Abstract
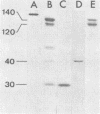
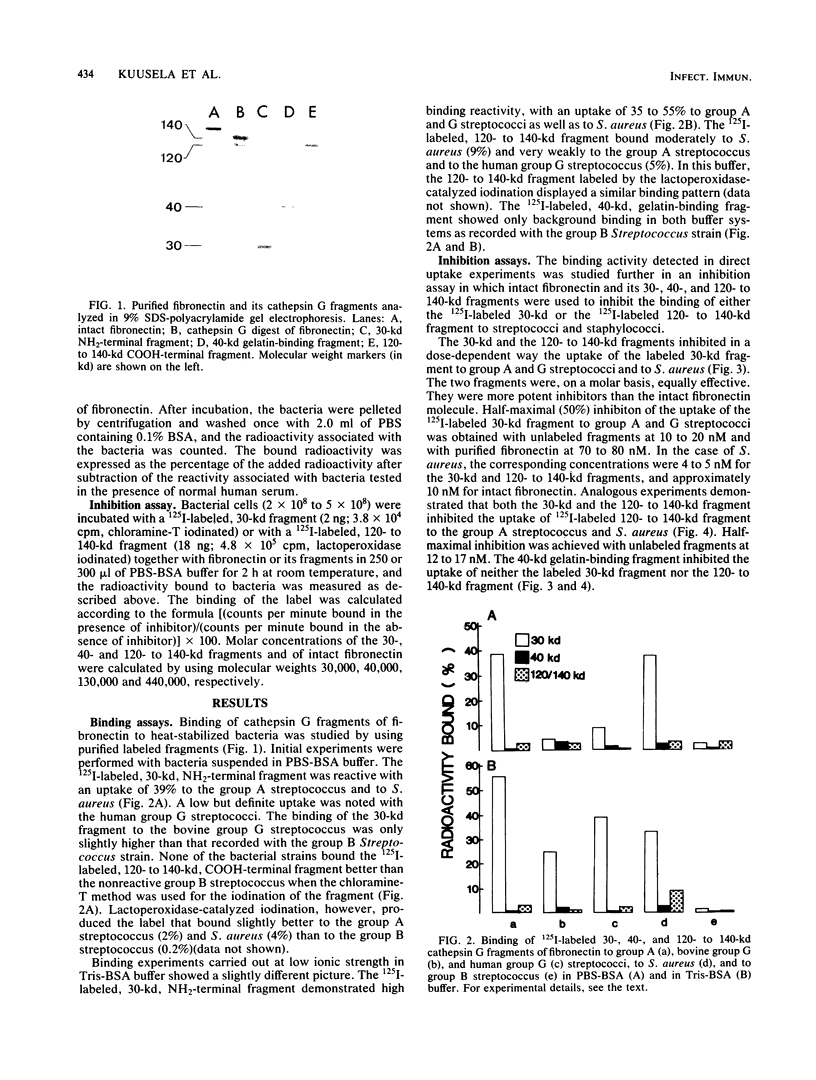
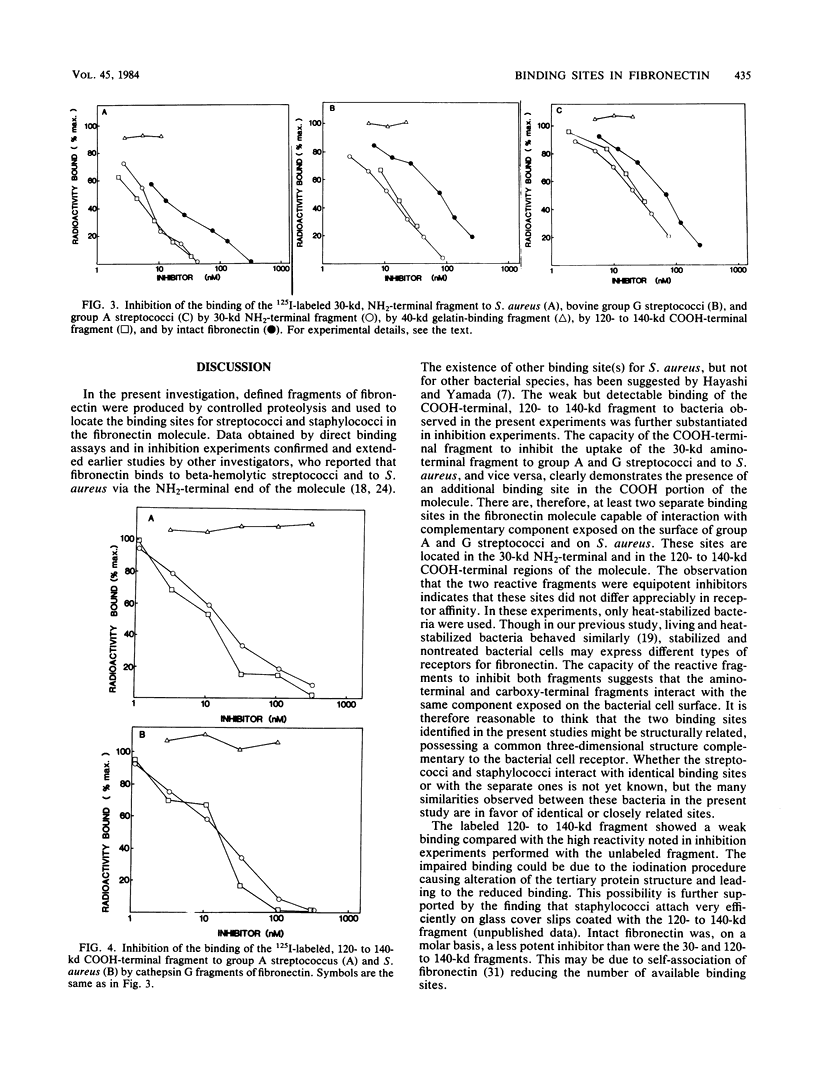
Purified cathepsin G fragments of fibronectin were used to locate the binding sites for streptococci and staphylococci in the fibronectin molecule. The iodinated, NH2-terminal, 30-kilodalton (kd) fragment bound to group A and G streptococci and to Staphylococcus aureus. The 125I-labeled, COOH-terminal, 120- to 140-kd fragment bound weakly to group A streptococcus strain and to S. aureus when tested in a buffer of low ionic strength. The 30- and 120- to 140-kd fragments inhibited the binding of iodinated fragments to bacteria. The two fragments were, on a molar basis, equally effective, and they were more potent inhibitors than intact fibronectin. The gelatin-binding 40-kd fragment neither bound to any of the bacterial strains nor inhibited the binding of 125I-labeled 30-kd or 125I-labeled 120- to 140-kd fragments to bacteria. The results indicate that fibronectin has at least two separate binding sites for streptococci and staphylococci, one in the NH2-terminal region and another in the COOH-terminal region of the molecule, both capable of specific interaction with a complementary structure exposed on streptococcal and staphylococcal cell surfaces.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Beachey E. H., Simpson W. A. Adherence of streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983 Sep;41(3):1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu J. P., Simpson W. A., Courtney H. S., Beachey E. H. Interaction of human plasma fibronectin with cariogenic and non-cariogenic oral streptococci. Infect Immun. 1983 Jul;41(1):162–168. doi: 10.1128/iai.41.1.162-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. B., Mosesson M. W., Solish G. I. Identification of the cold-insoluble globulin of plasma in amniotic fluid. Am J Obstet Gynecol. 1976 Aug 1;125(7):958–961. doi: 10.1016/0002-9378(76)90495-6. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Differences in domain structures between plasma and cellular fibronectins. J Biol Chem. 1981 Nov 10;256(21):11292–11300. [PubMed] [Google Scholar]
- Karonen S. L., Mörsky P., Siren M., Seuderling U. An enzymatic solid-phase method for trace iodination of proteins and peptides with 125-iodine. Anal Biochem. 1975 Jul;67(1):1–10. doi: 10.1016/0003-2697(75)90266-3. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Vartio T., Vaheri A. Polypeptides of human plasma fibronectin are similar but not identical. Biochim Biophys Acta. 1980 Aug 21;624(2):490–498. doi: 10.1016/0005-2795(80)90090-2. [DOI] [PubMed] [Google Scholar]
- Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature. 1978 Dec 14;276(5689):718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- Kuusela P., Vaheri A., Palo J., Ruoslahti E. Demonstration of fibronectin in human cerebrospinal fluid. J Lab Clin Med. 1978 Oct;92(4):595–601. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F., Furcht L. T. Fibronectin: review of its structure and possible functions. J Invest Dermatol. 1981 Aug;77(2):175–180. doi: 10.1111/1523-1747.ep12479791. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Myhre E. B., Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983 Apr;40(1):29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E., Hayman E. G. Fibronectin: current concepts of its structure and functions. Coll Relat Res. 1981;1(1):95–128. doi: 10.1016/s0174-173x(80)80011-2. [DOI] [PubMed] [Google Scholar]
- Simpson W. A., Beachey E. H. Adherence of group A streptococci to fibronectin on oral epithelial cells. Infect Immun. 1983 Jan;39(1):275–279. doi: 10.1128/iai.39.1.275-279.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switalski L. M., Ljungh A., Rydén C., Rubin K., Hök M., Wadström T. Binding of fibronectin to the surface of group A, C, and G streptococci isolated from human infections. Eur J Clin Microbiol. 1982 Dec;1(6):381–387. doi: 10.1007/BF02019939. [DOI] [PubMed] [Google Scholar]
- Vartio T. Characterization of the binding domains in the fragments cleaved by cathepsin G from human plasma fibronectin. Eur J Biochem. 1982 Apr 1;123(2):223–233. doi: 10.1111/j.1432-1033.1982.tb19757.x. [DOI] [PubMed] [Google Scholar]
- Vartio T., Salonen E. M., De Petro G., Barlati S., Miggiano V., Stähli C., Virgallita G., Takács B., Vaheri A. Monoclonal antibody against the N-terminal end of human plasma fibronectin. Biochem J. 1983 Oct 1;215(1):147–151. doi: 10.1042/bj2150147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Vaheri A., Von Essen R., Isomäki H., Stenman S. Fibronectin in synovial fluid and tissue in rheumatoid arthritis. Eur J Clin Invest. 1981 Jun;11(3):207–212. doi: 10.1111/j.1365-2362.1981.tb01842.x. [DOI] [PubMed] [Google Scholar]
- Vuento M., Salonen E., Koskimies A., Stenman U. H. High concentrations of fibronectin-like antigens in human seminal plasma. Hoppe Seylers Z Physiol Chem. 1980 Sep;361(9):1453–1456. [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuento M., Vartio T., Saraste M., von Bonsdorff C. H., Vaheri A. Spontaneous and polyamine-induced formation of filamentous polymers from soluble fibronectin. Eur J Biochem. 1980 Mar;105(1):33–42. doi: 10.1111/j.1432-1033.1980.tb04471.x. [DOI] [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Bass J. A. Role of fibronectin in the prevention of adherence of Pseudomonas aeruginosa to buccal cells. J Infect Dis. 1981 Jun;143(6):784–790. doi: 10.1093/infdis/143.6.784. [DOI] [PubMed] [Google Scholar]