Abstract
For patients with colorectal liver metastases, hepatic resection is the treatment of choice, and the 5-year survival rate following surgery now exceeds 50%. Timely multidisciplinary and multimodality approaches that may include preoperative systemic chemotherapy, preoperative portal vein embolization, extended hepatic resection, and two-stage hepatectomy, have enabled a large proportion of patients to undergo potentially curative treatment. The definition of resectability has shifted from a focus on tumor characteristics, such as tumor number and size, to determination of whether both intrahepatic and extrahepatic disease can be completely resected and whether such an approach is appropriate from an oncologic standpoint for a given patient. Future identification of molecular factors may aid in predicting prognosis of patients with colorectal liver metastases and in improving the selection of patients most likely to benefit from surgery. Hepatobiliary surgeons and medical oncologists should work together to individualize treatment strategies to maximize long-term survival in patients with colorectal liver metastases.
Colorectal cancer is the second leading cause of cancer-related mortality worldwide. Every year in the United States, more than 140,000 patients are diagnosed and 56,000 die of this disease.1 Although 85% of patients with colorectal carcinoma have tumors amenable to curative resection at the time of diagnosis, the disease recurs in more than half of patients within 5 years. The most frequent sites of colorectal cancer metastases are the liver (in 30% to 60% of cases), and the lung (in 20% to 30% of the cases). Up to 25% of colorectal cancer patients are found to have liver metastases at presentation, and a further 30% develop liver metastases at a later point in the disease course, usually within the first 2 years following primary tumor resection.2
Without treatment, median survival of patients with colorectal livermetastases (CLM) is 12 to 15 months, and 5-year survival is less than 5%. Rather disappointingly, despite the introduction of a wide range of new agents, median survival for patients with stage IV disease treated with the best available chemotherapy remains only 25 months.3,4 Although many different treatment treatment modalities have been investigated, at present, liver resection remains the best option for achieving long-term survival. Not all authors agree that aggressive surgery for CLM is appropriate; some argue that the benefit in terms of survival after this procedure could be the result of better patient selection rather than of the treatment strategy. Although tumor biology is likely to prevail regardless of treatment, the natural history of stage IV colon cancer is clearly altered in many patients who undergo complete hepatic resection of CLM. Thus, integrated therapy with surgery and systemic chemotherapy is of increased importance. Thanks to advances in multimodality treatment, many patients with CLM who would have been considered unresectable just a few years ago are today candidates for resection. The current 5-year overall survival rate after surgery has reached a new benchmark of 58%.5–10
ASSESSMENT OF HEPATIC INVOLVEMENT
A systematic and careful preoperative assessment of hepatic involvement is mandatory for accurate selection of patients for surgery.
The detection of CLM has significantly improved over the past decade, and several different imaging techniques are now available for follow-up of patients with colorectal cancer. Generally, thin-cut multiple-phase spiral computed tomography (CT) is the preferred imaging modality for detection of CLM because it is the most widely available technique. It allows study of the thorax, liver, abdomen, and pelvis in the same examination, and through the individual phases of the scan, provides anatomic details of tumor and vessel associations necessary for hepatic resection planning. A comparable alternative preferred by some centers is contrast-enhanced magnetic resonance imaging (MRI) using a combination of gadolinium and super-paramagnetic iron oxide.
Fluorodeoxyglucose positron emission tomography (FDG-PET) appears to be a useful tool for the detection of extrahepatic disease5, however, its ability to assess the liver itself is limited, as intrahepatic lesion detection is poor, especially after chemotherapy. 11 A recent meta-analysis provided evidence that FDG-PET has higher sensitivity in the detection of colorectal liver metastases (94.6%) than does helical CT (64.7%) or 1.5T MRI (75.8%).12 However, the resolution of FDG-PET remains inferior to that of CT or MRI, as does its specificity, especially in patients who undergo preoperative chemotherapy. Finally, FDG-PET does not provide anatomic details necessary for surgical planning. Thus, the role of FDG-PET in patients with CLM remains to be determined.
Although some groups advocate diagnostic laparoscopy to improve patient selection for hepatic resection of CLM,13 improved preoperative imaging has limited the role of laparoscopy in staging of this disease. A more important application of laparoscopy may be the evaluation of underlying liver disease, such as cirrhosis, steatosis, and chemotherapy-related liver injuries, for which preoperative diagnosis currently remains challenging.
In addition to careful assessment of hepatic and extrahepatic involvement, accurate measurement of liver volume is mandatory when assessing patient candidates for extensive hepatectomy to determine the future liver remnant and the need for preoperative portal vein embolization. 14 Volumetric data are obtained from multiphase CT imaging used for staging and surgical planning. The concept of liver volumetry and its clinical significance are discussed later in this paper.
DEFINITION OF RESECTABILITY
The major oncologic contraindications to liver surgery are the presence of unresectable liver disease and the presence of extrahepatic disease (Table 1).
Table 1.
Contraindications to resection of colorectal liver metastases
| Relative | Absolute |
|---|---|
| Extrahepatic metastases | Peritoneal carcinomatosis |
|
| |
| Colonic recurrence | Multiple extrahepatic metastases |
|
| |
| Solitary resectable peritoneal metastasis | Inability to perform hepatic R0 resection |
|
| |
| Hilar lymph nodes metastases | |
In the past, resection of CLM was not performed in patients with more than three metastases, an anticipated negative resection margin of less than 1 cm, or extrahepatic disease. However, recent studies have shown that long-term survival is possible even in patients with these clinicopathologic factors, and as such, the definition of resectability has shifted from one based on tumor characteristics, such as tumor number and size, to one based on whether both intrahepatic and extrahepatic disease can be completely resected (R0 resection). An alternative interpretation is that there has been a change from concentrating on what is removed (tumor) to a focus on what will remain after resection (liver remnant). Currently, CLM should be considered resectable when the patient has no underlying liver disease, and at least two adjacent liver segments (representing not less than 20% of the standardized future liver remnant) can be spared with adequate vascular inflow, outflow, and biliary drainage.14,15 The following sections review factors historically considered contraindications to hepatic resection for CLM and data supporting or refuting these contraindications, including discussion of prognostic factors vs. selection factors to define resectability.
Number of Metastases
Multiple metastases and the presence of bilobar disease are correlated with a less favorable prognosis, especially in cases where more than four metastatic sites are involved, since these features are associated with higher risk of extrahepatic disease and of systemic recurrence after surgery. Indeed, such patients generally undergo an extensive preoperative workup. The application of FDG-PET may be particularly useful to detect extrahepatic disease in such high-risk patients.5
Recently, Pawlik et al 6 reported on a cohort of patients with more than four metastases treated with multimodality therapy, including preoperative chemotherapy. The 5-year disease-free and overall survival rates were 22% and 51%, respectively, after hepatic resection. Similarly, Kokudo et al,16 in a review of patients treated in Tokyo, found that a high number of metastases, although a grim prognostic factor, should not be considered a contraindication per se to hepatic resection, because surgery for CLM is still the only curative treatment. Removal of up to 70% to 80% of the liver parenchyma can be accomplished safely, and the mortality rate after hepatic resection is almost nil.17 Therefore, the number of metastases should no longer be considered a contraindication for curative hepatic resection but rather a prognostic factor, which will be overcome by surgery and systemic chemotherapy in selected patients.
Surgical Margin
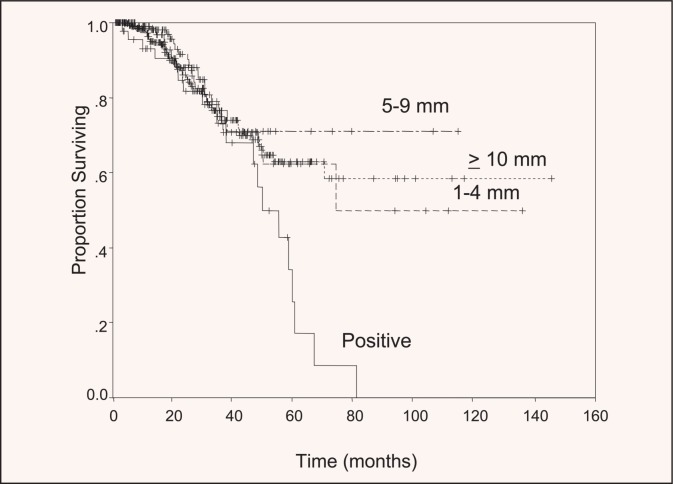
Historically, 1 cm was considered the minimum safe surgical margin for hepatic resection. Resections with an anticipated margin less than 1 cm were often defined as “not radical,” and the scenario was sometimes considered a contraindication to resection. Studies of true margin recurrence have shown that a 1-cm margin is not necessary to maximize survival probability. In a multicenter series of 557 patients reported by Pawlik et al, although a positive surgical margin (R1) was associated with a modestly increased risk of local tumor recurrence (11%), the width of the resected margin did not predict increased risk for margin recurrence or survival (Figure 1).7 Similarly, other studies have shown that nonanatomic resections, which are usually associated with a minimal margin, are not associated with an increased risk of local recurrence after surgery.18,19 These studies have clearly shown that the anticipated minimal margin should not be considered a contraindication for resection.
Figure 1.
Survival after curative resection for colorectal liver metastases, stratified by margin status. No significant survival differences were seen in patients with negative surgical margins, regardless of the width of the margin. Reprinted with permission from Pawlik.7
Hilar Lymph Node Metastases
Whether resection of CLM is indicated in patients with hilar and perihepatic lymph node metastases is an area of controversy. Such lymph node metastases may predict poor outcome after surgery for CLM. Jaeck et al20 recently showed that hilar and perihepatic lymph node metastases have a stronger negative influence on prognosis than do multiple and bilobar liver metastases, elevation of carcinoembryonic antigen level, or even the presence of a solitary site of resectable peritoneal disease. Therefore, the presence of hilar lymph node metastases is generally considered a contraindication to resection of CLM and could be a good indicator of the need for preoperative chemotherapy, even in patients with resectable disease at presentation. However, the precise role of lymphadenectomy during surgery for CLM has yet to be clarified.14
Extrahepatic Disease
Several authors have reported long-term survival in patients with CLM and resectable extrahepatic disease. Elias et al21 found a 28% 5-year overall survival rate in patients with more than five metastases and multiple extrahepatic disease sites treated with radical surgery. Other studies have shown that long-term survival can be expected after complete resection of pulmonary metastases from colorectal cancer, even when such metastases are detected at the same time as CLM. The criteria to select patients for pulmonary resection are currently under investigation. It is generally accepted, however, that patients can be considered for pulmonary resection as long as it is technically feasible and there is no evidence of hilar or paracardiac lymphadenopathy. With strict selection criteria, surgery for CLM with extrahepatic sites can result in survival rates of 78% at 3 years and 56% at 5 years.22
Molecular Markers
The utility of clinicopathologic features to predict prognosis after complete resection of CLM is limited, and molecular markers are sought to improve prediction of prognosis.
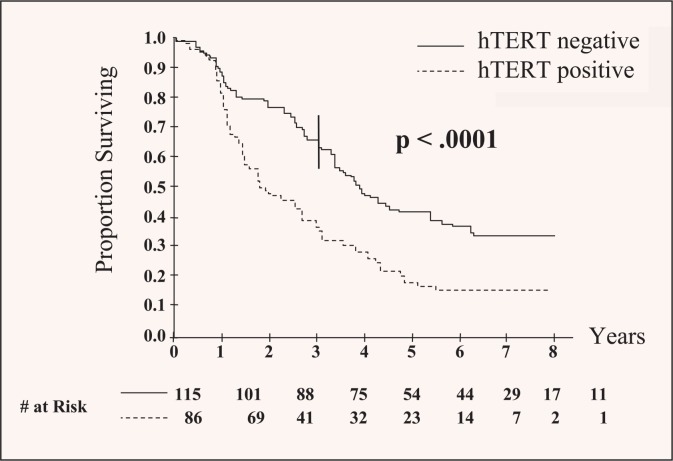
Specific molecular markers have been linked with clinical outcomes. Human telomerase reverse transcriptase (hTERT) expression has been shown to be independently associated with poor prognosis after curative resection for CLM, regardless of traditional clinicopathologic predictors. In fact, patients with hTERT-positive CLM have twice the risk of death than patients without hTERT expression (Figure 2). This important finding provides evidence that molecular markers can predict outcome after resection and may be used to plan adjuvant therapy for high-risk patients.23 More efforts are needed to understand the potential roles of hTERT or other markers and in which patients they should be applied.
Figure 2.
Negative effect of human telomerase reverse transcriptase (hTERT) expression on overall survival in patients undergoing curative resection for colorectal liver metastases. Patients with nucleolar hTERT staining had significantly shortened survival. Reprinted with permission from Dômont et al.23
ADVANCES IN SURGICAL TECHNIQUE
The current technique for liver resection is based on liver segmental anatomy described by Couinaud in 1957.24 Several attempts have been made to elucidate the significance of anatomic and nonanatomic (limited) hepatic resections for CLM. A recent systematic study that compared outcomes after limited vs. anatomic resections for CLM showed no differences in terms of tumor clearance, recurrence, or long-term survival.25 In other words, anatomic resections are not superior to limited resections for CLM from an oncologic standpoint. Thus, the principle that outcome depends on complete resection of metastatic disease applies whether anatomic or nonanatomic resection (or a combination) is used to extirpate disease. This analysis corroborates the finding that tumor biology, not resection type, predicts prognosis.
Intraoperative Ultrasonography
Intraoperative ultrasonography (IOUS) remains one of the most important tools in liver surgery. Since its introduction more than 20 years ago, IOUS has come into widespread use by hepatobiliary surgeons.26
Several studies have shown that IOUS complements the preoperative crosssectional imaging. In expert hands, IOUS can reveal additional lesions in 10% to 15% of patients, although improving preoperative staging studies may reduce this aspect of the IOUS advantage. More important, IOUS is essential to define the parenchymal transection plane and the relationship between the tumor and the intrahepatic vascular and biliary structures to assure complete tumor resection and preservation of critical vasculobiliary structures.27 The recent introduction of contrast-enhanced IOUS may further improve intraoperative staging.28
Hemorrhage Control
One of the most powerful, independent determinants of outcome after hepatic resection is the amount of intraoperative blood loss.29,30
Maintenance of low central venous pressure, usually less than 5 mm Hg, has been shown to reduce blood loss from hepatic veins and hepatic parenchyma during liver transection.31,32 Different clamping methods to reduce intraoperative hemorrhage have also been developed, such as continuous or intermittent pedicle clamping (Pringle’s maneuver), clamping of hemi-liver, and total vascular exclusion of the liver with or without clamping of the inferior vena cava.33 In general, most elective resections can be performed safely with intermittent pedicle clamping; use of total vascular exclusion techniques is rarely necessary34–36 and is usually associated with hemodynamic changes and the consequent increases in postoperative morbidity and mortality rates.
Recently developed devices including radiofrequency coagulators, saline-linked cautery, and ultrasonic dissectors allow hemostatic parenchymal transection with minimal blood loss.37 However, a prospective randomized trial found no significant differences in blood loss when surgery was performed using such new transection devices and the traditional crush technique.38
The contribution of postoperative pain control is also worthy of note. Use of continual epidural analgesia provides optimal pain control, allowing improved respiratory function (which was previously a problem in patients with upper abdominal incisions) and early patient mobilization, thus significantly reducing morbidity rates.
SHORT-TERM AND LONG-TERM RESULTS OF RESECTION
Liver resection is a well-established procedure, with a mortality rate of less than 5% and morbidity rates of less than 30% to 40%. Important determinants of poor outcome after liver resection are intraoperative bleeding, perioperative blood transfusions, insufficient remnant liver, and development of infective complications. These conditions can lead to hepatic failure, which, although it occurs in fewer than 4% of cases, can be devastating. Proper patient selection, meticulous intraoperative technique, and careful postoperative management are essential to minimize surgical complications.
Table 2 reports long-term results from major published series of liver resection for CLM and includes the main predictors of recurrence.5,7–9,19,39–48 Despite expanding indications for resection of CLM, 5-year overall survival is now consistently reported as 51% to 58% in single-and multi-institutional studies.5–10 Of note, recent studies included patients with advanced multiple and bilateral disease, who were not considered for resection until a few years ago, and patients treated with preoperative systemic chemotherapy, which has a definite role in achieving long-term survival.
Table 2.
Predictors of recurrence and long-term survival after resection for colorectal liver metastases
| Author, Year | R1 Status | Synchronous Presentation | Primary Nodes + | Size of Metastases | No. Metastases | Preoperative CEA | Extrahepatic Disease | 5-Year Survival |
|---|---|---|---|---|---|---|---|---|
| Fernandez, 20045 | – | – | – | + | – | 58% | ||
|
| ||||||||
| Pawlik, 20057 | + | – | – | + | + | + | 58% | |
|
| ||||||||
| Abdalla, 20048 | + | – | + | + | 58% | |||
|
| ||||||||
| Choti, 20029 | + | – | – | – | + | + | 58% | |
|
| ||||||||
| Elias, 199819 | + | + | – | – | – | – | – | 28% |
|
| ||||||||
| Gayowski, 199439 | + | + | + | – | + | – | + | 32% |
|
| ||||||||
| Scheele, 199540 | + | + | + | + | – | – | – | 40% |
|
| ||||||||
| Nordlinger, 199641 | + | + | + | + | + | + | 28% | |
|
| ||||||||
| Jaeck, 199742 | + | + | + | + | + | + | – | 26% |
|
| ||||||||
| Jamison, 199743 | – | – | – | 32% | ||||
|
| ||||||||
| Jenkins, 199744 | + | – | – | + | 25% | |||
|
| ||||||||
| Ambiru, 199945 | + | – | + | + | + | 23% | ||
|
| ||||||||
| Fong, 199946 | + | + | + | + | + | 46% | ||
|
| ||||||||
| Minagawa, 200047 | – | – | – | + | – | – | 38% | |
|
| ||||||||
| Figueras , 200148 | + | – | + | + | + | 53% | ||
CEA, Carcinoembryonic antigen.
The main clinicopathologic factors that are useful to predict prognosis after hepatic resection for CLM are margin status, stage of the primary colon tumor, preoperative carcinoembryonic antigen level, size and number of lesions, and presence or absence of extrahepatic metastases. These prognostic factors were determined before the advent of effective systemic chemotherapy, however, so their utility as prognostic indicators in this new era of CLM management is unknown. With advances in molecular techniques, biologic factors such as hTERT are emerging as potential prognostic indicators and may prove more accurate than clinical factors.23
STRATEGIES TO IMPROVE RATES OF RESECTABILITY
Progress in systemic chemotherapy combinations, in conjunction with advances in surgical technique and patient selection, has significantly expanded the population of patients for whom potentially curative hepatic resection of CLM is possible. Examples of relatively new techniques include preoperative chemotherapy, portal vein embolization, two-stage hepatectomy for bilateral liver metastases, extended hepatectomy, and repeat hepatectomy. Many patients who would not have been considered for resection just a few years ago are now treated with preoperative, cytoreductive chemotherapy followed by liver surgery.
Preoperative Systemic Chemotherapy
The development of new, more effective chemotherapy agents has led to a significant survival increase for patients with unresectable stage IV colon cancer. Oxaliplatin and irinotecan, agents that are commonly used in conjunction with 5-fluorouracil/folinic acid–based therapies, can downsize liver metastases and control potential sites of extrahepatic disease49 to permit subsequent resection of residual disease.
The indications for preoperative systemic chemotherapy are generally based on risk factors for disease recurrence, such as tumor size, tumor number, disease-free interval, and presence or absence of extrahepatic disease.
The increased use of preoperative chemotherapy poses a clinical dilemma; whether to prescribe or not to prescribe preoperative chemotherapy in patients with initially resectable disease. This dilemma is not merely academic, because recent reports indicate an increased risk of adverse post-resection events in patients treated with preoperative systemic chemotherapy. Liver injury, such as hepatic steatosis and steatohepatitis, has been described in irinotecan-treated patients, and intravascular damage, such as sinusoidal obstruction, has been reported in oxaliplatin-treated patients.50,51 These chemotherapy-related liver injuries may reduce the regenerative capacity of hepatocytes in response to major hepatectomy through alterations of nuclear factors, such as the nuclear factor-kappa B (NF-κB), which is crucial for the priming phase of liver regeneration, and increase postoperative morbidity and even mortality.
Although resection has been shown to be safe after preoperative chemotherapy,52,53 mortality is increased in certain types of chemotherapy-related hepatic injuries—specifically, steatohepatitis in association with irinotecan therapy.54 Thus, use of preoperative chemotherapy should be carefully considered in patients with resectable disease at presentation; development of intrahepatic complications could necessitate modification of the surgical strategy or even rule out surgery altogether as a treatment option. In light of this clinical dilemma, molecular markers are needed to help predict which patients are most likely to respond to preoperative chemotherapy and which drugs or drug classes will be more effective and better tolerated by a given patient.
Portal Vein Embolization
Portal vein embolization (PVE) in preparation for major hepatic resection has been shown to induce hypertrophy of the future liver remnant and reduce risk of postoperative liver failure after major hepatectomy.55 First described by the Japanese for patients with primary liver cancer, PVE has become part of clinical practice and contributes to improvements in resectability rates of liver cancer.56,57
PVE is usually performed through a percutaneous transhepatic ipsilateral approach, which uses ultrasound-guided puncture of a portal branch followed by embolization of the entire lobar portal territory to be resected. A variety of substances have been used for embolization, including absolute alcohol, ethiodized oil, and cyanoacrylate, none of which has been shown superior to another.58 PVE is a wellestablished and well-tolerated procedure. In our series of 112 cases, the complication rate is 8.9%, and includes hematoma, partial portal vein thrombosis, esophageal hemorrhage, and migration of embolizing material. Only one patient was deemed unresectable as a result of a PVE-related complication, but he also had tumor progression, which presented a contraindication to resection (The University of Texas M. D. Anderson Cancer Center, unpublished data, 11/2/2006).
Indications for PVE are based on the standardized future liver remnant volume (sFLR) and the presence or absence of underlying liver disease. The sFLR is calculated using the ratio between FLR and total liver volume (TLV). The FLR is directly measured with CT volumetry of the liver, and TLV is calculated using a formula derived from the association between TLV and body surface area, which estimates the hepatic metabolic demand for each patient.59 The presence of underlying liver disease is important because severely damaged livers may not have the capacity to regenerate. Both cirrhosis and severe steatosis have been found to impair liver regeneration significantly after major hepatectomy. Use of PVE in these settings will allow hypertrophy of the non-tumor–bearing liver, albeit at a slower rate than for healthy liver, thus reducing the risk of subsequent hepatectomy.
Hypertrophy of the remnant liver follows a nonlinear kinetic profile during the first 2 months after PVE. The greatest increase in liver volume (75%) occurs within 3 weeks after PVE, after which a plateau phase of minimal regeneration is reached (The University of Texas M. D. Anderson Cancer Center, unpublished data, 11/2/2006). This is in agreement with recent data showing that hypertrophy of the nonembolized liver is regulated mainly by transforming growth factor-alpha (TGF-α),60 the serum level of which peaks within 20 days after PVE and then reaches a plateau.61
It has recently been shown that hypertrophy of the remnant liver following PVE occurs through two complementary mechanisms—namely, increased proliferation and hypertrophy of hepatocytes in the remnant liver.62 Thus, the optimum interval of time to assess hypertrophic response to PVE is 3 to 4 weeks. At this time, repeat CT volumetry can provide two crucial pieces of information: (1) whether an adequate liver volume has been reached; and (2) rate of growth, which is indicative of the regenerative reserve of the liver. We found that patients with slow liver growth during the first month had a significantly worse clinical outcome, regardless of whether the target sFLR was reached.
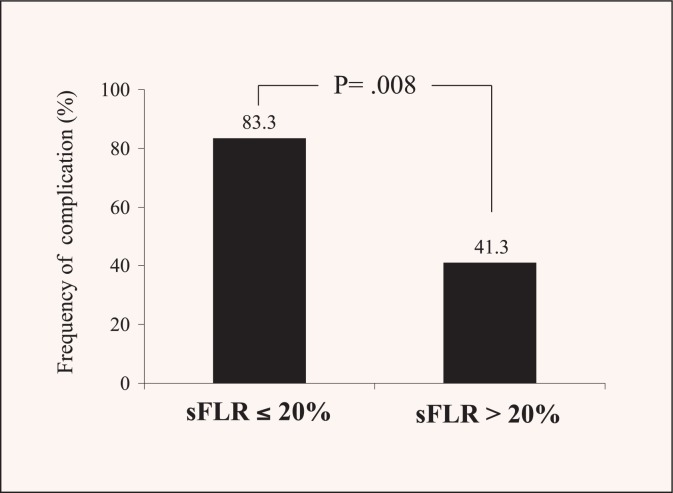
The liver volume limit for safe resection varies from patient to patient. In patients with an otherwise normal liver, PVE is indicated when the sFLR is ≤20%. In our experience, an sFLR less than 20% is associated with a significant increase in postoperative morbidity (Figure 3). In patients who have received extensive preoperative chemotherapy or with extensive steatosis, which can affect liver regeneration, a larger liver volume cut-off of ≤30% has been proposed. Finally, among patients with underlying cirrhosis, PVE is indicated when sFLR is ≤40%.14
Figure 3.
Complications rate stratified by standardized future liver remnant (sFLR) volume. Patients with sFLR less than 20% had significantly more complications than patients with sFLR greater than 20% (The University of Texas M. D. Anderson Cancer Center, unpublished data, 11/2/2006).
Contraindications to PVE include tumor invasion of the portal vein, portal thrombosis, uncorrectable coagulopathy, biliary dilation in the FLR, severe portal hypertension, and renal failure.
Two-Stage Hepatectomy
The resection of multiple bilobar CLM can cause an excessive reduction of the FLR, which may in turn lead to postoperative liver failure. In 2000, Adam et al63 proposed a novel two-stage approach for initially unresectable liver tumors. The highest possible number of tumors is removed in the first operation, and a second operation is performed after a period of liver regeneration to remove the remaining tumors. The rationale for two-stage hepatectomy is to minimize the risk of liver failure associated with massive hepatectomy in patients with bilateral metastases.
At our institution, we have inverted the sequence to perform minor resections as the first stage, usually in the future remnant liver, and later major or extended resections as the second stage with or without PVE, according to the FLR volume. This approach allows us to perform additional major procedures, such as resection of the primary tumor, at the first stage with low morbidity, as well as subsequent major resection. The need for interim chemotherapy or PVE can therefore be assessed before the subsequent major resection. This approach has been validated by Jaeck et al,64 who recently proposed a systematic approach based on the two-stage hepatectomy with or without preoperative PVE to permit curative resection for CLM. They reported 1- and 3-year overall survival rates of 70% and 54.4%, respectively.
The two-stage strategy should be considered in patients not eligible to undergo R0 resection in one procedure. However, selection criteria and use of preoperative chemotherapy in patients with multiple and bilobar CLM remain to be clarified.
Repeat Hepatectomy
Most patients who undergo hepatic resection for CLM develop disease recurrence, and one third of those develop isolated intrahepatic recurrence. Selected patients with isolated hepatic recurrence can undergo repeat hepatectomy and attain long-term survival. After third hepatectomy, a 5-year overall survival rate of 32% can be expected, and postoperative morbidity and mortality rates are no higher than after first hepatectomy.65 As multiple bilobar CLM are associated with high rates of hepatic recurrence, early diagnosis of recurrent disease is essential to maximize the number of patients eligible for repeat resection, because long-term survival can be achieved with this approach.
Radiofrequency Ablation
Unfortunately, not all patients are candidates for hepatic resection for CLM and alternative therapies have therefore been proposed. The most common alternative therapy used to treat CLM is radiofrequency ablation (RFA). RFA consists of placement of an electrode within the liver tumor under radiologic guidance (ultrasound [US], CT, or MRI) that generates thermal (radiofrequency) energy to destroy the tumor and a margin of normal parenchyma. RFA can be performed percutaneously, laparoscopically, or during laparotomy.
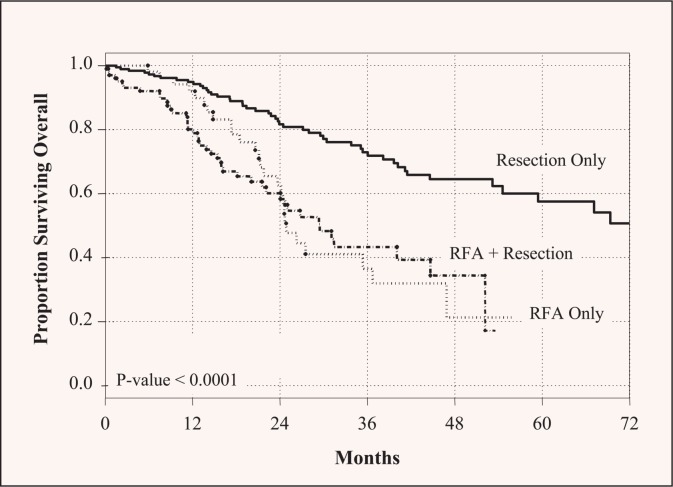
Use of RFA for liver metastases has been reported to be effective and safe by many authors. Larger follow-up data confirm the procedure’s safety, but suggest that RFA may not be equivalent to resection as a local modality. Abdalla et al 8 compared results using surgical resection vs. RFA vs. a combined procedure (resection plus ablation) for CLM, and found a higher 5-year recurrence rate after RFA than after the combined procedure or resection only (84%, 64%, and 52%, respectively). Liveronly recurrence following RFA was four times that of resection, as was true local recurrence. Consequently, long-term survival was better after resection than after local ablation (65% vs. 22%) (Figure 4).8 The same group subsequently studied solitary CLM and showed that hepatic resection is associated with superior survival rates.66 The local recurrence rate was significantly lower after resection (5%) than after RFA (37%) for solitary CLM, and 5-year overall survival rate was markedly longer after resection (71%) than after RFA (27%).
Figure 4.
Overall survival of patients with colorectal liver metastases stratified by type of treatment. Long-term survival was significantly higher in resected patients. Reprinted with permission from Abdalla et al.8
In a large study reported by de Baere et al, procedure-related mortality associated with RFA was approximately 2%.67 Some authors have reported altered growth patterns of recurrent disease after RFA, including sarcomatous and disseminated spreading patterns.68 If such recurrences are amenable to resection, a more aggressive approach is usually needed, which increases postoperative morbidity and mortality.
Based on experience to date with RFA, surgical resection should be considered the treatment of choice for CLM, with RFA restricted to patients who are not candidates for resection because of general contraindications, severe underlying liver disease that could impair postsurgical recovery, or technically unresectable disease. Therefore, because of inferior results with RFA and the availability of multiple resection modalities, patients should always be referred to surgeons with hepatobiliary expertise to determine their eligibility for resection before RFA is considered.
CONCLUSIONS
Hepatobiliary surgeons and medical oncologists should work together to evaluate patients with CLM to individualize treatment strategies and maximize the chances of long-term survival. Today, through a multidisciplinary and multimodality approach, and use of a wide variety of available techniques, it is possible to manage CLM successfully and offer many patients longterm survival.
Increasing acceptance of the role of liver resection in the management of CLM by the medical community as well as the public will no doubt lead to development of standardized population-wide screening protocols for patients undergoing colonic resection, similar to those currently used in specialized units. Improved screening and earlier identification of CLM will lead to increasing resectability rates and improved outcomes for more patients.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. J. N. Vauthey disclosed financial interests and/or professional relationships with Sanofi-Aventis and Genentech.
References
- 1.Jemal A, Tiwari RC, Murray T, et al. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. doi: 10.1002/bjs.1800771115. [DOI] [PubMed] [Google Scholar]
- 3.Venook A. Critical evaluation of current treatments in metastatic colorectal cancer. Oncologist. 2005;10:250–261. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlik TM, Abdalla EK, Ellis LM, et al. Debunking dogma: surgery for four or more colorectal liver metastases is justified. J Gastrointest Surg. 2006;10:240–248. doi: 10.1016/j.gassur.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Figueras J, Valls C, Rafecas A, et al. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg. 2001;88:980–985. doi: 10.1046/j.0007-1323.2001.01821.x. [DOI] [PubMed] [Google Scholar]
- 11.Akhurst T, Kates TJ, Mazumdar M, et al. Recent chemotherapy reduces the sensitivity of [18F]fluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol. 2005;23:8713–8716. doi: 10.1200/JCO.2005.04.4222. [DOI] [PubMed] [Google Scholar]
- 12.Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—meta-analysis. Radiology. 2005;237:123–131. doi: 10.1148/radiol.2371042060. [DOI] [PubMed] [Google Scholar]
- 13.D'Angelica M, Fong Y, Weber S, et al. The role of staging laparoscopy in hepatobiliary malignancy: prospective analysis of 401 cases. Ann Surg Oncol. 2003;10:183–189. doi: 10.1245/aso.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 14.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 15.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 16.Kokudo N, Imamura H, Sugawara Y, et al. Surgery for multiple hepatic colorectal metastases. J Hepatobiliary Pancreat Surg. 2004;11:84–91. doi: 10.1007/s00534-002-0754-2. [DOI] [PubMed] [Google Scholar]
- 17.Vauthey JN, Pawlik TM, Abdalla EK, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–732. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto J, Sugihara K, Kosuge T, et al. Pathologic support for limited hepatectomy in the treatment of liver metastases from colorectal cancer. Ann Surg. 1995;221:74–78. doi: 10.1097/00000658-199501000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias D, Cavalcanti A, Sabourin JC, et al. Results of 136 curative hepatectomies with a safety margin of less than 10 mm for colorectal metastases. J Surg Oncol. 1998;69:88–93. doi: 10.1002/(sici)1096-9098(199810)69:2<88::aid-jso8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Jaeck D. The significance of hepatic pedicle lymph nodes metastases in surgical management of colorectal liver metastases and of other liver malignancies. Ann Surg Oncol. 2003;10:1007–1011. doi: 10.1245/aso.2003.09.903. [DOI] [PubMed] [Google Scholar]
- 21.Elias D, Sideris L, Pocard M, et al. Results of R0 resection for colorectal liver metastases associated with extrahepatic disease. Ann Surg Oncol. 2004;11:274–280. doi: 10.1245/aso.2004.03.085. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe I, Arai T, Ono M, et al. Prognostic factors in resection of pulmonary metastasis from colorectal cancer. Br J Surg. 2003;90:1436–1440. doi: 10.1002/bjs.4331. [DOI] [PubMed] [Google Scholar]
- 23.Domont J, Pawlik TM, Boige V, et al. Catalytic subunit of human telomerase reverse transcriptase is an independent predictor of survival in patients undergoing curative resection of hepatic colorectal metastases: a multicenter analysis. J Clin Oncol. 2005;23:3086–3093. doi: 10.1200/JCO.2005.06.944. [DOI] [PubMed] [Google Scholar]
- 24.Couinaud C. Le foie. Etudes anatomiques et churgicales. In: Masson, Cie, editors. Paris: 1957. pp. 469–479. [Google Scholar]
- 25.Zorzi D, Mullen JT, Abdalla EK, et al. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006;10:86–94. doi: 10.1016/j.gassur.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Makuuchi M, Hasegawa H, Yamazaki S. Intraoperative ultrasonic examination for hepatectomy. Jpn J Clin Oncol. 1981;11:367–390. [PubMed] [Google Scholar]
- 27.Torzilli G, Montorsi M, Donadon M, et al. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005;201:517–528. doi: 10.1016/j.jamcollsurg.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Torzilli G, Del Fabbro D, Palmisano A, et al. Contrast-enhanced intraoperative ultrasonography during hepatectomies for colorectal cancer liver metastases. J Gastrointest Surg. 2005;9:1148–1153. doi: 10.1016/j.gassur.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860–869. doi: 10.1097/01.SLA.0000072371.95588.DA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okano T, Ohwada S, Nakasone Y, et al. Blood transfusion causes deterioration in liver regeneration after partial hepatectomy in rats. J Surg Res. 2001;101:157–165. doi: 10.1006/jsre.2001.6284. [DOI] [PubMed] [Google Scholar]
- 31.Hasegawa K, Takayama T, Orii R, et al. Effect of hypoventilation on bleeding during hepatic resection: a randomized controlled trial. Arch Surg. 2002;137:311–315. doi: 10.1001/archsurg.137.3.311. [DOI] [PubMed] [Google Scholar]
- 32.Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 33.Abdalla EK, Noun R, Belghiti J. Hepatic vascular occlusion: which technique? Surg Clin North Am. 2004;84:563–585. doi: 10.1016/S0039-6109(03)00231-7. [DOI] [PubMed] [Google Scholar]
- 34.Makuuchi M, Mori T, Gunven P, et al. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155–158. [PubMed] [Google Scholar]
- 35.Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg. 1995;229:369–375. doi: 10.1097/00000658-199903000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torzilli G, Makuuchi M, Midorikawa Y, et al. Liver resection without total vascular exclusion: hazardous or beneficial? An analysis of our experience. Ann Surg. 2001;233:167–175. doi: 10.1097/00000658-200102000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aloia TA, Zorzi D, Abdalla EK, et al. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lesurtel M, Selzner M, Petrowsky H, et al. How should transection of the liver be performed?: a prospective randomized study in 100 consecutive patients: comparing four different transection strategies. Ann Surg. 2005;242:814–822. doi: 10.1097/01.sla.0000189121.35617.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gayowski TJ, Iwatsuki S, Madariaga JR, et al. Experience in hepatic resection for metastatic colorectal cancer: analysis of clinical and pathologic risk factors. Surgery. 1994;116:703–710. [PMC free article] [PubMed] [Google Scholar]
- 40.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 41.Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases of the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 42.Jaeck D, Bachellier P, Guiguet M, et al. Longterm survival following resection of colorectal hepatic metastases. Association Française de Chirurgie. Br J Surg. 1997;84:977–980. doi: 10.1002/bjs.1800840719. [DOI] [PubMed] [Google Scholar]
- 43.Jamison RL, Donohue JH, Nagorney DM, et al. Hepatic resections for metastatic colorectal cancer. Results in cure for some patients. Arch Surg. 1997;132:505–511. doi: 10.1001/archsurg.1997.01430290051008. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins LT, Millikan KW, Bines SD, et al. Hepatic resection for metastatic colorectal cancer. Am Surg. 1997;63:605–610. [PubMed] [Google Scholar]
- 45.Ambiru S, Miyazaki M, Isono T, Ito H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–639. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 46.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minagawa M, Makuuchi M, Torzilli G, et al. Extension of the frontiers of surgical indications in the treatment of liver metastases from colorectal cancer: long-term results. Ann Surg. 2000;231:487–499. doi: 10.1097/00000658-200004000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Figueras J, Valls C, Rafecas A, Fabregat J, Ramos E, Jaurrieta E. Resection rate and effect of postoperative chemotherapy on survival after surgery for colorectal liver metastases. Br J Surg. 2001 Jul;88(7):980–985. doi: 10.1046/j.0007-1323.2001.01821.x. [DOI] [PubMed] [Google Scholar]
- 49.Poston GJ. The use of irinotecan and oxaliplatin in the treatment of advanced colorectal cancer. Eur J Surg Oncol. 2005;31:325–330. doi: 10.1016/j.ejso.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Fernandez FG, Ritter J, Goodwin JW, et al. Effect of steatohepatitis associated with irinotecan or oxaliplatin pretreatment on resectability of hepatic colorectal metastases. J Am Coll Surg. 2005;200:845–853. doi: 10.1016/j.jamcollsurg.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 51.Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol. 2004;15:460–466. doi: 10.1093/annonc/mdh095. [DOI] [PubMed] [Google Scholar]
- 52.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Parikh AA, Gentner B, Wu TT, et al. Perioperative complications in patients undergoing major liver resection with or without neoadjuvant chemotherapy. J Gastrointest Surg. 2003;7:1082–1088. doi: 10.1016/j.gassur.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 54.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 55.Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675–680. doi: 10.1001/archsurg.137.6.675. [DOI] [PubMed] [Google Scholar]
- 56.Azoulay D, Castaing D, Smail A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. 2000;231:480–486. doi: 10.1097/00000658-200004000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165–175. doi: 10.1046/j.1365-2168.2001.01658.x. [DOI] [PubMed] [Google Scholar]
- 58.Madoff DC, Abdalla EK, Vauthey JN. Portal vein embolization in preparation for major hepatic resection: evolution of a new standard of care. J Vasc Interv Radiol. 2005;16:779–790. doi: 10.1097/01.RVI.0000159543.28222.73. [DOI] [PubMed] [Google Scholar]
- 59.Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 60.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 61.Kusaka K, Imamura H, Tomiya T, et al. Factors affecting liver regeneration after right portal vein embolization. Hepatogastroenterology. 2004;51:532–535. [PubMed] [Google Scholar]
- 62.Komori K, Nagino M, Nimura Y. Hepatocyte morphology and kinetics after portal vein embolization. Br J Surg. 2006;93:745–751. doi: 10.1002/bjs.5332. [DOI] [PubMed] [Google Scholar]
- 63.Adam R, Laurent A, Azoulay D, et al. Two-stage hepatectomy: A planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–785. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaeck D, Oussoultzoglou E, Rosso E, et al. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004;240:1037–1049. doi: 10.1097/01.sla.0000145965.86383.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238:508–518. doi: 10.1097/01.sla.0000090449.87109.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–467. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 67.de Baere T, Risse O, Kuoch V, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 68.Nikfarjam M, Muralidharan V, Christophi C. Altered growth patterns of colorectal liver metastases after thermal ablation. Surgery. 2006;139:73–81. doi: 10.1016/j.surg.2005.07.030. [DOI] [PubMed] [Google Scholar]