Abstract
Previous work examining the neurobiological substrates of social cognition in healthy individuals has reported modulation of a social cognitive network such that increased activation of the amygdala, fusiform gyrus, and superior temporal sulcus are evident when individuals judge a face to be untrustworthy as compared with trustworthy. We examined whether this pattern would be present in individuals with schizophrenia who are known to show reduced activation within these same neural regions when processing faces. Additionally, we sought to determine how modulation of this social cognitive network may relate to social functioning. Neural activation was measured using functional magnetic resonance imaging with blood oxygenation level dependent contrast in 3 groups of individuals—nonparanoid individuals with schizophrenia, paranoid individuals with schizophrenia, and healthy controls—while they rated faces as either trustworthy or untrustworthy. Analyses of mean percent signal change extracted from a priori regions of interest demonstrated that both controls and nonparanoid individuals with schizophrenia showed greater activation of this social cognitive network when they rated a face as untrustworthy relative to trustworthy. In contrast, paranoid individuals did not show a significant difference in levels of activation based on how they rated faces. Further, greater activation of this social cognitive network to untrustworthy faces was significantly and positively correlated with social functioning. These findings indicate that impaired modulation of neural activity while processing social stimuli may underlie deficits in social cognition and social dysfunction in schizophrenia.
Keywords: schizophrenia, paranoia, amygdala, fusiform gyrus, trustworthiness, fMRI
Introduction
Social cognition, a construct broadly referring to the cognitive processes involved in how individuals perceive, interpret, and process social information,1,2 has become of increasing interest within schizophrenia research. Prompting this interest is a considerable body of work demonstrating that individuals with schizophrenia are impaired across a number of social cognitive domains including emotion perception, theory of mind (the ability to infer the intentions of others), and attributional style.3 Further, these deficits have been directly linked to social functioning4 and social behavior.5–7 Several studies suggest that social cognition mediates the relationship between neurocognition and social functioning,8–10 and others suggest that social cognitive abilities may be a better predictor of social functioning than cognitive abilities.5,11,12 These studies underscore the vital importance of social cognition in schizophrenia and indicate that work exploring the underlying mechanisms of social cognitive dysfunction is necessary.
Based on work with healthy individuals, neurobiological models of social cognition have confirmed that an interactive network of specific neural regions is recruited for the processing of social information.1,2,13 This network primarily includes the fusiform gyrus (FG) and superior temporal sulcus (STS), which underlie face processing14,15; the medial prefrontal cortex (MPFC), which underlies theory of mind16,17; and the amygdala (AMYG), which is integral to detecting threat, recognizing emotion, and making complex social judgments.18,19 These models suggest that impairments in this neural network may be related to the behavioral deficits in social cognition evidenced in schizophrenia, and indeed, several studies have demonstrated that individuals with schizophrenia show abnormal activation of this social cognitive circuit while processing social stimuli.20
Interestingly, these abnormalities, particularly in AMYG functioning, appear to vary across symptom-defined subgroups based on the presence or absence of specific symptoms such as flat affect or paranoid ideation (reviewed in Pinkham et al.21). Of importance here, recent studies have demonstrated that paranoid individuals with schizophrenia show reduced activation of the AMYG relative to nonparanoid individuals and healthy controls during tasks of emotion recognition.22–24 We recently replicated and extended this finding by assessing neural activation in healthy individuals, individuals with an autism spectrum disorder, nonparanoid individuals with schizophrenia, and paranoid individuals with schizophrenia while they made complex social judgments (ie, trustworthiness judgments) of faces.25 We found that the paranoid group not only showed less AMYG activation than the healthy and nonparanoid groups but also reduced activation of the fusiform face area of the FG and the ventrolateral prefrontal cortex (VLPFC), a region implicated in evaluative judgments and modulation of AMYG activity while viewing faces.26–28
Because this first analysis emphasized a comparison between individuals with schizophrenia and individuals with autism spectrum disorders, we assessed group differences in neural activation across the entire task of rating trustworthiness. However, 2 recent studies assessing trustworthiness evaluations in healthy individuals have demonstrated that the degree of activation within social cognitive neural regions is modulated by properties of the stimulus, namely how trustworthy a face appears.29,30 Both studies found greater activation of the AMYG when a face was rated as untrustworthy compared with trustworthy, and Winston et al.30 also reported greater activation of bilateral FG and bilateral STS for untrustworthy faces relative to trustworthy faces. In this follow-up analysis, we sought to determine if a similar pattern of greater activation for untrustworthy faces would be evident in individuals with schizophrenia and if this pattern would differ between paranoid and nonparanoid subgroups. Further, given that the previously reported differences between schizophrenia subgroups could be driven by neural responses to trustworthy faces, untrustworthy faces, or both, this new analysis is a critical next step for understanding the reported dysfunction.
Based on neurobiological models of social cognition and our previous work, the AMYG, FG, STS, VLPFC, and MPFC formed the regions of interest (ROIs) for our statistical analyses. Given that nonparanoid individuals tend to show comparable levels of neural activation relative to controls, we predicted that both the control and nonparanoid groups would show greater activation of this social cognitive network when a face was rated as untrustworthy. Paranoia, however, may involve the inability to correctly differentiate threatening from nonthreatening information, particularly when stimuli are ambiguous.31,32 We thus hypothesized that this lack of differentiation would be reflected in reduced modulation of this social cognitive network in paranoid individuals relative to the other groups.
Moreover, as a primary focus of this current analysis, we examined whether modulation of this social cognitive network would relate to social functioning. An important goal of functional neuroimaging is to elucidate brain-behavior relationships, and here, we sought to further our previous work by linking neural activation to a clinically meaningful outcome. Recent works utilizing continuum-based models of paranoia have examined the relationship between social behaviors and paranoia and found that both paranoid individuals with schizophrenia and healthy individuals with subclinical levels of paranoid ideation show more behaviors indicative of mistrust such as sitting further away from research personnel and taking longer to read consent forms.33,34 It is possible that these behavioral tendencies may be related to specific patterns of neural activation. In light of this work and the aforementioned associations between social cognition and social functioning, we anticipated that a greater degree of modulation would be positively correlated with better social functioning across all groups.
Methods
Raw imaging data and behavioral measures of task performance were obtained from a previously reported study examining the neural bases of trustworthiness judgments in schizophrenia and autism spectrum disorders.25 Assessments of social functioning were collected as part of this larger study but have not previously been reported.
Subjects
Participants were 12 individuals with schizophrenia (n = 9) or schizoaffective disorder (n = 3) without paranoid symptoms (nonparanoid schizophrenia, NP-SCZ), 12 individuals with schizophrenia (n = 8) or schizoaffective disorder (n = 4) with prominent paranoid symptoms (paranoid schizophrenia, P-SCZ), and 12 nonclinical healthy control individuals. All participants were male, were 18–35 years old, reported no history of head injury, self-identified as right-handed, had a visual acuity of 20/20 (natural or corrected via contact lenses), and did not currently meet criteria for substance abuse or dependence. Additional inclusion criteria for controls were a lack of psychotic or affective disorders in themselves and first-degree relatives. Individuals in the schizophrenia groups were recruited from the Schizophrenia Treatment and Evaluation Program at the University of North Carolina Neurosciences Hospital, and healthy participants were recruited via informational e-mails to university staff soliciting participation in research and from other research studies conducted in our laboratory. Prior to participation, all individuals provided written, informed consent, and the University of North Carolina Behavioral Institutional Review Board approved the research protocol.
Diagnoses for individuals in the schizophrenia groups were confirmed with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (SCID-P) and chart review. Symptomatology at the time of participation was assessed with the Positive and Negative Syndrome Scale.35 Participants experiencing marked symptoms of paranoia at the time of scanning, scoring at least a 4 or above on the suspiciousness/persecution item, constituted the P-SCZ group. Participants who demonstrated an absence or only subclinical levels of paranoia, by scoring a 2 or below on this item, constituted the NP-SCZ group. Across other symptoms, the P-SCZ group received higher ratings for both positive (F1,22 = 33.2, P < .001) and general (F1,22 = 6.69, P = .017) symptom clusters; however, these differences did not remain statistically different after controlling for paranoia. The groups did not differ in negative symptoms. Of particular relevance for the comparison of P-SCZ and NP-SCZ groups, all SCZ participants were taking atypical antipsychotic medications, and the 2 groups did not differ in chlorpromazine equivalents (F1,22 = 1.51, P = .232).36
Groups did not differ significantly in ethnicity (χ2 = .465, P = .793), age (F2,33 = .386, P = .683) or premorbid verbal IQ as assessed by the Wide Range Achievement Test (WRAT) reading subscale (F2,33 = 2.67, P = .084).37 Expectedly, education significantly differed between groups (F2,33 = 10.12, P < .001); controls completed more years of education than both SCZ groups (P = .001 for both comparisons), who did not differ from each other. All demographic information is presented in table 1.
Table 1.
Demographic Information and Behavioral Task Performance
| Control (n = 12), mean (SD) | NP-SCZ (n = 12), mean (SD) | P-SCZ (n = 12), mean (SD) | |
| Demographic information | |||
| Ethnicity | |||
| Caucasian | 10 | 11 | 10 |
| African American | 2 | 1 | 2 |
| Age | 27.08 (3.99) | 28.0 (3.93) | 26.42 (5.25) |
| WRAT reading | 112.58 (9.28) | 100.5 (15.37) | 103.83 (14.24) |
| Educationa | 16.92 (1.98) | 13.29 (2.05) | 13.29 (2.73) |
| Chlorpromazine equivalent | 297.22 (173.03) | 404.86 (249.2) | |
| PANSS | |||
| Positiveb | 9.83 (2.41) | 18.08 (4.34) | |
| Negative | 10.67 (3.28) | 11.83 (6.16) | |
| Generalb | 25.0 (4.65) | 31.0 (6.55) | |
| Behavioral tasks | |||
| Trustworthiness ratingsc | |||
| Number of faces rated as trustworthy | 25.33 (4.98) | 25.25 (5.49) | 19.42 (6.01) |
| Number of faces rated as untrustworthy | 14.62 (5.02) | 16.67 (5.61) | 22.5 (5.98) |
| Reaction time | |||
| Faces rated as trustworthy | 1.92 (0.77) | 2.70 (1.72) | 2.81 (1.06) |
| Faces rated as untrustworthy | 2.20 (1.13) | 2.88 (1.45) | 2.91 (1.34) |
| SFS total scorea | 163.25 (17.91) | 136.5 (21.1) | 126.67 (24.92) |
Note: NP-SCZ, nonparanoid schizophrenia; P-SCZ, paranoid schizophrenia; PANSS, Positive and Negative Syndrome Scale; SFS, Social Functioning Scale.
Controls significantly different from NP-SCZ and P-SCZ at P < .05.
P-SCZ significantly different from NP-SCZ at P < .05.
P-SCZ significantly different from controls and NP-SCZ at P < .05.
Tasks
Imaging Stimuli and Functional Magnetic Resonance Imaging Experiment
Functional magnetic resonance imaging (fMRI) was utilized while individuals completed the abbreviated Trustworthiness/Approachability Task.38 In this task, individuals viewed 42 grayscale frontal images of faces and made dichotomous decisions regarding the trustworthiness (ie, trustworthy or untrustworthy) of the individual in each photo. This procedure was based on Winston et al.30 Participants responded by pushing a button corresponding to their rating, and these determinations, as well as reaction time, were recorded as behavioral indexes of task performance.
The imaging session included 2 functional runs in which participants made trustworthiness judgments, with each run containing 21 photographs. Each photograph was displayed for 2 s, followed by a 16-s interstimulus interval, during which participants were instructed to keep their eyes focused on a white fixation cross presented in the middle of the screen. The imaging session also contained 2 additional runs of a different task, performed after the trustworthiness runs (described in Pinkham et al.25).
Social Functioning Assessment
Following scanning, social functioning was assessed with the Social Functioning Scale (SFS).39 The SFS is a self-report measure that assesses strengths and weaknesses in 7 areas of functioning: social engagement, interpersonal communication, prosocial activities, recreation, independence-competence, independence-performance, and employment/occupation. A full-scale score is calculated by summing across all subscales, and higher scores indicate better social functioning (range: 0–246).
Image Acquisition
Data were collected using a Siemens Allegra 3T MRI scanner to acquire echo planar T2*-weighted images with BOLD (blood oxygenation level dependent) contrast (echo planar imaging [EPI] free induction decay, 2D; 32 transverse slices, voxel size 3.8 × 3.8 × 3.8 mm, matrix = 64 × 64; field of view [FOV] = 243 × 243, repetition time [TR] = 2 s, echo time [TE] = 30 ms, flip angle = 80). All functional runs were collected using an interleaved sequence (bottom to top), and each functional run was preceded by 4 volumes that were discarded to allow for equilibration effects. A structural scan sequence (MPRAGE) was also conducted to obtain a T1-weighted anatomical image (128 slices, voxel size 1 × 1 × 1 mm, matrix = 256 × 256, FOV = 208 × 256, TR = 1520 ms, TE = 4.38 ms) for coregistration and display of functional data. Cushioned head restraints were used to control for movement.
Image Preprocessing and Inferential Testing
Data were preprocessed and analyzed using SPM2 (Wellcome Department of Cognitive Neurology, Queen Square, London, UK). Images were slice time corrected, motion corrected to the first image using b-spline interpolation, and high-pass filtered (128 s). Functional and anatomical images were coregistered and then transformed into a standard anatomical space (EPI Montreal Neurological Institute template) using trilinear interpolation.40 Normalized functional images were then spatially smoothed (8-mm full width at half maximum, Gaussian isotropic kernel). Subject-level statistical analysis was done using General Linear Model as implemented in SPM2. The events comprising each condition (trustworthy and untrustworthy) were parsed according to the idiosyncratic judgments of each participant (ie, the subject's individual responses rather than average ratings or categorization based on stimulus qualities), and the 2 conditions were then modeled using a canonical hemodynamic response function with a temporal derivative. Parameter estimates of event-related activity were estimated voxelwise for each condition relative to fixation baseline.
ROI Analyses of BOLD Signal Change
Coordinates for regions of the examined social cognitive network were derived from previous fMRI studies of social cognition and of trustworthiness evaluations. Coordinates for bilateral AMYG (8-mm radius; left: x, y, z = −16, −5, −19; right x, y, z = 24, −1, −18) and bilateral FG (12-mm radius; left: x, y, z = −48, −48, −24; right: x, y, z = 44, −46, −22) were taken from previous studies showing modulation of these regions with trustworthiness.29,30 Coordinates for bilateral STS (14-mm radius; left: x, y, z = −51, −44, 6; right: x, y, z = 54, −45, 6) were the center of face-related activations from several studies reviewed in Pelphrey et al.41 ROIs for bilateral VLPFC (14-mm radius; x, y, z = ±44, 32, −12) were derived using coordinates presented in a study of social evaluation demonstrating that this region activated specifically to evaluative judgments vs nonevaluative judgments.26 Finally, consistent with Ashwin et al.,42 the MPFC ROI (14-mm radius; x, y, z = −4, 42, 36) was taken from Calder et al.,17 wherein average coordinates were calculated from previous studies of theory of mind.
The mean percent signal change for each ROI ((parameter estimates (beta > 0)/baseline) × 100) was estimated with custom MATLAB scripts using the model described above, and these data were subject to further analyses. Only the activated voxels (Z > 0, contiguous) were used for both conditions across the 2 runs. These values were then entered into a repeated-measures analysis of variance (ANOVA) with ROI (R AMYG, L AMYG, R FG, L FG, R STS, L STS, R VLPFC, L VLPFC, and MPFC) and condition (trustworthy and untrustworthy) as within-subject factors and group (control, NP-SCZ, P-SCZ) as the between-subject factor. The threshold for statistical significance was set at P < .05 (2-tailed), and where Mauchly test indicated that the assumption of sphericity had been violated, Greenhouse-Geisser corrections were utilized. Finally, significant effects involving group were followed up with Tukey Honestly Significant Difference post hoc tests.
Neural Response–Social Functioning Correlations
To examine the relationships between neural activation and social functioning, we first calculated a change score representing modulation of social cognitive activation by subtracting the mean percent signal change for trustworthy faces from the mean percent signal change for untrustworthy faces within each ROI. Change scores were implemented as we sought to investigate the pattern previously reported in healthy individuals of greater activation for untrustworthy relative to trustworthy faces rather than just absolute amounts of activation for either condition. Spearman rank correlations (1-tailed) were then used to examine the relationships across all groups between these change scores and the SFS total score. To control type I error, a Bonferroni-corrected alpha level of P < .0055 was utilized to determine significance. Additionally, as a follow-up analysis, these relationships were also examined for each group independently. Given the small sample size of each group and the number of neural regions included, these follow-up analyses were considered exploratory, and the significance level was set at P < .05.
Results
Behavioral Data
A 1-way (group: control vs NP-SCZ vs P-SCZ) ANOVA conducted on behavioral ratings of trustworthiness during scanning revealed significant group differences in the number of faces rated as trustworthy (F2,33 = 4.58, P = .018). Tukey's HSD post hoc comparisons indicated that the P-SCZ group rated significantly fewer faces as trustworthy relative to both the control and NP-SCZ groups (P = .031 and P = .037, respectively). Importantly, despite these differences, the ratings for each photo across groups tended to vary similarly and were generally in agreement with each other (intraclass correlation coefficient = .924), demonstrating that no group appeared to respond in an arbitrary manner.
Response times did not account for group differences in judgments, as a repeated-measures ANOVA on reaction time with type of judgment (trustworthy vs untrustworthy) as the within-subject factor and group as the between-subject factor revealed only a trend-level main effect of type of judgment (F1,33 = 4.08, P = .052), indicating that participants responded more slowly when a face was rated as untrustworthy. There was no main effect for group (F2,33 = .35, P = .707), nor was there a significant interaction between group and type of judgment (F2,33 = 1.51, P = .235).
For social functioning, a 1-way ANOVA on SFS total score demonstrated significant differences between groups (F2,33 = 9.30, P = .001). Post hoc tests revealed that the control group showed better social functioning abilities than both SCZ groups (P = .012 for the comparison with NP-SCZ and P = .001 for the comparison with P-SCZ) and that whereas the NP-SCZ group scored higher than the P-SCZ group, the 2 SCZ groups did not significantly differ from each other. All behavioral data are presented in table 1.
ROI Analyses of BOLD Signal Change
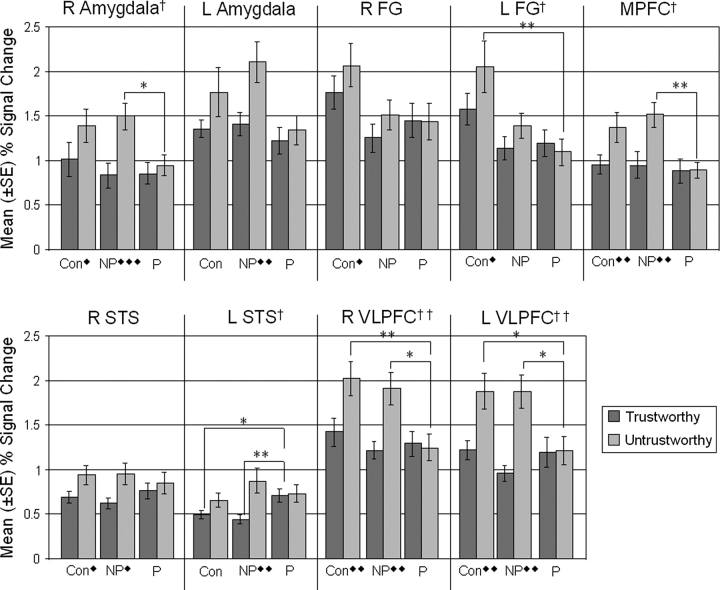
The repeated-measures ANOVA on mean percent signal change revealed a statistically significant main effect for condition (F1,33 = 22.53, P < .001), indicating greater activation of the examined social cognitive network when faces were rated as untrustworthy relative to trustworthy. A significant main effect was also evident for ROI (F4.48,147.66 = 26.64, P < .001) such that some regions of this social cognitive network (ie, AMYG, FG, and VLPFC) showed greater signal change than others (ie, STS and MPFC). The main effect of group was also significant (F2,33 = 3.46, P = .043) with post hoc tests demonstrating that the control group showed significantly more activation across this social cognitive network than the P-SCZ group (P = .035). The NP-SCZ group did not significantly differ from either comparison group.
The condition by group (F2,33 = 5.23, P = .011), ROI by group (F8.95,147.66 = 2.14, P = .03), and condition by ROI (F4.3,141.87 = 4.94, P = .001) interactions were all significant, and more importantly, the 3-way interaction between ROI, condition, and group (F8.60,141.87 = 2.17, P = .029) was also significant, indicating that the degree of neural modulation between trustworthy and untrustworthy ratings varied significantly between groups and regions within this social cognitive network. Subsequent repeated-measures analyses of mean percent signal change in the individual ROIs revealed significant condition by group interactions in the R AMYG (F2,33 = 4.34, P = .021), L FG (F2,33 = 3.58, P = .039), MPFC (F2,33 = 3.80, P = .033), L STS (F2,33 = 4.1, P = .026), and bilateral VLPFC (left: F2,33 = 7.25, P = .002; right: F2,33 = 6.87, P = .003). Consistent with our predictions, in each of these regions, the interactions indicated that both controls and NP-SCZ showed a greater increase in activation than P-SCZ when a face was perceived as untrustworthy relative to trustworthy. Specific differences between groups in each condition and in each ROI are detailed in figure 1.
Fig. 1.
Signal Change in Each Region of Interest (ROI) for Faces Rated as Trustworthy and Untrustworthy. ROI abbreviations: R = right, L = left, FG = fusiform gyrus, MPFC = medial prefrontal cortex, STS = superior temporal sulcus, VLPFC = ventrolateral prefrontal cortex. Group abbreviations: Con = control, NP = nonparanoid schizophrenia, P = paranoid schizophrenia. *Between-group differences significant at P < .05. **Between-group differences significant at P < .01. ♦Within-group differences across condition significant at P < .05. ♦♦Within-group differences across condition significant at P < .01. ♦♦♦Within-group differences across condition significant at P < .001. †Significant interaction between group and condition significant at P < .05. ††Significant interaction between group and condition significant at P < .01
Correlations Between Neural Response and Social Functioning
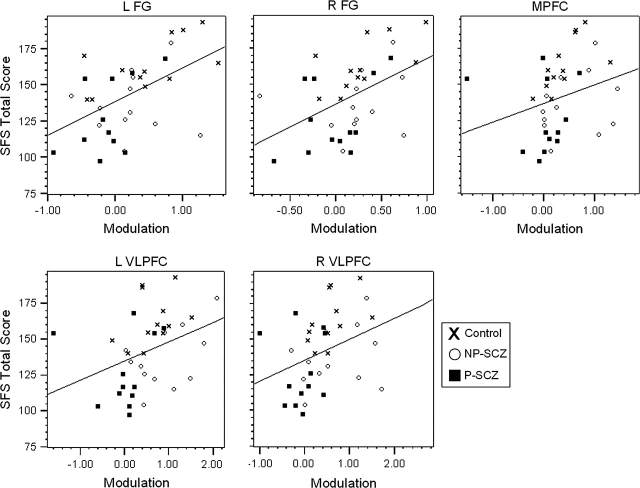
Correlations between the change scores representing modulation of this social cognitive network in response to the trustworthiness of each face and total score on the SFS revealed a significant and positive relationship between increased activation to untrustworthy faces and better social functioning. These relationships were evident in bilateral FG (left: Spearman ρ = .513, P = .001; right: Spearman ρ = .530, P < .001), MPFC (Spearman ρ = .420, P = .005), and bilateral VLPFC (left: Spearman ρ = .477, P = .002; right: Spearman ρ = .486, P = .001). Notably, increased modulation of activation in bilateral STS (left: Spearman ρ = .287, P = .045; right: Spearman ρ = .408, P = .007) was also correlated to better social functioning; however; these relationships did not survive correction for multiple tests. Significant correlations are shown in figure 2, and as can be seen from the scatterplots, these correlations do not appear to be due to group differences in mean SFS score or neural activation.
Fig. 2.
Association Between Brain Activation and Social Functioning. Scatterplots of the associations between change scores representing modulation of the social cognitive network in response to the trustworthiness of each face and total score on the Social Functioning Scale (SFS). Region of interest abbreviations: R = right, L = left, FG = fusiform gyrus, MPFC = medial prefrontal cortex, STS = superior temporal sulcus, VLPFC = ventrolateral prefrontal cortex.
The follow-up exploratory analysis of these relationships within each group revealed significant positive correlations between increased activation to untrustworthy faces and better social functioning in R AMYG, bilateral FG, MPFC, and R VLPFC in the healthy control group only. Interestingly, although primarily positive and therefore going in the same direction, the only correlation reaching statistical significance among the clinical groups was in the L AMYG for the P-SCZ group. No group differences in correlation strengths were statistically significant. All correlations are provided in table 2.
Table 2.
Correlations Between Neural Activation and Social Functioning
| ROI | Control (n = 12) |
NP-SCZ (n = 12) |
P-SCZ (n = 12) |
Combined (n = 36) |
||||
| Spearman ρ | P | Spearman ρ | P | Spearman ρ | P | Spearman ρ | P | |
| R AMYG | 0.546 | .033 | −0.028 | .466 | 0.355 | .129 | 0.245 | .075 |
| L AMYG | 0.329 | .148 | −0.098 | .381 | 0.503 | .048 | 0.215 | .104 |
| R FG | 0.662 | .01 | 0.351 | .159 | 0.439 | .076 | 0.53 | <.001 |
| L FG | 0.557 | .03 | 0.21 | .356 | 0.453 | .069 | 0.513 | .001 |
| MPFC | 0.76 | .002 | 0.259 | .208 | 0.264 | .204 | 0.42 | .005 |
| R STS | 0.483 | .056 | 0.07 | .415 | 0.39 | .105 | 0.408 | .007 |
| L STS | 0.252 | .215 | −0.053 | .436 | 0.397 | .101 | 0.287 | .045 |
| R VLPFC | 0.633 | .014 | 0.224 | .242 | 0.116 | .36 | 0.486 | .001 |
| L VLPFC | 0.417 | .089 | 0.336 | .143 | 0.366 | .121 | 0.477 | .002 |
Note:ROI, region of interest; NP-SCZ, nonparanoid schizophrenia; P-SCZ, paranoid schizophrenia; R, right; L, left; FG, fusiform gyrus; MPFC, medial prefrontal cortex; STS, superior temporal sulcus; VLPFC, ventrolateral prefrontal cortex.
Discussion
Both individuals with NP-SCZ and healthy comparison participants showed significantly greater activation of the examined social cognitive network when a face was judged to be untrustworthy relative to trustworthy, thus demonstrating neural sensitivity to threatening social stimuli. Paranoid individuals with schizophrenia, however, failed to show any modulation of neural activation for untrustworthy faces relative to trustworthy faces. These findings further our previous work by demonstrating that reduced neural activation for paranoid individuals during trustworthiness evaluations are specific to faces perceived as untrustworthy. The fact that groups showed comparable levels of activation for trustworthy faces indicates that the impairments seen in P-SCZ are not due to global reductions in activation but rather to a lack of normative increases in activation to threatening stimuli.
Results of this study are also consistent with previous work highlighting fundamental distinctions in neural activation between schizophrenia subgroups. Earlier studies showed relatively intact AMYG and MPFC activation for nonparanoid individuals during implicit processing of emotion,22–24 and here, we replicate those findings with a task of complex social cognition and extend them to the VLPFC. More normative activation for NP-SCZ (relative to P-SCZ) in several regions of the examined social cognitive circuit demonstrates that these differences between subgroups are widespread and emphasize the distributed and interactive nature of this social cognitive network.
In contrast, the only region where P-SCZ and NP-SCZ resembled each other was in bilateral FG. Here, neither group showed a significant increase in activation for untrustworthy faces, although it should be noted that the increases evident in the NP-SCZ approached significance (L FG: P = .115, and R FG: P = .073). Although speculative, this may suggest that all individuals with schizophrenia have some degree of impairment in the direct feedback connections from AMYG to FG43 that would increase FG activation once the AMYG has designated a stimulus to be threatening. This finding may also shed light on previous studies reporting reduced activation of the right lateral FG in schizophrenia during emotion perception tasks as compared with healthy individuals.44–46 It is possible that heightened FG responses to threatening emotions (ie, anger and fear) in controls may have driven these group differences.
Further, examination of behavioral responses revealed that the groups generally agreed in their assessments of trustworthiness but that paranoid individuals were more likely to rate a face as untrustworthy. The agreement between groups indicates that the majority of stimuli were included in the same category across groups and that the apparent differences in neural activation are not a function of random responding or largely different categorizations of faces. Rather, the integration of the behavioral and neural data may provide insights into the nature of paranoia. The lack of modulation seen in P-SCZ demonstrates that, at the neural level, all faces were processed similarly despite the fact that these individuals were able to behaviorally categorize faces as trustworthy or untrustworthy in a manner that was largely consistent with control and NP-SCZ participants (albeit while rating more faces as untrustworthy). Increased behavioral ratings of mistrust without concurrent increases in neural activation are consistent with findings of a disconnect between autonomic arousal systems and neural response in which paranoid individuals show enhanced arousal coupled with reduced AMYG activation when viewing threat-related stimuli.23 These findings may indicate that the neural mechanisms of threat appraisal are ineffective in paranoid individuals and that while retaining the ability to make gross distinctions between stimuli at a behavioral level, paranoid individuals may be unable to make fine-grained distinctions, which may contribute to the over attribution of threat seen in paranoid ideation. Alternately, these findings could reflect habituation of AMYG activity in paranoid individuals, suggesting that threatening stimuli may lose saliency over time; however, this interpretation is somewhat contradictory to the noted findings of increased autonomic arousal in P-SCZ. Nevertheless, these findings certainly require further investigation.
In addition to having implications for schizophrenia, the present study also furthers our understanding of the examined social cognitive neural network. Increased AMYG, FG, and STS activation to untrustworthy faces in healthy individuals was expected and is consistent with previous work.29,30 Greater AMYG activation to untrustworthy faces is likely related to this region's role in detecting threat19 and orienting to salient information,47,48 and as noted previously, increased FG activation is likely due to modulatory influences from the AMYG via back projections.43 Differential activation of the STS may be explained by its involvement in theory of mind inferences.30 Given that one may attempt to infer the intentions of another as a means of evaluating whether they can be trusted and that uncertainty about these intentions may lead to a judgment of untrustworthiness, such a process may explain the differential activation seen here.
Findings from this study also extend those of Winston et al.30 by demonstrating increased activation of frontal regions, specifically the MPFC and bilateral VLPFC, in nonclinical controls for untrustworthy faces. These findings suggest that both regions are sensitive to differing levels of perceived threat. For the MPFC increased activation for untrustworthy faces may reflect more emphasis on determining the intentions of pictured individuals who appear more likely to pose a threat. For VLPFC, greater activation during untrustworthiness judgments may reflect this region's role in modulating and regulating emotional responses.27,49 Such an interpretation is consistent with work showing that extended cognitive evaluation of emotional stimuli is associated with relative decreases in AMYG response and correlated increases in VLPFC activation, as compared with brief stimulus presentations.27,28
Of primary importance, across all groups, neural activation within this social cognitive network, and in particular the degree of modulation between trustworthy and untrustworthy faces, was positively correlated with social functioning. These relationships were evident in both frontal regions investigated (ie, MPFC and VLPFC) and also in the FG. Unexpectedly, a significant relationship with social functioning was not found for AMYG activation when groups were examined conjointly. This may be explained by work with healthy individuals demonstrating that AMYG response during trustworthiness evaluations is more closely related to consensus ratings of trustworthiness rather than idiosyncratic judgments.29 Thus, we may not have assessed activation in a way that maximally measures the AMYG response. Our decision to examine participant responses was made a priori based on anticipated differences between the judgments of schizophrenia subgroups and a desire to link neural activation to behavioral responses. Also unexpectedly, the clinical groups did not show the same strength of relationships between modulation of neural activation and social functioning. This may be partially explained by the increased variability in the clinical groups (eg, SFS range: controls 140–193, NP-SCZ 104–179, and P-SCZ 97–168) and the small sample sizes. Nevertheless, the significant correlations between greater modulation of activation and better social functioning across groups and the positive (albeit nonsignificant) correlations within the clinical groups indicate that the amount of neural modulation while processing social stimuli has potential to become a predictive marker of real-world social behavior. These relationships also highlight the direct connections between social cognition and social functioning.
Although the present study elucidates the nature of neural abnormalities in P-SCZ, a number of questions require further clarification. First, although the SCZ subgroups did not differ in chlorpromazine equivalents, the effects of medication were not assessed. Second, given recent work in autism demonstrating that neural activation in the AMYG is associated with visual scanning of the eye region of faces50 but work in schizophrenia showing reductions in AMYG activation even when faces are not consciously perceived,51 it is unknown what role visual face scanning patterns may have played in the present results. Likewise, it is unclear what effect autonomic arousal may have had on both neural activation and behavioral ratings. Future work would benefit from including concurrent eye-tracking and physiological monitoring to investigate these effects. Third, the 2 schizophrenia subgroups did not significantly differ from each other on social functioning, which is unexpected given the reported differences in neural activation. It is possible that the self-report format and the wide scope of functioning assessed by the SFS may have limited our ability to discern group differences in social behaviors that may be more proximal to social cognition (ie, social skill as opposed to independent living skills). Additionally, all clinical participants in this study were stable outpatients, which may have also contributed to the lack of significant group differences in overall social functioning. Finally, in order to assess multiple groups, the number of individuals who could be enrolled in each group was necessarily limited, and only right-handed male participants were recruited. These factors may limit the generalizability of the results and indicate that replication is needed.
Notwithstanding these limitations, the present study reports a novel observation related to the processing of threat-related social information in schizophrenia subgroups. The finding that paranoid individuals with schizophrenia failed to show normative increases in neural activation when they judged a face to be untrustworthy reveals an important distinction between schizophrenia subgroups and may shed light on the nature of paranoid ideation by demonstrating impairments in the neural mechanisms of threat appraisal in paranoid individuals. Moreover, the amount of neural modulation between trustworthy and untrustworthy stimuli within the examined social cognitive network was significantly and positively correlated to social functioning. These findings suggest that remediation of this response may aid in improving social behavior and emphasize the importance of social cognition for functional outcome.
Funding
The National Alliance for Research on Schizophrenia and Depression (to D.P.); Johnson and Johnson Pharmaceutical Research and Development, LLC, USA (to D.P.); The Foundation of Hope (to D.P.); National Institute of Mental Health (R01 MH66034 to J.H.); National Institute of Mental Health (T32 MH019112 to A.P.)
Acknowledgments
We are grateful to Kathy Wilber, BS, RT(R)(MR), and Weili Lin, PhD, of the University of North Carolina Magnetic Resonance Imaging Research Center for their invaluable assistance with data collection. We would also like to thank Dr Ralph Adolphs who provided us with the Trustworthiness Task, and finally, would like to thank all the individuals who participated in this study.
References
- 1.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 2.Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- 3.Penn DL, Addington J, Pinkham A. Social cognitive impairments. In: Lieberman JA, Stroup TS, Perkins DO, editors. The American Psychiatric Publishing Textbook of Schizophrenia. Washington, DC: American Psychiatric Publishing; 2006. pp. 261–274. [Google Scholar]
- 4.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl. 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brune M, Abdel-Hamid M, Lehmkamper C, Sonntag C. Mental state attribution, neurocognitive functioning, and psychopathology: what predicts poor social competence in schizophrenia best? Schizophr Res. 2007;92(1–3):151–159. doi: 10.1016/j.schres.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Ihnen GH, Penn DL, Corrigan PW, Martin J. Social perception and social skill in schizophrenia. Psychiatry Res. 1998;80(3):275–286. doi: 10.1016/s0165-1781(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 7.Mueser KT, Doonan R, Penn DL, et al. Emotion recognition and social competence in chronic schizophrenia. J Abnorm Psychol. 1996;105(2):271–275. doi: 10.1037//0021-843x.105.2.271. [DOI] [PubMed] [Google Scholar]
- 8.Addington J, Saeedi H, Addington D. Influence of social perception and social knowledge on cognitive and social functioning in early psychosis. Br J Psychiatry. 2006;189:373–378. doi: 10.1192/bjp.bp.105.021022. [DOI] [PubMed] [Google Scholar]
- 9.Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia. Schizophr Res. 2005;80(2–3):213–225. doi: 10.1016/j.schres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Nienow TM, Docherty NM, Cohen AS, Dinzeo TJ. Attentional dysfunction, social perception, and social competence: what is the nature of the relationship? J Abnorm Psychol. 2006;115(3):408–417. doi: 10.1037/0021-843X.115.3.408. [DOI] [PubMed] [Google Scholar]
- 11.Pinkham AE, Penn DL. Neurocognitive and social cognitive predictors of interpersonal skill in schizophrenia. Psychiatry Res. 2006;143(2–3):167–178. doi: 10.1016/j.psychres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Roncone R, Falloon IR, Mazza M, et al. Is theory of mind in schizophrenia more strongly associated with clinical and social functioning than with neurocognitive deficits? Psychopathology. 2002;35(5):280–288. doi: 10.1159/000067062. [DOI] [PubMed] [Google Scholar]
- 13.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 14.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4(6):223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- 15.Winston JS, Henson RN, Fine-Goulden MR, Dolan RJ. fMRI-adaptation reveals dissociable neural representations of identity and expression in face perception. J Neurophysiol. 2004;92(3):1830–1839. doi: 10.1152/jn.00155.2004. [DOI] [PubMed] [Google Scholar]
- 16.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 17.Calder AJ, Lawrence AD, Keane J, et al. Reading the mind from eye gaze. Neuropsychologia. 2002;40(8):1129–1138. doi: 10.1016/s0028-3932(02)00008-8. [DOI] [PubMed] [Google Scholar]
- 18.Adolphs R. Social cognition and the human brain. Trends Cogn Sci. 1999;3(12):469–479. doi: 10.1016/s1364-6613(99)01399-6. [DOI] [PubMed] [Google Scholar]
- 19.Amaral DG, Bauman MD, Schumann CM. The amygdala and autism: implications from non-human primate studies. Genes Brain Behav. 2003;2(5):295–302. doi: 10.1034/j.1601-183x.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- 20.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160(5):815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- 21.Pinkham AE, Gur RE, Gur RC. Affect recognition deficits in schizophrenia: neural substrates and psychopharmacological implications. Expert Rev Neurother. 2007;7(7):807–816. doi: 10.1586/14737175.7.7.807. [DOI] [PubMed] [Google Scholar]
- 22.Williams LM, Das P, Harris AW, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry. 2004;161(3):480–489. doi: 10.1176/appi.ajp.161.3.480. [DOI] [PubMed] [Google Scholar]
- 23.Williams LM, Das P, Liddell BJ, et al. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry Res. 2007;155(1):29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Russell TA, Reynaud E, Kucharska-Pietura K, et al. Neural responses to dynamic expressions of fear in schizophrenia. Neuropsychologia. 2007;45(1):107–123. doi: 10.1016/j.neuropsychologia.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99(1-3):164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham WA, Johnson MK, Gatenby JC, Gore JC, Banaji MR. Neural components of social evaluation. J Pers Soc Psychol. 2003;85(4):639–649. doi: 10.1037/0022-3514.85.4.639. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham WA, Johnson MK, Raye CL, Chris Gatenby J, Gore JC, Banaji MR. Separable neural components in the processing of black and white faces. Psychol Sci. 2004;15(12):806–813. doi: 10.1111/j.0956-7976.2004.00760.x. [DOI] [PubMed] [Google Scholar]
- 28.Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- 29.Engell AD, Haxby JV, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. J Cogn Neurosci. 2007;19(9):1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- 30.Winston JS, Strange BA, O'Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nat Neurosci. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- 31.Freeman D, Garety PA, Kuipers E, Fowler D, Bebbington PE. A cognitive model of persecutory delusions. Br J Clin Psychol. 2002;41(pt 4):331–347. doi: 10.1348/014466502760387461. [DOI] [PubMed] [Google Scholar]
- 32.Green MJ, Phillips ML. Social threat perception and the evolution of paranoia. Neurosci Biobehav Rev. 2004;28(3):333–342. doi: 10.1016/j.neubiorev.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Combs DR, Penn DL. The role of subclinical paranoia on social perception and behavior. Schizophr Res. 2004;69(1):93–104. doi: 10.1016/S0920-9964(03)00051-3. [DOI] [PubMed] [Google Scholar]
- 34.Gay NW, Combs DR. Social behaviors in persons with and without persecutory delusions. Schizophr Res. 2005;80(2–3):361–362. doi: 10.1016/j.schres.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale: Manual. Toronto, Canada: Multi-Health Systems, Inc; 1992. [Google Scholar]
- 36.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 37.Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive impairments in patients with schizophrenia displaying preserved and compromised intellect. Arch Gen Psychiatry. 2000;57(9):907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- 38.Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- 39.Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S. The Social Functioning Scale. The development and validation of a new scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry. 1990;157:853–859. doi: 10.1192/bjp.157.6.853. [DOI] [PubMed] [Google Scholar]
- 40.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapp. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelphrey KA, Morris JP, Michelich CR, Allison T, McCarthy G. Functional anatomy of biological motion perception in posterior temporal cortex: an fMRI study of eye, mouth and hand movements. Cereb Cortex. 2005;15(12):1866–1876. doi: 10.1093/cercor/bhi064. [DOI] [PubMed] [Google Scholar]
- 42.Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2007;45(1):2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 43.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45(1):174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64(12):1356–1366. doi: 10.1001/archpsyc.64.12.1356. [DOI] [PubMed] [Google Scholar]
- 45.Quintana J, Wong T, Ortiz-Portillo E, Marder SR, Mazziotta JC. Right lateral fusiform gyrus dysfunction during facial information processing in schizophrenia. Biol Psychiatry. 2003;53(12):1099–1112. doi: 10.1016/s0006-3223(02)01784-5. [DOI] [PubMed] [Google Scholar]
- 46.Streit M, Ioannides A, Sinnemann T, et al. Disturbed facial affect recognition in patients with schizophrenia associated with hypoactivity in distributed brain regions: a magnetoencephalographic study. Am J Psychiatry. 2001;158(9):1429–1436. doi: 10.1176/appi.ajp.158.9.1429. [DOI] [PubMed] [Google Scholar]
- 47.Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- 48.Sasson N, Tsuchiya N, Hurley R, et al. Orienting to social stimuli differentiates social cognitive impairment in autism and schizophrenia. Neuropsychologia. 2007;45(11):2580–2588. doi: 10.1016/j.neuropsychologia.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- 50.Dalton KM, Nacewicz BM, Johnstone T, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Das P, Kemp AH, Flynn G, et al. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90(1–3):284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]