Abstract
Gastric cancer is the second most common cause of cancer death in the world. In Japan, it is the most commonly diagnosed cancer and the second leading cause of cancer death. Standard treatment for advanced gastric cancer has not been established and prognosis remains poor worldwide. Numerous phase II studies have demonstrated promising results in overall response rate for new agents such as docetaxel, irinotecan, oxaliplatin, capecitabine, and S-1; some of these new agents may have improved toxicity profiles. Dozens of hase III studies comparing chemotherapy regimens have been conducted globally, several of which are ongoing in Japan. Results will be available in the near future. However, potential gains seem limited with the new regimens: a less than 1- to 2-month improvement in survival and a 10% or less improvement in response rate. This article reviews the history of, and recent progress in, chemotherapy for advanced gastric cancer globally, with a detailed look at investigations in Japan.
Gastric cancer is the fourth most common cancer worldwide, with an estimated 934,000 new cases diagnosed in 2002 (8.6% of total new cancer cases), and the second most common cause of cancer death (700,000 deaths annually). In Japan, it is the most commonly diagnosed cancer (an estimated 110,000 cases per year) and the second leading cause of cancer death (54,000 deaths annually), after lung cancer.1 Standard treatment for advanced gastric cancer has not been established, and prognosis remains poor. Although phase II studies have demonstrated promising results in overall response rate with new agents such as docetaxel, irinotecan, oxaliplatin, capecitabine, and S-1, and some of the new agents may have improved toxicity profiles, progress in chemotherapy for advanced gastric cancer has been slow in comparison to colorectal cancer.
This article reviews the history of, and recent progress in, establishing a standard chemotherapy regimen for unresectable advanced or recurrent gastric cancer globally, with a focus on Japanese investigations.
CHEMOTHERAPY FOR ADVANCED GASTRIC CANCER: GLOBAL PERSPECTIVE
History
In 1980, MacDonald et al reported the results of a study of the FAM combination regimen for advanced gastric cancer.2 The FAM regimen included three drugs: 5-fluorouracil (5-FU),3 doxorubicin, and mitomycin C. The overall response rate was 42%, and the median survival time was 5.5 months. Since the report was published, 5-FU–based combination chemotherapies have been used most frequently for the treatment of advanced gastric cancer (Table 1).
Table 1.
Phase III studies in advanced gastric cancer.
| Regimen | No. pts. | Antitumor activity
|
Survival
|
Author (year) | ||
|---|---|---|---|---|---|---|
| Response rate | P value | Median | P value | |||
| FAMTX | 105 | 41% | < .001 | 10.5 mo | .004 | Wils4 (1991) |
| FAM | 103 | 9% | 7.3 mo | |||
| FAMTX | 30 | 33% | NS | 7.3 mo | NS | Kelsen5 (1992) |
| EAP | 30 | 20% | 6.1 mo | |||
| FP | 103 | 51% | < .01 | 9.2 mo | NS | Kim7 (1993) |
| FAM | 98 | 25% | 7.3 mo | |||
| 5F - U | 94 | 26% | 7.7 mo | |||
| ECF | 126 | 45% (n=111) | < .001 | 8.9 mo | < .001 | Webb8 (1997) |
| FAMTX | 130 | 21% (n=108) | 5.7 mo | |||
| FAMTX | 133 | 12% | NS | 6.7 mo | NS | Vanhoefer9 (2000) |
| FP | 134 | 20% | 7.2 mo | |||
| ELF | 132 | 9% | 7.2 mo | |||
| DCF | 221 | 37% | .0106 | 9.2 mo | .0201 | Moiseyenko10 (2005) |
| FP | 224 | 25% | 8.6 mo | |||
| IF | 170 | 32% | NS | 9.0 mo | NSa | Dank11 (2005) |
| FP | 163 | 26% | 8.7 mo | |||
| FLO | 112 | 34% | NS | – | Not maturedb | Al-Batran12 (2006) |
| FLP | 106 | 25% | – | |||
| ECF | 263 | 38% | NS v ( s. ECF) | 9.9 mo | Noninferiority of X over Fand O over C was demonstrated | Cunningham13 (2006) |
| EOF | 245 | 40% | 9.3 mo | |||
| ECX | 250 | 41% | 9.9 mo | |||
| EOX | 144 | 47% | 11.2 mo | |||
| XP | 139 | 41% (n=160) | .03 | 10.5 mo | NSc | Kang14 (2006) |
| FP | 137 | 29% (n=156) | 9.3 mo | |||
Abbreviations: FAMTX = 5-FU (5-fluorouracil)/doxorubicin/methotrexate; FAM = 5-FU/doxorubicin/mitomycin C; EAP = etoposide/doxorubicin/cisplatin; FP = 5-FU/cisplatin; ECF = epirubicin/cisplatin/5-FU; ELF = etoposide/leucovorin/5-FU; DCF = docetaxel/cisplatin/5-FU; IF = irinotecan/leucovorin/5-FU; FLO = 5-FU/leucovorin/oxaliplatin; FLP = 5-FU/leucovorin/cisplatin; EOF = epirubicin/oxaliplatin/5-FU; ECX = epirubicin/cisplatin/capecitabine; EOX = epirubicin/oxaliplatin/capecitabine; XP = capecitabine/cisplatin; NS = not significant.
a Primary end point was superiority in time to progression: 5.0 months for IF, 4.2 months for FP; NS.
b Primary end point was superiority in time to progression: 5.7 months for FLO, 3.8 months for FLP; NS.
c Primary end point was noninferiority in progression-free survival: 5.6 months for XP, 5.0 months for FP; noninferiority was demonstrated.
In 1991, Wils et al reported the results of a randomized phase III study comparing FAMTX (5-FU/doxorubicin [Adriamycin]/methotrexate) with FAM.4 The median survival time was longer with FAMTX than with FAM (7.3 months for FAM and 10.5 months for FAMTX). In 1992, Kelsen et al compared FAMTX with EAP (etoposide/doxorubicin/cisplatin).5 The results demonstrated that FAMTX was superior to EAP in terms of safety. However, the median survival time did not differ significantly between EAP (6.1 months) and FAMTX (7.3 months). In 1993, Murad et al compared FAMTX with best supportive care and reported that the median survival time was longer with FAMTX (10 months) than with best supportive care (3 months), hence demonstrating activity of chemotherapy in patients with advanced gastric cancer.6 On the basis of these results, FAMTX therapy had been considered standard treatment for unresectable advanced or recurrent gastric cancer in Western countries by the late 1990s.
In 1993, a Korean study group reported the results of a three-arm comparison study: 5-FU monotherapy vs. FAM vs. FP (5-FU/cisplatin).7 The survival time was slightly longer for FP, but the difference was not statistically significant. The overall response rate was significantly higher for FP (51%) than for FAM (25%) and 5-FU (26%), and time to progression (TTP) was significantly longer for FP than for the other two groups.
Further comparison studies with FAMTX as a control arm were conducted. In 1997, Webb et al compared FAMTX with ECF (epirubicin/cisplatin/5-FU).8 They reported that the median survival time was longer with ECF (8.9 months) than with FAMTX (5.7 months) and suggested that the ECF regimen may serve as a new standard therapy. However, the median survival time for FAMTX reported in 1997 (5.7 months) was shorter than those of other comparative studies carried out in 1991 (10.5months) and 1995 (7 months). Furthermore, the 8.9-month ECF median survival time reported by Webb and colleagues in 1997 cannot be considered sufficiently long to conclude that ECF offers a true survival benefit when compared with other combination regimens.
In 2000, Vanhoefer reported the results of a phase III study comparing FAMTX with ELF (etoposide/leucovorin [LV]/5-FU) and FP.9 No difference was observed in overall response rate and overall survival (median survival times; 6.7, 7.2, and 7.2 months for FAMTX, ELF, and FP, respectively) among the three regimens, and all three were associated with comparable incidence, type, and degree of toxicity. These authors concluded that none of the regimens, including FP, should be considered standard therapy for advanced gastric cancer. They pointed out the importance of considering new strategies with better clinical efficacy in the treatment of advanced gastric cancer; eg, new drug combinations and analysis of molecular prognostic factors to identify responsive patients.
Through this history, a promising overall response rate did not always translate into a survival benefit. Time to progression and progression-free survival are considered more appropriate surrogate end points than overall response rate in predicting survival benefit. The evaluation of these end points in well-designed clinical studies is encouraged.
Recent Progress
In 2005, the final results of two large randomized phase III studies were presented at the 41st annual meeting of the American Society of Clinical Oncology (ASCO).10,11 Both studies used the same FP regimen (cisplatin 100 mg/m2 on day 1 plus 5-FU 1,000 mg/m2/day as a continuous infusion on days 1–5 every 4 weeks) as a reference arm.
The V-325 trial10 randomized 457 chemotherapy-naïve patients to receive docetaxel, cisplatin, and 5-FU (DCF) chemotherapy or FP. Response rate, time to progression, and median survival time were significantly superior with DCF (37%, 5.6, and 9.2 months, respectively) as compared to FP (25%, 3.7, and 8.6 months, respectively). The 2-year survival was 18% with DCF and 9% with FP. Grade 3/4 treatment-related adverse events occurred more frequently with DCF than with FP (81% vs. 75%), and neutropenia was more frequent with DCF than FP (82% vs. 57%). The authors concluded that docetaxel combined with FP and appropriate risk management represents a new option in advanced gastric cancer.
The V-306 trial11 randomized 337 chemotherapy-naïve patients to receive irinotecan/LV/5-FU (IF) or FP. Although IF demonstrated a favorable trend in time to progression, which was the primary end point, the results were not statistically significant (5.0 months for IF vs. 4.2 months for FP), and the median survival time was 9.0 months for IF and 8.7 months for FP. The major differences between the two arms (IF vs. FP) in grade 3/4 drug-related toxicity were diarrhea (21.6% vs. 7.2%), neutropenia (25% vs. 52%), febrile neutropenia or neutropenic infection (4.8% vs. 10.2%), stomatitis (2.4% vs.16.9%), and nausea (4.8% vs. 9.0%). More patients withdrew from the study due to drug-related adverse events with FP than IF (21.5% vs. 10.0%; P = .004). The IF regimen may be considered as an alternative non-cisplatin–based firstline treatment option.
In 2006, the results of three randomized phase III studies investigating the potential use of oxaliplatin or capecitabine were presented at the 42nd ASCO annual meeting.12–14 The results of a randomized phase III trial comparing 5-FU/LV/oxaliplatin (FLO, or the so-called modified FOLFOX6 regimen) with 5-FU/LV/cisplatin (FLP) were reported.12 A total of 220 chemotherapynaïve patients (112 FLO/108 FLP) were randomized. Time to progression, the primary end point, was 5.7 months for FLO compared with 3.8 months for FLP, but this was not statistically significant (logrank P = .081).
The major differences between the two arms (FLO vs. FLP) as far as grade 3/4 adverse events were in neutropenia (5% vs. 12%), anemia (5% vs. 10%), and peripheral neuropathy (13% vs. 3%). FLO and FLP, which are administered in a colorectal-cancer–like chemotherapy schedule, showed favorable safety profiles in comparison to the conventional FP regimen. Such dosing and scheduling of 5-FU plus cisplatin or oxaliplatin represents a better tolerated alternative to the conventional 4 to 5 days of continuous-infusion 5-FU in combination with higher doses of cisplatin administered every 3 to 4 weeks.
The REAL 2 study13 used a 2 × 2 design to evaluate several modifications of the ECF regimen, including the substitution of capecitabine for 5-FU and oxaliplatin for cisplatin. The primary end point was to demonstrate noninferiority in overall survival for capecitabine compared to 5-FU and for oxaliplatin compared to cisplatin. A total of 1,002 chemotherapy-naïve patients with histologically confirmed adenocarcinoma, squamous, or undifferentiated carcinoma of the esophagus, esophagogastric junction, or stomach were randomized to one of four regimens: ECF (epirubicin/cisplatin/5-FU), EOF (epirubicin/oxaliplatin/5-FU), ECX (epirubicin/cisplatin/capecitabine), or EOX (epirubicin/oxaliplatin/capecitabine). The median survival times for the 5-FU regimens (ECF and EOF), the capecitabine regimens (ECX and EOX), the cisplatin regimens (ECF and ECX), and the oxaliplatin regimens (EOX and EOF) were 9.6, 10.9, 10.1, and 10.4 months, respectively. The results met predefined noninferiority criteria for both comparisons; ie, capecitabine was not inferior to 5-FU and oxaliplatin was not inferior to cisplatin.
Another multinational randomized phase III trial of capecitabine in combination with cisplatin (XP) compared with FP was reported.14 The primary end point was to demonstrate noninferiority in progressionfree survival. A total of 316 chemotherapynaïve patients with previously untreated measurable advanced gastric cancer enrolled from Asia, Latin America, and Eastern European countries were randomized to either XP (160 patients) or FP (156 patients). Per protocol population (n = 276) was used for the primary efficacy analysis (139 patients in XP, 137 patients in FP). Progression-free survival, which was 5.6 months for XP and 5.0 months for FP, satisfied the predefined noninferiority criteria. The median survival times were 10.5 months for XP and 9.3 months for FP. Grade 3/4 stomatitis was less frequent for XP (2%) than for FP (7%), whereas anygrade hand-foot syndrome was more frequent for XP (22%) than for FP (4%). The trials of capecitabine and oxaliplatin demonstrated the noninferiority of these agents in comparison to 5-FU and cisplatin, with potentially less hematologic toxicity.
CHEMOTHERAPY FOR ADVANCED GASTRIC CANCER: JAPANESE PERSPECTIVE
Similar to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines (http://www.nccn.org/), the Japanese Gastric Cancer Association (JGCA) gastric cancer treatment guideline states that,
Although either CDDP [cisplatin] and/or 5-FU (or its derivatives) containing regimens are promising, no one specific regimen is recommended based on the results of clinical studies currently available in unresectable advanced or recurrent gastric cancer.
The history of, and recent progress in, developing chemotherapy for advanced gastric cancer in Japan are discussed in this section.
Single Agents
In Japan, oral fluoropyrimidines, such as tegafur (FT), UFT, carmofur (HCFU), and doxifluridine (5′-DFUR), were used frequently, in addition to intravenous 5-FU, cisplatin, mitomycin C, and methotrexate. Phase II studies in primarily chemotherapy-naïve patients demonstrated that overall response rates for these agents were about 20%.15–19 New anticancer agents such as S-1, capecitabine, irinotecan, and the taxanes showed promising activity in phase II studies (Table 2).
Table 2.
Overall response rates for single agents in advanced gastric cancer (Japanese phase II studies).
| Drug | No. pts. | Response rate | Author |
|---|---|---|---|
| UFT | 188 | 27.7% | Ota15 |
| 5′ -DFUR | 140 | 14.3% | Nitani17 |
| Epi-ADM | 31 | 16.1% | Sakata 18 |
| Cisplatin | 68 | 19.1% | Ishibiki19 |
| S-1 | 51 | 49.0% | Sakata23 |
| 50 | 40.0% | Koizumi24 | |
| Capecitabine | 31 | 19.4% | Koizumi28 |
| 55 | 25.5% | Sakamoto29 | |
| Irniotecan | 60 | 23.3% | Futatsuki 30 |
| Docetaxel | 59 | 23.7% | Taguchi31 |
| 59 | 23.7% | Mai32 | |
| Paclitaxel | 32 | 28.1% | Yamaguchi33 |
| 60 | 23.3% | Yamada34 | |
Abbreviations: Epi = epirubicin; ADM = doxorubicin (Adriamycin).
S-1 is an oral fluoropyrimidine composed of a mixture of FT (a prodrug of 5-FU) and two modulators—gimeracil (CDHP) and oteracil potassium (Oxo)—at a molar ratio of 1:0.4:1.20,21 CDHP causes reversible competitive inhibition of dihydropyrimidine dehydrogense (DPD), which serves as a rate-limiting enzyme in the metabolism of 5-FU. CDHP thus allows high 5-FU levels in blood and tumor tissue to be kept for prolonged periods of time. Oxo distributes locally in the digestive tract at high concentrations following oral administration and inhibits phosphorylation in the digestive tract, and thus can reduce the gastrointestinal toxicity of 5-FU.
An early phase II study of S-1 was conducted in patients with advanced gastric cancer. Prior chemotherapy was allowed in the study. The overall response rate was 53.6% (15/28 patients).22 Following these promising results, two late phase II studies of S-1 in chemotherapy-naïve patients with advanced gastric cancer were conducted.23,24 S-1 was administered twice daily at 80 mg/m2/day for 4 weeks followed by 2 weeks of recovery, repeated every 6 weeks. The overall response rates in these studies were 49% (25/51), and 40% (20/50). In a combined analysis of the two studies, the overall response rate was 44.6% (45/101), and the median survival time, 1-year survival rate, and 2-year survival rate were 244 days, 37%, and 17%, respectively.25
In Europe, a phase II study of S-1 in chemotherapy-naïve patients with advanced gastric cancer was conducted.26 The results showed a lower overall response rate (26%) and higher gastrointestinal toxicities than the Japanese phase II results. The dosing of S-1 for Western patients was recently reexamined due to possible ethnic differences in tolerability; ultimately, a relatively lower dose was recommended for Western patients than is generally administered to Japanese patients.27
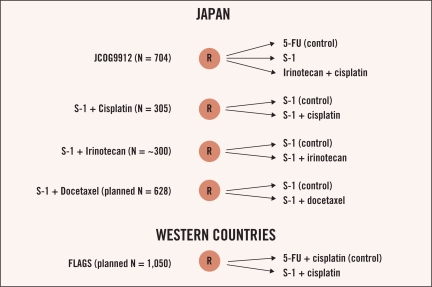
Thus, S-1 demonstrated promising antitumor activity. Several phase III studies with S-1–based chemotherapies are ongoing in Japan (Figure 1). The Japanese Clinical Oncology Group (JCOG) is performing a three-arm comparison study (JCOG9912)—continuous infusion 5-FU vs. irinotecan/cisplatin vs. S-1 alone—in chemotherapynaïve patients with inoperable advanced or recurrent gastric cancer. Study enrollment was completed at 704 patients and survival data will mature in 2007.
Figure 1.
Summary of ongoing S-1 phase III studies for advanced gastric cancer.
Capecitabine, an oral fluoropyrimidine derivative, was also evaluated in Japan. An early phase II study demonstrated that the overall response rate was 19.4% (6/31) and the median survival time was 8.25 months; prior chemotherapy was allowed in the study.28 In the subgroup analysis, the response rate in patients who received prior chemotherapy was 24.0% (6/25). A late phase II study was conducted in chemotherapy-naïve patients. The overall response rate was 25.5% (14/55) and the median survival time was 10.0 months (95% confidence interval [CI], 6.4–13.6 months).29 At the moment, this agent is only indicated for breast cancer in Japan.
Irinotecan, a topoisomerase I inhibitor, has also been evaluated in Japan. A phase II study that allowed prior chemotherapy demonstrated that the overall response rate was 23.3% (14/60). A 16.1% (9/45) response rate was observed in the subgroup of patients who received prior chemotherapy such as 5-FU, cisplatin, or other agents.30
As the taxanes docetaxel and paclitaxel are promising agents in advanced gastric cancer, phase II studies were conducted. The overall response rates for docetaxel in two phase II studies were 23.7% (14/59) and 23.7% (14/59).31,32 Those for paclitaxel were demonstrated to be 28.1% (9/32) and 23.3% (14/60) in two phase II studies.33,34 In the Yamada study,34 the overall response rate for paclitaxel was 26.9% (7/26) in patients who received prior chemotherapy. Taxanes are promising agents for first- and second-line therapy in advanced gastric cancer.
Combination Therapy
Combination chemotherapy with 5-FU (or oral fluoropyrimidines) and mitomycyn C was frequently used in Japan until cisplatin was available. Once cisplatin became available, combinations of cisplatin with fluoropyrimidines or irinotecan have been frequently used for the treatment of advanced gastric cancer.
Mitomycin C Plus Fluoropyrimidine
A randomized phase III study comparing mitomycin C plus FT with mitomycin C plus UFT (UFTM) was carried out by the JCOG.35 The response rate for UFTM (25.3%, 20/79) was significantly higher than that for mitomycin C plus FT (7.8%, 7/90). Based on these results, UFTM was a popular combination chemotherapy regimen for advanced gastric cancer in Japan until cisplatin-based chemotherapy emerged.
Cisplatin Plus Fluoropyrimidine
The preclinical synergy for fluoropyrimidines and cisplatin has supported ongoing interest in the clinical development of 5-FU and cisplatin-based chemotherapy. The combination of cisplatin with 5-FU or oral fluoropyrimidines was evaluated in phase II studies in the first-line setting, yielding overall response rates of about 40% and median survival times in the range of 8 to 9 months (Table 3). Kurihara reported that the overall response rate of 5′-DFUR plus cisplatin was 36.4%(16/44), and themedian survival time was 9 months.36 We reported that the overall response rate for this regimen was 50% (14/28).37 The combination of UFT and cisplatin (UFTP) showed an encouraging overall response rate (51.2%, 21/41) in our multicenter phase II study.38
Table 3.
Combination therapy: Cisplatin plus fluoropyrimidines for advanced gastric cancer in Japan.
| Treatment schedule | No. pts. | Response rate | Median survival (months) | Author (year) | |||
|---|---|---|---|---|---|---|---|
| Cisplatin | IV | 20 mg/m2/d | days 1–5 | 40 | 43% | 9 | Ohtsu39 (1994) |
| 5-FU | CI | 800 mg/m2/d | days 1–5, q4w k | ||||
| Cisplatin | IV | 80 mg/m2/d | day 5 days 1–5,15–18, q4wk | 44 | 50% | 9.1 | Koizumi37 (1993) |
| 5′ -DFUR | po | 1,400 mg/m2/d | |||||
| Cisplatin | IV | 80 mg/m2/d | day 8 | 41 | 51% | 8.6 | Sato38 (2000) |
| UFT | po | 350 mg/m2/d | days 1–21, q4wk | ||||
| Cisplatin* | IV | 60 mg/m2/d | day 8 | 25 | 76% | 12.8 | Koizumi41 (2003) |
| S-1 | po | 80 mg/m2/d | days 1–21, q4wk | ||||
Abbreviations: CI = continuous infusion; 5-FU = 5-fluorouracil; IV = intravenous; po = orally.
Phase I/II study.
Ohtsu et al conducted a phase II study of FP combination chemotherapy in Japan in which 5-FU was administered as a protracted infusion at 800 mg/m2/day for 5 days (days 1–5) and cisplatin was administered at 20 mg/m2/day for 5 days (days 1–5), with the regimen repeated every 4 weeks.39 The overall response rate was 43% (17/40), and the median survival time was 7 months. Grade 3/4 leukopenia occurred in 10/55 patients (18%) and grade 3/4 thrombocytopenia was observed in four patients (7%).
The JCOG conducted a randomized phase III study (JCOG9205) comparing FP with 5-FU alone and UFTM in chemotherapy-naïve patients with unresectable or recurrent gastric cancer.40 Overall response rate and time to progression were better with FP, while survival time did not differ among the three arms, and 5-FU alone had a favorable safety profile. Thus, the control arm in the JCOG’s ongoing phase III study (JCOG9912) is 5-FU alone.
S-1 Plus Cisplatin
We conducted a phase I/II clinical study of S-1 in combination with cisplatin to potentially enhance the antitumor activity of UFTP therapy by replacing UFT with S-1.41 S-1 was administered orally twice daily at a fixed dose of 80 mg/m2/day on days 1–21 with 1 to 2 weeks of recovery, repeated every 4 to 5 weeks. Cisplatin was administered on day 8 every cycle starting at 60 mg/m2 and escalated to 80 mg/m2 in 10-mg increments. The maximum tolerated dose for cisplatin was 70 mg/m2 and its recommended dose for further studies was determined to be 60 mg/m2. The overall response rate for all dosing levels was 76% (19/25) and 73.7% (14/19) at the recommended dosing level. The incidence of grade 3/4 hematologic and nonhematologic toxicities was 16% and 26%, respectively, indicating that this is a tolerable therapy.
The small sample size and the promising response rate and survival data (median survival time, 383 days) led us to conduct a phase III clinical study comparing S-1 plus cisplatin with S-1 alone in chemotherapy-naïve patients to clarify the benefit of adding of cisplatin to S-1 monotherapy. The enrollment of the study completed at 305 patients and the survival data will mature in 2007.
Ajani et al also conducted a phase I/II study of this combination in a Western population and found the overall response rate to be 51% (21/41).42,43 The recommended dose for further studies was lower (50 mg/m2/day) than in the Japanese studies. The First Line Advanced Gastric Cancer Study (FLAGS), a multinational phase III study of S-1 plus cisplatin compared with FP, is ongoing in 26 countries including North America, Latin America, Europe, South Africa, and Australia. The dosing regimen for S-1/cisplatin was established for a Western population by Ajani et al, and the schedule for FP is identical to that used in the V-325 and V-306 studies. The primary end point of the study is to demonstrate superiority in overall survival with a planned sample size of 1,050 patients. Survival results are anticipated in 2008.
S-1 Plus Irinotecan
Komatsu and colleagues conducted a phase I/II study of S-1 in combination with irinotecan in advanced gastric cancer patients.44 S-1 was administered orally, twice daily at 80 mg/m2/day, on days 1–14, and with three dose-escalation levels of irinotecan ranging from 100 to 150 mg/m2 administered on days 1 and 15, repeated every 4 weeks. The recommended dose of irinotecan for further phase II studies was determined to be 125 mg/m2. A 54% response rate was observed.
Narahara et al investigated the combination with a different schedule.45 S-1 was administered twice daily at a fixed dose of 80 mg/m2/day for 3 weeks and irinotecan was administered on days 1 and 15, repeated every 4 weeks. Irinotecan was increased from 40 to 100 mg/m2 in 20-mg increments. The recommended dose of irinotecan in the combination for further studies was 80 mg/m2; the response rate was 55.6%.
A phase III study comparing S-1 plus irinotecan with S-1 alone in chemotherapynaïve patients completed enrollment at approximately 300 patients; an overall survival result is anticipated in the near future. The development of a cisplatin-free regimen is important, especially for cisplatin-contraindicated patients.
Irinotecan Plus Cisplatin
Boku et al conducted a phase II study of irinotecan in combination with cisplatin in patients with metastatic gastric cancer; one or no prior chemotherapy was allowed.46 Irinotecan was administered on days 1 and 15 at a 70-mg/m2 dose and cisplatin was administered on day 1 at 80mg/m2, repeated every 4 weeks. The overall response rate was 47.7% (21/44) for all patients and 59% (17/29) for chemotherapy-naïve patients. The median survival time was 272 days for all patients and 322 days for the 29 patients who had not received prior chemotherapy. Grade 4 neutropenia was observed in 25 patients (57%), and grade 3/4 diarrhea developed in 9 patients (20%).
A modified irinotecan plus cisplatin regimen was evaluated to potentially develop a regimen with less toxicity and to allow outpatient treatment. In a phase I study, the cisplatin dose level was fixed at 30 mg/m2 and the irinotecan dose was escalated from 30 to 70 mg/m2 in 10-mg increments. The recommended irinotecan dose was 60 mg/m2.47 The results obtained to date indicate that this therapy could afford adequate efficacy even at the low dose level, and can be administered safely in outpatient clinics.
The promising irinotecan/cisplatin regimen is also one of the three arms in a JCOG phase III study (JCOG9912). The hypothesis of the study is that irinotecan/cisplatin is superior to 5-FU alone in overall survival.
S-1 Plus Docetaxel
The combination of S-1 with docetaxel was evaluated in phase I and II studies. The recommended dose of docetaxel on day 1 was 40 mg/m2 when combined with S-1 twice daily at a fixed dose of 80 mg/m2/day on days 1–14 with a 1- or 2-week recovery period.48,49 Yamaguchi investigated this combination in a phase I/II study.48 The overall response rate was 46% (21/46) and the median survival time was 14.0 months (95% CI, 8.3–17.3 months). The most common grade 3/4 toxicity was neutropenia (67%). Yoshida reported the results of a phase II study of the combination in 48 patients.49 The overall response rate was 56.3% and the median survival time was 14.3 months (95% CI, 10.7–20.3 months). The most common grade 3/4 hematologic toxicity was neutropenia (58.3%). The approximately 14-month median survival time is encouraging. A phase III study comparing S-1 plus docetaxel with S-1 alone in chemotherapy-naïve patients is ongoing in Japan and Korea, with a planned sample size of 628 patients.
DISCUSSION
Standard treatment for advanced gastric cancer has not been established, and the disease continues to be associated with a poor prognosis worldwide. Dozens of randomized phase III studies comparing chemotherapy regimens have been conducted globally. Several phase III studies are ongoing in Japan, most of which include an S-1 arm; results will be available in the near future. Among recent phase III studies, only the V-325 study demonstrated a survival benefit so far—adding docetaxel to FP showed superior overall survival to FP. New therapy options have the potential for either improved efficacy or reduced therapy-related toxicity: irinotecan-based regimens, including irinotecan and 5-FU; the potential use of capecitabine or oxaliplatin as a substitute for 5-FU or cisplatin; and the modeling of the 5-FU dose and schedule based on the better-tolerated colorectal cancer paradigms.
The potential gain, however, seems to be limited. Even with these new therapies, less than a 1- to 2-month survival improvement has been made in the past decade. Progress in developing effective chemotherapy for advanced gastric cancer has been slow in comparison to colorectal cancer. As a next step, adding the promising newer targeted, biologic agents to traditional chemotherapy needs to be investigated in clinical studies.
Footnotes
Disclosures of Potential Conflicts of Interest
Dr. Koizumi indicated no potential conflicts of interest.
REFERENCES
- 1.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald JS, Schein PS, Woolley PV, et al. 5-fluorouracil, mitomycin-C, and Adriamycin (FAM): a new combination chemotherapy program for advanced gastric carcinoma. Ann Intern Med. 1980;93:533–536. doi: 10.7326/0003-4819-93-4-533. [DOI] [PubMed] [Google Scholar]
- 3.Duschinsky R, Pleven E, Heidelberger C, et al. The synthesis of 5-fluoropyrimidines. J Am Chem Soc. 1957;79:4559–4560. [Google Scholar]
- 4.Wils J, Klein DJ, Wagener TH, et al. Sequential high dose methotrexate and 5-fluorouracil combined with doxorubicin—a step ahead in the treatment of advanced gastric cancer: a trial of the EORTC Gastrointestinal Tract Cooperative Group. J Clin Oncol. 1991;9:827–831. doi: 10.1200/JCO.1991.9.5.827. [DOI] [PubMed] [Google Scholar]
- 5.Kelsen D, Atiq OT, Saltz L, et al. FAMTX versus etoposide, doxorubicin, and cisplatin: a random assignment trial in gastric cancer. J Clin Oncol. 1992;10:541–548. doi: 10.1200/JCO.1992.10.4.541. [DOI] [PubMed] [Google Scholar]
- 6.Murad AM, Santiago FF, Petroianu A, et al. Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer. 1993;72:37–41. doi: 10.1002/1097-0142(19930701)72:1<37::aid-cncr2820720109>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Kim NK, Park YS, Heo DS, et al. A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer. 1993;71:3813–3818. doi: 10.1002/1097-0142(19930615)71:12<3813::aid-cncr2820711205>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol. 1997;15:261–267. doi: 10.1200/JCO.1997.15.1.261. [DOI] [PubMed] [Google Scholar]
- 9.Vanhoefer UV, Rougier P, Wilke H, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 10.Moiseyenko V, Ajani J, Tjulanclin S, et al. Final results of a randomized controlled phase III trial (TAX 325) comparing docetaxel (T) combined with cisplatin (C) and 5-fluorouracil (F) to CF in patients (pts) with metastatic gastric adenocarcinoma (MGC). 2005 ASCO Annual Meeting Proceedings J Clin Oncol 2316S.2005. (abstr 4002) [Google Scholar]
- 11.Dank M, Zaluski J, Baorone C, et al. Randomized phase 3 trial of irinotecan (CPT-11) + 5FU/folinic acid (FA) vs CDDP + 5FU in 1st-line advanced gastric cancer patients. 2005 ASCO Annual Meeting Proceedings J Clin Oncol 2316S.2005. (abstr 4003) [Google Scholar]
- 12.Al-Batran S, Hartmann JT, Probst S, et al. A randomized phase III trial in patients with advanced adenocarcinoma of the stomach receiving first-line chemotherapy with fluorouracil, leucovorin and oxaliplatin (FLO) versus fluorouracil, leucovorin and cisplatin (FLP). 2006 ASCO Annual Meeting Proceedings J Clin Oncol 2418S.2006. (abstr LBA4016) [Google Scholar]
- 13.Cunningham D, Rao S, Starling N, et al. Randomised multicentre phase III study comparing capecitabine with fluorouracil and oxaliplatin with cisplatin in patients with advanced oesophagogastric (OG) cancer: the REAL 2 trial. 2006 ASCO Annual Meeting Proceedings J Clin Oncol 2418S.2006. (abstr LBA4017) [Google Scholar]
- 14.Kang Y, Kang WK, Shin DB, et al. Randomized phase III trial of capecitabine/cisplatin (XP) vs. continuous infusion of 5-FU/cisplatin (FP) as first-line therapy in patients (pts) with advanced gastric cancer (AGC): efficacy and safety results. 2006 ASCO Annual Meeting Proceedings J Clin Oncol 2418S.2006. (abstr LBA4018) [Google Scholar]
- 15.Ota K, Taguchi T, Kimura K, et al. Report on nationwide pooled data and cohort investigation in UFT phase II study. Cancer Chemother Pharmacol. 1988;22:333–338. [PubMed] [Google Scholar]
- 16.Takiuchi H, Ajani JA. Uracil-Tegafur in gastric carcinoma: a comprehensive review. J Clin Oncol. 1998;16:2877–2885. doi: 10.1200/JCO.1998.16.8.2877. [DOI] [PubMed] [Google Scholar]
- 17.Niitani H, Kurihara M, Saito T, et al. Phase II study of 5′-deoxy-5-fluorouridine (5′ -DFUR) on patients with malignant cancer—multiinstitutional cooperative study. Jpn J Cancer Chemother. 1989;12:2044–2051. [PubMed] [Google Scholar]
- 18.Sakata Y, Yoshida Y. Phase II study of epirubicin in inoperable or recurrent gastric cancer. Gan To Kagaku Ryoho. 1986;13:1887–1892. [PubMed] [Google Scholar]
- 19.Ishibiki K, Kumai K, Kodaira S, et al. Phase II study with cisplatin in advanced stomach and colon cancer. Jpn J Cancer Chemother. 1989;16:3185–3193. [PubMed] [Google Scholar]
- 20.Shirasaka T, Shimamoto Y, Ohshimo H, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulation. Anticancer Drugs. 1996;7:548–557. doi: 10.1097/00001813-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Schoeffscii P. The modulated oral fruoropyrimidine prodrug S-1, and its use in gastrointestinal cancer and other solid tumors. Anticancer Drugs. 2004;15:85–106. doi: 10.1097/00001813-200402000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Sugimachi K, Maehara Y, Hirokoshi N, et al. An early phase II study of oral S-1, a newly developed 5-fluorouracil derivative for advanced and recurrent gastrointestinal cancers. Oncology. 1999;57:202–210. doi: 10.1159/000012032. [DOI] [PubMed] [Google Scholar]
- 23.Sakata Y, Ohtsu A, Horikoshi N, et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–1720. doi: 10.1016/s0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 24.Koizumi W, Kurihara M, Nakano S, et al. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. Oncology. 2000;58:191–197. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 25.Maehara Y. S-1 in gastric cancer: a comprehensive review. Gastric Cancer. 2003;6(suppl 1):2–8. doi: 10.1007/s10120-003-0232-9. [DOI] [PubMed] [Google Scholar]
- 26.Chollet P, Schoffski P, Weigang-Kohler K, et al. Phase II trial of S-1 in chemotherapy-naïve patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG) Eur J Cancer. 2003;39:1264–1270. doi: 10.1016/s0959-8049(03)00237-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhu AX, Clark JW, Ryan DP, et al. Phase I and pharmacokinetic study of S-1 administered for 14 days in a 21-day cycle in patients with advanced upper gastrointestinal cancer. Cancer Chemother Pharmacol. 2007;59:285–293. doi: 10.1007/s00280-006-0265-y. [DOI] [PubMed] [Google Scholar]
- 28.Koizumi W, Saigenji K, Ujiie S, et al. A pilot phase II study of capecitabine in advanced or recurrent gastric cancer. Oncology. 2003;64:232–236. doi: 10.1159/000069313. [DOI] [PubMed] [Google Scholar]
- 29.Sakamoto J, Chin K, Kondo K, et al. Phase II study of a 4-week capecitabine regimen in advanced or recurrent gastric cancer. Anticancer Drugs. 2006;17:231–236. doi: 10.1097/00001813-200602000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Futatsuki K, Wakui A, Nakao I, et al. Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer. Jpn J Cancer Chemother. 1994;21:1033–1038. [PubMed] [Google Scholar]
- 31.Taguchi T, Sakata Y, Kanamaru R, et al. Late phase II clinical study of RP56976 (docetaxel) in patients with advanced/recurrent gastric cancer: a Japanese Cooperative Study Group Trial (Group A) Jpn J Cancer Chemother. 1998;25:1915–1924. [PubMed] [Google Scholar]
- 32.Mai M, Sakata Y, Kanamaru R, et al. A late phase II clinical study of RP56976 (docetaxel) in patients with advanced or recurrent gastric cancer: a Japanese Cooperative Study Group Trial (Group B) Jpn J Cancer Chemother. 1999;26:487–496. [PubMed] [Google Scholar]
- 33.Yamaguchi K, Tada M, Horikoshi N, et al. Phase II study of paclitaxel with 3-hour infusion for advanced gastric cancer. Gastric Cancer. 2002;5:90–95. doi: 10.1007/s101200200015. [DOI] [PubMed] [Google Scholar]
- 34.Yamada Y, Shirao K, Ohtasu A, et al. Phase II trial of paclitaxel by 3-hour infusion for advanced gastric cancer with short premedication for prophylaxis of paclitaxel associated hypersensitivity reactions. Ann Oncol. 2001;12:1133–1137. doi: 10.1023/a:1011680507956. [DOI] [PubMed] [Google Scholar]
- 35.Kurihara M, Izumi T, Yoshida S, et al. A cooperative randomized study on tegafur plus mitomycin C versus combined tegafur and uracil plus mitomycin C in the treatment of advanced gastric cancer. Jpn J Cancer Res. 1991;82:613–620. doi: 10.1111/j.1349-7006.1991.tb01894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurihara M. Relationship between quality of life and survival in patients undergoing two therapeutic regimens with 5′-DFUR and CDDP for inoperable advanced gastric cancer—report of a phase II study. Proceedings of the Society for Survival Time Studies on Cancer Patients. 1993;13:39–42. [Google Scholar]
- 37.Koizumi W, Kurihara M, Sasai T, et al. A phase II study of combination therapy with 5′-deoxy-5-fluorouridine and cisplatin in the treatment of advanced gastric cancer with primary foci. Cancer. 1993;72:658–662. doi: 10.1002/1097-0142(19930801)72:3<658::aid-cncr2820720306>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 38.Sato A, Kurihara M, Koizumi W, et al. A phase II study of UFT plus cisplatin (UFTP) therapy in patients with advanced gastric cancer Proc Am Soc Clin Oncol 19279A.2000. (abstr 1087) [Google Scholar]
- 39.Ohtsu A, Shimada Y, Yoshida S, et al. Phase II study of protracted infusional 5-fluorouracil combined with cisplatinum for advanced gastric cancer: report from the Japan Clinical Oncology Group (JCOG) Eur J Cancer. 1994;30A:2091–2093. doi: 10.1016/0959-8049(94)00350-e. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsu A, Shimada Y, Shirao K, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus cisplatin plus tegafur plus mitomycin C in patients with unresectable, advanced gastric cancer: The Japan Clinical Oncology Group study (JCOG9205) J Clin Oncol. 2003;21:54–59. doi: 10.1200/JCO.2003.04.130. [DOI] [PubMed] [Google Scholar]
- 41.Koizumi W, Tanabe S, Saigenji K, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207–2212. doi: 10.1038/sj.bjc.6601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol. 2005;23:6957–6965. doi: 10.1200/JCO.2005.01.917. [DOI] [PubMed] [Google Scholar]
- 43.Ajani JA, Lee FC, Singh DA, et al. Multicenter phase II trial of S-1 plus cisplatin in patients with untreated advanced gastric or gastroesophageal junction adenocarcinoma. J Clin Oncol. 2006;24:663–667. doi: 10.1200/JCO.2005.04.2994. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu Y, Takei M, Takeda H, et al. Phase II study of CPT-11 plus S-1 in patients with advanced gastric cancer Proc Am Soc Clin Oncol 22319.2003. (abstr 1282) [Google Scholar]
- 45.Narahara H, Takiuchi H, Tsujinaka T, et al. Phase I study of CPT-11 plus S-1 in patients with metastatic gastric cancer Proc Am Soc Clin Oncol 21170a2002. (abstr 677) [Google Scholar]
- 46.Boku N, Ohtsu A, Shimada Y, et al. Phase II study of combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol. 1999;17:319–323. doi: 10.1200/JCO.1999.17.1.319. [DOI] [PubMed] [Google Scholar]
- 47.Takiuchi H, Kurihara M, Koizumi W, et al. Phase I/II study of CPT-11 plus CDDP in patients with advanced gastric carcinoma Proc Am Soc Clin Oncol 19275a2000. (abstr 1074) [Google Scholar]
- 48.Yamaguchi K, Shimamura T, Hyodo I, et al. Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer. Br J Cancer. 2006;94:1803–1808. doi: 10.1038/sj.bjc.6603196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida K, Ninomiya M, Takakura N, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402–3407. doi: 10.1158/1078-0432.CCR-05-2425. [DOI] [PubMed] [Google Scholar]