Abstract
While genetic factors account for a significant proportion of liability to schizophrenia, a body of evidence attests to a significant environmental contribution. Understanding the mechanisms through which genetic and environmental factors coalesce in influencing schizophrenia is critical for elucidating the pathways underlying psychotic illness and for developing primary prevention strategies. Although obstetric complications (OCs) remain among the most well-documented environmental indicators of risk for schizophrenia, the pathogenic role they play in the etiology of schizophrenia continues to remain poorly understood. A question of major importance is do these factors result from a genetic diathesis to schizophrenia (as in gene-environment covariation), act additively or interactively with predisposing genes for the disorder in influencing disease risk, or independently cause disease onset? In this review, we evaluate 3 classes of OCs commonly related to schizophrenia including hypoxia-associated OCs, maternal infection during pregnancy, and maternal stress during pregnancy. In addition, we discuss several mechanisms by which OCs impact on genetically susceptible brain regions, increasing constitutional vulnerability to neuromaturational events and stressors later in life (ie, adolescence), which may in turn contribute to triggering psychosis.
Keywords: schizophrenia, obstetric complications, gene-environment interaction, covariation, hypoxia, infection, stress, rGE, G × E
Introduction
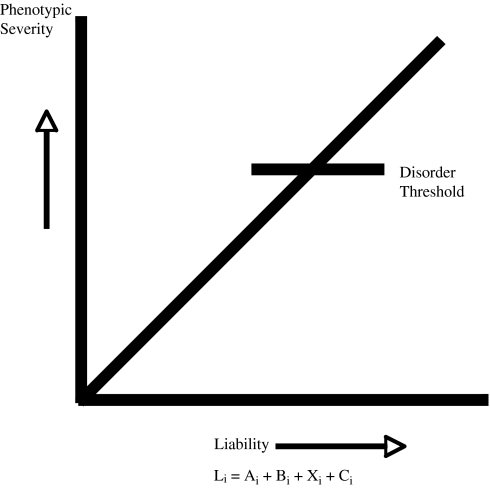
Although genetic factors account for 80%–85% of liability to schizophrenia,1,2 findings that the disorder occurs in only 50% of monozygotic co-twins of an affected proband indicate that the environment plays a nontrivial role. Risk for schizophrenia is thus assumed to represent an integrated function of multiple predisposing genes as well as environmental risk factors, as instantiated in the multifactorial polygenic threshold model (MPTM) (see figure 1). A question of major importance, and the focus of this review, is how do these 2 classes of risk factors—genetic and nongenetic—contribute to susceptibility for disease onset?
Fig. 1.
Multifactorial Polygenic Threshold Model of Schizophrenia.
Given the relative magnitude of the genetic contribution to schizophrenia, it is tempting to speculate that most or all environmental contributors have a gene-dependent relevance, as in genotype × environment interaction (G × E).3 However, the G × E model is only one of several possible mechanisms by which such factors could aggregate; other models including direct environmental influences4 and genotype-environment covariation (rGE) are also plausible.5 In fact, the answer to the question of how genetic and nongenetic factors aggregate in influencing risk for schizophrenia could vary according to the particular nongenetic and genetic factors in question. Highlighting the correct model for each contributing factor is critical given that each model makes different predictions concerning the etiological relevance of the nongenetic effects, therefore affecting ascertainment of populations at risk and prevention/intervention strategies.
Across environmental variables, obstetric complications (OCs) are among the most well-documented predictors of risk for schizophrenia.6–8 In this article, we evaluate 3 classes of OCs—hypoxia-associated obstetric events, maternal infection during pregnancy, and maternal stress during pregnancy—in relation to risk for schizophrenia, addressing the question how each OC coalesces with genetic influences.
Mechanisms of Genetic and Environmental Influence
Phenocopy Model
Some theorists have proposed that there may be a subgroup of schizophrenia patients who acquired the disorder from entirely nongenetic origins; ie, for some patients, an OC could cause schizophrenia without the participation of genetic influences.4 In the framework of the MPTM described in figure 1, the phenocopy model would imply that the weight of a particular environmental risk factor on disease risk is so substantial as to cause an individual exposed to it to cross the threshold on the liability continuum for overt expression of schizophrenia, even though the individual lacks genetic risk factors that account for a lion's share of liability to the disorder in the population.
This model provides the clearest rationale for primary prevention because reducing the rate of exposure to the environmental influence in question would therefore result in a reduction in the incidence of schizophrenia in the population. The phenocopy model does not predict that all schizophrenic patients should have the exposure, just that all individuals with a history of the exposure would develop schizophrenia. One test of this model is to compare the rate of the exposure in the population with the rate of schizophrenia, such that increases in rates of the exposure should lead to measurable increases in disease risk. Using this strategy, we evaluate the phenocopy model within the framework of 3 prominent classes of OC.
Gene-Environment Covariation Model
The gene-environment covariation model posits that OCs are associated with the genes for the disorder. In this model, the roles of genes and OCs are confounded such that OCs may not actually play a role in the etiology of the disorder. In the framework of the MPTM, each covarying term (rGE) influences liability to the disorder, but because the G and E components are covariant with each other, it is unclear whether both causally influence liability or whether only one does, with the other representing an epiphenomenon. This model provides the least clear rationale for prevention because it is ambiguous whether reducing exposure to the environmental factor in question will result in reductions in liability to the disorder. The chief question when evaluating the covariation model is “does the exposure occur more frequently in groups at elevated genetic risk for schizophrenia than in groups without a known genetic risk?” A null result would weaken the gene-environment covariation model because gene carriers, such as patients with schizophrenia and unaffected relatives, would be no more likely to have the exposure than nongene carriers. For example, the gene-environment covariation model is weakened by the finding that mothers with a first-degree schizophrenia relative showed no significant increase in rates of OCs, suggesting that the genetic liability for schizophrenia, on its own, does not confer increased risk for OCs.9 Although mothers with a schizophrenia diagnosis show increased rates of OCs, these findings are difficult to interpret given that smoking during pregnancy was found to mediate the relationship between maternal schizophrenic status and decreased birth weight, suggesting that an observed increase in OCs among these women may be more related to health risk behaviors occurring during pregnancy than to genetic liability to schizophrenia.9 Given the aforementioned findings, examination of OCs among schizophrenic mothers is confounded by multiple intervening variables. Most studies of rGE use unaffected first-degree relatives of probands to index genetic liability, which is reasonable given that this group likely has some of the disease-producing genes. However, there is a cost in statistical power in detecting rGE to the extent that such individuals are not expected to have all the relevant predisposing genes.
Gene-Environment Interaction and Additive Influences Models
The gene-environment interaction model asserts that the environmental influence on risk for disorder depends on the presence of disease-promoting genes.10,11 According to this model, the occurrence of an OC in a genetically vulnerable individual would increase the likelihood of that individual developing the disorder. In the framework of the MPTM (figure 1), the effects of a particular G term and a particular E term are multiplicative in influencing liability to the disorder. It also is possible that genetic and obstetric risk factors for schizophrenia occur independently of each other but additively influence risk for disease expression. In both models, reducing the rate of exposure to the environmental factor in question will result in a reduction in liability to the disorder in the population, with the magnitude of this reduction depending on the weights of the direct effects of the particular E and G terms or the multiplicative effects of the particular G and E terms. Both the gene-environment interaction model and the additive influences model predict a relative increase in the rate of OCs among individuals who develop schizophrenia; therefore, the 2 models can be difficult to parse apart.8 Specifically, it is very difficult to segregate multiplicative vs additive gene-environment influences for a low-frequency disorder such as schizophrenia unless information on both family history and OC exposures are available on a very large population (tens of thousands) or unless one has directly measured the environmental and genetic influences in question.12,13 The latter approach has become more feasible with the recent availability of serological data from pregnancy and the identification of candidate genes involved in schizophrenia.12 Nevertheless, while the list is growing, at the present time only a handful of candidate genes have been identified, and there is clearly a plurality of susceptibility genes for schizophrenia, which further complicates detecting gene-environment interactions.12
In the absence of direct measures of the genetic effects, it is still possible to test a gene-environment interaction vs additive influences model if a continuous measure of liability to the disorder is available. For example, certain structural brain abnormalities have been shown to covary with degree of genetic liability to schizophrenia.1 In this situation, one can perform a factorial analysis of the main effects of genetic and environmental risk and then test whether the multiplicative (interactive) term between the 2 classes of risk factors accounts for a significant proportion of the variation in the liability indicator (in this example, in the severity of structural brain abnormalities).
Multiple Models
In a recent review, Ellman and Cannon12 proposed that multiple models might be operating simultaneously in relation to a given genetic and/or environmental risk factor. Specifically, the authors point out that a genetic polymorphism associated with a magnified inflammatory response to infection has been linked to schizophrenia, such that in the presence of infection there is an exaggerated inflammatory response (gene-environment interaction), possibly leading to fetal neuronal injury, as discussed below.14,15 The authors also note that this polymorphism is associated with increased incidence of certain OCs, such as preterm delivery (gene-environment covariation); this relationship raises the possibility that a gene associated with schizophrenia could have multiple functions, some of which may contribute to the etiology of the disorder and some of which may be unrelated to etiology.12,16
Obstetric Complications
Hypoxia-Associated OCs
A number of studies and reviews have concluded that fetal hypoxia likely is involved in a variety of OCs associated with risk for schizophrenia.17–21 Specifically, OCs associated with schizophrenia, such as emergency cesarean section, bleeding during pregnancy, and preeclampsia, have all been associated with fetal hypoxia.10,22,23 In order to test the strength of the relationship between hypoxia-associated OCs and schizophrenic onset in offspring, one series of investigations used a hypoxia-associated OCs scale including both indirect and direct indicators of hypoxia.24,25 Direct hypoxia-associated OCs included events such as blue at birth, required resuscitation, neonatal cyanosis, and neonatal apnea.24,25 Indirect complications were selected based on validation with direct measures of hypoxia and from previous studies. OCs associated with hypoxia were related to schizophrenia onset, especially among the cases who had an earlier age of onset.24,25
Phenocopy Model of Hypoxia-Associated OCs
Are hypoxia-associated OCs capable of causing schizophrenia on their own, without the participation of genetic factors? Animal models (sheep and rats) indicate that prenatal exposure to hypoxia is associated with multiple brain abnormalities that have been found in schizophrenia patients, including decreased N-methyl-D-asparate (NMDA) receptor binding; increased transcript expression of NR1 subunit in frontal and temporal regions, nucleus accumbens, and hippocampus (while NR2A subunit expression was found to be downregulated in hippocampal subregions)26; increased dopamine (DA) release including persistent alterations in function of mesolimbic and mesostriatal pathways as well as the way dopaminergic function is regulated by stress later in adulthood27; neuronal death (cerebellum, hippocampus, and cerebral cortex); white matter damage; reduced growth of neural processes28; disrupted development of mesotelencephalic pathways modulating the expression of sleep/wakefulness; and cognitive deficits in areas such as working memory and locomotion.29 Therefore, even in the absence of a genetic liability for schizophrenia, there are independent contributions of fetal hypoxia on neuronal and behavioral alterations similar to those found among schizophrenic patients.
Nevertheless, the prevailing evidence suggests that a phenocopy model is unlikely because the rate of hypoxia-associated OCs in the general population (10%–15%) is far higher than the rate of schizophrenia (approximately 1% of the population).18 That is, the vast majority of individuals who suffer hypoxia-associated OCs (97%) do not develop psychosis.30 It could be argued that a more limited phenocopy model might apply to the most severe cases of perinatal hypoxia. However, even among the most severely cyanotic infants who require prolonged efforts to invoke breathing, the lifetime prevalence of schizophrenia does not exceed 6%.30 Although the phenocopy model is unlikely, we cannot fully reject the model because the currently available data are only relevant to OCs that are presumed to be related to hypoxia; to our knowledge there is yet to be a published study that directly measures fetal hypoxia using molecular indicators.
Gene-Environment Covariation Model of Hypoxia-Associated OCs
The preponderance of studies has found no increase in the frequency of hypoxia-associated OCs in groups at high vs low genetic risk for schizophrenia.8 One such study examined the incidence of hypoxia-associated OCs among individuals with schizophrenia, their nonschizophrenic siblings, and demographically matched nonpsychiatric controls drawn from the National Collaborative Perinatal Project cohort in the United States.25 The authors found a higher incidence of hypoxia-associated OCs in schizophrenia patients than in their nonpsychotic siblings and in nonpsychiatric controls. In contrast to what would be expected under a gene-environment covariation model, there was no difference in the incidence of such OCs between the nonschizophrenic siblings and controls, suggesting that the incidence of OCs does not increase with genetic loading.25 If the covariation model were correct, siblings of schizophrenic patients, who share some of the disease-promoting genes, would have an increased risk of hypoxia-associated OCs. It is also possible that siblings have less of the disease-promoting genes, and therefore, the covariation is obscured due to decreased power to detect effects. Nevertheless, the prevailing evidence does not support the covariation model.
Gene-Environment Interaction/Additive Influences Models of Hypoxia-Associated OCs
In a prior review of the hypoxia-associated OC literature, Cannon8 found that regardless of whether risk is based on having an affected parent,5,31 sibling,32–35 or co-twin,36 studies using objective birth records have found that those high-risk subjects with a history of hypoxia-associated OCs are significantly more likely to become schizophrenic than those without such a history.
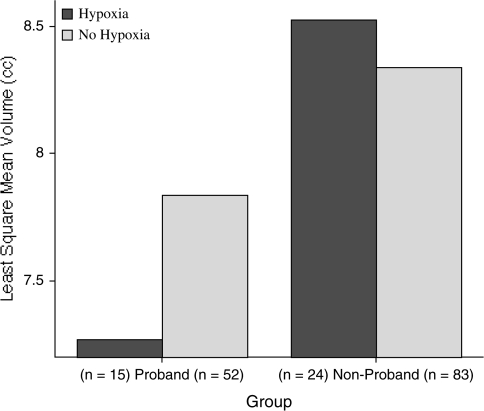
In magnetic resonance imaging studies of siblings from a Finnish birth cohort, hypoxia-associated OCs were significantly related to more severe brain abnormalities among patients with schizophrenia.37,38 Specifically, hypoxia-associated OCs were related to reduced gray matter and increased cerebrospinal fluid throughout the cortex in cases and their nonaffected siblings, as well as ventricular enlargement among the cases.37 Similarly, a stepwise decrease in hippocampal volumes according to genetic liability was found among cases, nonaffected siblings, and controls, with the most pronounced decreases among the cases exposed to hypoxia-associated OCs38 (see figure 2).
Fig. 2.
Total Hippocampus Volumes in Probands and Nonprobands With and Without Fetal Hypoxia.
A new generation of high-risk studies combining clinical and family history criteria may also be relevant to the gene-environment interaction model of the role of OCs in schizophrenia. In this approach, individuals exhibiting a recent onset of subthreshold psychotic symptoms are followed over time; this “prodromal” group has a 35% risk for converting to psychosis over a 2.5-year follow-up, and the risk is greatest among those prodromal subjects who also have a first-degree relative with psychosis.39,40 Utilizing this methodology, Ballon et al41 examined 52 clinical high-risk individuals and compared the incidence of OCs (including hypoxia-associated OCs) with nonpsychiatric and schizophrenia comparison groups using the Lewis and Murray Scale42 with subjects. The authors found comparable and significant elevations in OCs in the clinical ultrahigh-risk and schizophrenia groups; however, it is still unclear whether this result is specific to hypoxia.
A similar study evaluated OC histories of 47 ultrahigh-risk individuals and prospectively followed the participants for diagnostic status for a 2-year period (V.A.M., R. W. Willhite, MA, T. Niendam, M., PhD, Daley, C., MD, Bearden, T.D.C., PhD, unpublished data). The presence of OCs (including hypoxia-associated OCs) was gauged utilizing the Lewis-Murray Scale42, and course of illness was closely monitored during 5 diagnostic assessments over the 2-year study, during which 9 of the 47 individuals converted to an Axis I psychotic disorder. When the converted group was compared with the group of ultrahigh-risk individuals who did not convert, a history of OCs was associated with increased odds of conversion (odds ratio = 4.90, confidence interval [CI]: 1.04–22.20). Further, the authors found that the frequency of OCs was strongly associated with the severity and frequency of positive and negative symptomatology. It is important that both studies are limited in that they utilized maternal and patient recall of OCs, and although both studies included hypoxia-associated OCs, it is unclear whether hypoxia is the primary mechanism of action underlying these epidemiological associations.
Prenatal Maternal Infection
In a landmark study, Mednick et al43 found that individuals whose mothers were exposed to an influenza epidemic in Finland during the second trimester of pregnancy exhibited substantially higher rates of schizophrenia. A multitude of studies have replicated this finding, and subsequent epidemiological studies have also linked fetal exposure to upper respiratory infections, genital tract infections, and rubella to risk for schizophrenia.44–50 In addition, a new line of research utilizing serological confirmation of infection from archived maternal sera (not susceptible to retrospective biases) has indicated that in addition to influenza infection, Toxoplasma gondii (T. gondii), toxoplasmosis, and herpes simplex virus type 2 are linked to psychosis.51–53
With the exception of the parasitic infection by T. gondii, most viral infections rarely cross the placenta, and evidence suggests that the teratogenic effects of fetal exposure to infection involves other mechanisms, such as the mother's immune response to infection.54 This idea is supported by 2 studies that have found that fetal exposure to increases in proinflammatory cytokines during pregnancy resulted in increased incidence of psychosis in offspring.51,53
Phenocopy Model of Prenatal Infection
A phenocopy model predicts that prenatal exposure to a viral teratogen would result in a schizophrenia outcome independently of genetic contributions. Consequently, prenatal virus exposure would reliably lead to expression of schizophrenia. This model is supported by animal studies that have found neuronal and behavioral abnormalities similar to those found among schizophrenic patients after exposure to infection and/or proinflammatory cytokines.55–57 For example, pregnant mice exposed to the human influenza virus yielded offspring displaying deficits in prepulse inhibition, as well as responses to acute administration of antipsychotic and psychomimetic drugs similar to adults with schizophrenia (ameliorations and exacerbations, respectively); further, at least some of these behavioral changes were attributable to the maternal immune response, given that a group of mice injected with sham viruses yielded offspring with acoustic startle response deficits.56 In a related rodent study, investigators examined the role of maternal exposure to human influenza virus on day 9 of pregnancy and found altered pyramidal and nonpyramidal cell density values as well as atrophy of pyramidal cells (despite normal cell proliferation) indicative of both acute and long-lasting effects characteristic to what would be observed in individuals with schizophrenia.55 Further, rat models of perinatal viral infection have revealed a pattern suggestive of disruption in developing neurocircuitry, yielding eventual death of dentate granule cells secondary to excitotoxity.58 In humans, researchers have also linked proinflammatory cytokines to neonatal white matter damage during prenatal maternal infection.59
Despite these findings, only a small minority of mothers who are exposed to infection give birth to schizophrenic offspring.43,46,60 Even studies that have found increased rates of schizophrenia following flu epidemics, T. gondii, toxoplasmosis, and herpes simplex virus type 2 note only an incremental increase.60–62 The consistent findings showing that a large majority of individuals prenatally exposed to maternal infection do not develop schizophrenia weaken the phenocopy model.
Gene-Environment Covariation Model of Prenatal Infection
A gene-environment covariation model posits that prenatal infection is associated with the genes for schizophrenia but that the exposure itself may not exert any etiological role. If this model were correct, there would be an increased incidence of prenatal virus exposure in individuals carrying a genetic loading for schizophrenia, regardless of whether or not the individuals developed the disorder.
One strong area of support for gene-environment covariation comes from recent studies examining the incidence of genetic variation in genes associated with immune responses. Common proinflammatory cytokine polymorphisms (eg, interleukin IL-1 complex [IL-1α (-889) allele 2, IL-1β (-511) allele 1, and IL-1RA allele 1] and tumor necrosis factor alpha [TNF-α]) have been found to be associated with schizophrenia outcome63,64 and associated with particular OCs.65 This is a particularly powerful methodology; where we were once only able to index genetic risk using high-risk designs, the recent ability to examine specific genetic variations holds promise for elucidating mechanisms behind this complex phenomenon. In addition, these cytokines are associated with premature delivery,66 an OC that has been consistently reported to be elevated in the birth histories of schizophrenia patients.
As noted, preterm delivery has been found to be associated with aberrant developmental processes also implicated in the etiology of schizophrenia12; these findings provide support for covariation but do not rule out the possibility of other mechanism of gene-environment influence. For example, a particular gene or gene cluster may contribute to both OCs and schizophrenia separately (as in pleitropy), but OCs may not have a causal effect on schizophrenia. For example, if a hypothetical gene is associated with low birthweight, and this same gene, independently of its effects on low birthweight, also results in an irregular DA metabolism that contributes significantly to psychotic symptoms, then it may appear as if low birthweight is associated with psychotic symptoms when in fact it is not. In this hypothetical example, it appears as though schizophrenia and low birthweight are related, whereas, they in fact represent different expressions of the same gene.
Some genes may contribute to schizophrenia not directly, but indirectly, by causing certain OCs that in turn affect brain structure and function in a way that increases risk for schizophrenia. For example, a hypothetical gene might increase rates of hypoxia, subsequently damaging the hippocampus, and this insult may participate with other risk factors resulting in the onset of psychotic symptoms. Such a gene would not be causally related to schizophrenia independently of its effects on OCs.
Each of these 2 models of rGE may apply in relation to different sets of OCs and schizophrenia susceptibility genes. OCs are heterogeneous in origin and mechanisms of effect and can therefore have a number of different casual relationships with schizophrenia, each through a different gene-environment mechanism of origin.12 Using the 2 examples noted above, the same person could carry a hypothetical gene resulting in low birthweight and independently of this effect, dysregulation of the dopamine system (which in turn increases risk for psychosis), and a second hypothetical gene that results in hypoxia-related hippocampal damage, increasing vulnerability for schizophrenia. Because schizophrenia is likely to be the result of numerous interacting genetic and environmental factors, this hypothetical person may in fact represent the rule rather than the exception.
Another likely possibility also serves as an illustration of the complexities faced in OC and biobehavioral genetics research. More specifically, OCs that directly effect etiological processes (like hypoxia in the second example above) might cause secondary OCs that are not directly related to etiological processes. For example, a gene related to hypoxia (which can indeed affect hippocampal development and subsequently play a significant vulnerability factor) may also indirectly lead to low birthweight, which may or may not have an independent etiological role in schizophrenia. Such interactions between OCs have been rarely studied to date. Consistent with the notion of multiple cooccurring mechanisms, in the following section we also discuss how these polymorphisms also support an interactive model.
Gene-Environment Interaction/Additive Influences Models of Prenatal Exposure to Virus
This model would predict that prenatal virus exposure in genetically high-risk individuals would increase the odds of a schizophrenia outcome. In addition to genetic polymorphisms leading to OCs, like preterm delivery, polymorphisms in both interleukin IL-1 complex [IL-1α (-889) allele 2, IL-1β (-511) allele 1, and IL-1RA allele 1] and TNF-α have been associated with increased inflammation in response to infection, as well as increased basal levels of proinflammatory cytokines.12,67 Thus, carriers of these polymorphisms are likely to be more vulnerable to the deleterious effects of prenatal infection, which could increase the likelihood of psychotic onset in adulthood, although there have been no published studies directly testing this potential gene-environment interaction.12
One strong area of support comes from Brown et al,68 who found that the rubella-exposed individuals who eventually developed schizophrenia demonstrated a decline in IQ from childhood to adolescence as well as increased neuromotor and behavioral abnormalities when compared with rubella-exposed controls. Because rubella exposure uniquely affected the premorbid functioning of the schizophrenia group, it is likely that the genetic or environmental vulnerability associated with schizophrenia made the fetal brain more susceptible to the effects of rubella with observable cognitive effects appearing prior to the onset of the disorder.68
Similarly, a recent study found that among ultrahigh-risk individuals, maternal recall of prenatal viral exposure was reported in the histories of 50% of those patients who converted to psychosis compared with only 20% of the high-risk individuals who did not convert to psychosis.69 Even though this study is limited by retrospective reports of prenatal virus exposure and the use of clinical high-risk status exclusively (without consideration of family history of psychosis in modeling genetic risk), it is suggestive of a link between prenatal viral exposure and increased risk of conversion among individuals at high risk for psychosis.
Our understanding of gene-environment interaction influence and prenatal exposure to infection remains limited. Studies looking at specific genetic polymorphisms that play a key role in the immune response combined with serological exposure data are needed to directly test gene-environment interactions.12
Maternal Stress Exposure
Exposure to war,70 unwanted pregnancy,71 and death of a loved one or spouse72,73 are among the list of maternal stressors during pregnancy that have been associated with increased risk of schizophrenia among offspring. It is likely that prenatal stress triggers the release of maternal stress hormones, which have been found to disturb fetal neurodevelopment and subsequent functioning of the hypothalamic-pituitary-adrenal (HPA) axis, which in turn influences behavior and cognition.74 This mechanism is complex however, as effects appear to vary upon timing and duration of the stressor.75–77 Nevertheless, this effect has been demonstrated in numerous animal models, such that prenatal exposure to maternal stress and the administration of glucocorticoids (GCs) and other substances leads to changes in the structure/function of the HPA system in offspring that often persist through adulthood.78,79
Findings from several lines of investigation (animal and human) indicate that persistently elevated GC levels can affect multiple neuronal regions that have been implicated in schizophrenia research. Animal studies have shown that, at heightened levels, GCs can inhibit neurogenesis in the dentate gyrus and contribute to neuronal death80,81 In rodents, prenatal exposure to stress hormones has been associated with abnormalities in the adult brain including increases in dopamine D2-like receptors in the dorsal frontal cortex, medial prefrontal cortex, hippocampus, and nucleus accumbens.82,83 In addition, animal models have shown prenatal stress to be associated with decreased dopamine turnover84 and alteration of genes responsible for dopaminergic function in the midbrain.85
In humans, research suggestive of possible alterations in learning beginning in utero point to the vulnerability and susceptibility of this delicate period.76 For example, salivary cortisol in late pregnancy has been associated with lower mental scores at 3 months.86 In addition, persistently heightened GC secretion also has implications for cognitive functions, in that the hippocampus plays a critical role in memory and research has shown that volumetric reductions of the hippocampus are associated with memory deficits.87–89 Prenatal inhibition of hippocampal neurogenesis has also been associated with persistent learning deficits.90 Further, fetal exposure to increases in stress hormones have been associated with OCs found in the history of schizophrenic patients, including shortened gestational length, fetal growth restriction, and decreased infant physical and neuromuscular maturation.84 These effects appear to depend on the gestational timing of the exposure, and emerging evidence suggests that the deleterious effects of fetal exposure to stress hormones may be moderated by gender.75,76 Taken together, the evidence suggest that prenatal stress exposure can result in lasting synaptic and neurotransmitter alterations, potentially altering personality and predisposing individuals to attention deficits as well as mental illness.91
Phenocopy Model of Maternal Stress
Studies supporting a phenocopy model would yield results suggesting that exposure to prenatal stress in itself, without any genetic contribution, would reliably result in offspring with schizophrenia. As stated above, fetal exposure to stress hormones affects multiple areas of the brain implicated in schizophrenia. Nevertheless, while studies examining rates of schizophrenia in cohorts of individuals who experience a stressful event such as a war70 or a death of a spouse or close relative72 show an incremental increase in schizophrenia rates following stressful events, the findings are presented within the framework of small percentages. For example, van Os and Selten70 found a significant increase in the incidence of schizophrenia among individuals prenatally exposed to stress during a Nazi invasion, but the relative risk (1.15, 95% CI 1.03-1.28) indicates only an incremental increase in rates of schizophrenia. These results indicate that stress exposure is likely to play an etiological role, but other etiological events are likely to be necessary for a schizophrenia outcome.
Gene-Environment Covariation Model of Maternal Stress
Research supporting a gene-environment covariation model would yield results suggesting that maternal susceptibility to stress is associated with a genetic loading for schizophrenia but that the stress itself may not be a contributing etiological factor. The most appropriate basis for examining this question would be to select unaffected siblings or offspring of schizophrenics and then to compare severity of maternal stress during pregnancy in this group with that in a sample of unaffected individuals without affected relatives. However, the idiosyncratic nature of stress adds a level of complexity that renders such a design difficult to implement and interpret.92 Given the methodological limitations, testing a gene-environment covariation model in humans is difficult. Prospective studies, examining cortisol levels of schizophrenic and nonschizophrenic individuals, who have experienced a stressful event (naturalistically), and following their offspring stand to help elucidate our understanding of this complicated issue.
Gene-Environment Interaction/Additive Influences Model of Maternal Stress
A gene-environment interaction model predicts that individuals at genetic high risk for developing schizophrenia who also were exposed to acute or chronic prenatal stress would be at an increased risk of developing the disorder, when compared with genetically high-risk individuals not exposed to maternal stress or with healthy controls regardless of their exposure status. The best design study for investigating this model would consist of a prospective longitudinal study, assessing maternal cortisol levels throughout the pregnancy, following high-risk offspring (including multiple siblings) from the prenatal period to adulthood. If the high-risk children who developed schizophrenia were exposed to a greater degree of prenatal stress than their unaffected siblings, this would provide strong direct evidence for an interaction. However, this design is prohibitive in terms of cost and time and involves many uncontrollable naturalistic factors, and there have been no such studies to date.
How Do OCs Influence the Pathophysiology of an Adult-Onset Disorder?
Given the foregoing, it is imperative to identify the mechanisms through which OCs interact with the developing brain to increase the likelihood of schizophrenia in late adolescence and early adulthood. One theory is that OCs confer a latent constitutional vulnerability that become evident when interacting with later neuromaturational processes.93–95 Within this framework, we review evidence suggesting that early hippocampal lesions (associated with OCs) can interact with normative adolescent increases in (1) synaptic pruning and (2) stress hormone levels and thereby contribute to the onset of psychosis.
In healthy development, the peak of synaptic density occurs at 2 years of age and is followed by a slow drop during childhood followed by a steep decline in adolescence.96,97 Early in development many synaptic connections are superfluous, and the increase in synaptic pruning occurring during adolescence, particularly in the prefrontal brain regions, is thought to be related to efficiency and adult reasoning capabilities.96 Indeed, abilities to solve abstract and complex problems coincide with the pruning during this period.98 McGlashan and Hoffman99 proposed that an abnormally aggressive synaptic pruning process, which would lead to a reduction in synaptic connectivity, may be a strong contributing factor to psychosis. This idea is supported by postmortem studies, which have shown reduced neuropil, representing a loss of connections between neurons, without neural loss.100
In continuing within the framework of this model, OCs disrupting a particular neural structure(s) (eg, hypoxia affecting the hippocampus) would, in effect, reduce the amount of synaptic pruning necessary to pass a threshold of synaptic loss, resulting in psychosis. This prediction is congruent with research of individuals with a history of OCs, who typically have an earlier age of onset and more pronounced neuroanatomical abnormalities.101
An example through which OCs could fit within this framework also relates to the effects of early hippocampal damage. More specifically, a body of animal literature suggests that particular structures such as the hippocampus, and particular neural systems such as the HPA axis, are particularly susceptible to OCs, as discussed above.102,103 This work is of interest to our understanding of the etiology of psychotic disorders because the hippocampus regulates the HPA axis that governs the release of cortisol.104 Several lines of research suggest that prenatal insult can produce hippocampal damage in the fetus, and this can render the organism hypersensitive to stress postnatally.105 During adolescence, a time period also associated with the synaptic pruning noted above, there is also a well-documented normative increase in cortisol.93,106 Although the mechanisms are not yet clearly understood, it is clear that GC secretion augments dopamine activity in certain brain regions, especially the mesolimbic system.107–109 Because cortisol is known to augment dopamine activity, it is believed that the hippocampus and HPA axis act as a nonspecific moderating system potentiating expression of psychotic disorders as well as mediating the effects of stress on symptom expression.95,110 Within this framework, OCs resulting in hippocampal and/or HPA axis abnormality could result in a constitutional vulnerability or lesion, which may later interact with normative maturational processes leading to psychosis.95
By acting through these distinct pathways, OCs may result in neural changes that place individuals at higher risk for schizophrenia. The brain may be “preprogrammed,” in part via the impact of OCs on genetically susceptible brain regions such as the hippocampus, to be vulnerable to later life (ie, adolescent) stress and/or neurobiological influences (ie, circulating neurohormonal surges during pubertal maturation), which may subsequently trigger psychosis.
Conclusion and Future Directions
This present review provides support for the notion that different forms of OCs can influence liability to schizophrenia through disparate but potentially overlapping mechanisms of gene-environment coalescence. The findings have several broad-ranging implications. First, it may be possible to decrease the risk of schizophrenia in some genetically at-risk individuals with careful prenatal and perinatal monitoring.111 Further, a new generation of high-risk studies suggests that including OCs in models combining multiple risk indicators holds promise in enhancing our ability to identify those individuals who stand to most benefit from early intervention.39,112,113
Second, some component of the genetic diathesis to schizophrenia would appear to render the fetal brain particularly susceptible to the neurotoxic consequences of OCs, and thus, search for candidate genes that mediate the brain's vulnerability to early injury continues to be highly warranted. The emergence of candidate disease genes, as well as the advances in mapping out molecular pathways, will likely elucidate our understanding and treatment of psychosis. Although the list of candidates is long, prominent suspects might be located within the glutamatergic NMDA receptor system because overstimulation of such receptors represents an early event in the sequence leading from hypoxia to neuronal death.114,115 Moreover, as mentioned previously, genes that may be involved in mechanisms linking OCs to the etiology of schizophrenia have been identified, such as IL-1B and TNF-α polymorphisms.67 This research can lead to a new depth of epidemiological understanding and by linking the disease, environmental insult, and specific genetic polymorphisms help to highlight new promising gene clusters.
Although we have thus far focused on G × E interactions, another possibility is that OCs could alter the function and structure of genes leading to pathogenic consequences, such as schizophrenia. Specifically, in analyzing the phenomena of increased concordance rates of schizophrenia following population-sized famines such as the Dutch Hunger Winter and the Chinese famine of 1959–1961, McClellan et al116 introduce the theory that a potential molecular basis for the increased risk may be the occurrence of new mutations in genes critical for brain development and that famine-induced folate deficiency might be a mediator by impairing DNA repair. The authors point out that identifying disease-causing de novo mutations is particularly informative to the larger population of individuals with schizophrenia because a gene that harbors one disease-associated mutation is likely to harbor other illness-associated mutations.116 Further, because folate is necessary for normal DNA methylation, and folate regulates the expression of many genes involved in neurodevelopment, a deficiency could also lead to epigenetic alterations (stable changes in gene expression not dependent on mutations) that could predispose an individual to the later development of schizophrenia.116 This area of inquiry represents a significant future direction for OC research as well as epidemiological and behavioral genetics.
Funding
National Institute of Mental Health (Grant MH52857 to T.D.C.); National Center for Research Resources (Center for Computational Biology Grant U54 RR021813, P41 Resource Grant RR013642). Funding was also provided by a gift to the UCLA Foundation from the Staglin Music Festival for Mental Health.
References
- 1.Cannon TD, Kaprio J, Lonnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort. A population-based modeling study. Arch Gen Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- 2.Kendler KS, Diehl SR. The genetics of schizophrenia: a current, genetic-epidemiologic perspective. Schizophr Bull. 1993;19:261–285. doi: 10.1093/schbul/19.2.261. [DOI] [PubMed] [Google Scholar]
- 3.Cannon TD, Van Erp TG, Bearden CE, et al. Early and late neurodevelopmental influences in the prodrome to schizophrenia: contributions of genes, environment, and their interactions. Schizophr Bull. 2003;29:653–669. doi: 10.1093/oxfordjournals.schbul.a007037. [DOI] [PubMed] [Google Scholar]
- 4.Torrey EF, Yolken RH. Could schizophrenia be a viral zoonosis transmitted from house cats? Schizophr Bull. 1995;21:167–171. doi: 10.1093/schbul/21.2.167. [DOI] [PubMed] [Google Scholar]
- 5.Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neutrointegrative defect. Arch Gen Psychiatry. 1992;49:221–235. doi: 10.1001/archpsyc.1992.01820030053007. [DOI] [PubMed] [Google Scholar]
- 6.Cannon TD, Hennah W, van Erp, et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–1213. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 7.Brown AS, Susser ES. In utero infection and adult schizophrenia. Ment Retard Dev Disabil Res Rev. 2002;8:51–57. doi: 10.1002/mrdd.10004. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD. On the nature and mechanisms of obstetric influences in schizophrenia: a review and synthesis of epidemiologic studies. Int Rev Psychiatry. 1997;9:387–397. [Google Scholar]
- 9.Ellman LM, Huttunen M, Lonnqvist J, Cannon TD. The effects of genetic liability for schizophrenia and maternal smoking during pregnancy on obstetric complications. Schizophr Res. 2007;93:229–236. doi: 10.1016/j.schres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- 11.Van Erp TG, Saleh PA, Rosso IM, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520. doi: 10.1176/appi.ajp.159.9.1514. [DOI] [PubMed] [Google Scholar]
- 12.Ellman LM, Cannon TD. Environmental Pre- and Perinatal Influences. The Clinical Handbook of Schizophrenia. New York: Guilford Press; [Google Scholar]
- 13.Moffitt TE, Caspi A, Rutter M. Strategy for investigation interactions between measured genes and measured environments. Arch Gen Psychiatry. 2005;62:473–481. doi: 10.1001/archpsyc.62.5.473. [DOI] [PubMed] [Google Scholar]
- 14.Boin F, Zanardini R, Pioli R, Altamura CA, Maes M, Gennarelli M. Association between –G308A tumor necrosis factor alpha gene polymorphism and schizophrenia. Mol Psychiatry. 2001;6:79–82. doi: 10.1038/sj.mp.4000815. [DOI] [PubMed] [Google Scholar]
- 15.Katila H, Hanninen K, Hurme M. Polymorphisms of the interleukin-1 gene complex in schizophrenia. Mol Psychiatry. 1999;4:179–181. doi: 10.1038/sj.mp.4000483. [DOI] [PubMed] [Google Scholar]
- 16.Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16:469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- 17.Byrne M, Agerbo E, Bennedsen B, Eaton WW, Mortensen PB. Obstetric conditions and risk of first admission with schizophrenia: a Danish national register based study. Schizophr Res. 2007;97:51–59. doi: 10.1016/j.schres.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Clarke MC, Harlery M, Cannon M. The role of obstetric events in schizophrenia. Schizophr Bull. 2006;32:3–8. doi: 10.1093/schbul/sbj028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boog G. Obstetrical complications and subsequent schizophrenia in adolescent and young adult offspring: is there a relationship? Eur J Obstet Gynecol Reprod Biol. 2004;114:130–136. doi: 10.1016/j.ejogrb.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202. doi: 10.1176/appi.ajp.157.2.196. [DOI] [PubMed] [Google Scholar]
- 21.McNeil TF. Obstetric factors and perinatal injuries. In: Tsaung MT, Simpson JC, editors. Handbook of Schizophrenia Vol. 3: Nosology, Epidemiology and Genetics. New York, NY: Elsevier Science Pub. Co; 1988. pp. 319–343. [Google Scholar]
- 22.McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212. doi: 10.1176/appi.ajp.157.2.203. [DOI] [PubMed] [Google Scholar]
- 23.Dalman C, Allebeck P, Cullberg J, Grunewald C, Koester M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- 24.Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lonnqvist J, Gasperoni TL. Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry. 2000;157:801–807. doi: 10.1176/appi.ajp.157.5.801. [DOI] [PubMed] [Google Scholar]
- 25.Cannon TD, Rosso IM, Hollister J, Bearden CE, Sanchez LE, Hadley T. A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull. 2000;26:351–366. doi: 10.1093/oxfordjournals.schbul.a033458. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt A, Fendt M, Zink M, et al. Altered NMDA receptor expression and behavior following postnatal hypoxia: potential relevance to schizophrenia. J Neural Transm. 2007;114:239–248. doi: 10.1007/s00702-006-0440-7. [DOI] [PubMed] [Google Scholar]
- 27.Boksa P, El-Khodor BF. Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neurosci Biobehav Rev. 2003;27:91–101. doi: 10.1016/s0149-7634(03)00012-5. [DOI] [PubMed] [Google Scholar]
- 28.Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–761. doi: 10.1016/j.earlhumdev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Decker MJ, Rye DB. Neonatal intermittent hypoxia impairs dopamine signaling and executive functioning. Sleep Breath. 2002;6:205–210. doi: 10.1007/s11325-002-0205-y. [DOI] [PubMed] [Google Scholar]
- 30.Buka SL, Tsuang MT, Lipsitt LP. Pregnancy/delivery complications and psychiatric diagnosis: a prospective study. Arch Gen Psychiatry. 1993;50:151–156. doi: 10.1001/archpsyc.1993.01820140077009. [DOI] [PubMed] [Google Scholar]
- 31.Cannon TD, Mednick SA, Parnas J. Antecedents of predominantly negative and predominantly positive-symptom schizophrenia in a high-risk population. Arch Gen Psychiatry. 1990;47:622–632. doi: 10.1001/archpsyc.1990.01810190022003. [DOI] [PubMed] [Google Scholar]
- 32.Kinney DK, Levy DL, Yurgelun-Todd DA, Medoff D, LaJonchere CM, Radford-Paregol M. Season of birth and obstetrical complications in schizophrenics. J Psychiatr Res. 1994;28:499–509. doi: 10.1016/0022-3956(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 33.Eagles JM, Gibbon I, Bremner MH, Clunie F, Ebmeier KP, Smith NC. Obstetric complications in DSM-III schizophrenics and their siblings. Lancet. 1990;335:1139–1141. doi: 10.1016/0140-6736(90)91136-x. [DOI] [PubMed] [Google Scholar]
- 34.Woerner MG, Pollack M, Klein DF. Pregnancy and birth complications in psychiatric patients: a comparison of schizophrenic and personality disorder patients with their siblings. Acta Psychiatr Scand. 1973;49:712–721. doi: 10.1111/j.1600-0447.1973.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 35.Pollack M, Woerner MG, Goodman W, Greenberg IM. Childhood development patterns of hospitalized adult schizophrenic and nonschizophrenic patients and their siblings. Am J Orthopsychiatry. 1966;36:510. doi: 10.1111/j.1939-0025.1966.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 36.Pollin W, Stabenau JR. Biological, psychological and historical differences in a series of monozygotic twins discordant for schizophrenia. In: Rosenthal D, Kety SS, editors. The Transmission of Schizophrenia. London, UK: Pergammon Press; 1968. pp. 317–332. [Google Scholar]
- 37.Cannon TD, Thompson PM, van Erp TG, et al. Cortex mapping reveals regionally specific patterns of genetic and disease-specific gray-matter deficits in twins discordant for schizophrenia. Proc Natl Acad Sci USA. 2002;99:3228–3233. doi: 10.1073/pnas.052023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannon TD, van Erp TG, Rosso IM, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- 39.Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittal VA, Walker EF. Movement abnormalities predict conversion to Axis I psychosis among prodromal adolescents. J Abnorm Psychol. 2007;116:796–803. doi: 10.1037/0021-843X.116.4.796. [DOI] [PubMed] [Google Scholar]
- 41.Ballon JS, Dean KA, Cadenhead KS. Obstetrical complications in people at risk for developing schizophrenia. Schizophr Res. 2008;98:307–311. doi: 10.1016/j.schres.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis SW, Murray RM. Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J Psychiatr Res. 1987;21:413–421. doi: 10.1016/0022-3956(87)90088-4. [DOI] [PubMed] [Google Scholar]
- 43.Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- 44.Mortensen PB, Pedersen CB, Westergaard T, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–608. doi: 10.1056/NEJM199902253400803. [DOI] [PubMed] [Google Scholar]
- 45.O'Callaghan E, Sham P, Takei N, Glover G, Murray RM. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet. 1991;337:1248–1250. doi: 10.1016/0140-6736(91)92919-s. [DOI] [PubMed] [Google Scholar]
- 46.Barr CE, Mednick SA, Munk-Jorgensen P. Exposure to influenza epidemics during gestation and adult schizophrenia. Arch Gen Psychiatry. 1990;47:869–874. doi: 10.1001/archpsyc.1990.01810210077012. [DOI] [PubMed] [Google Scholar]
- 47.Murray RM, O'Callaghan E, Castle DJ, Lewis SW. A neurodevelopmetal approach to the classification of schizophrenia. Schizophr Bull. 1992;18:319–332. doi: 10.1093/schbul/18.2.319. [DOI] [PubMed] [Google Scholar]
- 48.Limosin F, Rouillon F, Payan C, Cohen J, Strub N. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr Scand. 2003;107:331–335. doi: 10.1034/j.1600-0447.2003.00052.x. [DOI] [PubMed] [Google Scholar]
- 49.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163:927–929. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 50.Brown AS, Schaefer CA, Wyatt RJ, et al. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 51.Brown AS, Hooton J, Schaefer CA, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 52.Brown AS, Schaefer CA, Quesenberry CP, Jr., Liu L, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 53.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- 54.Shi L, Tu N, Patterson PH. Maternal influenza infection is likely to alter fetal brain development indirectly: the virus is not detected in the fetus. Int J Dev Neurosci. 2005;23:299–305. doi: 10.1016/j.ijdevneu.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Fatemi SH, Earle J, Kanodia R, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pearce BD, Valadi NM, Po CL, Miller AH. Viral infection of developing GABAergic neurons in a model of hippocampal disinhibition. Neuroreport. 2000;11:2433–2447. doi: 10.1097/00001756-200008030-00019. [DOI] [PubMed] [Google Scholar]
- 59.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Izumoto Y, Inoue S, Yasuda N. Schizophrenia and the influenza epidemics of 1957 in Japan. Biol Psychiatry. 1999;46:119–124. doi: 10.1016/s0006-3223(98)00359-x. [DOI] [PubMed] [Google Scholar]
- 61.Takei N, Lewis S, Jones P, Harvey I, Murray RM. Prenatal exposure to influenza and increased cerebrospinal fluid spaces in schizophrenia. Schizophr Bull. 1996;22:1521–1534. doi: 10.1093/schbul/22.3.521. [DOI] [PubMed] [Google Scholar]
- 62.Sham PC, O'Callaghan E, Takei N, Murray G, Hare E, Murray RM. Schizophrenia following pre-natal exposure to influenza epidemics between 1939 and 1960. Br J Psychiatry. 1992;160:461–466. doi: 10.1192/bjp.160.4.461. [DOI] [PubMed] [Google Scholar]
- 63.Lencz T, Morgan T, Athanasiou M, et al. Converging evidence for a pseudoautosomal cytokine receptor gene locus in schizophrenia. Mol Psychiatry. 2007;12:572–580. doi: 10.1038/sj.mp.4001983. [DOI] [PubMed] [Google Scholar]
- 64.Brown AS. Prenatal infection as a risk factor for schizophrenia. Schizophr Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Crider KS, Whitehead N, Buus RM. Genetic variation associated with preterm birth: a HuGE review. Genet Med. 2005;7(9):593–604. doi: 10.1097/01.gim.0000187223.69947.db. [DOI] [PubMed] [Google Scholar]
- 66.Engel SA, Erichsen HC, Savitz DA, Thorp J, Chanock SJ, Olshan AF. Risk of spontaneous preterm birth is associated with common proinflammatory cytokine polymorphisms. Epidemiology. 2005;16:469–477. doi: 10.1097/01.ede.0000164539.09250.31. [DOI] [PubMed] [Google Scholar]
- 67.Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992;1:353. doi: 10.1093/hmg/1.5.353. [DOI] [PubMed] [Google Scholar]
- 68.Brown AS, Cohen P, Harkavy-Friedman J, et al. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol Psychiatry. 2001;49:473–486. doi: 10.1016/s0006-3223(01)01068-x. [DOI] [PubMed] [Google Scholar]
- 69.Mittal VA, Sacazawa M, Walder DJ, Willhite R, Walker EF. Prenatal viral teratogen exposure and conversion among adolescents at high-risk for psychosis. Schizophr Res. 2008;99:375–376. doi: 10.1016/j.schres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 71.Herman DB, Brown AS, Opler Mark G, et al. Does unwantedness of pregnancy predict schizophrenia in the offspring? Findings from a prospective birth cohort study. Soc Psychiatry Psychiatr Epidemiol. 2006;41:605–610. doi: 10.1007/s00127-006-0078-7. [DOI] [PubMed] [Google Scholar]
- 72.Khashan AS, Abel KM, McNamee R, et al. High risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 73.Huttunen MO. Maternal stress during pregnancy and the behavior of the offspring. In: Doxiadis S, editor. Early Influence Shaping the Individual. New York, NY: Plenum Press; 1989. pp. 175–182. [Google Scholar]
- 74.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;2:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 75.Ellman LM, Schetter CD, Hobel CJ, Chicz-Demet A, Glynn LM, Sandman CA. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sandman CA, Wadhwa P, Hetrick W, Porto M, Peeke HV. Human fetal heart rate dishabituation between thirty and thirty-two weeks gestation. Child Dev. 1997;68:1031–1040. [PubMed] [Google Scholar]
- 77.Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 78.Koenig JL, Elmer GI, Shepard PD, et al. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156:251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 79.Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Van Reeth O. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27:119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 80.Brunson KL, Avishai-Eliner S, Hatalski CG, Baram TZ. Neurobiology of the stress response early in life: evolution of a concept and the role of corticotropin releasing hormone. Mol Psychiatry. 2001;6:647–656. doi: 10.1038/sj.mp.4000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hennessy MB, Davis HN, McCrea AE, Harvey AT, Williams MT. Short- and long-term consequences of corticotropin-releasing factor in early development. Ann N Y Acad Sci. 1999;897:76–91. doi: 10.1111/j.1749-6632.1999.tb07880.x. [DOI] [PubMed] [Google Scholar]
- 82.Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC. Long-term effects of prenatal stress on dopamine and glutamate receptors in adult rat brain. Neurochem Res. 2002;27:1525–1533. doi: 10.1023/a:1021656607278. [DOI] [PubMed] [Google Scholar]
- 83.McArthur S, McHale E, Dalley JW, Buckingham JC, Gillies GE. Altered mesencephalic dopaminergic populations in adulthood as a consequence of brief perinatal glucocorticoid exposure. J Neuroendocrinol. 2005;17:475–482. doi: 10.1111/j.1365-2826.2005.01331.x. [DOI] [PubMed] [Google Scholar]
- 84.Weinstock M. Alterations induced by gestational stress in brain morphology and behavior of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 85.Son GH, Chung S, Geum D, et al. Hyperactivity and alteration of the midbrain dopaminergic system in maternally stressed male mice offspring. Biochem Biophys Res Commun. 2007;352:823–829. doi: 10.1016/j.bbrc.2006.11.104. [DOI] [PubMed] [Google Scholar]
- 86.Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- 87.Maguire EA, Vargha-Khadem F, Mishkin M. The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain. 2001;124:1156–1170. doi: 10.1093/brain/124.6.1156. [DOI] [PubMed] [Google Scholar]
- 88.O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161:2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 89.Squire LR, Zola SM. Episodic memory, semantic memory, and amnesia. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 90.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Phillips LJ, Francey SM, Edwards J, McMurray N. Stress and psychosis: towards the development of new models of investigation. Clin Psychol Rev. 2007;27:307–317. doi: 10.1016/j.cpr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Walker EF, Mittal VA, Tessner KD. HPA activity and the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008 doi: 10.1146/annurev.clinpsy.4.022007.141248. doi:10.1016/j.cpr.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Corcoran C, Walker E, Huot R, et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull. 2003;29(4):671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 95.Walker E, Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol Rev. 1997;104:1–19. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- 96.Spear LP. Neurodevelopment during adolescence. In: Cicchetti D, Walker E, editors. Neurodevelopmental Mechanisms in Psychopathology. New York, NY: Cambridge University Press; 2003. pp. 62–83. [Google Scholar]
- 97.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1982;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 98.Huttenlocher PR. Synaptic density in human frontal cortex: developmental changes and effects of again. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 99.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–647. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 100.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 101.Verdoux H, Geddes JR, Takei N, et al. Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry. 1997;154:1220–1227. doi: 10.1176/ajp.154.9.1220. [DOI] [PubMed] [Google Scholar]
- 102.Raff H, Jacobson L, Cullinan W. Augmented hypothalamic corticotrophin-releasing hormone mRNA and corticosterone response to stress in adult rats exposed to perinatal hypoxia. J Neuroendocrinol. 2007;19:907–912. doi: 10.1111/j.1365-2826.2007.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Dev Brain Res. 2005;156:202–209. doi: 10.1016/j.devbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 104.de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- 105.Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- 106.Mittal VA, Dhruv S, Tessner KD, Walder DJ, Walker EF. The relations among putative bio risk markers in schizotypal adolescents: minor physical anomalies, movement abnormalities and salivary cortisol. Biol Psychiatry. 2007;61:1179–1186. doi: 10.1016/j.biopsych.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 107.Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- 108.Czyrak A, Makowiak M, Chocyk A, Fijal K, Wedzony K. Role of glucocorticoids in the regulation of dopaminergic neurotransmission. Pol J Pharmacol. 2003;55:667–674. [PubMed] [Google Scholar]
- 109.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implications for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 110.Weinstein D, Diforio D, Schiffman J, Walker E, Bonsall B. Minor physical anomalies, dermatoglyphic abnormalities and cortisol levels in adolescents with schizotypal personality disorder. Am J Psychiatry. 1999;154:617–623. doi: 10.1176/ajp.156.4.617. [DOI] [PubMed] [Google Scholar]
- 111.Warner R. Time trends in schizophrenia: changes in obstetric risk factors with industrialization. Schizophr Bull. 1995;21:483–500. doi: 10.1093/schbul/21.3.483. [DOI] [PubMed] [Google Scholar]
- 112.Mittal VA, Neumann C, Saczawa M, Walker EF. The longitudinal progression of movement abnormalities and psychotic symptoms in adolescents at high-risk for psychosis. Arch Gen Psychiatry. 2008;65:165–170. doi: 10.1001/archgenpsychiatry.2007.23. [DOI] [PubMed] [Google Scholar]
- 113.Mittal VA, Tessner KD, Trottman HD, et al. Movement abnormalities and the progression of prodromal symptomatology in adolescents at risk for psychotic disorders. J Abnorm Psychol. 2007;116:260–267. doi: 10.1037/0021-843X.116.2.260. [DOI] [PubMed] [Google Scholar]
- 114.Vij S, Vannucci S, Gurd JW. Differential effects of hypoxia-ischemia on phosphorylation of the N-methyl-D-aspartate receptor in one-and three-week-old rats. Dev Neurosci. 2005;27:211–219. doi: 10.1159/000085994. [DOI] [PubMed] [Google Scholar]
- 115.Spandou E, Karkavelas G, Soubasi V, Avgovstides-Savvopoulou P, Loizidis T, Guiba-Tziampiri O. Effect of ketamine on hypoxic-ischemic brain damage in newborn rats. Brain Res. 1999;819:1–7. doi: 10.1016/s0006-8993(98)01333-x. [DOI] [PubMed] [Google Scholar]
- 116.McClellan JM, Susser E, King MC. Maternal famine, de novo mutations, schizophrenia. JAMA. 2006;296:582–584. doi: 10.1001/jama.296.5.582. [DOI] [PubMed] [Google Scholar]