, et al. Evidence for a putative bipolar disorder locus on 2p13-16 and other potential loci on 4q31, 7q34, 8q13, 9q31, 10q21-24, 13q32, 14q21 and 17q11-12. Mol Psychiatry. 2003;8(3):333–342. doi: 10.1038/sj.mp.4001254. [DOI] [PubMed] [Google Scholar]
- 43.Egeland JA, Gerhard DS, Pauls DL, et al. Bipolar affective disorders liked to DNA markers on chromosome 11. Nature. 1987;325(6107):783–787. doi: 10.1038/325783a0. [DOI] [PubMed] [Google Scholar]
- 44.Berrettini WH, Ferraro TN, Goldin LR, et al. Chromosome 18 DNA markers and manic-depressive illness: evidence for a susceptibility gene. Proc Natl Acad Sci U S A. 1994;91(13):5918–5921. doi: 10.1073/pnas.91.13.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron M, Risch N. The spectrum concept of schizophrenia: evidence for a genetic-environmental continuum. J Psychiatr Res. 1987;21(3):257–267. doi: 10.1016/0022-3956(87)90027-6. [DOI] [PubMed] [Google Scholar]
- 46.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol Psychiatry. 2002;7(4):405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 47.Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Curr Psychiatry Rep. 2001;3(4):332–337. doi: 10.1007/s11920-001-0030-1. [DOI] [PubMed] [Google Scholar]
- 48.Sklar P, Gabriel SB, McInnis MG, et al. Family-based association study of 76 candidate genes in bipolar disorder: BDNF is a potential risk locus. Brain-derived neutrophic factor. Mol Psychiatry. 2002;7(6):579–593. doi: 10.1038/sj.mp.4001058. [DOI] [PubMed] [Google Scholar]
- 49.Park N, Juo SH, Cheng R, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry. 2004;9(12):1091–1099. doi: 10.1038/sj.mp.4001541. [DOI] [PubMed] [Google Scholar]
- 50.Hamshere ML, Bennett P, Williams N, et al. Genomewide linkage scan in schizoaffective disorder: significant evidence for linkage at 1q42 close to DISC1, and suggestive evidence at 22q11 and 19p13. Arch Gen Psychiatry. 2005;62(10):1081–1088. doi: 10.1001/archpsyc.62.10.1081. [DOI] [PubMed] [Google Scholar]
- 51.Ekelund J, Hovatta I, Parker A, et al. Chromosome 1 loci in Finnish schizophrenia families. Hum Mol Genet. 2001;10:1611–1617. doi: 10.1093/hmg/10.15.1611. [DOI] [PubMed] [Google Scholar]
- 52.Ekelund J, Hennah W, Hiekkalinna T, et al. Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Mol Psychiatry. 2004;9(11):1037–1041. doi: 10.1038/sj.mp.4001536. [DOI] [PubMed] [Google Scholar]
- 53.Millar JK, Wilson-Annan JC, Anderson S, et al. Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum Mol Genet. 2000;9:1415–1423. doi: 10.1093/hmg/9.9.1415. [DOI] [PubMed] [Google Scholar]
- 54.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shifman S, Bronstein M, Sternfeld M, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71(6):1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malhotra AK, Kestler LJ, Mazzanti CM, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- 57.Bray NJ, Buckland PR, Owen MJ, O'Donovan MC. Cis-acting variation in the expression of a high proportion of genes in human brain. Hum Genet. 2003;113:149–153. doi: 10.1007/s00439-003-0956-y. [DOI] [PubMed] [Google Scholar]
- 58.Funke B, Finn CT, Plocik AM, et al. Association of the DTNBP1 locus with schizophrenia in a U.S. population. Am J Hum Genet. 2004;75(5):891–898. doi: 10.1086/425279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Numakawa T, Yagasaki Y, Ishimoto T, et al. Evidence of novel neuronal functions of dysbindin, a susceptibility gene for schizophrenia. Hum Mol Genet. 2004;13(21):2699–2708. doi: 10.1093/hmg/ddh280. [DOI] [PubMed] [Google Scholar]
- 60.Schwab SG, Knapp M, Mondabon S, et al. Support for association of schizophrenia with genetic variation in the 6p22.3 gene, dysbindin, in sib-pair families with linkage and in an additional sample of triad families. Am J Hum Genet. 2003;72(1):185–190. doi: 10.1086/345463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straub RE, Jiang Y, MacLean CJ, et al. Genetic variation in the 6p22.3 gene DTNBP1, the human ortholog of the mouse dysbindin gene, is associated with schizophrenia. Am J Hum Genet. 2002;71:337–348. doi: 10.1086/341750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams NM, Preece A, Morris DW, et al. Identification in 2 independent samples of a novel schizophrenia risk haplotype of the dystrobrevin binding protein gene (DTNBP1) Arch Gen Psychiatry. 2004;61:336–344. doi: 10.1001/archpsyc.61.4.336. [DOI] [PubMed] [Google Scholar]
- 63.Corfas G, Roy K, Buxbaum J. Neureguline 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7(6):575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 64.Hall D, Gogos JA, Karayiorgou M. The contribution of three strong candidate schizophrenia susceptibility genes in demographically distinct populations. Genes Brain Behav. 2004;3:240–248. doi: 10.1111/j.1601-183X.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 65.Petryshen TL, Middleton FA, Kirby A, et al. Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol Psychiatry. 2005;10:366–374. doi: 10.1038/sj.mp.4001608. [DOI] [PubMed] [Google Scholar]
- 66.Stefansson H, Sarginson J, Kong A, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tosato S, Dazzan P, Collier D. Association between the neuregulin 1 gene and schizophrenia: a systematic review. Schizophr Bull. 2005;31(3):613–617. doi: 10.1093/schbul/sbi043. [DOI] [PubMed] [Google Scholar]
- 68.Korostishevsky M, Kaganovich M, Cholostoy A, et al. Is the G72/G30 locus associated with schizophrenia?—Single nucleotide polymorphisms, haplotypes, and gene expression analysis. Biol Psychiatry. 2004;56(3):169–176. doi: 10.1016/j.biopsych.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Schumacher J, Jamra RA, Freudenberg J, et al. Examination of G72 and D-amino-acid oxidase as genetic risk factors for schizophrenia and bipolar affective disorder. Mol Psychiatry. 2004;9:203–207. doi: 10.1038/sj.mp.4001421. [DOI] [PubMed] [Google Scholar]
- 70.Wang X, He G, Gu N, et al. Association of G72/G30 with schizophrenia in the Chinese population. Biochem Biophys Res Commun. 2004;319:1281–1286. doi: 10.1016/j.bbrc.2004.05.119. [DOI] [PubMed] [Google Scholar]
- 71.Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotropic factor in first-episode psychosis. Schizophr Res. 2007;91(1–3):1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gratacos M, Gonzalez JR, Mercader JM, de Cid R, Urretavizcaya M, Estivill X. Brain-derived neurotrophic factor Val66Met and psychiatric disorders: meta-analysis of case-control studies confirm association to substance-related disorders, eating disorders, and schizophrenia. Biol Psychiatry. 2007;61(7):911–922. doi: 10.1016/j.biopsych.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 73.Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007;164(12):1890–1899. doi: 10.1176/appi.ajp.2007.05111903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen X, Dunham C, Kendler S, et al. Regulator of G-protein signaling 4 (RGS4) gene is associated with schizophrenia in Irish high density families. Am J Med Genet B Neuropsychiatr Genet. 2004;129:23–26. doi: 10.1002/ajmg.b.30078. [DOI] [PubMed] [Google Scholar]
- 75.Chowdari KV, Mirnics K, Semwal P, et al. Association and linkage analyses of RGS4 polymorphisms in schizophrenia. Hum Mol Genet. 2002;11(12):1373–1380. doi: 10.1093/hmg/11.12.1373. [DOI] [PubMed] [Google Scholar]
- 76.Morris DW, Rodgers A, McGhee KA, et al. Confirming RGS4 as a susceptibility gene for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2004;125:50–53. doi: 10.1002/ajmg.b.20109. [DOI] [PubMed] [Google Scholar]
- 77.Williams NM, Preece A, Spurlock G, et al. Support for RGS4 as a susceptibility gene for schizophrenia. Biol Psychiatry. 2004;55:192–195. doi: 10.1016/j.biopsych.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 78.Green EK, Dimitrova A, Grozeva D, et al. Evidence for linkage disequilibrium at both G72/G30 and D-amino acid oxidase with genetic risk for bipolar disorder (abstract) Am J Med Genet B Neuropsychiatr Genet. 2004;130(1):26. [Google Scholar]
- 79.Hattori E, Liu C, Badner JA, et al. Polymorphisms at the G72/G30 gene locus, on 13q33, are associated with bipolar disorder in two independent pedigree series. Am J Hum Genet. 2003;72:1131–1140. doi: 10.1086/374822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen YS, Akula N, Detera-Wadleigh SD, et al. Findings in an independent sample support an association between bipolar affective disorder and the G72/G30 locus on chromosome 13q33. Mol Psychiatry. 2004;9:87–92. doi: 10.1038/sj.mp.4001453. [DOI] [PubMed] [Google Scholar]
- 81.Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH., Jr Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161(9):1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- 82.Neves-Pereira M, Mundo E, Muglia P, King N, Macciardi F, Kennedy JL. The brain-derived neurotrophic factor gene confers susceptibility to bipolar disorder: evidence from a family-based association study. Am J Hum Genet. 2002;71(3):651–655. doi: 10.1086/342288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Macgregor S, Visscher PM, Knott SA, et al. A genome scan and follow-up study identify a bipolar disorder susceptibility locus on chromosome 1q42. Mol Psychiatry. 2004;9(12):1083–1090. doi: 10.1038/sj.mp.4001544. [DOI] [PubMed] [Google Scholar]
- 84.Green EK, Raybould R, Macgregor S, et al. The schizophrenia susceptibility gene, Neuregulin 1 (NRG1), operates across traditional diagnostic boundaries to increase risk for bipolar disorder. Arch Gen Psychiatry. 2005;62(6):642–648. doi: 10.1001/archpsyc.62.6.642. [DOI] [PubMed] [Google Scholar]
- 85.Kato T. Molecular genetics of bipolar disorder and depression. Psychiatry Clin Neurosci. 2007;61(1):3–19. doi: 10.1111/j.1440-1819.2007.01604.x. [DOI] [PubMed] [Google Scholar]
- 86.Xu C, Macciardi F, Li PP, et al. Association of the putative susceptibility gene, transient receptor potential protein melastatin type 2, with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141(1):36–43. doi: 10.1002/ajmg.b.30239. [DOI] [PubMed] [Google Scholar]
- 87.Thomson PA, Wray NR, Thomson AM, et al. Sex-specific association between bipolar affective disorder in women and GPR50, an X-linked orphan G protein-coupled receptor. Mol Psychiatry. 2005;10(5):470–478. doi: 10.1038/sj.mp.4001593. [DOI] [PubMed] [Google Scholar]
- 88.Lyons-Warren A, Chang JJ, Balkissoon R, et al. Evidence of association between bipolar disorder and Citron on chromosome 12q24. Mol Psychiatry. 2005;10(9):807–809. doi: 10.1038/sj.mp.4001703. [DOI] [PubMed] [Google Scholar]
- 89.McNabb LD, Moore KW, Scena JE, Buono RJ, Berrettini WH. Association analysis of CHMP1.5 genetic variation and bipolar disorder. Psychiatr Genet. 2005;15(3):211–214. doi: 10.1097/00041444-200509000-00013. [DOI] [PubMed] [Google Scholar]
- 90.Kealey C, Roche S, Claffey E, McKeon P. Linkage and candidate gene analysis of 14q22-24 in bipolar disorder: support for GCHI as a novel susceptibility gene. Am J Med Genet B Neuropsychiatr Genet. 2005;136(1):75–80. doi: 10.1002/ajmg.b.30192. [DOI] [PubMed] [Google Scholar]
- 91.Verma R, Mukerji M, Grover D, et al. MLC1 gene is associated with schizophrenia and bipolar disorder in Southern India. Biol Psychiatry. 2005;58(1):16–22. doi: 10.1016/j.biopsych.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 92.Otani K, Ujike H, Tanaka Y, et al. The GABA type A receptor alpha5 subunit gene is associated with bipolar I disorder. Neurosci Lett. 2005;381(1–2):108–113. doi: 10.1016/j.neulet.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 93.Hashimoto R, Okada T, Kato T, et al. The breakpoint cluster region gene on chromosome 22q11 is associated with bipolar disorder. Biol Psychiatry. 2005;57(10):1097–1102. doi: 10.1016/j.biopsych.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 94.Glaser B, Kirov G, Green E, Craddock N, Owen MJ. Linkage disequilibrium mapping of bipolar affective disorder at 12q23-q24 provides evidence for association at CUX2 and FLJ32356. Am J Med Genet B Neuropsychiatr Genet. 2005;132(1):38–45. doi: 10.1002/ajmg.b.30081. [DOI] [PubMed] [Google Scholar]
- 95.Weller AE, Dahl JP, Lohoff FW, Ferraro TN, Berrettini WH. Analysis of variations in the NAPG gene on chromosome 18p11 in bipolar disorder. Psychiatr Genet. 2006;16(1):3–8. doi: 10.1097/01.ypg.0000180678.88169.b0. [DOI] [PubMed] [Google Scholar]
- 96.Mansour HA, Wood J, Logue T, et al. Association study of eight circadian genes with bipolar I disorder, schizoaffective disorder and schizophrenia. Genes Brain Behav. 2006;5(2):150–157. doi: 10.1111/j.1601-183X.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 97.Nievergelt CM, Kripke DF, Barrett TB, et al. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141(3):234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benedetti F, Serretti A, Colombo C, et al. Influence of CLOCK gene polymorphism on circadian mood fluctuation and illness recurrence in bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2003;123(1):23–26. doi: 10.1002/ajmg.b.20038. [DOI] [PubMed] [Google Scholar]
- 99.Benedetti F, Dallaspezia S, Fulgosi MC, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144(5):631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 100.Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007;9(3):333–342. doi: 10.31887/DCNS.2007.9.3/elamont. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McClung CA. Clock genes and bipolar disorder: implications for therapy. Pharmacogenomics. 2007;8(9):1097–1100. doi: 10.2217/14622416.8.9.1097. [DOI] [PubMed] [Google Scholar]
- 102.Bailer U, Wiesegger G, Leisch F, et al. No association of clock gene T3111C polymorphism and affective disorders. Eur Neuropsychopharmacol. 2005;15(1):51–55. doi: 10.1016/j.euroneuro.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 103.Shiino Y, Nakajima S, Ozeki Y, Isono T, Yamada N. Mutation screening of the human period 2 gene in bipolar disorder. Neurosci Lett. 2003;338(1):82–84. doi: 10.1016/s0304-3940(02)01290-9. [DOI] [PubMed] [Google Scholar]
- 104.Craddock N, O'Donovan MC, Owen MJ. Genes for schizophrenia and bipolar disorder? Implications for psychiatric nosology. Schizophr Bull. 2006;32(1):9–16. doi: 10.1093/schbul/sbj033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hodgkinson CA, Goldman D, Jaeger J, et al. Disrupted in schizophrenia 1 (DISC1): association with schizophrenia, schizoaffective disorder, and bipolar disorder. Am J Hum Genet. 2004;75(5):862–872. doi: 10.1086/425586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raybould R, Green EK, Macgregor S, et al. Bipolar disorder and polymorphisms in the dysbindin gene (DTNBP1) Biol Psychiatry. 2005;57(7):696–701. doi: 10.1016/j.biopsych.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 107.Owen MJ, Craddock N, Jablensky A. The genetic deconstruction of psychosis. Schizophr Bull. 2007;33(4):905–911. doi: 10.1093/schbul/sbm053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williams NM, Green EK, Macgregor S, et al. Variation at the DAOA/G30 locus influences susceptibility to major mood episodes but not psychosis in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2006;63(4):366–373. doi: 10.1001/archpsyc.63.4.366. [DOI] [PubMed] [Google Scholar]