Abstract
Objective: The effects of estrogen on comprehension of metaphoric speech, word fluency, and verbal ability were investigated in women suffering from schizophrenia. The issue of estrogen-dependent neuropsychological performance could be highly relevant because women with schizophrenia frequently suffer from hypoestrogenism. Method: A placebo-controlled, double-blind, crossover study using 17β-estradiol for replacement therapy and as an adjunct to a naturalistic maintenance antipsychotic treatment was carried out over a period of 8 months. Nineteen women (mean age = 38.0 years, SD = 9.9 years) with schizophrenia were included in the study. Comprehension of metaphoric speech was measured by a lexical decision paradigm, word fluency, and verbal ability by a paper-and-pencil test. Results: Significant improvement was seen for the activation of metaphoric meaning during estrogen treatment (P = .013); in contrast, no difference was found for the activation of concrete meaning under this condition. Verbal ability and word fluency did not improve under estrogen replacement therapy either. Conclusions: This is the very first study based on estrogen intervention instead of the physiological hormone changes to examine the estrogen effects on neuropsychological performance in women with schizophrenia. In addition, it is the first time that the effect of estrogen on metaphoric speech comprehension was investigated in this context. While in a previous study estrogen therapy as adjunct to a naturalistic maintenance treatment with antipsychotics did not show an effect on psychopathology measured by a rating scale, a significant effect of estrogen on the comprehension of metaphoric speech and/or concretism, a main feature of schizophrenic thought and language disturbance, was found in the present study. Because the improvement of formal thought disorders and language disturbances is crucial for social integration of patients with schizophrenia, the results may have implications for the treatment of these individuals.
Keywords: schizophrenia, estrogen, women, metaphoric speech, concrete thinking, concretism, verbal ability, word fluency, formal thought disorder, language disturbances
Inroduction
Estrogen affects many actions in the brain putatively implicated in multiple processes involving the regulation of mood, cognition, and behavior in women with and without neuropsychiatric disorders.1–6
In normal subjects, gender differences in various aspects of behavior have been studied extensively. They have been attributed to some extent to the putative effect of the different endogenous sex steroids in men and women, which play a role in neurodevelopment and sexual differentiation. In regard to cognitive function, men have been shown to perform better than women at abstract reasoning, spatial visualization, and orientation tasks whereas women show better results in verbal ability and perceptual speed and accuracy tasks.1,7,8 Among the verbal abilities, women are better in spelling, grammar, rate of speech acquisition in childhood, and ability to comprehend or decode language. A prominent task in which women outperform men is verbal fluency. Women also perform better than men in certain nonverbal tasks such as tests of short-term memory that use either verbal or pictorial stimuli and in tests of perceptual speed and accuracy.9,10 Although numerous empirical data provide evidence for sex differences for the aforementioned cognitive functions, the fact that the frequency distributions overlap considerably and the difference in means for the 2 sexes is not large—even if statistically significant—must be considered.1
Studies of replacement therapy in postmenopausal women also provide evidence for an effect of sex hormones on cognitive functions. Estrogen has been shown to prevent the normal age-related decline in cognition, especially in memory.11–18 In surgically menopausal patients and in women with pharmacologically induced estrogen deficiency, a significant benefit of estrogen replacement on cognition and/or memory was also demonstrated.19–21 However, not all studies found an improvement in cognition, which might be partly the result of methodological shortcomings and differences in the paradigms that were employed.22–24 Although there is evidence of an endocrinological effect on cognition, there are also data demonstrating that ethnic differences, socioeconomic status, experiential factors, training, and attitudes have an impact on the performance in cognitive tasks.25–31
In patients suffering from schizophrenia, gender differences have consistently been shown with regard to age at onset, course of the disease, and expression of psychopathology. Compared with men, the onset in women is 4–5 years later, when a flat peak for the first onset of schizophrenia is seen between 25 and 29 years of age, and another, less pronounced first-onset peak can be observed in the age group between 45 and 49 years. Furthermore, premenopausal women with schizophrenia respond better to treatment and present with fewer negative symptoms and more affective and positive symptoms than men. However, the findings in regard to gender differences in neuropsychological functioning in schizophrenia are inconsistent.32–40 It has been hypothesized that estrogen raises the vulnerability threshold due to its putative antipsychotic-like effect and postulated to exert a protective effect in women with schizophrenia.41–43 Therefore, schizophrenia occurs less often in premenopausal women than in men because women physiologically produce more estrogen. There is some evidence from studies in women with schizophrenia of disease onset being observed predominantly when estrogen levels drop, ie, before or during menstruation, after childbirth, and in the pre- or perimenopausal period when estrogen production decreases—although direct evidence is still limited in this area and only very few controlled studies have been conducted.44
Studies have also shown that psychopathological symptoms vary during the menstrual cycle and that the therapeutically required dose of antipsychotics negatively correlates with the cycle phase and estrogen levels.45–47 In addition, a therapeutic effect of estrogen has been reported; however, the results from the few clinical trials in which estradiol is administered in women with schizophrenia are not consistent in this regard.48,49
Clinical and animal studies have demonstrated what could be the basis of the protective effect of estrogen in schizophrenia. In addition to their genuine, hormonal function in the gonadal axis, estrogens exert various effects on the brain.50–53 Various research groups have shown that estrogens modulate certain neurotransmitter systems, not only the dopaminergic system, but also the serotonergic, GABAergic, noradrenergic, and cholinergic systems.4 Estrogen also exerts neurotrophic and neuroprotective effects that are mediated by nongenomic as well as by direct and indirect genomic pathways.54,55 Estrogens circulate freely and are bound to albumin or to sex hormone–binding globulin or conjugated as sulfates or glucuronates. Free estrogens—about 3% of the circulating estrogens—are lipophilic, freely traverse cell membranes, and are therefore biologically active.
In schizophrenia, widespread deficits in comprehension, production, attention, and cerebral lateralization of language are evident.56,57 A main feature of schizophrenic language and thought disturbance is concretism, the inability to understand the meaning of metaphors and proverbs. Schizophrenic patients typically understand the literal but not the underlying metaphoric meaning of proverbs and metaphors.58–67 Allthough concretism is routinely tested during clinical interviews, it is rarely described in modern psychiatric textbooks and only few studies focusing on this issue can be found in the recent literature.63–65 Indeed, impaired language comprehension skills are likely to have significant impact on social interaction in patients with schizophrenia.66
In the clinical context, concretism is tested by asking the patient to explain a proverb presented by the interviewer67–69; however, this method has limitations for research as regards reliability and practicability.64,70 Therefore, tests for assessing concretism were developed, and experimental approaches were defined.63,64,71
Our study is based on these research approaches. In this context, we introduced the lexical decision paradigm to study comprehension of metaphoric speech and/or concretism in women with schizophrenia using estrogen as an independent variable. We hypothesized that estrogen replacement in women suffering from schizophrenia is associated with better performance in comprehension of metaphoric speech, ie, a reduction in concrete thinking (“concretism”). Furthermore, we postulated that estrogen improves verbal ability and word fluency in women with schizophrenia, as was shown in healthy subjects.
Methods
Study Design
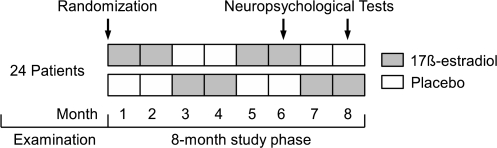
To address these questions, neuropsychological tests were applied as part of a previously reported intervention study in which patients received an estrogen-progestin combination drug.49 A placebo-controlled, double-blind, 3-time crossover study with 17β-estradiol as a hormone replacement therapy (HRT) and adjunct therapy to a naturalistic antipsychotic maintenance treatment was carried out over a period of 8 months. Figure 1 shows the study design. The crossover design was chosen because it is assumed that estrogen has a short-term effect on psychopathology and neuropsychological tasks.45 This design also has advantages in terms of increasing the statistical power.
Fig. 1.
Study Design.
Neuropsychological tests were conducted during the periovulatory phase (day 10–12) of months 6 and 8. The differences in the serum estrogen levels between drug and placebo phases are presumed to be the greatest during this period. In this mid-cycle period, under physiological conditions in healthy, menstruating women, 17β-estradiol increases to up to 350 ng/ml; under HRT, an increase in the 17β-estradiol levels of at least over 150 ng/ml was expected. In contrast, in women with schizophrenia not receiving HRT, low levels of 17β-estradiol have been observed in various studies.45,73,74
The study was performed in accordance with the Declaration of Helsinki of 1964 (edition from the 41st World Medical Assembly in Hong Kong in 1989), the Good Clinical Practice on Medicinal Products in the European Community (111/3976/88-EN-July 1990), Germany's Guidelines for Clinical Trials (“Grundsätze für die ordnungsgemäße Durchführung der klinischen Prüfung von Arzneimitteln,” Bundesanzeiger 243: 16618; 1987), and the stipulations of German Law on Medicinal Products (“Arzneimittelgesetz,” AMG §§ 40–42). The protocol was approved by the local Research Ethics Committee at the University of Heidelberg. The study was registered with the Regional Government Administration (“Regierungspräsidium”; No. 63-28/3404.9-6) in Karlsruhe and the German Research Council (“Deutsche Forschungsgemeinschaft,” SFB 258).
Patients
Twenty-four patient women suffering from schizophrenia who were consecutively admitted to the Department of General Psychiatry, Heidelberg University Hospitals, and in whom disease had sufficiently remitted after the acute phase participated in the trial. The earliest point of sufficient remission was reached 6 weeks after acute exacerbation of the schizophrenic disorder and when the patient achieved a sum-score of <30 on the Brief Psychiatric Rating Scale (BPRS).75 In addition, the following inclusion criteria were defined for study entry: first or a further episode of schizophrenia sensu International Statistical Classification of Diseases, 10th Revision, and Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, criteria; hypoestrogenism (17β-estradiol plasma level ≤ 30 pg/ml in the follicular phase on cycle day 2–4 and ≤100 pg/ml in the periovulatory phase on cycle day 10–12); woman between 16 and 67 years of age; cooperation; and informed consent. The following exclusion criteria were defined: alcohol and drug dependence, family history of breast carcinoma, oral contraceptives within the past year, estrogen replacement therapy longer than 3 months within the past year, mastodynia, pregnancy and breast feeding, treatment-resistant schizophrenia, and relevant comorbidity such as Parkinson disease or severe respiratory, renal, hepatic, hematological, or endocrinological disease.
The mean age of the women was 37.3 years (SD = 9.6; range: 23–60 years). The mean duration of illness was 7.4 years (SD = 8.0), and all patients suffered from paranoid schizophrenia. Nine patients were unemployed, 2 were fully employed, 3 were employed part-time, 3 were in occupational therapy, 5 were housewives, 2 were retired, and 1 was a student. Fifteen patients were single, 4 married, 3 divorced, and 2 widowed. Nineteen patients lived under normal conditions at home, 4 lived in therapeutic settings, and 1 patient had been hospitalized for a longer period of time. The sample was reduced by 2 dropouts who did not participate in both sessions. Another 3 patients were excluded because they did not show the expected relation between the 17β-estradiol plasma levels during drug treatment and/or placebo phase (see below), most likely because of noncompliance. The mean age of the 19 patients who completed the study was 38.0 years (SD = 9.9; range: 24−60 years). The mean age at onset of the disease was 29.9 years (SD = 10.5). On average, they had suffered from schizophrenia for 8.4 years (SD = 8.4; range: 0.5−24 years) and were hospitalized 1.9 times (SD = 3.4; range: 1−14 years).
Medication
During the drug (estrogen) treatment phase, a relatively high dose of 17β-estradiol was to be given in addition to the antipsychotic medication. To meet this requirement, Trisequens plus Estrifam (both by Novo Nordisk Pharma, Bagsværd, Denmark) were administered. Trisequens is an HRT preparation with a 3-phase estrogen-progestin combination including the following compounds:
blue-coated tablet: 17β-estradiol 2 mg (follicular phase),
white-coated tablet: 17β-estradiol 2 mg + norethisterone acetate (NETA) 1 mg (periovulatory phase),
red-coated tablet: 17β-estradiol 1 mg (luteal phase).
17β-estradiol is a naturally occurring form of estrogen, and NETA is a synthetic form of progesterone. A progestogen—in this case in the form of NETA—is required as part of HRT to prevent endometrial cancer. Trisequens is a sequential form of a combined HRT, ie, this estrogen is taken continuously and progesterone is added for a small portion each month. The blue-coated tablets taken in the first 12 days of each cycle contain only estradiol; the white-coated tablets taken for the next 10 days of each cycle contain both estradiol and norethisterone; and the red-coated tablets taken for the last 6 days contain just estradiol. This type of HRT is more suitable for women who still have a period, most likely irregularly as it usually results in a monthly withdrawal bleeding toward the end of the white-coated tablet phase or while taking the red-coated tablets.
Estrifam contains 2 mg of 17β-estradiol as the medically active component. It was added to Trisequens in the follicular and periovulatory phases to obtain a higher dose of estradiol during these phases.
During the placebo phases, a placebo medication of the same appearance was administered. In accordance with a naturalistic study design, the antipsychotic medication was given without predefining the drug or dose. It included clozapine in 7 cases, risperidone in 5, flupenthixol and fluphenazine in 2 cases each, and perphenazine, haloperidol, and sertindole were administered in 1 case each. The patients received this nonstandardized antipsychotic medication as required by the individual psychopathological condition and all other state-of-the-art interventions in terms of care, diagnosis, and therapy.
Blood Specimens and Laboratory Analyses
Blood samples were taken in the morning between 8:00 and 9:00 AM. The following laboratory parameters were assessed: 17β-estradiol, luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin (PRL), testosterone, progesterone, and dehydroepiandrosterone sulfate (DHEA-S). An electrochemical luminescence immunoassay was used for laboratory analysis of all parameters (Roche Elecsys 2010 immunoassay analyzer). In addition, for safety reasons routine laboratory parameters were monitored—complete blood count, liver enzyme profile, renal plasma clearance, thyroid-stimulating hormone—and an electrocardiogram and electroencephalogram were carried out.
Paradigms and Material
We selected verbal abilities for analysis because of their functional relevance and because they are sensitive to estrogen. Verbal ability was measured by 3 different tests from the “Leistungsprüfsystem” (LPS), a well-known German-language paper-and-pencil performance test developed by Horn.76 The LPS is a standardized neuropsychological test with extensive normative data. It measures a “verbal factor” with the aspects of recognition vocabulary and “word fluency” with its components “word activation” and “word recognition,” partly equivalent to subtests by Thurstone.77 In LPS subtests 1 and 2, the subject needs to find the misspelled letter in given words; the 2 tests are scored together. In LPS subtest 5, words are presented in which the letters are in the wrong order, and the subject needs to recognize the true word and mark its first letter. LPS subtest 6 measures “word fluency”; the subject needs to produce as many words as possible with a given letter within a defined period of time.
Concrete thinking was measured by a lexical decision paradigm according to Spitzer et al.63 This experimental procedure is supposed to be more sensitive than other methods of testing concrete thinking, such as questionnaires. The comprehension of auditorily presented sentences with metaphoric content (“primes”) was measured according to the lexical decision subsequently made between a word and a nonword (“targets”), assuming that the “semantic priming” of the metaphoric content affects the time span under the various experimental conditions, ie, concrete, metaphoric, nonrelated words, and nonwords. Sixty metaphoric sentences that are part of standard language were selected from the dictionary. The metaphoric sentences had the general structure “subject-predicate-object” (or adverbial adjunct) and, in the majority of cases, ended with a concrete noun. The patients had to decide whether a word is a German word with meaning or a nonword by pushing different buttons on the keyboard of the computer. The word was shown for 400 milliseconds on the screen 1200 milliseconds subsequently to an orally presented metaphoric sentence. The nonwords were orthographically possible words; however, they did not exist in the German language. In most cases, they were created by changing 2 letters of an existing German word. The words either related to the concrete meaning or to the literal meaning of the metaphoric sentence or they had no relationship to it. The time was measured between the presentation of the word until the patient made the decision “word or nonword.”
Three words and 3 nonwords were attached to each of the 60 sentences so that comparable stimulus material was available for both conditions, tests during the active drug phase and the placebo phase. The concrete and abstract priming effects were operationalized as follows.
Concrete priming effect: reaction time to the nonrelated word minus reaction time to the concrete related word.
Abstract priming effect: reaction time to the nonrelated word minus reaction time to the abstract related word.
In previous experiments with this paradigm, no effect of gender and education on concrete or abstract priming was found. Similarly, a systematic effect of the various target words within each condition on the reaction time could be excluded.63
Furthermore, a computerized version of the Continuous Performance Test (CPT)78 was used to assess concentration in the patients because concentration might act as a moderator variable and should be controlled.
Statistical Analyses
Besides standard descriptive statistics, various inference statistical procedures were applied. The effect of the drug treatment on hormone levels and on test performance was assessed using random-effect regressions with a dummy variable for drug (0 = no, 1 = yes) and time (0 = first assessment, 1 = second assessement) and a random effect for each patient as explanatory variables. To test whether concentration affects verbal abilities and priming performance, the CPT d-prime index was included as a covariate in the random-effect regression in a second step. The statistics were calculated using the statistical program Stata 9.2.79
With the crossover design applied, 19 patients, 2-sided testing, and a significance level of 5% an effect size of 1 within-patient SD can be detected with a probability of 83%. This is a small effect size because even without any effect, the probability that 2 consecutive measurements of one patient differ by more than 1 within-patient SD is 48%.
Results
Hormone Parameters
First, the experimental manipulation of estradiol was evaluated. Three patients showed higher estrogen plasma concentrations in the placebo phase than in the active drug phase and were therefore excluded from the analyses. Table 1 shows the hormone parameters during the active drug and placebo phases. As expected, 17β-estradiol was significantly higher during the active drug than during the placebo phase (P < .001); during the active drug phase estradiol was within the normal range, during the placebo phase far below the lower limit of the normal range.72,80
Table 1.
Mean Values and SDs (M ± SD) of 17β-Estradiol and Other Hormones in Women With Schizophrenia (n = 19) During the Periovulatory Phase of the Menstrual Cycle Under Active Drug (Estrogen Replacement) and Placebo Conditions; Normal Reference Values for the Periovulatory Phase According to Klinga72; Differences Between Active Drug and Placebo Conditions and the Effect of Time Were Calculated by Means of Random-Effect Regression Analysis
| Hormone | Normal Reference Values | Active Drug | Placebo | P |
| 17β-Estradiol (pg/mL) | 150–350 | 192 ± 91 | 56 ± 57 | <.001 |
| LH (mU/mL) | 30–80 | 4.3 ± 6.4 | 10.6 ± 11.4 | .011 |
| FSH (mU/mL) | 2–14 | 6.8 ± 10.7 | 16.1 ± 22.8 | .044 |
| Prolactin (mU/mL) | 70–410 | 1169 ± 1060 | 891 ± 933 | .009 |
| Testosterone (pg/mL) | 200–600 | 399 ± 155 | 469 ± 247 | .466 |
| DHEA-S (ng/mL) | 350–4300 | 2439 ± 967 | 2448 ± 997 | .717 |
Note: LH, luteinizing hormone; FSH, follicle-stimulating hormone; DHEA-S, dehydroepiandrosterone sulfate.
The P values in the table are the results of Wald tests of the drug effect.
In addition, the values for LH, FSH, and PRL were significantly different under active drug and placebo phases. As expected, both gonadotropins increased when estrogen replacement was interrupted and/or placebo was administered. However, LH was far below the lower limit of the normal range under both conditions. PRL was far above the normal range under both conditions as expected under medication with predominantly conventional antipsychotics. A significant time effect was only shown for PRL. In contrast to the aforementioned hormones, testosterone and DHEA-S show no difference under active drug and placebo conditions, and under both conditions these parameters were within the normal range.
Comprehension of Metaphoric Speech and Verbal Abilities
There was a statistically significant effect of estradiol on metaphoric priming (P = .013). This means that the metaphoric meaning was more rapidly activated under estrogen replacement than under placebo. In contrast, no significant differences were observed in the concrete priming between the 2 conditions (table 2).
Table 2.
Results of the Tests for Assessing the Comprehension of Metaphoric Speech, Verbal Ability (LPS 1 + 2, 5, 6), and Concentration (CPT) in Women With Schizophrenia (n = 19) Under Active Drug (Estrogen Replacement) and Placebo Conditions—Mean Values and SDs (M ± SD); Differences Between Active Drug and Placebo Conditions, and the Effect of Time (Practice Effect) Were Calculated by Means of Random-Effect Regression Analysis
| Tests | Active Drug | Placebo | P |
| Abstract priming (ms) | 60.6 ± 95.53 | −7.1 ± 108.5 | .013 |
| Concrete priming (ms) | 65.4 ± 170.0 | 34.4 ± 142.0 | .400 |
| LPS 1 + 2 (verbal factor) | 37.9 ± 17.9 | 39.3 ± 16.9 | .294 |
| LPS 5 (word recognition) | 20.2 ± 11.1 | 18.4 ± 10.7 | .253 |
| LPS 6 (word fluency) | 31.9 ± 11.5 | 32.8 ± 10.4 | .450 |
| CPT (d-prime index) | 4.87 ± 1.25 | 4.57 ± 1.40 | .149 |
Note: LPS, Leistungsprüfsystem; CPT, Continuous Performance Test.
The P values in the table are the results of Wald tests of the drug effect.
These findings correspond to results reported by Spitzer et al63 in a group of 35 patients with schizophrenia (24 men, 11 women; mean age = 34.4 years, SD = 12.4 years) and a group of 43 healthy controls (13 men, 30 women; mean age = 28.1 years, SD = 10.8 years). The patients in this trial showed a mean abstract priming effect of −17.5 milliseconds (SD = 113.4 milliseconds) that is close to the results in our study; in contrast, the group of healthy controls showed a mean value of 15.5 milliseconds (SD = 56.5 milliseconds). The concrete priming effect amounts to 27.5 milliseconds (SD = 90.1 milliseconds) and/or 25.1 milliseconds (SD = 54.9 milliseconds), respectively. However, this comparison does not account for the fact that both sexes were included in the trial by Spitzer et al.63
No statistically significant difference was found for any of the verbal ability tests under the estrogen and placebo conditions. Furthermore, estrogen replacement had no effect on the concentration measured by the CPT. No learning effect was found for any of the LPS subtests, CPT, or concrete and abstract priming, except for LPS 5 (P = .009). The addition of concentration as a covariate had no effect on abstract or concrete priming, LPS 1 + 2, and LPS 5. Only the performance on LPS 6 was significantly influenced by concentration (P = .007).
Discussion
In a previous study by Bergemann et al49 in women suffering from schizophrenia, during a naturalistic maintenance therapy with adjuvant antipsychotics, estradiol treatment did not show any significant, additional effect on psychopathology measured by the Positive and Negative Syndrome Scale,81 the BPRS,75 the Clinical Global Impressions,82 and the Beck Depression Inventory83 in a German version.84 The overall condition was assessed by using a 100-mm Analogue Scale. Neither the required antipsychotic doses nor the side effects measured by various standardized scales differed between the 2 phases.
In contrast, in the present study a significant effect of estrogen on the comprehension of metaphoric speech and/or concretism, a main feature of schizophrenic thought and language disturbance, was found. However, the verbal ability tests and concentration as measured by the CPT did not show a significant difference between the estrogen and placebo conditions.
The results regarding the effect of estrogen on “concretism” are in line with our hypotheses; however, the expected effect of estrogen on word fluency and verbal performance could not be confirmed.
Only a few other trials studying neuropsychological performance in women with schizophrenia have used physiological hormonal changes during the menstrual cycle as an independent variable,40,85–87 and to our knowledge our study is the very first one to be based on an estrogen intervention strategy. In addition, it is the first time that the effect of estrogen on metaphoric speech comprehension has been investigated in such a context.
The findings of the aforementioned studies are inconsistent. Thompson et al85 found, as hypothesized, that patients with schizophrenia and healthy controls perform better on spatial tasks in the low estrogen phase of the menstrual cycle (follicular phase); however, no effect of estrogen was found in regard to verbal and articulatory motor tasks. In contrast to these findings, Hoff et al86 discovered that higher levels of estrogen over a 4-week period correlated with better cognitive performance in language, executive functioning, verbal memory, spatial memory, concentration/speed, and perceptual speed in a group of women suffering from schizophrenia. Halari et al40 investigated the effects of serum levels of gonadal hormones and cortisol on neuropsychological functioning—attention, verbal abilities, language, memory, executive functioning, motor, and speed of information processing—and psychopathology in female and male patients suffering from schizophrenia. They did not find significant sex differences in any of the cognitive tests, and except for a moderate inverse correlation between estrogen and positive symptoms, no other correlations were found between estrogen and any of the cognitive measures and symptom scores. In contrast to these findings, Ko et al87 found lower levels of estrogen associated with more severe negative symptoms and reduced performance in cognitive function, especially verbal performance and executive functioning, in 35 women of reproductive age suffering from schizophrenia. The reason for the inconsistency may be the differences in methodology, such as the assessment of cognitive functioning and psychopathology, or differences in the samples and the small sample sizes.
In our study, we did not find an effect of estrogen on verbal abilities either; however, by means of a sophisticated experimental assessment we found an effect of estrogen on the comprehension of metaphoric meaning in this group of women with schizophrenia. Because improvement in formal thought disorders and language disturbances is crucial for social interaction and therefore for social integration of patients with schizophrenia, the results may be of particular interest.88 Lysaker et al89, eg, have shown that concretism and cognition impairments measured by the Gorham Proverb Test71 and/or the Wisconsin Card Sorting Test90, respectively, are associated with a reduction in the clinical effects of rehabilitation. Further studies provide empirical evidence for the association between concretism measured with the Gorham Proverb Test and difficulties in social integration.91–93
However, there are limitations to this study. The naturalistic design of our study allowed all different antipsychotics and other psychotropic drugs at various doses. The naturalistic medication given might rule out an effect of estrogen on some of the cognition tests. Thirteen patients of our sample received atypical antipsychotics; evidence is provided in the literature that atypical antipsychotics improve cognition in patients with schizophrenia to a greater or lesser extent compared with conventional antipsychotics. However, more recent studies did not find such a difference between conventional and atypical antipsychotics or any differences between the atypical antipsychotics.94–96
A further limitation of this study was the small size and the broad age range of the sample; the latter is particularly relevant because performance in neurocognitive tasks is likely to be age dependent. Nevertheless, the data presented here clearly indicate that changes in estrogen levels have a significant impact on concretism in women with schizophrenia. The question of possible age effects should be addressed in further studies on this topic.
Furthermore, the effects of these various medications on reproductive hormones were not controlled. However, it is well known that conventional and some of the atypical antipsychotics have a PRL-raising effect.97 In our sample, 11 patients received PRL-raising antipsychotics and 8 PRL-sparing antipsychotics. Due to hypothalamic feedback mechanisms, hyperprolactinemia induce hypoestrogenemia. In addition to this mechanism, there is evidence for a primary hypoestrogenemia in women with schizophrenia.74 Furthermore, potential effects of estrogen on PRL must be discussed as regards the differences in the PRL levels during estrogen and placebo phases.98 The data on this issue, however, are controversial. Some studies have shown that estrogen-containing oral contraceptives can indeed cause hyperprolactinemia in percentages ranging from 13% to 30% of treated women; others have shown either a minimal or no increase in PRL levels. In contrast, most studies have shown either no or a minimal effect of estrogen replacement therapy on PRL levels after oophorectomy or at menopause.98
In addition, it is not yet clear whether the paradigms used for assessing verbal abilities or their operationalizations are appropriate for the hypotheses in question. Further studies on the topic presented here should elucidate these unresolved issues.
Estrogen-dependent neuropsychological performance and/or formal thought disorders could be of particular interest because women with schizophrenia frequently suffer from hypoestrogenism.74 Previous studies suggested that estradiol has specific antipsychotic-like effects on the symptoms of schizophrenia, and an estrogen supplement might be effective for treatment and prophylaxis in a subgroup of women suffering from schizophrenia. Therefore, our results may have implications for the treatment of patients with schizophrenia receiving adjunctive estrogen replacement therapy. Hence, it may be worthwhile to further investigate the therapeutic possibilities of estrogen. Due to potential side effects and contraindications, particularly addressed in the Women's Health Initiative study,99–103 the individual risk and benefit should be assessed and taken into account.
Funding
German Research Council (“Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 258: Indikatoren und Riskomodelle für Entstehung und Verlauf psychischer Störungen,” Indications and risk models for the development and course of mental disorders).
Acknowledgments
We thank Dirk Kaiser for his excellent help in patient recruitment and sample collection and Sherryl Sundell who assisted with the proofreading of the manuscript. The authors have no conflict of interest with any commercial or other associations in connection with the submitted article.
References
- 1.Hampson E. Sex differences in human brain and cognition: the influence of sex steroids in early and adult life. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. 2nd ed. Cambridge, Mass: MIT Press; 2002. pp. 579–628. [Google Scholar]
- 2.Halbreich U, Lumley LA. The multiple interactional biological processes that might lead to depression and gender differences in its appearance. J Affect Disord. 1993;29:159–173. doi: 10.1016/0165-0327(93)90030-n. [DOI] [PubMed] [Google Scholar]
- 3.Sherwin BB. Estrogen and cognitive functioning in women. Proc Soc Exp Biol Med. 1998;217:17–22. doi: 10.3181/00379727-217-44200. [DOI] [PubMed] [Google Scholar]
- 4.Fink G, Sumner BEH, McQueen JK, Wilson H, Rosie R. Sex steroids control of mood, mental state and memory. Clin Exp Pharmacol Physiol. 1998;25:764–775. doi: 10.1111/j.1440-1681.1998.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 5.Norbury R, Craig M, Cutter WJ, Whitehead M, Murphy DGM. Oestrogen: brain ageing, cognition and neuropsychiatric disorder. J Br Menopause Soc. 2004;10:118–122. doi: 10.1258/1362180042003857. [DOI] [PubMed] [Google Scholar]
- 6.Vedder H, Behl C. Estrogens in neuropsychiatric disorders: from physiology to pathophysiology. In: Bergemann N, Riecher-Rössler A, editors. Estrogen Effects in Psychiatric Disorders. New York, NY: Springer; 2005. pp. 1–30. [Google Scholar]
- 7.Hampson E. Spatial cognition in humans: possible modulation by androgens and estrogens. J Psychiatry Neurosci. 1995;20:397–404. [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura D. Sex and Cognition. Cambridge, Mass: MIT Press; 1999. [Google Scholar]
- 9.Halpern DF. Sex Differences in Cognitive Abilities. 3rd ed. Hillsdale, NJ: Erlbaum; 2000. [Google Scholar]
- 10.Kimura D, Clarke P. Womens's advantage on verbal memory is not restricted to concrete words. Psychol Rep. 2002;91:1137–1142. doi: 10.2466/pr0.2002.91.3f.1137. [DOI] [PubMed] [Google Scholar]
- 11.Hackman BW, Galbraith D. Replacement therapy and piperazine oestrone sulphate (‘Harmogen’) and its effect on memory. Curr Med Res Opin. 1976;4:303–306. doi: 10.1185/03007997609109322. [DOI] [PubMed] [Google Scholar]
- 12.Robinson D, Friedman L, Marcus R, Tinklenber J, Yesavage J. Estrogen replacement therapy and memory in older women. J Am Geriatr Soc. 1994;42:919–922. doi: 10.1111/j.1532-5415.1994.tb06580.x. [DOI] [PubMed] [Google Scholar]
- 13.Kampen DL, Sherwin BB. Estrogen use and verbal memory in healthy postmenopausal women. Obstet Gynecol. 1994;83:979–983. doi: 10.1097/00006250-199406000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Resnick SM, Metter EJ, Zonderman AB. Estrogen replacement therapy and longitudinal decline in visual memory. A possible protective effect? Neurology. 1997;49:1491–1497. doi: 10.1212/wnl.49.6.1491. [DOI] [PubMed] [Google Scholar]
- 15.Carlson LE, Sherwin BB. Steroid hormones, memory and mood in a healthy elderly population. Psychoneuroendocrinology. 1998;23:583–603. doi: 10.1016/s0306-4530(98)00025-0. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs DM, Tang MX, Stern Y, et al. Cognitive function in nondemented older women who took estrogen after menopause. Neurology. 1998;50:368–373. doi: 10.1212/wnl.50.2.368. [DOI] [PubMed] [Google Scholar]
- 17.Maki MP, Zonderman AB, Resnick SM. Enhanced verbal memory in nondemented elderly women receiving hormone-replacement therapy. Am J Psychiatry. 2001;158:227–233. doi: 10.1176/appi.ajp.158.2.227. [DOI] [PubMed] [Google Scholar]
- 18.Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- 19.Sherwin BB. Estrogen and/or androgen replacement therapy and cognitive functioning in surgically menopausal women. Psychoneuroendocrinology. 1988;13:345–357. doi: 10.1016/0306-4530(88)90060-1. [DOI] [PubMed] [Google Scholar]
- 20.Phillips SM, Sherwin BB. Effects of estrogen on memory function in surgically menopausal women. Psychoneuroendocrinology. 1992;17:485–495. doi: 10.1016/0306-4530(92)90007-t. [DOI] [PubMed] [Google Scholar]
- 21.Sherwin BB, Tulandi T. “Add-back” estrogen reverses cognitive deficits induced by a gonadotropin-releasing hormone agonist in women with leiomyomata uteri. J Clin Endocrinol Metab. 1996;81:2545–2549. doi: 10.1210/jcem.81.7.8675575. [DOI] [PubMed] [Google Scholar]
- 22.Haskell SG, Richardson ED, Horwitz RI. The effect of estrogen replacement therapy on cognitive function in women: a critical review of the literature. J Clin Epidemiol. 1997;50:1249–1264. doi: 10.1016/s0895-4356(97)00169-8. [DOI] [PubMed] [Google Scholar]
- 23.Sherwin BB. Can estrogen keep you smart? Evidence from clinical studies. J Psychiatry Neurosci. 1999;24:315–321. [PMC free article] [PubMed] [Google Scholar]
- 24.Sherwin BB. The clinical relevance of the relationship between estrogen and cognition in women. J Steroid Biochem Mol Biol. 2007;106:151–156. doi: 10.1016/j.jsbmb.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Mayes JT, Jahoda G, Neilson I. Patterns of visual-spatial performance and ‘spatial ability’: dissociation of ethnic and sex differences. Br J Psychol. 1988;79:105–119. doi: 10.1111/j.2044-8295.1988.tb02276.x. [DOI] [PubMed] [Google Scholar]
- 26.Quaiser-Pohl C, Lehmann W. Girls’ spatial abilities: charting the contributions of experiences and attitudes in different academic groups. Br J Educ Psychol. 2002;72:245–260. doi: 10.1348/000709902158874. [DOI] [PubMed] [Google Scholar]
- 27.Cherney ID, Jagarlamudi K, Lawrence E, Shimabuku N. Experiential factors in sex differences on mental rotation. Percept Mot Skills. 2003;96:1062–1070. doi: 10.2466/pms.2003.96.3c.1062. [DOI] [PubMed] [Google Scholar]
- 28.Devlin AS. Sailing experience and sex as correlates of spatial ability. Percept Mot Skills. 2004;98:1409–1421. doi: 10.2466/pms.98.3c.1409-1421. [DOI] [PubMed] [Google Scholar]
- 29.Levine SC, Vasilyeva M, Lourenco SF, Newcombe NS, Huttenlocher J. Socioeconomic status modifies the sex difference in spatial skill. Psychol Sci. 2005;16:841–845. doi: 10.1111/j.1467-9280.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 30.Ginn SR, Pickens SJ. Relationships between spatial activities and scores on the mental rotation test as a function of sex. Percept Mot Skills. 2005;100:877–881. doi: 10.2466/pms.100.3.877-881. [DOI] [PubMed] [Google Scholar]
- 31.Contreras MJ, Rubio VJ, Peña D, Colom R, Santacreu J. Sex differences in dynamic spatial ability: the unsolved question of performance factors. Mem Cognit. 2007;35:297–303. doi: 10.3758/bf03193450. [DOI] [PubMed] [Google Scholar]
- 32.Perlick D, Mattis S, Stastny P, Teresi J. Gender differences in cognition in schizophrenia. Schizophr Res. 1992;8:69–73. doi: 10.1016/0920-9964(92)90062-a. [DOI] [PubMed] [Google Scholar]
- 33.Goldberg TE, Gold JM, Torrey EP, Weinberger DR. Lack of sex differences in the neuropsychological performance of patients with schizophrenia. Am J Psychiatry. 1995;152:883–888. doi: 10.1176/ajp.152.6.883. [DOI] [PubMed] [Google Scholar]
- 34.Andia AM, Zisook S, Heaton RK, et al. Gender differences in schizophrenia. J Nerv Ment Dis. 1995;183:522–528. doi: 10.1097/00005053-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Lewine RR, Walker EF, Shurett R, Caudle J, Haden C. Sex differences in neuropsychological functioning among schizophrenic patients. Am J Psychiatry. 1996;153:1178–1184. doi: 10.1176/ajp.153.9.1178. [DOI] [PubMed] [Google Scholar]
- 36.Albus M, Hubmann W, Mohr F, et al. Are there gender differences in neuropsychological performance in patients with first-episode schizophrenia? Schizophr Res. 1997;28:39–50. doi: 10.1016/s0920-9964(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 37.Seidman LJ, Goldstein JM, Goodman JM, Koren D, Turner W, Tsuang MT. Sex differences in olfactory identification and Wisconsin card sorting performance in schizophrenia. Biol Psychiatry. 1997;42:104–115. doi: 10.1016/S0006-3223(96)00300-9. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein JM, Seidman LJ, Goodman JM, et al. Are there sex differences in neuropsychological functions among patients with schizophrenia? Am J Psychiatry. 1998;155:1358–1364. doi: 10.1176/ajp.155.10.1358. [DOI] [PubMed] [Google Scholar]
- 39.Hoff AL, Wieneke M, Faustman WO, et al. Sex differences in neuropsychological functioning of first-episode and chronically ill schizophrenic patients. Am J Psychiatry. 1998;155:1437–1439. doi: 10.1176/ajp.155.10.1437. [DOI] [PubMed] [Google Scholar]
- 40.Halari R, Kumari V, Mehrota R, Wheeler M, Hines M, Sharma T. The relationship of sex hormones and cortisol with cognitive functioning in schizophrenia. J Psychopharmacol. 2004;18:366–374. doi: 10.1177/026988110401800307. [DOI] [PubMed] [Google Scholar]
- 41.Seeman MV. Interaction of sex, age, and neuroleptic dose. Compr Psychiatry. 1983;24:125–128. doi: 10.1016/0010-440x(83)90100-1. [DOI] [PubMed] [Google Scholar]
- 42.Häfner H, Behrens S, De Vry J, Gattaz WF. Oestradiol enhances the vulnerability threshold for schizophrenia in women by an early effect on dopaminergic neurotransmission. Evidence from an epidemiological study and from animal experiments. Eur Arch Psychiatry Clin Neurosci. 1991;241:65–68. doi: 10.1007/BF02193758. [DOI] [PubMed] [Google Scholar]
- 43.Häfner H, Riecher-Rössler A, an der Heiden W, Maurer K, Fatkenheuer B, Loffler W. Generating and testing a causal explanation of the gender difference in age at first onset of schizophrenia. Psychol Med. 1993;23:925–940. doi: 10.1017/s0033291700026398. [DOI] [PubMed] [Google Scholar]
- 44.Bergemann N, Parzer P, Nagl I, et al. Acute psychiatric admission and menstrual cycle phase in women with schizophrenia. Arch Womens Ment Health. 2002;5:119–126. doi: 10.1007/s00737-002-0004-2. [DOI] [PubMed] [Google Scholar]
- 45.Riecher-Rössler A, Häfner H, Stumbaum M, Maurer K, Schmidt R. Can estradiol modulate schizophrenic symptomatology? Schizophr Bull. 1994;20:203–214. doi: 10.1093/schbul/20.1.203. [DOI] [PubMed] [Google Scholar]
- 46.Bergemann N, Parzer P, Runnebaum B, Resch F, Mundt C. Estrogen, menstrual cycle phase, and psychopathology in women suffering from schizophrenia. Psychol Med. 2007;37:1427–1436. doi: 10.1017/S0033291707000578. [DOI] [PubMed] [Google Scholar]
- 47.Gattaz WF, Vogel P, Riecher-Rössler A, Soddu G. Influence of the menstrual cycle phase on the therapeutic response in schizophrenia. Biol Psychiatry. 1994;36:137–139. doi: 10.1016/0006-3223(94)91195-9. [DOI] [PubMed] [Google Scholar]
- 48.Chua WL, Izquierdo de Santiago A, Kulkarni J, Mortimer A. Estrogen for schizophrenia. Cochrane Database Syst Rev. 2005;4:CD004719. doi: 10.1002/14651858.CD004719.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Bergemann N, Mundt C, Parzer P, et al. Estrogen as an adjuvant therapy to antipsychotics does not prevent relapse in women suffering from schizophrenia: results of a placebo-controlled double-blind study. Schizophr Res. 2005;74:125–134. doi: 10.1016/j.schres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 50.McEwen BS, Alves SE, Bulloch K, Weiland NG. Ovarian steroids and the brain: implications for cognition and aging. Neurology. 1997;48(suppl 7):8–15. doi: 10.1212/wnl.48.5_suppl_7.8s. [DOI] [PubMed] [Google Scholar]
- 51.Stevens JR. Schizophrenia: reproductive hormones and the brain. Am J Psychiatry. 2002;159:713–719. doi: 10.1176/appi.ajp.159.5.713. [DOI] [PubMed] [Google Scholar]
- 52.Bergemann N, Riecher-Rössler A, editors. Estrogen Effects in Psychiatric Disorders. New York, NY: Springer; 2005. [Google Scholar]
- 53.Mortimer AM. Relationship between estrogen and schizophrenia. Expert Rev Neurother. 2007;7:45–55. doi: 10.1586/14737175.7.1.45. [DOI] [PubMed] [Google Scholar]
- 54.Behl C, Widmann M, Trapp T, Holsboer F. 17-beta estradiol protects neurons from oxidative stress-induced cell death in vitro. Biochem Biophys Res Commun. 1995;216:473–482. doi: 10.1006/bbrc.1995.2647. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- 56.Crow TJ. Nuclear schizophrenia symptoms as a window on the relationship between thought and speech. Br J Psychiatry. 1998;173:303–309. doi: 10.1192/bjp.173.4.303. [DOI] [PubMed] [Google Scholar]
- 57.DeLisi LE. Speech disorder in schizophrenia: review of the literature and exploration of its relation to uniquely human capacity for language. Schizophr Bull. 2001;27:481–496. doi: 10.1093/oxfordjournals.schbul.a006889. [DOI] [PubMed] [Google Scholar]
- 58.Finckh J. Zur Frage der Intelligenzprüfung [Testing intelligence] Zentralbl Nervenheilkd Psychiatr. 1906;29:945–957. [Google Scholar]
- 59.Vigotsky LS. Thought in schizophrenia. Arch Neurol Psychiatry. 1934;31:1063–1077. [Google Scholar]
- 60.Muncie W. The psychopathology of metaphor. Arch Neurol Psychiatry. 1937;37:796–804. [Google Scholar]
- 61.Goldstein K. Methodological approach to the study of schizophrenic thought disorder. In: Kasanin JS, editor. Language and Thought in Schizophrenia. Berkeley, Calif: University of California Press; 1944. [Google Scholar]
- 62.Chapman LJ. Confusion of figurative and literal usages of words by schizophrenics and brain damaged patients. J Abnorm Soc Psychol. 1960;60:412–416. doi: 10.1037/h0043371. [DOI] [PubMed] [Google Scholar]
- 63.Spitzer M, Lukas M, Maier S, Hermle L. Das Verstehen metaphorischer Rede bei gesunden Probanden und schizophrenen Patienten [Comprehension of metaphoric speech by schizophrenic patients and healthy controls. An experimental contribution] Nervenarzt. 1994;65:282–292. [PubMed] [Google Scholar]
- 64.Barth A, Küfferle B. Die Entwicklung eines Sprichworttests zur Erfassung konkretistischer Denkstörungen bei schizophrenen Patienten [A proverb test to evaluate concretistic thought disorders in schizophrenia] Nervenarzt. 2001;72:853–858. doi: 10.1007/s001150170019. [DOI] [PubMed] [Google Scholar]
- 65.Kircher TTJ, Leube DT, Erb M, Grodd W, Rapp AM. Neural correlates of metaphor processing in schizophrenia. Neuroimage. 2007;34:281–289. doi: 10.1016/j.neuroimage.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 66.Langdon R, Coltheart M, Ward PB, Catts SV. Disturbed communication in schizophrenia: the role of poor pragmatics and poor mind-reading. Psychol Med. 2002;32:1273–1284. doi: 10.1017/s0033291702006396. [DOI] [PubMed] [Google Scholar]
- 67.Benjamin JD. A method for distinguishing and evaluating formal thinking disorders in schizophrenia. In: Kasanin JS, editor. Language and Thought in Schizophrenia. Berkeley, Calif: University of California Press; 1944. [Google Scholar]
- 68.Blaufarb H. A demonstration of verbal abstracting ability in chronic schizophrenics under enriched stimulus and instructional conditions. J Consult Psychol. 1962;26:471–475. doi: 10.1037/h0045408. [DOI] [PubMed] [Google Scholar]
- 69.Holm-Hadulla R-M, Haug F. Die Interpretation von Sprichwörtern als klinische Methode zur Erfassung schizophrener Denk-, Sprach- und Symbolisationsstörungen [Interpretation of proverbs as a clinical method to exploring schizophrenic thought and speech disorders] Nervenarzt. 1984;55:496–503. [PubMed] [Google Scholar]
- 70.Andreasen NC. Reliability and validity of proverb interpretation to assess mental status. Compr Psychiatry. 1977;18:465–472. doi: 10.1016/0010-440x(77)90046-3. [DOI] [PubMed] [Google Scholar]
- 71.Gorham DR. A proverb test for clinical and experimental use. Psychol Rep. 1956;2:1–2. [Google Scholar]
- 72.Klinga K. Determination of hormones and hormone receptors. In: Runnebaum B, Rabe T, editors. Gynecological Endocrinology and Reproductive Medicine. Vol. 1. Heidelberg, Germany: Springer; 1997. pp. 45–54. [Google Scholar]
- 73.Canuso CM, Goldstein JM, Wojcik J, et al. Antipsychotic medication, prolactin elevation, and ovarian function in women with schizophrenia and schizoaffective disorder. Psychiatry Res. 2002;111:11–20. doi: 10.1016/s0165-1781(02)00123-3. [DOI] [PubMed] [Google Scholar]
- 74.Bergemann N, Mundt C, Parzer P, et al. Plasma concentration of estradiol in women suffering from schizophrenia treated with conventional and atypical antipsychotics. Schizophr Res. 2005;73:357–366. doi: 10.1016/j.schres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 75.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 76.Horn W. Leistungsprüfsystem L-P-S [Performance Test System] Göttingen, Germany: Hogrefe; 1983. [Google Scholar]
- 77.Thurstone LL, Thurstone TG. Factorial Studies of Intelligence. Chicago, Ill: University of Chicago Press; 1941. [Google Scholar]
- 78.Conner CK. Continuous Performance Test—Version II. Tornoto, Canada: Multi Health System; 1992. [Google Scholar]
- 79.Stata Corporation. Stata Statistical Software: Release 9.2. College Station, Tex: Stata Corporation; 2005. [Google Scholar]
- 80.Rabe T, Runnebaum B. Hormones. In: Runnebaum B, Rabe T, editors. Gynecological Endocrinology and Reproductive Medicine. Vol 1. Heidelberg, Germany: Springer; 1997. pp. 1–43. [Google Scholar]
- 81.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 82.Guy U, editor. Rockville, Md: National Institute of Mental Health; 1976. ECDEU Assessment Manual for Psychopharmacology. revised. DHEU Publication; pp. 76–338. [Google Scholar]
- 83.Beck AT, Steer RA. Beck Depression Inventory—Manual. San Antonio, Tex: The Psychological Corporation; 1987. [Google Scholar]
- 84.Hautzinger M, Bailer M, Worall H, Keller F. 2nd ed. Bern, Switzerland: Huber; 1995. Beck-Depressions-Inventar (BDI) [Google Scholar]
- 85.Thompson K, Sergejew A, Kulkarni J. Estrogen affects cognition in women with psychosis. Psychiatry Res. 2000;94:201–209. doi: 10.1016/s0165-1781(00)00161-x. [DOI] [PubMed] [Google Scholar]
- 86.Hoff AL, Kremen WS, Wieneke MH, et al. Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry. 2001;158:1134–1139. doi: 10.1176/appi.ajp.158.7.1134. [DOI] [PubMed] [Google Scholar]
- 87.Ko Y-H, Joe S-H, Cho W, et al. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology. 2006;53:169–175. doi: 10.1159/000093780. [DOI] [PubMed] [Google Scholar]
- 88.Brekke JS, Hoe M, Long J, Green MF. How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophr Bull. 2007;33:1247–1256. doi: 10.1093/schbul/sbl072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lysaker PH, Bell MD, Bioty SM. Cognitive deficits in schizophrenia. Prediction of symptom change for participators in work rehabilitation. J Nerv Ment Dis. 1995;183:332–336. [PubMed] [Google Scholar]
- 90.Heaton R, Chelune GT, Talley JL, Kay GG, Curtiss GG. Wisconsin Card Sorting Test Manual Revised and Expanded. Odessa, Fla: Psychological Assessment Resources, Inc; 1993. [Google Scholar]
- 91.Lysaker PH, Bell MD. Work rehabilitation and improvements in insight in schizophrenia. J Nerv Ment Dis. 1995;183:107. doi: 10.1097/00005053-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 92.Spaulding WD, Storms L, Goodrich V, Sullivan M. Applications of experimental psychopathology in psychiatric rehabilitation. Schizophr Bull. 1986;12:560–577. doi: 10.1093/schbul/12.4.560. [DOI] [PubMed] [Google Scholar]
- 93.Lysaker PH, Bell MD, Goulet JL. The Wisconsin Card Sorting Test and work performance in schizophrenia. Psychiatry Res. 1995;56:45–51. doi: 10.1016/0165-1781(94)02641-u. [DOI] [PubMed] [Google Scholar]
- 94.Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry. 2002;159:1018–1028. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- 95.Keefe RS, Sweeney JA, Gu H, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- 96.Swartz MS, Perkins DO, Stroup TS, et al. Effects of antipsychotic medications on psychosocial functioning in patients with chronic schizophrenia: findings from the NIMH CATIE study. Am J Psychiatry. 2007;164:428–436. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]
- 97.Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: mechanisms, clinical features and management. Drugs. 2004;64:2291–2314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- 98.Molitch ME. Medication-induced hyperprolactinemia. Mayo Clin Proc. 2005;80:1050–1057. doi: 10.4065/80.8.1050. [DOI] [PubMed] [Google Scholar]
- 99.Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women, principal results from the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 100.Shumaker SA, Legault C, Kuller L, et al. Women's Health Initiative Memory Study. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 101.Schneider LS. Estrogen and dementia: insights from the Women's Health Initiative Memory Study. JAMA. 2004;291:3005–3007. doi: 10.1001/jama.291.24.3005. [DOI] [PubMed] [Google Scholar]
- 102.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone therapy, physiological complexity belies therapeutic simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 103.Resnick SM, Maki PM, Rapp SR, et al. Women's Health Initiative Study of Cognitive Aging Investigators. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]