Abstract
Chemotherapy agents available for the treatment of stage II and stage III colon cancer have changed substantially since the 1992 National Institutes of Health consensus report recommended that all stage III patients routinely receive adjuvant treatment with 5-fluorouracil/levamisole. Subsequent trials demonstrated superiority of 5-fluorouracil/leucovorin over 5-fluorouracil/levamisole in the adjuvant setting, and the recent addition of oxaliplatin to this regimen has further improved disease-free survival. While stage III colon cancer patients are routinely treated, the use of adjuvant chemotherapy in patients with stage II disease is still a subject of debate. Many trials that are assessing the potential role of biologics in the adjuvant setting will soon be completed. However, identifying molecular prognostic markers that accurately select patients with stage II or III cancers who are at risk of recurrence would be essential to select and individualize therapy.
Early detection strategies for colorectal cancer appear to be paying off, as suggested by the recently reported decrease in the annual death rate in the United States. Despite that, colorectal cancer remains both a significant health problem in the West and an increasingly common problem in Asia and South America. In the United States alone, approximately 112,340 new cases of colon cancer, representing 7.8% of total cancer cases, are projected for the year 2007.1 Approximately 8.5% of total cancer deaths are caused by colon cancer, based on data from 2003, the most recent year for which colon cancer (excluding rectum) mortality data are available.2 Because 77% of first diagnoses are characterized as localized or regional tumors, the majority of colon cancer patients will be candidates for adjuvant therapy.
The prognosis of colon cancer patients is most accurately predicted by the pathologic stage of the cancer. Surveillance, Epidemiology, and End Results (SEER) data accumulated for 1991 through 2000 indicated that the 5-year survival rates by stage were 93.2% for stage I, 82.5% for stage II, 59.5% for stage III, and 8.1% for stage IV, based on the 5th edition of the American Joint Committee on Cancer (AJCC) staging guidelines.3 However, a reevaluation of the same patient data using the updated AJCC staging guidelines (6th edition, published in 20034), which subdivided stage II and III cancers, found that the 5-year survival rates were 93.2% for stage I, 84.7% for stage IIa, 72.2% for stage IIb, 83.4% for stage IIIa, 64.1% for stage IIIb, 44.3% for stage IIIc, and 8.1% for stage IV patients.3 Interestingly, this restratification of patients diagnosed with colon cancer from 1991 to 2000 revealed that significantly fewer patients (P < .001) with stage IIb tumors survived 5 years than those with stage IIIa tumors. It is plausible that some of the notable difference in patient outcome resulted from the routine availability of adjuvant chemotherapy for patients with stage III disease, but not for those with stage II. This phenomenon supports the notion that former practice standards did not adequately address the treatment needs of a high-risk subpopulation of patients with stage II tumors.
The task of this panel was to create an updated consensus that integrates newly available data and trial findings, and to provide an expert analysis of new data relevant to the current adjuvant treatment setting.
ADJUVANT CHEMOTHERAPY OF COLON CANCER: A HISTORICAL PERSPECTIVE
Despite decades of clinical trials, it was not until the 1990s that the value of adjuvant chemotherapy for colon cancer was firmly established. Mature data published in 1995 of a large Intergroup phase III trial (INT 0035) demonstrated that adjuvant chemotherapy significantly reduced the recurrence rate in patients with stage III disease.5 In this trial, 929 patients with node-positive, resectable tumors were randomly assigned to observation (n = 315), levamisole alone (n = 310), or 5-fluorouracil (5-FU) plus levamisole (n = 304) after surgery. At a median follow-up of 6.5 years, median overall survival (OS) rates were 46.7% and 49%, respectively, in patients who received no chemotherapy or levamisole alone after surgery, and 60% in those treated with 5-FU/levamisole (5-FU/lev) (P = .0007). The survival advantage in the 5-FU–treated patients corresponded with a 40% reduction in recurrence rate.
In response to an early report of this work, a consensus panel convened by the National Institutes of Health (NIH) recommended that adjuvant chemotherapy with 5-FU plus levamisole be routinely offered to patients with stage III colon cancer.6–8 At that time, the NIH consensus panel was not prepared to make a similar recommendation for those with stage II disease. A subsequent NIH statement in 1992 reiterated that 5-FU/lev should be standard treatment for stage III disease but not stage II disease, as a separate, underpowered trial of 5-FU/lev compared to surgery alone for stage II disease failed to show a benefit for adjuvant treatment.5
The National Surgical Adjuvant Breast and Bowel Project (NSABP) concurrently evaluated the efficacy of 5-FU in combination with leucovorin (LV) rather than levamisole. Results from the NSABP C-03 trial indicated that 5-FU/LV was an active treatment regimen for colon cancer in the adjuvant setting, precipitating a head-to-head comparison between 5-FU/lev and 5-FU/LV.9 In the landmark NSABP C-04 trial, more than 2,000 patients with stage II and stage III colon cancer were randomized between treatment with 5-FU/LV (n = 691), 5-FU/lev (n = 691), or 5-FU/LV/lev (n = 696).10 For both primary end points of the trial, disease-free survival (DFS) and OS, 5-FU/LV demonstrated superiority to 5-FU/lev for the adjuvant treatment of both stages II and III colon cancer. For stage II patients, 5-FU/LV reduced the risk of recurrence by 22% according to 5-year DFS (75% vs. 71%) and by 29% according to 5-year OS (84% vs. 81%).
Interestingly, stage of disease had no apparent effect on the relative efficacy of treatment, as stage III patients treated with 5-FU/LV experienced a 13% relative risk reduction according to 5-year DFS (57% vs. 53%) and 10% by 5-year OS (67% vs. 63%) compared to those treated with 5-FU/lev. Findings of the trial supported the use of 5-FU/LV rather than 5-FU/lev as standard of-care adjuvant chemotherapy for patients with stage III colon cancer and, further, suggested a potential role of 5-FU/LV in patients with stage II disease.
Another pivotal phase III trial open during the early 1990s was the Intergroup 0089 trial (INT 0089), which directly compared the efficacy of 5-FU/LV and 5-FU/lev as well as the two most common dose/schedules for the administration of 5-FU/LV, the Roswell Park (5-FU and highdose LV) and the Mayo Clinic (5-FU and low-dose LV) regimens.11 A total of 3,561 eligible stage II and stage III patients were randomly assigned to one of four treatment arms and then followed for a median of 10 years. The four treatment arms were 5-FU/lev (12-month protocol), 5-FU/highdose LV (Roswell Park, 7 months), 5-FU/low-dose LV (Mayo, 6 months), and 5-FU/LV/lev (6 months). Results for both 5-year DFS and OS (after a 10-year followup) demonstrated equivalent efficacy for all treatment arms. These results provided a choice for patient treatment schedules based on toxicities and other existing factors rather than on maximization of survival outcome.
RECENT DEVELOPMENTS IN ADJUVANT CHEMOTHERAPY
Starting in the mid 1990s, bolus 5-FU/LV (the Roswell Park or Mayo Clinic regimen) was considered standard adjuvant therapy for stage III colon cancer. Since then, the introduction of new cytotoxic chemotherapy agents, reports of clinical trial findings using combination chemotherapy, and redefinition of clinical benefit for high-risk colon cancer have altered the landscape of adjuvant therapy for colon cancer (Table 1).
Table 1.
Key clinical trials in the adjuvant colorectal carcinoma setting.
| Clinical Trial | N | Primary End Points | Disease Stage Included | Trial Conclusions | Reference |
|---|---|---|---|---|---|
| INT-0035 | 929 | OS | III | 5-FU/levamisole superior to observation | Moertel et al, J Clin Oncol 19955 |
| NSABP C-04 | 2,078 | DFS, OS | Dukes B/C | 5-FU/LV superior to 5-FU/levamisole | Wolmark et al, J Clin Oncol 199910 |
| INT-0089 | 3,759 | DFS | II or III | Equivalency of 6 and 12 month treatment cycles and of high-dose vs. low-dose LV | Haller et al, J Clin Oncol 200511 |
| QUASAR | 3,238 | OS | II | 5-FU/LV superior to observation (improves stage II survival by 3.1%) | Gray et al, ASCO 200428 |
| GERCOR C96 | 905 | DFS | Dukes B2/C | Equivalency of LV5FU2 and monthly 5-FU/LV | Andre et al, ASCO 200537 |
| X-ACT | 1,987 | DFS | III | Capecitabine equivalency with LV5FU bolus; less toxic | Twelves et al, N Engl J Med 200519 |
| NSABP C-06 | 1,553 | DFS | II or III | Equivalency of UFT/LV and 5-FU/LV (UFT not approved in US) | Wolmark et al, ASCO 200438 |
| MOSAIC | 2,246 | DFS | II or III | Superiority of FOLFOX4 to LV5FU2 (improves DFS 3% for all stage II, 5% for high risk stage II) | Andre et al, N Engl J Med 200417 |
| NSABP C-07 | 1,407 | DFS | II or III | Bolus 5-FU/LV+oxaliplatin (FLOX) superior to 5-FU/LV | Kuebler et al, J Clin Oncol 200724 |
| CALGB 89803 | 1,264 | OS | III | No bolus IFL in stage III adjuvant CRC | Saltz et al, ASCO 200418 |
| PETACC-3 | 3,278 | DFS | II or III | LV5FU2 + CPT-11 not superior (statistically insignificant) | Van Cutsem et al, ASCO 200526 |
Abbreviations: 5-FU = 5-fluorouracil; CALGB = Cancer and Leukemia Group B; CPT-11 = irinotecan; CRC = colorectal carcinoma; DFS = disease-free survival; FOLFOX4 = 5-fluorouracil/leucovorin/oxaliplatin; GERCOR = Groupe d’Etude et de Recherche en Cancréologie Onco-Radiothérapic; IFL = irinotecan/fluorouracil/leucovorin; LV = leucovorin; LV5FU2 = leucovorin/5-fluorouracil; MOSAIC = Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer; NSABP = National Surgical Adjuvant Breast and Bowel Project; OS = overall survival; PETACC = Pan-European Trial in Adjuvant Colon Cancer; QUASAR = Quick and Simple and Reliable; UFT = uracil/tegafur; X-ACT = Xeloda in Adjuvant Colon Cancer Therapy.
Conventional Chemotherapeutic Agents
Prior to 2000, several new chemotherapeutic agents investigated for antitumor activity in the metastatic colorectal cancer (mCRC) setting were considered to hold promise for the adjuvant setting. The success in the late 1990s of irinotecan in US trials and of oxaliplatin in European trials in the treatment of mCRC resulted in the initiation of clinical studies assessing potential roles for these agents in the adjuvant setting. Several phase III trials of oxaliplatin and irinotecan investigated potential improvement of patient outcomes when these agents were administered in combination with 5-FU/LV.12–18 In addition, an orally bioavailable prodrug of 5-FU, capecitabine, was introduced into the adjuvant setting and investigated for noninferiority to bolus 5-FU/LV (the Mayo Clinic regimen).19 Results from trials of these three agents helped shape the current treatment approaches that are endorsed by this panel.
Elderly Benefit From Adjuvant Chemotherapy
The landmark meta-analysis of seven phase III trials, published by Sargent et al in 2001, compared the outcomes of 3,351 patients treated with 5-FU/LV or 5-FU/lev after surgery vs. those treated with surgery alone.20 The study revealed that adjuvant chemotherapy improved OS (hazard ratio [HR] = 0.76; 95% confidence interval [CI], 0.68–0.85; P < .001) and time to tumor recurrence (HR = 0.68; 95% CI, 0.60– 0.76; P < .001), with a 5-year OS of 71% for those who received chemotherapy and 64% for those who did not. Importantly, the improvement in patient outcome extended to patients older than 70 years of age, indicating that patients of all ages who were eligible for and enrolled in clinical trials receive the same benefit from adjuvant chemotherapy. Additionally, older patients did not suffer more toxic effects from chemotherapy than those who were younger, with the exception of asymptomatic neutropenia.
A key finding of this analysis was that patient age should not determine patient selection for treatment with adjuvant chemotherapy; rather, the presence or absence of comorbid conditions and patient preference were of paramount importance in decision making in the older person with colorectal cancer.
AJCC Subdivided Stage II and III
Updated staging characteristics released in the 6th edition of the AJCC tumor staging manual subdivided stage II and III tumors based on T stage and the number of positive lymph nodes.4 This development was central to improving stratification in trials and assessing the efficacy of drugs in particular patient populations.
Biomarkers of High-Risk Disease
Identifying molecular markers that correlate with poor prognosis has remained a priority in colon cancer research. Many markers have been investigated as potential aides in selecting patients at high risk of recurrence. Of particular interest to adjuvant chemotherapy of colon cancer is the loss of heterozygosity at chromosome 18q (LOH18q) and the presence of microsatellite instability (MSI) which are the first biomarkers to be used for patient stratification in a prospective clinical trial. While there is preliminary evidence suggesting the possible utility of these determinants, they are not ready for use in practice. The recent position paper from the American Society of Clinical Oncology (ASCO) provides us with a summary of the use of biomarkers in colon cancer.21 The following is the authors’ conclusion:
For colorectal cancer, it is recommended that carcinoembryonic antigen (CEA) be ordered preoperatively, if it would assist in staging and surgical planning. Postoperative CEA levels should be performed every 3 months for stage II and III disease for at least 3 years if the patient is a potential candidate for surgery or chemotherapy of metastatic disease. CEA is the marker of choice for monitoring the response of metastatic disease to systemic therapy. Data are insufficient to recommend the routine use of p53, ras, thymidine synthase, dihydropyrimidine dehydrogenase, thymidine phosphorylase, microsatellite instability, 18q loss of heterozygosity, or deleted in colon cancer (DCC) protein in the management of patients with colorectal cancer.21
Richard Goldberg, Jaffer Ajani
John Marshall
Heinz-Josef Lenz
ADJUVANT TREATMENT OF COLON CANCER
In the adjuvant setting, formulation of postsurgical therapeutic strategies should be individualized and depends on patient history, existing or competing comorbidities, and tumor characteristics. Adjuvant therapy is multidisciplinary in nature, and requires that community oncologists play a central role in integrating information from medical, surgical, and pathologic evaluation of disease. It is worth noting that adequate surgical intervention and accurate pathologic staging are paramount to devising appropriate postsurgical treatment avenues.
Adjuvant Chemotherapy for Stage III Colon Cancer
Recommendation
The panel recommends that chemotherapy with an oxaliplatin/fluoropyrimidine-containing regimen should routinely be offered to all patients for the adjuvant treatment of stage III colon cancer (Table 2). The efficacy of combining capecitabine and oxaliplatin in this disease setting are currently not supported by data, although this issue is under study in phase III trials. The value of 6 months of adjuvant chemotherapy compared to the risk of cumulative toxicity should be addressed on an individual basis. The panel supports discontinuing oxaliplatin and continuing with 5-FU/LV alone when oxaliplatin-related toxicities such as thrombocytopenia and neuropathy become problematic. This is particularly true if the cumulative oxaliplatin dose is in excess of 765 mg/m2.
Table 2.
Regimens currently appropriate for adjuvant chemotherapy of colon cancer
| Name | Protocol |
|---|---|
| 5-FU/LV (bolus) | Mayo or Roswell Park regimens |
| LV5FU2 | LV 200 mg/m2, 5-FU 400 mg/m2 bolus followed by 5-FU 600 mg/m2 22-hr infusion, given every 14 days for 12 cycles |
| Capecitabine | Capecitabine 1,250 mg/m2 twice daily for 14 days every 3 weeks |
| FLOX | 5-FU/LV: 5-FU 500 mg/m2 bolus every week for 6 weeks, LV 500 mg/m2 every week for 6 weeks of 8-week cycle, for 3 cycles + 85 mg/m2 oxaliplatin on week 1,3, and 5 of each cycle |
| mFOLFOX6 | LV 400 mg/m2 IV on day 1, 5-FU 400 mg/m2 IV bolus on day 1 followed by 2,400 mg/m2 by continuous IV infusion over 46 hours (day 1 and day 2); oxaliplatin 85 mg/m2 IV day 1; every 14 days for 12 cycles (6 months) |
| FOLFOX4 | LV5FU2 + oxaliplatin 85 mg/m2 IV on day 1 (with leucovorin) |
Abbreviations: 5-FU = 5-fluorouracil; IV = intravenous; LV = leucovorin.
Other treatment regimens appropriate for this disease setting in patients who are not candidates for combination therapy due to comorbidities or personal preference are bolus or infusional 5-FU/LV regimens or oral capecitabine. The panel recognizes that the majority of patients with stage II tumors are cured by surgery, and the value of chemotherapy may be mitigated by competing comorbidities. Ultimately, the decision to employ adjuvant chemotherapy must be reached through open discussion between the oncologist and patient, based on individual considerations, patient willingness, and tumor characteristics.
Literature Review
Oxaliplatin.—After oxaliplatin’s successful use in treating advanced colon cancer, a large, international phase III trial investigated a potential role for oxaliplatin in the adjuvant setting.17,22 In this trial— the Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC)— 2,246 patients with stage II (40%) and stage III (60%) colon cancer were randomly assigned to treatment with infusional 5-FU/LV (LV5FU2: 200 mg/m2 LV, bolus 400 mg/m2 5-FU followed by 22 hour infusion of 600 mg/m2 5-FU, given every 14 days for 12 cycles; n = 1,123), or FOLFOX4 (LV5FU2 + oxaliplatin 85 mg/m2; n = 1,123). The primary study end point was DFS; secondary end points included OS and safety.
Including data through January 2005, the addition of oxaliplatin to LV5FU2 in stage III patients improved DFS by 7.2% (73.0% vs. 65.8%; HR = 0.75; 95% CI, 0.62–0.89) over that in the LV5FU2-alone arm, which corresponded to a 25% relative risk reduction. Median OS, reported in an interim analysis, also improved by 3.2% in stage III patients who received oxaliplatin (HR = 0.86; 95% CI, 0.69–1.08). The noted improvement in patient outcome was accompanied by an increase in grade 3/4 toxicities. The addition of oxaliplatin to LV5FU2 was associated with an increase in neutropenia (41% vs. 4.7%), febrile neutropenia (1.8% vs. 0.2%), diarrhea (10.8% vs. 6.7%), vomiting (5.9% vs. 1.4%), allergy (3.0% vs. 0.2%), and neuropathy (12.4% vs. 0%), but no increase in mortality. The main safety concern with oxaliplatin use is peripheral neuropathy, which increases in duration and severity with increasing cumulative dose.
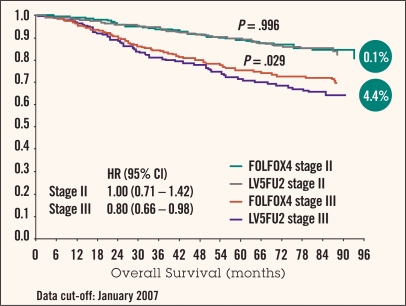
The 6-year update of the MOSAIC trial demonstrated a 2.6% (P = .057) OS benefit, suggesting that the DFS benefit is now translating into this key improvement in survival.23 Importantly, the stage III subgroup showed a 4.4% (P = .029) OS benefit with the addition of oxaliplatin (Figure 1).
Figure 1.
Data from 6-year update of the MOSAIC trial. Overall survival in stage II vs. stage III patients. From de Gramont et al.23
A second landmark phase III trial of adjuvant oxaliplatin (NSABP C-07) investigated its addition to weekly bolus 5-FU/LV for the treatment of patients with stage II and III colon cancer.24,25 Patients were randomly assigned to treatment with 5-FU/LV (500 mg/m2 bolus 5-FU every week for 6 weeks, 500 mg/m2 LV every week for 6 weeks of an 8-week cycle, for 3 cycles; n = 1,207) or FLOX (5-FU/LV + 85 mg/m2 oxaliplatin on weeks 1, 3, and 5 of each cycle; n = 1,200). The cumulative dose of oxaliplatin in this trial was 765 mg/m2, compared to a maximum of 1,020 mg/m2 in the MOSAIC trial. The primary study end point was DFS.
After a median of 34 months’ follow-up, FLOX-treated patients experienced a 21% relative risk reduction and a 4.9% improvement in DFS (76.5% vs. 71.6%; P < .004) compared with those treated with 5-FU/LV alone. The overall rates of grade 3/4 toxicities were 50% (41% grade 3) in the 5-FU/LV arm and 60% (50% grade 3) in the FLOX treatment arm. Similar to findings in the MOSAIC trial, oxaliplatininduced neurotoxicity was the main safety concern. The incidence of grade 3 neurotoxicity increased from 1% in 5-FU/LV– treated patients to 8% with the addition of oxaliplatin, and the rate of diarrhea/dehydration with bowel wall thickening increased from 2.7% to 4.5%, respectively. Administration of oxaliplatin had no effect on mortality (1% for both arms).
Irinotecan.—The success of irinotecan in the treatment of metastatic colon cancer has, interestingly, not translated into the adjuvant setting. Available data do not support a role for irinotecan in adjuvant chemotherapy and the panel currently recommends against its use in this setting.
The large Cancer and Leukemia Group B (CALGB) 89803 trial investigated the efficacy of irinotecan when added to bolus 5-FU/LV for the treatment of stage III colon cancer.18 A total of 1,260 patients were randomly assigned to treatment with bolus 5-FU/LV (Roswell Park) or IFL (125 mg/m2 irinotecan followed by 20 mg/m2 bolus LV and 500 mg/m2 bolus 5-FU, administered for 5 cycles of 4 weeks on/2 weeks off). After more than 4 years’ median follow-up, no statistically significant difference in the survival curves emerged between the two treatments (P = .81). Further, irinotecan treatment was associated with a significant increase in grade 3/4 neutropenia (43% vs. 5%, P < .00001), grade 3/4 febrile neutropenia (4% vs. 1%, P = .005), and mortality (2.8% vs. 1.0%, P = .008). The conclusions of this trial stated that weekly bolus IFL should not be used in the treatment of stage III colon cancer.
Both the Pan-European Trial in Adjuvant Colon Cancer (PETACC)-3 and the ACCORD1 trials investigated a potential role for irinotecan in stage II and III colon cancer in combination with infusional 5-FU (FOLFIRI).26,27 Neither trial demonstrated superiority for the irinotecan-containing regimen in terms of 3-year DFS.
Capecitabine.—An important new development in the treatment of adjuvant colon cancer was the demonstrated noninferiority of capecitabine compared with bolus 5-FU/LV (Mayo regimen) in the X-ACT (Xeloda [capecitabine] in Adjuvant Colon Cancer Therapy) study. The panel concurs with the interchangeable use of capecitabine and 5-FU/LV treatment regimens in stage III disease.
In the X-ACT trial, 1,987 patients who underwent curative resection of stage III colon cancer were randomly assigned to treatment with either capecitabine (1,250 mg/m2 twice daily for 14 days every 3 weeks; n = 1,004) or bolus 5-FU/LV (n = 983).19 The primary objective of the trial was equivalency in DFS. Secondary end points included relapse-free survival, OS, and safety. The findings of the trial demonstrated not only capecitabine noninferiority to bolus 5-FU/LV in terms of DFS (HR = 0.87; 95% CI, 0.75–1.00; P < .001), but revealed a trend in favor of capecitabine treatment. Treatment with capecitabine was also associated with longer relapse-free survival (HR = 0.86; 95% CI, 0.74– 0.99; P = .04). In addition, safety analysis indicated that the onset of predefined grade 3/4 toxicities was significantly reduced in patients treated with capecitabine compared to those treated with bolus 5-FU/LV (P < .001). These findings indicated that adjuvant treatment of stage III colon cancer with capecitabine at the recommended dose and schedule is at least equivalent to that of bolus 5-FU/LV by the Mayo Clinic technique, and is associated with reduced toxicity.
Adjuvant Chemotherapy for Stage II Colon Cancer
Recommendation
The panel does not recommend that adjuvant chemotherapy be routinely offered to all patients with stage II colon cancer, but advises that those who desire treatment receive it, preferably as part of a clinical trial. Risk assessment and patient selection for adjuvant chemotherapy for stage II disease requires accurate pathologic staging and should be based upon clinical prognostic markers indicative of a high risk of recurrence in this patient population: T4 tumor stage, inadequately sampled lymph nodes (<10–12), tumor grade showing poor differentiation, lymphatic or vascular invasion, tumor perforation, or obstructing tumors.
Daniel Haller, Richard Goldberg
Aimery de Gramont, Howard Hochster
The panel emphasizes that the existence of clinical markers of poorer prognosis in stage II disease should not be considered predictive of benefit from adjuvant chemotherapy. The panel additionally recognizes, however, that despite the persisting controversy over patient selection for chemotherapy for stage II disease, evidence is mounting that there is a subpopulation of these patients who are indeed at higher risk of recurrence and whose treatment needs were not fully addressed by previous treatment standards.
The challenge of counseling patients with stage II tumors regarding their individual treatment options depends on open and honest discussion concerning the risk:benefit ratio of chemotherapy. Patients should be made aware of the potentially small absolute benefit gained by treatment with 5-FU/LV, and the small increase in patient benefit resulting from the addition of oxaliplatin. Appropriate chemotherapeutic regimens available are the same for stage II as for stage III disease with the exception of capecitabine, whose efficacy and safety were not evaluated in the stage II disease setting. Again, the value of 6 months of adjuvant chemotherapy balanced against potential cumulative toxicity should be addressed on an individual basis. The panel supports deleting oxaliplatin from treatment and continuing with 5-FU/LV alone when related toxicities become problematic.
Other treatment regimens appropriate for this disease setting are bolus or infusional 5-FU/LV regimens or oral capecitabine. Ultimately, appropriate treatment choices for stage II colon cancer must be based on the existence of clinical markers of poor prognosis, individual concerns and patient willingness, and tumor characteristics.
Literature Review
Controversy has persisted for years over the validity of chemotherapy in the treatment of stage II colon cancer. The reports concerning the extent to which patients with stage II disease have benefited from adjuvant chemotherapy have varied. Two major European trials—one done by a team based in England called the QUASAR (Quick and Simple and Reliable) group, and the other performed by the French GERCOR (Groupe d’Etude et de Recherche en Cancréologie Onco-Radiothérapic) group, referred to as the MOSAIC study, are informative concerning the potential relative and absolute benefits of chemotherapy in this setting.22,28
To assess the survival benefit gained from adjuvant chemotherapy in patients with colon cancer, the QUASAR study randomized a total of 3,239 patients (91% stage IIB) to treatment with 5-FU/LV, with or without levamisole, (Mayo or Roswell Park, 6 months; n = 1,622) or observation only (n = 1,617) after curative resection.28 Treatment of patients with 5-FU/lev significantly increased 5-year survival by approximately 3 percentage points as compared with that in patients observed after surgery (80.3% vs. 77.4%; relative risk of death 0.83; 95% CI, 0.71–0.97; P = .04). Although the trial studied a somewhat heterogeneous population, including some patients who received radiation therapy, the findings of QUASAR marked the first demonstration of a statistically significant survival benefit for adjuvant chemotherapy for stage II patients.
In addition, 40% of the patients enrolled in the MOSAIC trial had stage II tumors (n = 899) and were randomized to receive FOLFOX4 (n = 451) or LV5FU2 (n = 448) treatment.22 A total of 87% of patients with stage II disease treated with FOLFOX4 were disease-free 3 years post therapy compared with 84.3% of those treated with LV5FU2 (HR = 0.80; 95% CI, 0.56–1.15); this represented an improvement of approximately 3% in DFS and a 20% recurrence risk reduction in the FOLFOX4 treatment arm. Subgroup analysis of patients with clinical markers of poor prognosis (T4 stage, bowel obstruction, tumor perforation, poorly differentiated tumor, lymphovascular invasion, fewer than 10 sampled lymph nodes) found that high-risk stage II patients treated with oxaliplatin experienced an improvement in DFS of 5.4% (HR = 0.76).
Importantly, findings of this trial revealed that patients with stage II disease with clinical prognostic markers of high risk of recurrence might benefit more from adjuvant chemotherapy than those with a better prognosis. However, unlike the result in stage III patients, the 6-year update of the MOSAIC trial showed no OS survival benefit for the stage II patients regardless of their risk stratification with the addition of oxaliplatin (Figure 1).23 This result does not say that 5-FU did not help patients with stage II disease, only that oxaliplatin did not in this subgroup analysis.
CLINICAL AND MOLECULAR PROGNOSTIC MARKERS FOR HIGH RISK
There are two types of potentially informative indicators when trying to identify stage II patients at higher risk of disease recurrence. The first, which has been well described in the literature, assesses risk by identifying tumor characteristics that correlate with poorer prognosis. In 1999, the College of American Pathologists convened a consensus conference to assess prognostic factors associated with various solid tumors.29–31 Some of the factors outlined in the consensus statement that the panel agreed were proven to be prognostic were T stage (depth of tumor penetration); nodal status assessed by at least 12 sampled nodes (with the recommendation to use visual enhancement if less than 12 are found, based on findings that an increasing number of lymph nodes sampled was significantly associated with an increasing percentage of specimens with lymph node metastases32); perioperative CEA level; and extramural venous invasion.
Other clinical factors listed as “promising” prognostic factors were histologic tumor grade and radial margin status. In the clinical setting, a higher risk of recurrence in stage II disease is indicated by advanced tumor stage, inadequate (<12) sampled nodes, poor cell differentiation, the presence of venous invasion, tumor perforation, and an obstructing tumor (Table 3).
Table 3.
Status of potential clinical and molecular prognostic markers for high-risk colon cancer.
| Prognostic marker | Recommended/proven | References |
|---|---|---|
| Cilnical | ||
| Tumor stage (T3 vs. T4) | Yes | 20, 28–30 |
| Inadequately sampled lymph nodes (<12) | Yes | 20, 28–30 |
| Poor tumor cell differentiation | Yes | 20, 28–30 |
| Extramural venous invasion | Yes | 20, 28–30 |
| Tumor perforation | Yes | 20, 28–30 |
| Molecular | ||
| CEA | Yes | 20, 28–30 |
| CA 19-9 | Insufficient data | 20 |
| DNA ploidy | Insufficient data | 20 |
| p53 | Insufficient data | 20 |
| ras | Insufficient data | 20 |
| TS, DPD and TP | Insufficient data | 20 |
| MSI | Insufficient data | 20,34 |
| 18q LOH/DCC | Insufficient data | 20 |
Abbreviations: CEA = carcinoembryonic antigen; DCC = deleted in colon cancer; DPD = dihydropyrimidine dehydrogenase; LOH = loss of heterozygosity; MSI = microsatellite instability; TP = thymidine phosphorylase; TS = thymidylate synthase.
A key future challenge in the adjuvant chemotherapy of stage II disease is the identification of molecular prognostic markers that indicate an individual’s risk of recurrence and, hence, allow clinicians to better select those for whom the risk:benefit ratio may be more favorable. A recently published ASCO consensus report could not recommend any tumor markers for assessing prognosis in colon cancer patients. 21 The report noted the correlation between elevated CEA and poor prognosis, but stated that the data remain insufficient to recommend its use in determining which patients should receive adjuvant chemotherapy.
The consensus panel agreed that data were insufficient to recommend CA 19-9, DNA ploidy, p53, ras, thymidylate synthase, dihydropyrimidine dehydrogenase, thymidine phosphorylase, MSI, and 18q LOH/DCC as markers of prognosis. Notwithstanding, 18q LOH and MSI remain of interest in colon cancer, and tumor status for these molecular markers is prospectively being used to randomize patients with stage II disease to treatment with adjuvant chemotherapy in the Eastern Cooperative Oncology Group (ECOG) 5202 trial.
FUTURE DIRECTIONS
The focus of ongoing and future clinical trials addresses the potential role of both molecular prognostic markers and biologic agents in the adjuvant treatment of colon cancer. Several trials warrant mention (Table 4).
Table 4.
Clinical trials key for the future of adjuvant chemotherapy in colon cancer.
| Trial name | Stage | Status | Trial schema |
|---|---|---|---|
| XELOXA NO16968 | III | Closed | XELOX vs. bolus 5-FU/LV (Mayo or Roswell Park) |
| NSABP C-08 | II and III | Closed | mFOLFOX6 ± bevacizumab (12 months) |
| AVANT | II and III | Recruiting | FOLFOX4 vs. FOLFOX4 + bevacizumab (12 months) vs. XELOX + bevacizumab (12months) |
| ECOG 5202 | II | Recruiting | High risk (MSS, LOH 18q)-> mFOLFOX6 ± bevacizumab; low risk-> observation |
| ECOG/N0147 | III | Recruiting | mFOLFOX6 ± cetuximab |
| PETACC-8 | III | Recruiting | FOLFOX4 ± cetuximab |
Abbreviations: 5-FU = 5-fluorouracil; ECOG = Eastern Cooperative Oncology Group; FOLFOX4 = 5-fluorouracil/leucovorin/oxaliplatin; LOH = loss of heterozygosity; LV = leucovorin; mFOLFOX6 = modified 5-fluorouracil/leucovorin/oxaliplatin; MSS = microsatellite stability; NSABP = National Surgical Adjuvant Breast and Bowel Project; PETACC = Pan-European Trial in Adjuvant Colon Cancer; XELOX = capecitabine/oxaliplatin.
Eastern Cooperative Oncology Group Study 5202
This trial by the ECOG is investigating bevacizumab, the vascular endothelial growth factor (VEGF)–targeted antibody, or placebo administered in combination with 5-FU/LV/oxaliplatin (modified [m] FOLFOX6) in high-risk stage II patients. The first of its kind, this trial prospectively stratifies patients based on the status of the putative high-risk molecular markers, 18q LOH and microsatellite stability (MSS). Notably, this trial is not designed to test the predictive value of these markers, which would require many thousands more patients.33
Interest in these two potential biomarkers stems from the investigation of MSI and 18q LOH/DCC status of 145 consecutively resected stage II/III colorectal carcinomas. 34,35 These findings demonstrated that a subgroup of patients with stage II tumors marked by MSI and 18q LOH had a risk of recurrence comparable to those with stage III disease. In ECOG 5202, patients deemed at high risk of recurrence using these two markers will be randomized to receive either mFOLFOX6 or mFOLFOX6 plus bevacizumab. Patients without these high-risk factors do not receive chemotherapy and are observed for the duration of follow-up.
NSABP C-08 and AVANT BO17920 Bevacizumab Trials
Two additional trials are investigating the potential role of bevacizumab in the adjuvant setting. In the NSABP C-08 phase III trial, which has already completed accrual, stage II and III patients were stratified by number of positive nodes (0, 1–3, >3) and then randomized between treatment with mFOLFOX6 every 2 weeks (oxaliplatin 85 mg/m2 IV day 1; LV 400 mg/m2 IV day 1; 5-FU 400 mg/m2 bolus day 1; 5-FU 2,400 mg/m2 continuous IV infusion over 46 hours) with or without bevacizumab (5 mg/kg every 2 weeks for 1 year). The primary study objective is to compare the relative efficacy of mFOLFOX6 plus bevacizumab with that of mFOLFOX6 alone in prolonging DFS and OS. Neither of these trials is prospectively evaluating the role of treatment in stage II patients.
The AVANT BO17920 study is an international phase III trial investigating the role of both bevacizumab and capecitabine in the adjuvant setting within one protocol. Patients with stage II and III cancer are randomized between the treatment arms FOLFOX4, FOLFOX4 plus bevacizumab (5 mg/kg every 2 weeks), and capecitabine/oxaliplatin (XELOX) plus bevacizumab (7.5mg/kg every 3 weeks). This treatment is continued for 24 weeks, and then the FOLFOX4 or XELOX discontinued, leaving the treatment arms of observation and bevacizumab (7.5 mg/kg every 3 weeks, for both bevacizumab-containing arms) for the last 24 weeks of intervention. The primary end point of the trial is DFS, with secondary end points that include safety, OS, pharmacogenomics, pharmacodynamics, convenience, and satisfaction with chemotherapy. The AVANT trial was stopped for 5 months (beginning in February 2006) due to a perceived elevated death rate in the XELOX plus bevacizumab arm, but has recontinued and is currently recruiting patients.
XELOXA NO16968 Capecitabine Study
Specifically addressing the contribution of capecitabine to adjuvant chemotherapy, the XELOXA trial randomized patients with stage III disease to treatment with either bolus 5-FU/LV (Mayo Clinic: LV 20 mg/m2 + 5-FU 425 mg/m2 on days 1–5, every 4 weeks for 6 cycles; or Roswell Park: LV 500 mg/m2 + 5-FU 500 mg/m2 day 1, every week for 6 weeks in each 8-week cycle, 4 cycles total) or XELOX (capecitabine 1,000 mg/m2 bid days 1–14 + oxaliplatin 130 mg/m2 day 1, every 3 weeks for 8 cycles). The primary end point of the trial is DFS and results are expected in 2007. A report of the safety of XELOX indicated that the incidences of grade 3/4 toxicities for 5-FU/LV (n = 924) and XELOX (n = 937) were as follows: diarrhea, 20% vs. 19%; stomatitis, <1% vs. 8%; nausea, 5% vs. 4%; vomiting, 6% vs. 3%; neurosensory, 11% vs. 0%; hand-foot syndrome, 5% vs. <1%; neutropenia, 8% vs. 15%; and febrile neutropenia, <1% vs. 4%, respectively.36
NCCTG N0147 and PETACC-8 Studies of Cetuximab
Both the North Central Cancer Treatment Group (NCCTG) N0147 (American) and the PETACC-8 (international) trials are investigating the role of cetuximab in the adjuvant setting. Both trials are currently recruiting stage III patients and will compare the efficacy of an oxaliplatincontaining regimen (mFOLFOX6 in N0147 and FOLFOX4 in PETACC-8) with and without cetuximab. The primary end point for both trials is DFS.
CONCLUSION
Adjuvant chemotherapy is considered a standard component of care for patients with stage III colon cancer, while this approach is still being debated for patients with stage II disease. The recommendation for or against adjuvant therapy requires an individualized approach based on multiple factors including patient medical history and health status and tumor-specific factors considered reliable indicators of recurrence risk. Patients with high-risk stage II disease as characterized by obstruction, perforation, or a surgical pathology report that includes less than 12 lymph nodes after diligent efforts on the part of the pathologist to isolate additional nodes, should likely be treated with adjuvant FOLFOX therapy. In patients without high-risk features, after review of the data on the potential benefits of therapy, patients and physicians should individualize their choice of therapy vs. follow-up.
The toxicities of treatment and patient preferences also play important roles in selecting postsurgical management approaches. Results of recently completed and ongoing trials will help define patient populations at high risk for recurrence who are therefore the most appropriate candidates for adjuvant therapy, and determine the role of new targeted therapies in this setting as well. In the future, with the identification of molecular prognostic and predictive markers, we may be able to identify patients likely to benefit from adjuvant chemotherapy, as well as which combination of agents would be most effective and least toxic.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Ajani has received research grants from sanofi-aventis, Taiho, Amgen, Adherex, and Ascenta. He also serves on speakers’ bureaus for sanofi-aventis and Amgen.
Dr. de Gramont has received honoraria from Roche, Genentech, and sanofi-aventis.
Dr. Goldberg has served as a consultant for Genentech, Bristol-Myers Squibb, sanofi-aventis, Pfizer, AstraZeneca, Amgen, ImClone, and Boehringer-Ingelheim.
Dr. Haller has served as a consultant to, and received honoraria and research funding from, Genentech, Roche, sanofi-aventis, and Pfizer.
Dr. Hochster has served as a consultant to sanofi-aventis.
Dr. Lenz has served as a consultant to Roche, Genentech, Bristol-Myers Squibb, and sanofi-aventis.
Dr. Marshall has received honoraria from Genentech, Bristol-Myers Squibb, sanofi-aventis, Pfizer, Amgen, and Roche.
REFERENCES
- 1.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 2003. Incidence and Mortality Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2007. Available at: www.cdc.gov/cancer/nper/uscs/ Accessed June 27, 2007.
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420–1425. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 4.Greene FL, Balch CM, Fleming ID, et al. ed 6. New York, NY: Springer; 2003. AJCC Cancer Staging Manual. [Google Scholar]
- 5.Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes' B2 colon cancer. J Clin Oncol. 1995;13:2936–2943. doi: 10.1200/JCO.1995.13.12.2936. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health Adjuvant therapy for patients with colon and rectum cancer. Consensus Statement. 1990;8:1–25. [PubMed] [Google Scholar]
- 7.NIH consensus conference Adjuvant therapy for patients with colon and rectal cancer. JAMA. 1990;264:1444–1450. [PubMed] [Google Scholar]
- 8.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 9.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 10.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes' B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 11.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol. 2005;23:8671–8678. doi: 10.1200/JCO.2004.00.5686. [DOI] [PubMed] [Google Scholar]
- 12.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–2947. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RM. N9741: a phase III study comparing irinotecan to oxaliplatin-containing regimens in advanced colorectal cancer. Clin Colorectal Cancer. 2002;2:81. doi: 10.1016/S1533-0028(11)70509-6. [DOI] [PubMed] [Google Scholar]
- 14.Grothey A, Deschler B, Kroening H, et al. Phase III study of bolus 5-fluorouracil (5-FU)/folinic acid (FA) (Mayo) vs. weekly high-dose 24h 5-FU infusion/FA + oxaliplatin (OXA) in advanced colorectal cancer (ACRC) Proc Am Soc Clin Oncol. 2002;21:129a. (abstr 512) [Google Scholar]
- 15.Tournigand C, Andre T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 16.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 17.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 18.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan plus fluorouracil/leucovorin (IFL) versus fluorouracil/leucovorin alone (FL) in stage III colon cancer (Intergroup trial CALGB C89803). 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:14S. (abstr 3500) [Google Scholar]
- 19.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 20.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 21.Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24:5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 22.de Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5FU/LV in the adjuvant treatment of stage II and stage III colon cancer: efficacy results with a median follow-up of 4 years. 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23:16S. (abstr 3501) [Google Scholar]
- 23.de Gramont A, Boni C, Navarro M, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. 2007 ASCO Annual Meeting Proceedings. J Clin Oncol. 2007;25:18S. (abstr 4007) [Google Scholar]
- 24.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP protocol C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 25.Land SR, Kopec JA, Cecchini RS, et al. Neurotoxicity from oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: NSABP C-07. J Clin Oncol. 2007;25:2205–2211. doi: 10.1200/JCO.2006.08.6652. [DOI] [PubMed] [Google Scholar]
- 26.Van Cutsem E, Labianca R, Hossfeld D, et al. Randomized phase III trial comparing infused irinotecan/5-fluorouracil (5-FU)/folinic acid (IF) versus 5-FU/FA (F) in stage III colon cancer patients (pts). (PETACC 3). 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23:16S. (abstr 8) [Google Scholar]
- 27.Ychou M, Raoul J, Douillard J, et al. A phase III randomized trial of LV5FU2+CPT-11 vs LV5FU2 alone in adjuvant high risk colon cancer (FNCLCC Accord02/FFCD9802). 2005 ASCO Annual Meeting Proceedings. J Clin Oncology. 2005;22:16S. (abstr 3502) [Google Scholar]
- 28.Gray RG, Barnwell J, Hills R, et al. QUASAR: A randomized study of adjuvant chemotherapy (CT) vs observation including 3238 colorectal cancer patients. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:14S. (abstr 3501) [Google Scholar]
- 29.Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer. 2000;88:1739–1757. doi: 10.1002/(sici)1097-0142(20000401)88:7<1739::aid-cncr30>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Compton CC, Fielding LP, Burgart LJ, et al. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979–994. doi: 10.5858/2000-124-0979-PFICC. [DOI] [PubMed] [Google Scholar]
- 31.Hammond ME, Fitzgibbons PL, Compton CC, et al. College of American Pathologists Conference XXXV: solid tumor prognostic factors-which, how and so what? Summary document and recommendations for implementation Cancer Committee and Conference Participants. Arch Pathol Lab Med. 2000;124:958–965. doi: 10.5858/2000-124-0958-COAPCX. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein NS. Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years: recommendations for a minimum number of recovered lymph nodes based on predictive probabilities. Am J Surg Pathol. 2002;26:179–189. doi: 10.1097/00000478-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Sargent DJ, Conley BA, Allera C, et al. Clinical trial design for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020–2027. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 34.Jen J, Kim H, Piantadosi S, et al. Allelic loss of chromosome 18q and prognosis in colorectal cancer. N Engl J Med. 1994;331:213–221. doi: 10.1056/NEJM199407283310401. [DOI] [PubMed] [Google Scholar]
- 35.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmoll H-J, Cartwright T, Tabernero J, et al. Phase III trial of capecitabine plus oxaliplatin as adjuvant therapy for stage III colon cancer: a planned safety analysis in 1,864 patients. J Clin Oncol. 2007;25:102–109. doi: 10.1200/JCO.2006.08.1075. [DOI] [PubMed] [Google Scholar]
- 37.Andre T, Quinaux E, Louvet C, et al. Updated results at 6 year of the GERCOR C96.1 phase III study comparing LV5FU2 to monthly 5FU-leucovorin (mFufol) as adjuvant treatment for Dukes B2 and C colon cancer patients. 2005 ASCO Annual Meeting Proceedings. J Clin Oncol. 2005;23:16S. (abstr 3522) [Google Scholar]
- 38.Wolmark N, Wieand S, Lembersky B, et al. A phase III trial comparing oral UFT to FULV in stage II and III carcinoma of the colon: results of NSABP Protocol C-06. 2004 ASCO Annual Meeting Proceedings. J Clin Oncol. 2004;22:14S. (abstr 3508) [Google Scholar]