Abstract
Purpose
To evaluate the association between dihydropyrimidine dehydrogenase (DPD) and thymidylate synthase (TS) levels in primary gastric tumors and clinical response to S-1 or S-1 plus irinotecan in patients with unresectable advanced gastric cancer, and to investigate the molecular mechanism of augmented antitumor activity of the combination using human gastric cancer xenografts with high TS activity.
Materials and Methods
TS mRNA expression and DPD mRNA expression were measured by reverse transcription polymerase chain reaction in initial primary cancer biopsy specimens in 29 patients with advanced gastric cancer who had received S-1 alone (n=18) or in combination with irinotecan (n=11). In an experimental study, antitumor effects of S-1, irinotecan, and the combination were assessed in mice bearing human gastric tumors with high TS expression (4-1-ST and AZ-521 tumors) and low TS expression (SC-2 tumors), and activities of 5-fluorouracil–metabolizing enzymes were measured.
Results
In the clinical study, a strong statistical association between high TS expression and clinical resistance to S-1 alone was found (P = .009). In the experimental studies, S-1 plus irinotecan showed augmented antitumor activity against tumors with high TS activity (P < .01) compared with either agent alone. A potential mechanism for this effect was suggested by the significant reduction in TS activity observed following irinotecan administration in tumors with high TS activity.
Conclusion
This study suggests that, via down-regulation of TS by irinotecan treatment, combination chemotherapy with S-1 and irinotecan could be effective in gastric cancer patients with high TS levels.
Tumors expressing high levels of thymidylate synthase (TS), the rate limiting enzyme of de novo DNA synthesis, have poor sensitivity to fluoropyrimidine based chemotherapy,1–4 and tumor levels of dihydropyrimidine dehydrogenase (DPD), a rate-limiting enzyme of 5-fluorouracil (5-FU) catabolism, have been reported to inversely correlate with sensitivity to 5-FU– based chemotherapy.5–9 These observations have led to attempts to predict efficacy of 5-FU treatment by assessing TS and DPD levels in gastrointestinal tumors.5, 8–11
S-1 is a new oral fluoropyrimidine with high activity in gastric and colorectal cancer.12–14 The drug contains the potent DPD inhibitor 5-chloro-2, 4-dihydroxypyrimidine (gimeracil, CDHP) and a potassium oxonate (oteracil potassium, Oxo) component that inhibits the phosphorylation of 5-FU, together with the 5-FU prodrug tegafur. The inclusion of CDHP as a component of S-1 is expected to reduce the effect of level of DPD expression on tumor response to fluoropyrimidine treatment. However, as with other fluoropyrimidines, tumors that express high levels of TS mRNA are expected to be relatively resistant to S-1. Ichikawa et al15 reported a positive correlation between TS mRNA expression and expression of topoisomerase I, and Danenberg et al16 have reported cases of metastatic colorectal cancer in which the topoisomerase I inhibitor irinotecan was effective in tumors with high levels of TS mRNA. Such findings suggest that the use of irinotecan in combination with S-1 might overcome tumor fluoropyrimidine resistance on the basis of high TS levels, and that selection of S-1 monotherapy or combined therapy could be made on the basis of tumor TS mRNA levels.
In the current study, we analyzed TS mRNA and DPD mRNA levels in initial biopsy samples from patients with unresectable advanced gastric cancer who had been treated with S-1 monotherapy or S-1 plus irinotecan to determine the association of expression levels with response to treatment. We further evaluated the antitumor activity of S-1 plus irinotecan using human gastric cancer xenografts with high or low TS activity in nude mice and investigated the molecular mechanism of the augmented activity seen with the combination.
MATERIALS AND METHODS
Clinical Study
Patient Characteristics and Treatment Outcome
The study population consisted of all 29 patients with unresectable advanced gastric cancer who were treated with first-line S-1 alone (n=18) or S-1 plus irinotecan (n=11) from January 2000 to December 2001 at the Second Department of Internal Medicine, Osaka Medical College (Osaka, Japan). This study was approved by the Institutional Review Board of Osaka Medical College, and all patients gave written informed consent. To be eligible, patients had to have a histologically confirmed diagnosis of gastric cancer; age of 20 to 75 years; performance status of 0, 1, or 2 on the Zubrod scale (Eastern Cooperative Oncology Group); no prior chemotherapy regimens before entry; adequate hematologic, hepatic, and renal function; and estimated survival of at least 3 months. Clinical characteristics of the patients are shown in Table 1.
Table 1.
Main clinical characteristics of patients.
| S-1 alone (n=18) | S-1 + irinotecan (n=11) | |||
|---|---|---|---|---|
| Sex | ||||
| Male | 12 | (67%) | 6 | (55%) |
| Female | 6 | (32%) | 5 | (45%) |
| PS | ||||
| 0–1 | 14 | (78%) | 8 | (73%) |
| 2 | 4 | (22%) | 3 | (27%) |
| Macroscopic type* | ||||
| Type 1 | 1 | (5%) | 0 | (0%) |
| Type 2 | 4 | (22%) | 0 | (0%) |
| Type 3 | 4 | (22%) | 2 | (18%) |
| Type 4 | 9 | (50%) | 9 | (82%) |
| Hisotology | ||||
| Differentiated | 10 | (56%) | 2 | (18%) |
| Undifferentiated | 8 | (44%) | 9 | (82%) |
| Response | ||||
| Responder | 6 | (33%) | 8 | (73%) |
| Nonresponder | 12 | (67%) | 3 | (27%) |
*Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma, 2nd English Edition. Gastric Cancer 1:10–24, 1998
Oral S-1 was given at 80 to 120 mg/day in two doses for 28 days, followed by a 14-day rest period for 1 course. S-1/irinotecan treatment consisted of S-1 at the same dose for 21 days followed by a 14-day rest period and irinotecan 80 mg/m2 IV on days 1 and 15 for 1 course.17 Objective response was determined by World Health Organization (WHO) criteria, and primary lesions were assessed by Japanese Research Society for Gastric Cancer criteria (originally established by WHO). Response was defined as complete or partial response; nonresponse was defined as no change or progressive disease.
Among 18 patients who received S-1 alone, 6 (33.3%) had partial response and 12 were nonresponders (8 with no change, 4 with progressive disease). Among 11 patients receiving S-1/irinotecan, 8 (72.7%) had partial response and 3 were nonresponders (all with no change).
Determination of TS mRNA and DPD mRNA in Tumor Specimens
Tumor samples were obtained from primary gastric tumors at the time of initial endoscopy. Immediately after biopsy, the tumor biopsy specimens were fresh frozen in liquid nitrogen until the time of RNA extraction. Semiquantitative reverse transcription polymerase chain reaction (RT-PCR)was performed using a previously described method, the reliability and validity of which have been reported in detail by Ishikawa et al.18 Total RNA for each sample was isolated using the RNeasy mini kit (Qiagen Inc., Chatsworth, CA) according to the manufacturer’s instructions. Reverse transcription using 10 g of total RNA was performed in a total volume of 100 L containing 250 pmol oligo (dT)18, 80 units of Rnasin (Promega, Madison, WI), 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, and 0.5 mM deoxynucleotide triphosphates solution. Initially, RNA and oligo (dT)18 were heated to 70ºC for 10 minutes and immediately chilled on ice; the remaining reagents were added and then incubated for 15 minutes at 30ºC and 60 minutes at 42ºC.
cDNA for genes of interest and an internal reference gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were quantified using a fluorescence-based realtime detection method (ABI PRISM 7700 Sequence Detection System [TaqMan]; Perkin-Elmer Applied Biosystems, Foster City, CA) as described previously.19
The PCR reaction mixture consisted of 600 nM of each primer, 200 nM probe, 2.5 units AmpliTaq Gold Polymerase, 200 μM each of dATP, dCTP, and dGTP, 400 μM dUTP, 5.5 mM MgCl2, and 1 X TaqMan Buffer A containing a reference dye, to a final volume of 25 μL (all reagents Perkin-Elmer Applied Biosystems). Primer and probe sequences were those previously described.20,21 Cycling conditions were 50ºC for 10 seconds, 95ºC for 10 minutes, followed by 46 cycles at 95ºC for 15 seconds and 60ºC for 1minute. TaqMan analyses yield values expressed as ratios between two absolute measurements (gene of interest/internal reference gene).
Statistical Analysis
The Mann-Whitney U test was used to compare the responders and nonresponders in terms of the related gene expressions. To evaluate the association of DPD or TS mRNA with response, DPD or TS mRNA was categorized into low or high values with the cutoff value determined by receiver operating characteristic (ROC) analysis. Association with response was analyzed by two-sided Fisher’s exact test.
Experimental Study
The antitumor effects of S-1 and irinotecan were evaluated in human gastric cancer xenografts with high TS activity (4-1-ST and AZ-521) and low TS activity (SC-2) in nude mice. Human gastric tumors AZ-521, 4-1-ST, and SC-2 were obtained from Dai-Nippon Pharmaceutical Co., Ltd. (Osaka, Japan) and maintained by implantation into the right axilla of nude mice at 3-week intervals. For enzyme assays, [6-3H]-5-FU (525 GBq/mmol), [6-3H]-thymidine (dThd; 2.41 TBq/mmol), [6-3H]-FdUMP (625 GBq/mmol), and [14C(U)]-cytidine-5′ -diphosphate (CDP; 2.04 GBq/mmol) were obtained from Moravek Biochemicals, Inc. (Brea, CA). For immunoblot analysis of proteins, anti-TS antibody was provided by Okabe,20 and all other antibodies used were purchased from Santa Cruz Biochemicals Inc. (San Diego, CA).
Xenografts and Treatments
For the antitumor experiments, 4-1-ST, AZ-521, and SC-2 tumors were prepared by subcutaneous implantation of approximately 3 mm3 fragments into the right axilla in groups of 5 mice each. After 7 days, animals received oral S-1 at 8.3 mg/kg for 14 consecutive days, irinotecan 40 mg/kg IV on days 1 and 8, or the combination. For studies of the effects of irinotecan on 5-FU–metabolizing enzymes, groups of 5 nude mice were prepared by the same methods and received saline or irinotecan alone 75 mg/kg once weekly for 2 weeks. In studies assessing dose response of TS activity, groups of 3 nude mice with AZ-521 tumors received no irinotecan or irinotecan 20, 40, or 60 mg/kg weekly for 2 weeks.
In the antitumor studies, tumor volume [½ × (the major axis) × (the minor axis)2] was measured in all groups twice a week throughout the experiments, and relative tumor volume (RTV) was calculated as follows: RTV = (mean tumor volume during therapy)/(mean tumor volume at the start of therapy). The antitumor effects of S-1, irinotecan, and both drugs combined were estimated by the following equation: mean tumor growth inhibition (TGI, %) = 1-(mean RTV of drug-treated group/mean RTV of control group) × 100.
Enzyme Assay
The effects of irinotecan treatment on 5-FU–metabolizing enzymes in AZ-521, 4-1-ST, and SC-2 tumors and the effect of different doses of irinotecan on TS activity in AZ-521 tumors were analyzed. AZ-521, 4-1-ST, and SC-2 tumors were homogenized with 3 volumes of 50 mM Tris-HCl (pH 7.6) containing 10 mM 2-mercaptoethanol, 25 mM KCl, and 5 mM MgCl2, centrifuged at 105,000g for 60 minutes, and the resulting supernatant was used to measure enzyme activity. The enzymes measured were TS, DPD, ribonucleotide reductase (RNR), orotate phosphoribosyltransferase (OPRT), thymidine kinase (TK), and thymidine phosphorylase (TP). TS was measured by [6-3H]-FdUMP binding assay based on the method of Spears et al.21 DPD and OPRT activity was determined according to the method of Shirasaka et al22 using [6-3H]-5-FU as the substrate. TK activity was measured by the method of Ikenaka et al23 except that the reaction product, [6-3H]-thymidine-5′ -monophosphate, was separated from [6-3H]-thymidine by Silica gel 60F254 (2 × 10 cm) thin layer chromatography with a mixture of chloroform, methanol, and acetic acid (17:3:1, v/v/v) as the mobile phase. TP was measured according to the modified method described by Maehara et al.24 Ribonucleotide reductase activity was determined using [14C(U)]-CDP as the substrate.25
Western Blot Analysis
For analysis of expression of TS proteins, Western blot analysis was performed using the ECL Western blotting detection system and protocol (Amersham Corp., Arlington Heights, IL). Briefly, cells were scraped from the plates, washed with PBS, and lysed in cell lysis buffer. Forty micrograms of protein were electrophoretically separated on SDS-polyacrylamide gels and transferred to polyvinylide difluoride membrane (Millipore, Bedford, MA). Anti-Rh TS polyclonal antibody was applied and the membranes were enhanced by chemiluminescence using ECLTM Western blot detection reagents (Amersham Corp.) according to the manufacturer’s instructions.
Statistical Analysis
The significance of differences between groups was assessed using Dunnett’s test and the IUT test.
RESULTS
Clinical Study
The relationship between tumor response and DPD mRNA and TS mRNA expression was assessed in 29 primary gastric tumor samples from patients with advanced gastric cancer treated with S-1 alone (n=18) or S-1/irinotecan (n=11). The cutoff values for high vs. low mRNA expression, determined by ROC analysis, were 6.76 (range, 0.19–19.77) for DPD mRNA and 4.53 (range, 0.75–17.27) for TS mRNA. Tumor response occurred in 6 of 18 patients receiving S-1 alone and in 8 of 11 receiving S-1/irinotecan.
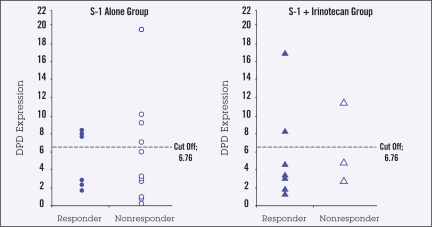
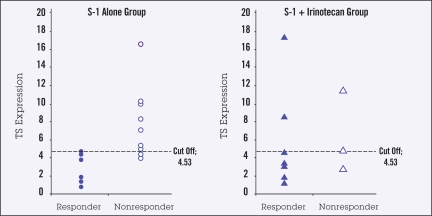
There was no relationship between high or low DPD mRNA expression and tumor response with either S-1 alone or with S-1/irinotecan (Figure 1). Response in patients receiving S-1 alone was significantly associated with TS mRNA expression, with none of the 6 responses occurring in tumors with high TS mRNA levels (P =.009) (Figure 2). There was no relationship between TS mRNA expression and tumor response in the S-1/irinotecan group, with responses being observed in some patients with high TS levels (Figure 2).
Figure 1.
DPD mRNA in responding (partial or complete response) and nonresponding (no change or disease progression) primary gastric tumors n i patients receiving S-1 (n=18) or S-1 plus irinotecan (n=11). Cutoff value demarcates high vs. low values.
Figure 2.
TS mRNA in responding (partial or complete response) and nonresponding (no change or disease progression) primary gastric tumors in patients receiving S-1 (n=18) or S-1 plus irinotecan (n=11). Cutoff value demarcates high vs. low values. P =.009 for relationship between tumor response and TS mRNA expression.
Experimental Study
Antitumor Effects of S-1 and Irinotecan
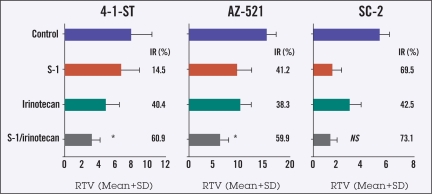
The antitumor activities of S-1 and irinotecan were evaluated in nude mice with human gastric cancer xenografts with high TS activity (4-1-ST and AZ-521) or low TS activity (SC-2). As shown in Figure 3, for 4-1-ST and AZ-521 tumors, the tumor growth inhibition rate (IR) with the combination of S-1/irinotecan was significantly augmented (P < .01) compared with either agent alone, reaching approximately 60% for both tumor types. For SC-2 tumors, S-1 alone had an IR of approximately 70%, with no further augmentation observed with the addition of irinotecan.
Figure 3.
Antitumor effect of S-1, irinotecan, and both drugs combined in human gastric cancer xenografts in mice (n = 5 for each xenograft). S-1 8.3 mg/kg was orally administered once daily for 14 days, and irinotecan 40 mg/kg was injected weekly for 2 weeks to tumor-bearing mice starting 2 weeks after tumor implantation. On treatment day 15, the antitumor activity of S-1, irinotecan, and their combination was evaluated as the inhibition rate of tumor growth (IR, %).
*For 4-1-ST and AZ-521 tumors, growth inhibition rate with S-1/irinotecan was significantly augmented (P < .01) compared with either agent alone by the IUT test.
Effects of Irinotecan on the Activity of 5-FU–Metabolizing Enzymes
To investigate the mechanism for augmented antitumor activity of the combination of S-1 and irinotecan on 4-1-ST and AZ-521 tumors, activities of the 5-FU–metabolizing enzymes TS, DPD, OPRT, TP, RNR, and TK were assessed in 4-1-ST, AZ-521, and SC-2 tumor xenografts in mice treated with irinotecan or saline. As shown in Table 2, irinotecan 75 mg/kg weekly for 2 weeks significantly reduced TS activity in 4-1-ST and AZ-521 xenografts compared with controls, and produced no change in TS activity in SC-2 tumors. Other 5-FU–metabolizing enzymes were not affected by treatment with irinotecan. Moreover, TS activity in AZ-521 tumor xenografts decreased dose-dependently following administration of 20, 40, and 60 mg/kg of irinotecan (Table 3). Overall, the doses of irinotecan examined in these studies were associated with a dose-dependent antitumor effect, with IR ranging from approximately 30% to 60% (data not shown). Doses of 20 to 40 mg/kg correspond to clinically applicable doses of irinotecan.
Table 2.
Effect of irinotecan administration on the activities of 5-FU–metabolizing enzymes in 4-1-ST, AZ-521, and SC-2 human gastric cancer xenografts in mice.
| Tumor | 4-1-ST
|
AZ-521
|
SC-2
|
|||
|---|---|---|---|---|---|---|
| Enzyme | Control | Irinotecan (75 mg/kg/wk × 2) | Control | Irinotecan (75 mg/kg/wk × 2) | Control | Irinotecan (75 mg/kg/wk × 2) |
| TS | 2.163±0.281 | 1.375±0.184* | 0.622±0.095 | 0.082±0.019* | 0.089±0.043 | 0.086±0.033 |
| DPD | 2.22 ± 0.27 | 1.84 ± 0.34 | 78.10±12.59 | 53.87±15.40† | 8.580±3.45 | 8.120±1.46 |
| OPRT | 8.119±0.663 | 7.599±0.806 | 6.649±0.772 | 4.726±0.600† | 21.01±2.68 | 22.10±4.00 |
| TP | 1.011±0.018 | 1.168±0.157 | 0.115±0.030 | 0.103±0.025 | 0.647±0.154 | 0.676±0.105 |
| RNR | 2.030±0.975 | 3.076±1.183 | 0.483±0.282 | 0.660±0.099 | 4.330±1.840 | 4.150±0.730 |
| TK | 15.28±1.90 | 15.29± 3.41 | 31.10± 3.75 | 35.97± 6.86 | 32.01± 4.20 | 68.70±13.61 |
For each tumor, mice received saline (n=5) or irinotecan 75 mg/kg (n=5) administered IV weekly for 2 weeks. At 24 hours after last treatment, tumors were removed and activities of 5-FU-metabolizing enzymes were measured. TS = thymidylate synthase; DPD = dihydropyrimidine dehydrogenase; OPRT = orotate phosphoribosyltransferase; TP = thymidine phosphorylase; RNR = ribonucleotide reductase; TK = thymidine kinase.
P < .01
P < .05 compared with control group by Dunnett’s test
Table 3.
Effect of irinotecan 20 to 60 mg/kg on TS activity in AZ-521 human gastric cancer xenografts in mice.
| Dose (mg/kg) | N | TS activity* (pmol/mg ± SD) | % of Control |
|---|---|---|---|
| Control | 3 | 0.994 ± 0.188 | 100.0 |
| 20 | 3 | 0.526 ± 0.118 | 52.7 |
| 40 | 3 | 0.292 ± 0.088 | 29.3 |
| 60 | 3 | 0.214 ± 0.054 | 21.4 |
| Control | 5 | 0.622 ± 0.095 | 100.0 |
| 75 | 5 | 0.082 ± 0.019 | 13.2* |
Mice received no irinotecan or irinotecan 20, 40, or 60 mg/kg on days 1 and 8; 24 hours after final treatment, tumors were dissected and TS activity was measured.
Data on 75 mg/kg treatment and control are from Table 2
Immunochemical Detection of TS Proteins and Rate-Limiting Proteins in G1/S Phase of Cell Cycle
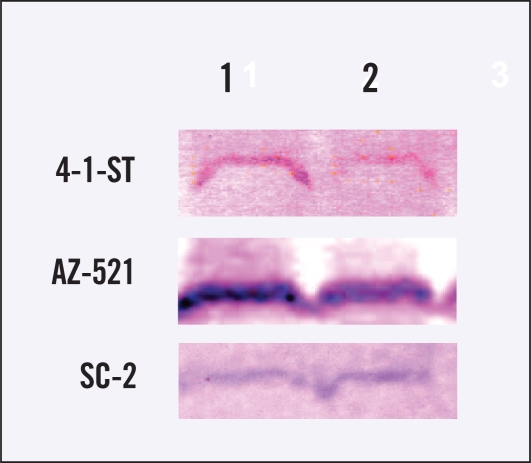
To confirm the decrease in TS activity in 4-1-ST and AZ-521 tumors treated with irinotecan, we assessed expression of TS proteins. The expression of TS proteins in 4-1-ST and AZ-521 tumors treated with irinotecan also decreased, whereas TS protein expression in SC-2 tumors did not change with irinotecan treatment (Figure 4).
Figure 4.
Expression of TS proteins in 3 human gastric cancer xenografts in mice treated with or without irinotecan. The TS aliquot (50 μg protein) was immunochemically analyzed using anti-TS polyclonal antibody by the Western blot method. The left lane shows nontreated tumors and the right lane shows irinotecan-treated tumors.
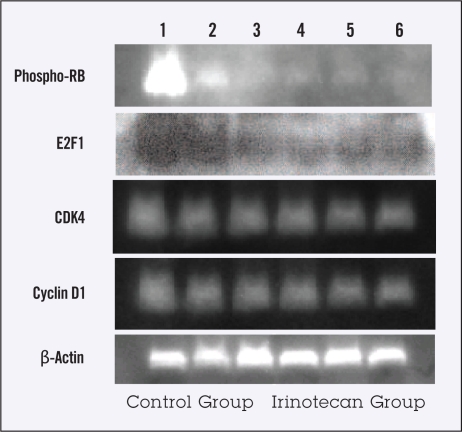
The expression of TS mRNA and its subsequent protein expression are known to be regulated by active E2F1 proteins released from the RB-R2F complex on which cell cycle-regulating proteins (eg, CDK4 and cyclin D1) would operate. We thus assessed expression of activated phospho-RB, free E2F1, CDK4, and cyclin D1 proteins in AZ-521 tumors treated with irinotecan. As shown in Figure 5, the expression of those proteins seemed to decrease in the tumors treated with irinotecan compared with untreated tumors. This result suggests that a decline in free E2F1 proteins in irinotecan-treated tumors may be connected with downregulation of TS expression.
Figure 5.
Expression of phospho-RB, E2F1, CDK4 and cyclin D1 proteins in AZ-521 tumors treated with or without irinotecan. Fifty microgram proteins of nuclear extracts from the tumors were immunochemically analyzed using anti-pRB, anti-E2F1, anti-CDK4, anti-cyclin D1, and anti-β-actin monoclonal antibodies, respectively. Lanes 1 to 3 show nontreated tumors and lanes 4 to 6 irinotecan-treated tumors.
DISCUSSION
High expression of TS mRNA and DPD mRNA has been associated with reduced gastrointestinal tumor sensitivity to 5-FU–based chemotherapy.1–8 Since the fluoropyrimidine S-1 contains the potent DPD inhibitor CDHP, its antitumor activity theoretically should be independent of level of DPD expression. It also has been reported that TS mRNA expression is positively correlated with topoisomerase I mRNA expression and that response can be achieved in tumors with high levels of TS activity by using the topoisomerase I inhibitor irinotecan.15,16 It is thus plausible to combine S-1 with irinotecan in patients with tumors with high expression of TSmRNA.
The findings in our study using samples from advanced gastric cancer patients receiving first-line therapy with either S-1 or S-1/irinotecan support the absence of effect of DPD level on tumor response to S-1, since there appeared to be no association between response and high or low level of DPD expression in primary tumors in patients receiving S-1 alone; level of DPD expression also did not affect response when S-1 and irinotecan were used together. However, tumor response to S-1 alone was observed only in patients with low TS mRNA expression, whereas response was observed in some patients with high TS expression with the addition of irinotecan.
The augmentation of antitumor activity with the addition of irinotecan to S-1 was confirmed by our findings in gastric cancer xenografts, with tumor growth inhibition being markedly greater with the combination of S-1/irinotecan than with S-1 alone in the high-TS-expressing 4-1-ST and AZ-521 tumors and no difference between S-1 alone and the combination being found in the low-TS-expressing SC-2 tumors.
These findings suggest that patients with advanced gastric cancer may derive greater benefit from the combination of S-1 and irinotecan when high levels of TS expression are present. Our experimental studies indicated that TS activity was down-regulated by irinotecan in a dose-dependent manner in xenografts with high levels of TS expression, suggesting that irinotecan resulted in an environment in which S-1 was more likely to exert its antitumor effect. This finding also suggests that order of treatment with S-1 and irinotecan might make some difference in achieving tumor response. Ongoing preliminary animal studies indeed suggest that treatment with irinotecan followed by S-1 may result in more prolonged tumor inhibition in GI tumors with high TS expression than that observed with S-1 alone, irinotecan alone, or both drugs in combination (data not shown).
Fluoropyrimidines are a mainstay of palliative treatment for unresectable advanced gastric cancer. S-1 has a theoretic advantage over 5-FU in terms of having antitumor activity that appears to be independent of level of DPD expression, suggesting that it should be active in tumors expected to be resistant to 5-FU on the basis of high DPD expression. The potential strategy of using S-1 alone or in combination with irinotecan based on absence or presence of high TS mRNA expression on initial biopsy of tumor tissue should be explored in a prospective clinical setting. Such a strategy might help avoid unnecessary combination therapy and unnecessary toxicity—a primary concern in palliative treatment—in patients with low TS expression, and the combination may improve response rates in patients with high TS expression.
Measuring TS mRNA expression would seem to be a first step toward the individualized treatment of gastric cancer using fluoropyrimidine-based chemotherapy, particularly S-1–based chemotherapy. However, it also needs to be acknowledged that fluoropyrimidine metabolism involves a large number of genes in addition to those examined in the current study, and the effects of different levels of expression of these genes and their products may also be of importance in modulating response to fluoropyrimidine-based treatment.
Acknowledgements
The authors are indebted to Prof. J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University for his review of this manuscript. The authors also thank Dr. Masakazu Fukushima of Taiho Pharmaceutical Company for his kind suggestions regarding the preclinical animal studies.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Lenz HJ, Leichman CG, Danenberg KD, et al. Thymidylate synthase mRNA level in adenocarcinoma of the stomach: a predictor for primary tumor response and overall survival. J Clin Oncol. 1996;14:176–182. doi: 10.1200/JCO.1996.14.1.176. [DOI] [PubMed] [Google Scholar]
- 2.Johnston PG, Lenz HJ, Leichman CG, et al. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407–1412. [PubMed] [Google Scholar]
- 3.Leichman L, Lenz HJ, Leichman CG, et al. Quantitation of intratumoral thymidylate synthase expression predicts for resistance to protracted infusion of 5-fluorouracil and weekly leucovorin in disseminated colorectal cancers: preliminary report from an ongoing trial. Eur J Cancer. 1995;31A:1306–1310. doi: 10.1016/0959-8049(95)00326-e. [DOI] [PubMed] [Google Scholar]
- 4.Leichman CG, Lenz HJ, Leichman L, et al. Quantitation of intratumoral thymidylate synthase expression predicts for disseminated colorectal cancer response and resistance to protracted-infusion fluorouracil and weekly leucovorin. J Clin Oncol. 1997;15:3223–3229. doi: 10.1200/JCO.1997.15.10.3223. [DOI] [PubMed] [Google Scholar]
- 5.Beck A, Etienne MC, Cheradame S, et al. A role for dihydropyrimidine dehydrogenase and thymidylate synthase in tumour sensitivity to fluorouracil. Eur J Cancer. 1994;30A:1517–1522. doi: 10.1016/0959-8049(94)00216-r. [DOI] [PubMed] [Google Scholar]
- 6.Etienne MC, Cheadame S, Fischel JL, et al. Response to fluorouracil therapy in cancer patients: the role of tumoral dihydropyrimidine dehydrogenase activity. J Clin Oncol. 1995;13:1663–1670. doi: 10.1200/JCO.1995.13.7.1663. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa Y, Kubota T, Otani Y, et al. Dihydropyrimidine dehydrogenase activity and messenger RNA level may be related to the antitumor effect of 5-fluorouracil on human tumor xenografts in nude mice. Clin Cancer Res. 1999;5:883–889. [PubMed] [Google Scholar]
- 8.Kirihara Y, Yamamoto W, Toge T, et al. Dihydropyrimidine dehydrogenase, multidrug resistance-associated protein, and thymidylate synthase gene expression levels can predict 5-fluorouracil resistance in human gastrointestinal cancer cells. Int J Oncol. 1999;14:551–556. doi: 10.3892/ijo.14.3.551. [DOI] [PubMed] [Google Scholar]
- 9.Salonga D, Danenberg KD, Johnson M, et al. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- 10.Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
- 11.Metzger R, Danenberg K, Leichman CG, et al. High basal level gene expression of thymidine phosphorylase (platelet-derived endothelial cell growth factor) in colorectal tumors is associated with nonresponse to fluorouracil. Clin Cancer Res. 1998;4:2371–2376. [PubMed] [Google Scholar]
- 12.Sakata Y, Ohtsu A, Horikoshi N, et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1M tegafur-0.4M gimestat-1M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–1720. doi: 10.1016/s0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 13.Koizumi W, Kurihara M, Nakano S, et al. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. Oncology. 2000;58:191–197. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 14.Ohtsu A, Baba H, Sakata Y, et al. Phase II study of S-1, a novel oral fluoropyrimidine derivative, in patients with metastatic colorectal carcinoma. Br J Cancer. 2000;83:141–145. doi: 10.1054/bjoc.2000.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ichikawa W, Uetake H, Nihei Z, et al. Topoisomerase I (Topo-I) expression correlates to thymidylate synthase (TS) expression in colorectal cancer (CRC) Proc Am Soc Clin Oncol 18246a1999. (abstract 947) [Google Scholar]
- 16.Johnston PG, Danenberg KD, Gilmore PM, et al. Thymidylate synthase (TS) expression from formalin fixed paraffin embedded (FFPE) tissues using quantitative RT-PCR correlates with frozen tissue data and predicts for response to 5-fluorouracil (5-FU) in metastatic colorectal cancers Proc Am Soc Clin Oncol 18617a1999. (abstr 2383) [Google Scholar]
- 17.Takiuchi H, Narahara H, Tsujinaka T, et al. Phase I study of S-1 combined with irinotecan (CPT-11) in patients with advanced gastric cancer (OGSG 0002) Jpn J Clin Oncol. 2005;35:520–525. doi: 10.1093/jjco/hyi148. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa Y, Kubota T, Ohtani Y, et al. Dihydropyrimidine dehydrogenase activity and messenger RNA level may be related to the antitumor effect of 5-fluorouracil on human tumor xenografts in nude mice. Clin Cancer Res. 1999;5:883–889. [PubMed] [Google Scholar]
- 19.Yoshinare K, Kubota T, Watanabe M, et al. Gene expression in colorectal cancer and in vitro chemosensitivity to 5-fluorouracil: a study of 88 surgical specimens. Cancer Sci. 2003;9:633–638. doi: 10.1111/j.1349-7006.2003.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okabe H, Tsujimoto H, Fukushima M. Preparation of the antibodies against recombinant human thymidylate synthase for the detection of its intratumoral levels and the application to sensitivity-study of 5-fluorouracil. Oncol Rep. 1997;4:685–690. doi: 10.3892/or.4.4.685. [DOI] [PubMed] [Google Scholar]
- 21.Spears CP, Shahinian AH, Moran RG. In vivo kinetics of thymidylate synthase inhibition in 5-fluorouracil-sensitive and -resistant murine colon adenocarcinomas. Cancer Res. 1982;42:450–456. [PubMed] [Google Scholar]
- 22.Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–4009. [PubMed] [Google Scholar]
- 23.Ikenaka K, Fukushima M, Nakamura H, et al. Metabolism of pyrimidine nucleotides in various tissues and tumor cells from rodents. Gann. 1981;72:590–597. [PubMed] [Google Scholar]
- 24.Maehara Y, Nakamura H, Nakane Y, et al. Activities of various enzymes of pyrimidine nucleotide and DNA synthesis in normal and neoplastic human tissues. Gann. 1982;73:289–298. [PubMed] [Google Scholar]
- 25.Fukushima M, Fijioka A, Uchida J, et al. Thymidylate synthase (TS) and ribonucleotide reductase (RNR) may be involved in acquired resistance to 5-fluorouracil (5-FU) in human cancer xenografts in vivo. Eur J Cancer. 2001;37:1681–1687. doi: 10.1016/s0959-8049(01)00174-5. [DOI] [PubMed] [Google Scholar]