Abstract
Background: Herpes family viruses can cause central nervous system inflammatory changes that can present with symptoms indistinguishable from schizophrenia and therefore are of interest in schizophrenia research. Most existing studies of herpes viruses have used small populations and postdiagnosis specimens. As part of a larger research program, we conducted a hypothesis-generating case-control study of selected herpes virus antibodies among individuals discharged from the US military with schizophrenia and pre- and postdiagnosis sera. Methods: Cases (n = 180) were servicemembers hospitalized and discharged from military service with schizophrenia. Controls, 3:1 matched on several factors, were members not discharged. The military routinely collects and stores members' serum specimens. We used microplate enzyme immunoassay to measure immunoglobulin G (IgG) antibody levels to 6 herpes viruses in pre- and postdiagnosis specimens. Conditional logistic regression was used, and the measure of association was the hazard ratio (HR). Results: Overall, we found a significant association between human herpes virus type 6 and schizophrenia, with an HR of 1.17 (95% confidence interval [CI] = 1.04, 1.32). Women and blacks had significant negative associations with herpes simplex virus type 2 and cytomegalovirus; among blacks, there was a significant positive association with herpes simplex virus type 1. Among men, there was a HHV-6 temporal effect with an HR of 1.41 (95% CI = 1.02, 1.96) for sera drawn 6–12 months before diagnosis. Discussion: Findings from previous studies of herpes family viruses and schizophrenia have been inconsistent. Our study is based on a larger population than most previous studies and used serum specimens collected before onset of illness. This study adds to the body of knowledge and provides testable hypotheses for follow-on studies.
Keywords: herpes, virus, schizophrenia, psychosis, antibodies, military
Introduction
Recent research has focused on infectious agents as potential players in the etiologic pathway of chronic diseases,1–3 including psychiatric illnesses such as schizophrenia.4–10 Due to their potential neurotropism and latency, viral organisms in particular are considered possible agents in many chronic central nervous system (CNS) disorders.2,11–13 Encephalitis and other conditions leading to CNS inflammatory changes often present with symptoms that are difficult to distinguish from new-onset schizophrenia. As significant causes of encephalitis, viruses in the Herpes Simplex Family (herpes simplex virus type 1 [HSV-1], herpes simplex virus type 2 [HSV-2], Epstein-Barr virus [EBV], cytomegalovirus [CMV], varicella-zoster virus [VZV], and human herpes virus type 6 [HHV-6]) are of prime interest in schizophrenia research.14
Although laboratory-based research into herpes family viruses as possible etiologic agents for schizophrenia goes back decades,15–22 ascertaining the nature of a possible etiologic association between infection and schizophrenia is highly challenging. There have been few consistent findings between studies, which could be due to many factors, including the heterogeneity of schizophrenia itself, the use of different immunologic assays across the studies and over time as technology changes, focusing attention on a variety of different infections, and the possibility that maternal infection occurring at the right time during pregnancy may be enough to increase the risk of psychosis in offspring.23 There is more information regarding risks associated with maternal, neonatal, or childhood infection and schizophrenia than for adult infection, with a number of studies reporting increased risk of schizophrenia among persons exposed in utero to a number of infections24–26 or born after epidemic.27
Little is known about potential mechanisms of action for herpes family virus infections and risk of schizophrenia. Studies of maternal infection provide some evidence that modulation of immune response28 and adverse effects on in utero maturation of critical brain structural and functional componenets24 may correlate with increased risk of schizophrenia in offspring. From animal models, maternal infection can alter interleukin 6, interleukin 1b, or tumor necrosis factor α (TNF-α) in amniotic fluid or placenta and TNF-α in the fetal brain.29 There is evidence that cytokines have an important function in the development of fetal neurons and glial cells, and abnormal levels of these (proinflammatory) cytokines may contribute to abnormal brain development,30–34 at least in animals.
Findings from epidemiologic studies of herpes family viruses and schizophrenia among adults are mixed. In their 1995 review article, Yolken and Torrey14 identified numerous published studies assessing viral (not just herpes family viruses) antibodies in serum of schizophrenia cases. Several of the studies were not interpretable because they lacked control groups or were evaluating changes in antibody titers over time. Of 21 interpretable studies, 3 reported positive significant associations with HSV-1 (or HSV unspecified),35–37 1 study reported an association with EBV,18 and 1 found a negative association with CMV.38
The Military New-Onset Psychosis Project hypothesis-generating studies afford unique opportunities to correct for some of the weaknesses identified in other studies of herpes viruses and schizophrenia. Because the US military routinely collects and stores serum samples and medical data from all active duty personnel, we are able to study large numbers of cases who have prediagnostic serum available and document the prevalence of infection prior to onset of disease. Because military members provide a serum sample at accession and about every 2 years thereafter while they remain on active duty, more than one prediagnostic sample will be available for many individuals. Sera from these routine samples are stored in the Department of Defense Serum Repository (DoDSR).39 Informed consent is not routinely obtained when the serum is drawn; however, the DoDSR is maintained for surveillance and research purposes. Information obtained from this study may lead to more effective means of preventing, identifying, and treating new-onset schizophrenia in this population. This project was reviewed and approved by the appropriate human protection committees at the authors' institutions.
Methods
We assayed the serum of 180 individuals diagnosed with schizophrenia who had been hospitalized in a military facility with a mental health diagnosis and subsequently medically discharged or retired from the military between 1992 and 2001 and the serum of 532 controls with no evidence of any mental illness. Diagnosis date for cases was estimated as the date of first mental health hospitalization, used as a proxy for onset. Controls were selected 3:1 to cases and matched on the following variables: date of birth (± 1 year), the corresponding case's accession date ± 6 months, sex, race (black, white, other), branch of military service, and the number of serum specimens available. We attempted to obtain the same number of specimens for cases and controls: the first available specimen (usually collected during the accession medical examination), a specimen collected in the 3- to 24-month period prior to the matched case's first mental health hospitalization, and the first available after the matched case's hospitalization. Additional details on study subject selection and inclusion, serum selection and shipping, and sources and types of ancillary data have been published.40 Sera were assayed for 6 herpes family viruses: HSV-1, HSV-2, EBV, CMV, VZV, and HHV-6.
Laboratory
IgG antibody to human herpes viruses were measured using microplate enzyme immunoassay. Reagents were obtained from the following sources: HSV-1 and HSV-2 from Focus Diagnostics, Cypress, CA; HHV-6 from Advanced Biotechnologies, Columbia, MD; VZV and EBV nuclear antigen from IBL Laboratories, Minneapolis, MN; and CMV from Viro-Immun Labor Diagnostika, Oberursel, Germany.
Enzyme immunoassay consists of binding serum to solid-phase antigen and subsequent reactions with enzyme-labeled antihuman IgG and enzyme substrate. The amount of color generated by the enzyme substrate reaction was measured in optical density (OD) units with a microplate colorimeter. This method of analysis was selected because it allows for high throughput measurement of antibodies using a common platform and requiring only small amounts of sample. Samples were run on plates under code in matched groups in which case and control status was not identified.
Quantitative Antibody Measurements Data Normalization
All matched case-control samples were tested on the same plates, but over the course of the study, samples were assayed on over 21 different plates per agent. To control for potential systematic error introduced by plate-to-plate variation and to ensure that observed differences in OD are due to differential expression (cases or controls) and not experimental artifacts, data were normalized using the robust median normalization method which combines the within-plate and between-plates variance41 using the following equation,
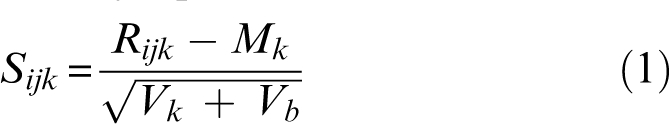 |
(1) |
where Rijk is the raw OD of ith subject's jth blood sample, Mk is the median of all the control samples in plate k, Vk is the variance of Rijk of control samples in plate k, and Vb is the variance of all Rijk between plates. Therefore, Sijk is the scaled score for the ith subject's jth blood sample on plate k.
To investigate the relationship between subject status (case or control) and antibody level in a matched study design, conditional logistic regression is used. Failure to account for matching in analysis may lead to biased results, usually toward the null. The conditional analysis, which has a higher (less negative) log likelihood, suggests a better “fit” for this data.42 A guiding concern in regression modeling is that the relationship between the independent and dependent variables (the latter assumed to be continuous) should be as inherently “linear” as possible; hence, OD was analyzed as a continuous, rather than dichotomous (positive vs negative) variable. This approach also preserves the power to detect a difference between cases and controls, particularly because infection with most of these herpes viruses is ubiquitous and differences cannot be detected based on prevalence data.
For this analysis, we chose the proportional hazards (PH) model for conditional logistic regression.42 The PH model is similar to regular conditional logistic regression but modified to allow for multiple and variable numbers of controls per case and specimens per subject. Dummy “survival time” to diagnosis was generated so that all samples for a given case had the same event time with corresponding controls censored at a later time. Proportional hazard regression was then used to study associations, reported as hazard ratios (HRs), between “survival time” and the risk factors. Because the PH assumption might not be true for all the data, but may be true among specific demographic subgroups, we performed stratified analysis on several of the matched variables.
First, we assessed the overall antibody effect for each agent separately. The analysis was stratified by the matched factors with case or control status as the outcome. Variables analyzed included antibody level, the time from serum collection to the case's diagnosis, and time in service for both cases and controls. Logistic models were developed to assess the antibody effect for modeling the agents separately as well as simultaneously.
To study the homogeneity of the agent effect across demographic levels and time, interaction terms were also evaluated. Where an interaction with demographic factor was observed, separate models were developed to explore the possibility of effect modification by those factors. For homogeneity of the agent effect across the time, we studied the interaction with time to diagnosis. Time to diagnosis was categorized as follows: greater than 2 years, 1–2 years, 0.5–1 year, less than 0.5 year before diagnosis, and after diagnosis. The interaction with time to diagnosis shows the temporal effect of antibody level and describes the consistency of risk across time periods. The interactions of the agents with demographic factors show the heterogeneity of the agent effect by those factors.
Because we used scaled values to represent measured antibody level, there is no recognized unit with which to describe increasing or decreasing levels. In this case, we chose the SD as the unit of measure. All results are reported as HRs for each increase of 1 SD of antibody level. This method also allows comparison between the effects of different antibody agents on schizophrenia. The SD of antibody level for the 6 agents ranged from 0.10 for HHV-6 to 0.59 for HSV-2.
Results
A total of 180 cases (contributing 404 serum specimens) and 532 controls (with 1180 specimens) were included in the study population. Eight cases could only be matched to 2 controls. Table 1 shows the distribution of cases and controls by demographic factors. Overall, about 83% were males, 49% were whites, 44% were blacks, over 57% were younger than 25 years, 10% were older than 35 years, about 12% were Hispanic, and over 56% were in the army. Approximately 35% of cases had greater than 3 years of military service.
Table 1.
Demographic and Military Characteristics of Study Subjects
| Characteristic | Percent of Cases (n = 180) | Percent of Controls (n = 532) |
| Gender | ||
| Male | 83.3 | 83.8 |
| Age at diagnosis categories (y) | ||
| 18–20 | 15.6 | 16.4 |
| 21–25 | 41.7 | 41.2 |
| 26–30 | 20.6 | 20.3 |
| 31–35 | 12.2 | 12.8 |
| >35 | 10.0 | 9.4 |
| Race | ||
| White | 48.9 | 49.1 |
| Black | 44.4 | 44.5 |
| Other | 6.7 | 6.4 |
| Hispanic | ||
| Yes | 11.7 | 6.0 |
| Branch of Military | ||
| Army | 56.1 | 56.0 |
| Air force | 11.1 | 11.3 |
| Marines | 4.4 | 4.5 |
| Navy | 28.3 | 28.2 |
| Time in service before discharge (y) | ||
| ≤1 | 41.1 | 39.7 |
| >1 to ≤ 3 | 24.4 | 24.6 |
| >3 to ≤ 5 | 16.7 | 16.7 |
| >5 to ≤ 10 | 18.3 | 9.4 |
| >10 | 9.4 | 9.6 |
Overall Antibody Effects
We found that antibody levels for all 6 agents were only weakly correlated (data not shown), and therefore, collinearity between agents would not likely be a source of bias or instability in the regression modeling. There was little difference in the results between the separate agent antibody models and the model with all agents considered simultaneously. For example, modeling agent separately, with increasing 1 SD, the HR for CMV was 0.87 (95% confidence interval [CI] = 0.77, 0.98), for HHV-6 was 1.13 (95% CI = 1.00, 1.27), comparing the HRs of 0.86 (95% CI = 0.76, 0.98) and 1.17 (95% CI = 1.04, 1.32), respectively, when modeling all 6 agents together. Therefore, only the simultaneous models are presented in table 2, which presents the overall estimation from conditional logistic modeling. No consistent pattern emerged: a significant protective HR (0.86, 95% CI = 0.76, 0.98) was observed for CMV, a significant HR (1.17, 95% CI = 1.04, 1.32) was demonstrated for HHV-6, while no other agents were significantly associated with schizophrenia.
Table 2.
Schizophrenia Hazard Ratio associated with Antibody Levels in Multiagent Modela
| Factor | Hazard Ratiob | 95% Confidence Interval | P Value |
| HSV-1 | 1.00 | 0.89, 1.14 | .94 |
| HSV-2 | 0.91 | 0.80, 1.03 | .13 |
| CMV | 0.86 | 0.76, 0.98 | .02 |
| EBV | 0.93 | 0.82, 1.04 | .20 |
| VZV | 1.01 | 0.90, 1.13 | .93 |
| HHV-6 | 1.17 | 1.04, 1.32 | .01 |
Note: HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; CMV, cytomegalovirus; EBV, Epstein-Barr virus; VZV, varicella-zoster virus; HHV-6, human herpes virus type 6.
Adjusted for age, race, sex, hospitalization status, years of service, and temporal relationship to diagnosis.
Hazard ratio expressed per 1 SD increase of each agent antibody level.
Stratified Analysis
No significant effect modification was noted for sex (male vs female) or age (<25 vs ≥25) for any of the infectious agents (P values > .10). A significant interaction effect with race (black vs white) was found for HSV-1 (P < .01) and for CMV (P = .03), suggesting that significant differences exist in these antibody effects between black and white cases. As seen in table 3, the HR among black cases was significant for HSV-1 (1.23, 95% CI = 1.02, 1.47), HSV-2 (0.83, 95% CI = 0.70, 0.99), and CMV (0.69, 95% CI = 0.57, 0.84), but no significant findings were observed among white cases. There were no significant interactions between agents and gender, likely due to the small number of female cases (n = 30) in the analyses, but there were differences in antibody effect between men and women. Among women, significant negative HRs were noted for 2 agents, HSV-2 (0.69, 95% CI = 0.52, 0.92) and CMV (0.62, 95% CI = 0.44, 0.89). The HHV-6 effect among males (HR of 1.17, 95% CI = 1.03, 1.33) was almost the same as for all subjects because the majority of subjects were male (83%). For females, the HHV-6 HR was lower (1.08, 95% CI = 0.70, 1.66) but not significant and with a broader CI, reflecting less precision in the estimate. There was no significant difference in antibody effect by age.
Table 3.
Schizophrenia Hazard Ratio associated with Antibody Levels in Multiagent Models by Sex and Racea
| Factor | Hazard Ratiob | 95% Confidence Interval | P Value |
| Men | |||
| HSV-1 | 1.03 | 0.90, 1.17 | .71 |
| HSV-2 | 0.97 | 0.84, 1.12 | .63 |
| CMV | 0.90 | 0.78, 1.03 | .12 |
| EBV | 0.92 | 0.81, 1.04 | .17 |
| VZV | 0.99 | 0.87, 1.13 | .91 |
| HHV-6 | 1.17 | 1.03, 1.33 | .01 |
| Women | |||
| HSV-1 | 1.01 | 0.70, 1.47 | .95 |
| HSV-2 | 0.69 | 0.52, 0.92 | .01 |
| CMV | 0.62 | 0.44, 0.89 | .01 |
| EBV | 1.02 | 0.70, 1.48 | .93 |
| VZV | 1.11 | 0.79, 1.57 | .55 |
| HHV-6 | 1.08 | 0.70, 1.66 | .74 |
| White | |||
| HSV-1 | 0.84 | 0.70, 1.01 | .07 |
| HSV-2 | 0.97 | 0.80, 1.19 | .80 |
| CMV | 1.05 | 0.88, 1.25 | .60 |
| EBV | 0.94 | 0.80, 1.11 | .47 |
| VZV | 0.98 | 0.83, 1.16 | .80 |
| HHV-6 | 1.14 | 0.97, 1.33 | .12 |
| Black | |||
| HSV-1 | 1.23 | 1.02, 1.47 | .03 |
| HSV-2 | 0.83 | 0.70, 0.99 | .03 |
| CMV | 0.69 | 0.57, 0.84 | .00 |
| EBV | 0.95 | 0.79, 1.14 | .56 |
| VZV | 1.09 | 0.92, 1.29 | .31 |
| HHV-6 | 1.17 | 0.97, 1.42 | .10 |
Note: HSV-1, herpes simplex virus type 1; HSV-2, herpes simplex virus type 2; CMV, cytomegalovirus; EBV, Epstein-Barr virus; VZV, varicella-zoster virus; HHV-6, human herpes virus type 6.
aAdjusted for age, hospitalization status, years of service, and temporal relationship to diagnosis.
Hazard ratio expressed per 1 SD increase of each agent antibody level.
Among men, only HHV-6 showed weak temporal variability, with the highest HR for sera drawn 12–6 months before onset (1.41, 95% CI = 1.02, 1.96) and the lowest HR of for sera drawn 2 years or longer before diagnosis (1.08, 95% CI = 0.88, 1.33) as shown in table 4. No temporal effect was observed for women and other demographic groups due to small sample size.
Table 4.
Hazard Ratios for HHV-6 Antibody Levels by Temporal Relationship to Diagnosis Among Men
| Time to Diagnosis (y) | Hazard Ratioa | 95% Confidence Interval | P Value |
| Before ≥2 | 1.08 | 0.88, 1.33 | .47 |
| 1–2 | 1.09 | 0.75, 1.59 | .66 |
| 0.5–1 | 1.41 | 1.02, 1.96 | .04 |
| ≤0.5 | 1.15 | 0.82, 1.61 | .43 |
| After diagnosis | 1.09 | 0.87, 1.38 | .46 |
Hazard ratio expressed per 1 SD increase of the human herpes virus type 6 (HHV-6) immunoglobulin G antibody level.
Discussion
Our hypothesis-generating study found a statistically significant positive HR between HHV-6 and schizophrenia among men and between HSV-1 and schizophrenia among blacks discharged or retired from the military with a diagnosis of schizophrenia and a history of mental health hospitalization.
A negative association with HSV-2 and CMV was noted among women and blacks. Blacks dominated the results for women. These findings should be interpreted with caution, however, because they are driven by a small number of subjects (n = 80 for black cases, n = 30 for female cases, n = 22 for black females) and may be the result of type I error. Further analysis is warranted with a larger sample size. No significant associations were observed for HSV-2, EBV, or VZV among men or women. No significant association was found among whites for any agent.
Our subanalysis of HHV-6 IgG levels by time period for males around diagnosis is obviously limited by the sample size. We conducted this analysis to replicate the analytic modeling in our previous, related work on Toxoplasma gondii IgG level and risk of schizophrenia.40 The P values of .04 in this hypothesis-generating study warrant further evaluation in the hypothesis-testing phase of our research.
More recently, studies of antibody levels in serum and cerebrospinal fluid demonstrate mixed findings. One analysis of untreated subjects with recent-onset schizophrenia found increased IgG antibody levels to CMV, decreased levels of antibodies to HHV-6 and VZV, and no differences in antibody level to HSV-1 and -2 and EBV.9 Several other studies of cerebrospinal fluid yielded conflicting results with some reporting increased CMV antibody titers38,43–45 while others demonstrate no association.46–48 Increased levels of HSV-1 antibody were demonstrated in one group of schizophrenic patients compared with normal controls, and cases with higher levels of antibody also demonstrated decreased gray matter in 2 areas of the brain.49 Another study noted that deficit schizophrenics were more likely to have antibodies to CMV than were nondeficit patients.50
A recent review of the literature regarding CMV and schizophrenia identified a number of studies reporting more frequent infection or higher levels of antibody in serum or cerebrospinal fluid.51 The authors noted that studies conducted in 1992 were all null but that the serum assays utilized had been complement fixation or other less sensitive methods. They note 3 unpublished studies (M. J. Schwarz and N. Mueller, S. Bachmann; J. Schröder; and R. H. Yolken, unpublished data) in which patients with schizophrenia were more likely to have antibodies to CMV, or had higher levels of antibodies, than did the control subjects. One of these studies (F. B. Dickerson, C. Stallings, A. Origoni, J. J. Boronow, R. H. Yolken, unpublished data) was of 415 outpatients with schizophrenia and 164 matched controls, in which the odds ratio for CMV positivity was 2.1. The authors note that patients who were seropositive were more likely to be female, black, older, and less educated. Leweke et al9 found that CMV IgG antibody levels, but not HSV-1, HSV-2, EBV, HHV-6, or VZV, were higher among patients with schizophrenia.9
Given the limited amount of research reported and the discordant findings among the existing articles regarding herpes viruses and schizophrenia, interpretation of our findings is challenging. Recent work has implicated HHV-6 in acute52 and chronic53,54 neurologic diseases. We note the negative association with HSV-2 and CMV among women and blacks and the positive association with HSV-1 among blacks. Although speculative, and limited by sample size, there is a potential for underlying genetic differences that could explain some portion of the racial differences.
There are a number of factors that could potentially account for the discrepancies observed between the various reports above and the present study. These include but are not limited to differences in diagnostic criteria for schizophrenia, all cases were adult onset, different time frames of serum collection related to illness onset, differences in laboratory assays, and multiple vs single serum specimens. In addition, our sample was drawn from the military population that differs from the general US population in several important ways. The male to female ratio in the military is much higher than in the general population, making it difficult to achieve adequate power when analyzing females separately. Comprehensive medical screening prior to entry into the military creates a healthy worker effect in the population and skews our sample toward individuals with later onset of schizophrenia. Also, our cases are a convenience sample of individuals with schizophrenia in the military. A small degree of bias introduced by any of these factors could account for the significance and direction of our findings.
This study has 2 important strengths. First, we used cases that were diagnosed and discharged from the military after a careful evaluation process.40 A record review conducted on a sample of study subjects demonstrated a high level of concordance between the diagnoses documented in the military records and those assigned by 2 psychiatrist reviewers.55 Also, because military applicants receive an extensive administrative and medical examination prior to accession, are directly supervised while on active duty, and have access to health care, we assumed that cases were not psychotic on accession and that the onset of psychosis would generally result in a mental health hospitalization for an expedited psychiatric evaluation. This assumption was validated by the record review.55 Therefore, we are confident that diagnostic misclassification is not a major source of error in our findings.
In addition, the current study obtained multiple (between 1 and 3) specimens for most subjects both prior to and after onset of illness in the matched case. The second specimen, collected in the 3- to 24-month period prior to first mental health hospitalization was chosen as preonset of psychosis. The ability to analyze longitudinal specimens may be important if illness alters behaviors in a way that could impact antibody levels or if medical treatment for illness changes antibody responses.
Although this hypothesis-generating study does not resolve the issue of diverse findings between studies, we feel that our study has advantages over other studies with our high degree of diagnostic validity, adequate numbers of appropriate controls, and multiple serum specimens. It is clear that additional studies are needed to clarify the many remaining questions, particularly regarding HHV-6, CMV, and HSV-1. As part of our broad research program, we will be conducting a much larger case-control study with approximately 1600 cases and 2100 controls. Although herpes family viruses will not be the primary focus of this follow-on study, we intend to further explore the associations identified in this hypothesis-generating study.
Funding
Stanley Medical Research Institute, Bethesda, MD; Department of the Army; Funding Cooperative Research and Development Agreement (DAMD control, No: 17-04-0041).
Acknowledgments
The views expressed are those of the authors and should not be construed to represent the positions of the Department of the Army or Department of Defense. None of the authors have any associations, financial or otherwise, that may present a conflict of interest.
References
- 1.Diomedi M, Leone G, Renna A. The role of chronic infection and inflammation in the pathogenesis of cardiovascular and cerebrovascular disease. Drugs Today (Barc) 2005;41:745–753. doi: 10.1358/dot.2005.41.11.917342. [DOI] [PubMed] [Google Scholar]
- 2.Levin LI, Munger KL, Rubertone MV, et al. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293:2496–2500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- 3.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 4.Amminger GP, McGorry PD, Berger GE, et al. Antibodies to infectious agents in individuals at ultra-high risk for psychosis. Biol Psychiatry. 2007;61(10):1215–1217. doi: 10.1016/j.biopsych.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Brown AS, Begg MD, Gravenstein S, et al. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 6.Brown AS, Cohen P, Harkavy-Friedman J, et al. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Schaefer CA, Quesenberry CP, Jr, Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 8.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 9.Leweke FM, Gerth CW, Koethe D, et al. Antibodies to infectious agents in individuals with recent onset schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2004;254:4–8. doi: 10.1007/s00406-004-0481-6. [DOI] [PubMed] [Google Scholar]
- 10.Yolken RH, Bachmann S, Ruslanova I, et al. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin Infect Dis. 2001;32:842–844. doi: 10.1086/319221. [DOI] [PubMed] [Google Scholar]
- 11.Forton DM, Taylor-Robinson SD, Thomas HC. Central nervous system changes in hepatitis C virus infection. Eur J Gastroenterol Hepatol. 2006;18:333–338. doi: 10.1097/00042737-200604000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Jacomy H, Fragoso G, Almazan G, Mushynski WE, Talbot PJ. Human coronavirus OC43 infection induces chronic encephalitis leading to disabilities in BALB/C mice. Virology. 2006;349:335–346. doi: 10.1016/j.virol.2006.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komaroff AL, Jacobson S, Ablashi DV, Yamanishi K. Highlights from 5th International Conference on HHV-6 and -7. Herpes. 2006;13:81–82. [PubMed] [Google Scholar]
- 14.Yolken RH, Torrey EF. Viruses, schizophrenia, and bipolar disorder. Clin Microbiol Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander RC, Cabirac G, Lowenkopf T, et al. Search for evidence of herpes simplex virus, type 1, or varicella-zoster virus infection in postmortem brain tissue from schizophrenic patients. Acta Psychiatr Scand. 1992;86:418–420. doi: 10.1111/j.1600-0447.1992.tb03290.x. [DOI] [PubMed] [Google Scholar]
- 16.Bartova L, Rajcani J, Pogady J. Herpes simplex virus antibodies in the cerebrospinal fluid of schizophrenic patients. Acta Virol. 1987;31:443–446. [PubMed] [Google Scholar]
- 17.Delisi LE, Smith SB, Hamovit JR, et al. Herpes simplex virus, cytomegalovirus and Epstein-Barr virus antibody titers in sera from schizophrenic patients. Psychol Med. 1986;16:757–763. doi: 10.1017/s0033291700011764. [DOI] [PubMed] [Google Scholar]
- 18.Gotlieb-Stematsky T, Zonis J, Arlazoroff A, Mozes T, Sigal M, Szekely AG. Antibodies to Epstein-Barr virus, herpes simplex type 1, cytomegalovirus and measles virus in psychiatric patients. Arch Virol. 1981;67:333–339. doi: 10.1007/BF01314836. [DOI] [PubMed] [Google Scholar]
- 19.King DJ, Cooper SJ, Earle JA, et al. A survey of serum antibodies to eight common viruses in psychiatric patients. Br J Psychiatry. 1985;147:137–144. doi: 10.1192/bjp.147.2.137. [DOI] [PubMed] [Google Scholar]
- 20.Rimon R, Halonen P, Puhakka P, Laitinen L, Marttila R, Salmela L. Immunoglobulin G antibodies to herpes simplex type 1 virus detected by radioimmunoassay in serum and cerebrospinal fluid of patients with schizophrenia. J Clin Psychiatry. 1979;40:241–243. [PubMed] [Google Scholar]
- 21.Rimon R, Nishmi M, Halonen P. Serum and CSF antibody levels to herpes simplex type 1, measles and rubella viruses in patients with schizophrenia. Ann Clin Res. 1978;10:291–293. [PubMed] [Google Scholar]
- 22.Stevens JR, Langloss JM, Albrecht P, Yolken R, Wang YN. A search for cytomegalovirus and herpes viral antigen in brains of schizophrenic patients. Arch Gen Psychiatry. 1984;41:795–801. doi: 10.1001/archpsyc.1984.01790190069009. [DOI] [PubMed] [Google Scholar]
- 23.Pearce BD. Schizophrenia and viral infection during neurodevelopment: a focus on mechanisms. Mol Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- 24.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 25.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001;15:411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- 26.Suvisaari J, Haukka J, Tanskanen A, Hovi T, Lonnqvist J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am J Psychiatry. 1999;156:1100–1102. doi: 10.1176/ajp.156.7.1100. [DOI] [PubMed] [Google Scholar]
- 27.Rantakallio P, Jones P, Moring J, Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997;26:837–843. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- 28.Hinze-Selch D. Infection, treatment and immune response in patients with bipolar disorder versus patients with major depression, schizophrenia or healthy controls. Bipolar Disord. 2002;4(suppl 1):81–83. doi: 10.1034/j.1399-5618.4.s1.32.x. [DOI] [PubMed] [Google Scholar]
- 29.Urakubo A, Jarskog LF, Lieberman JA, Gilmore JH. Prenatal exposure to maternal infection alters cytokine expression in the placenta, amniotic fluid, and fetal brain. Schizophr Res. 2001;47:27–36. doi: 10.1016/s0920-9964(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 30.Mehler MF, Kessler JA. Hematolymphopoietic and inflammatory cytokines in neural development. Trends Neurosci. 1997;20:357–365. doi: 10.1016/s0166-2236(96)01045-4. [DOI] [PubMed] [Google Scholar]
- 31.Mehler MF, Marmur R, Gross R, et al. Cytokines regulate the cellular phenotype of developing neural lineage species. Int J Dev Neurosci. 1995;13:213–240. doi: 10.1016/0736-5748(94)00060-g. [DOI] [PubMed] [Google Scholar]
- 32.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 33.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Mehler MF, Kessler JA. Cytokines in brain development and function. Adv Protein Chem. 1998;52:223–251. doi: 10.1016/s0065-3233(08)60437-4. [DOI] [PubMed] [Google Scholar]
- 35.Halonen PE, Rimon R, Arohonka K, Jantti V. Antibody levels to herpes simplex type I, measles and rubella viruses in psychiatric patients. Br J Psychiatry. 1974;125:461–465. doi: 10.1192/bjp.125.5.461. [DOI] [PubMed] [Google Scholar]
- 36.Libikova H. Schizophrenia and viruses: principles of etiologic studies. In: Morozov P, editor. Research on the Viral Hypothesis of Mental Disorders. Basel, Switzerland: Karger; 1983. pp. 20–51. [Google Scholar]
- 37.Pelonero AL, Pandurangi AK, Calabrese VP. Serum IgG antibody to herpes viruses in schizophrenia. Psychiatry Res. 1990;33:11–17. doi: 10.1016/0165-1781(90)90144-t. [DOI] [PubMed] [Google Scholar]
- 38.Albrecht P, Torrey EF, Boone E, Hicks JT, Daniel N. Raised cytomegalovirus-antibody level in cerebrospinal fluid of schizophrenic patients. Lancet. 1980;2:769–772. doi: 10.1016/s0140-6736(80)90386-4. [DOI] [PubMed] [Google Scholar]
- 39.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense Serum Repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900–1904. doi: 10.2105/ajph.92.12.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niebuhr DW, Millikan AM, Cowan DN, Yolken R, Li Y, Weber NS. Selected infectious agents and risk of schizophrenia among U.S. military personnel: an hypothesis-generating study of Toxoplasma gondii. Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2007.06081254. doi: 10.1176\APPI.AJP.2007.06081254. [DOI] [PubMed] [Google Scholar]
- 41.Wit E, McClure JD. Statistics for Microarrays Design, Analysis, and Inference. Chichester, England: John Wiley & Sons; 2004. [Google Scholar]
- 42.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. Wiley Series in Probability and Statistics. Texts and Reference Section. New York, NY: Wiley; 1999. [Google Scholar]
- 43.Kaufmann CA, Weinberger DR, Yolken RH, Torrey EF, Pofkin SG. Viruses and schizophrenia. Lancet. 1983;2:1136–1137. doi: 10.1016/s0140-6736(83)90645-1. [DOI] [PubMed] [Google Scholar]
- 44.Torrey EF, Yolken RH, Winfrey CJ. Cytomegalovirus antibody in cerebrospinal fluid of schizophrenic patients detected by enzyme immunoassay. Science. 1982;216:892–894. doi: 10.1126/science.6281883. [DOI] [PubMed] [Google Scholar]
- 45.van Kammen DP, Mann L, Scheinin M, van Kammen WB, Linnoila M. Spinal fluid monoamine metabolites and anti-cytomegalovirus antibodies and brain scan evaluation in schizophrenia. Psychopharmacol Bull. 1984;20:519–522. [PubMed] [Google Scholar]
- 46.Ahokas A, Rimon R, Koskiniemi M, Vaheri A, Julkunen I, Sarna S. Viral antibodies and interferon in acute psychiatric disorders. J Clin Psychiatry. 1987;48:194–196. [PubMed] [Google Scholar]
- 47.Rimon R, Ahokas A, Palo J. Serum and cerebrospinal fluid antibodies to cytomegalovirus in schizophrenia. Acta Psychiatr Scand. 1986;73:642–644. doi: 10.1111/j.1600-0447.1986.tb02737.x. [DOI] [PubMed] [Google Scholar]
- 48.Shrikhande S, Hirsch SR, Coleman JC, Reveley MA, Dayton R. Cytomegalovirus and schizophrenia. A test of a viral hypothesis. Br J Psychiatry. 1985;146:503–506. doi: 10.1192/bjp.146.5.503. [DOI] [PubMed] [Google Scholar]
- 49.Prasad KM, Shirts BH, Yolken RH, Keshavan MS, Nimgaonkar VL. Brain morphological changes associated with exposure to HSV1 in first-episode schizophrenia. Mol Psychiatry. 2007;12:105–113. doi: 10.1038/sj.mp.4001915. 101. [DOI] [PubMed] [Google Scholar]
- 50.Dickerson F, Kirkpatrick B, Boronow J, Stallings C, Origoni A, Yolken R. Deficit schizophrenia: association with serum antibodies to cytomegalovirus. Schizophr Bull. 2006;32:396–400. doi: 10.1093/schbul/sbi054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torrey EF, Leweke MF, Schwarz MJ, et al. Cytomegalovirus and schizophrenia. CNS Drugs. 2006;20:879–885. doi: 10.2165/00023210-200620110-00001. [DOI] [PubMed] [Google Scholar]
- 52.Isaacson E, Glaser CA, Forghani B, et al. Evidence of human herpesvirus 6 infection in 4 immunocompetent patients with encephalitis. Clin Infect Dis. 2005;40:890–893. doi: 10.1086/427944. [DOI] [PubMed] [Google Scholar]
- 53.Alvarez-Lafuente R, De Las Heras V, Bartolome M, Garcia-Montojo M, Arroyo R. Human herpesvirus 6 and multiple sclerosis: a one-year follow-up study. Brain Pathol. 2006;16:20–27. doi: 10.1111/j.1750-3639.2006.tb00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fotheringham J, Donati D, Akhyani N, et al. Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 2007;4:e180. doi: 10.1371/journal.pmed.0040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Millikan AM, Weber NS, Niebuhr DW, et al. Evaluation of data obtained from military disability medical administrative databases for service members with schizophrenia or bipolar disorder. Mil Med. 2007;172:1032–1038. doi: 10.7205/milmed.172.10.1032. [DOI] [PubMed] [Google Scholar]


