Abstract
This selective review combines cognitive models and biological models of psychosis into a tentative integrated neuropsychiatric model. The aim of the model is to understand better, how pharmacotherapy and cognitive-behavior therapy come forward as partners in the treatment of psychosis and play complementary and mutually reinforcing roles. The article reviews the dominant models in literature. The focus in this review is on one hand on neural circuits that are involved in cognitive models and on the other hand on cognitive processes and experiences involved in biological models. In this way, a 4-component neuropsychiatric model is tentatively constructed: (1) a biological component that leads to aberrant perceptions and salience of stimuli, (2) a cognitive component that attempts to explain the psychic abnormal events, (3) a mediating component with psychological biases which influences the reasoning process in the direction of the formation of (secondary) delusions, and (4) a component of psychological processes that maintains delusions and prevents the falsification of delusional ideas. Remission consists actually of 2 processes. Biological remission consists of the dampening of mesolimbic dopamine releases with antipsychotic medication and decreases the continuous salient experiences. Psychological remission consists of the reappraisal of primary psychotic experiences. Both forms of remission are partially independent. We expect that a full remission including biological and psychological remission could prevent relapse.
Keywords: schizophrenia, severe mental illness, cognitive-behavior therapy, symptoms, psychosis, antipsychotic medication
Introduction
Schizophrenia is characterized by 3 symptom syndromes: positive, disorganization, and negative symptoms.1 The development of (neuro)cognitive and structural models on disorganization and negative symptoms is just starting. Recent research on positive symptoms such as delusions and hallucinations has progressed markedly, and models are developed and tested. The evidence shows that psychosis is best understood with a neuropsychiatric model that integrates heredity, vulnerability, social adversities, and cognitive appraisal processes. The stages of development of psychosis are based on a neurodevelopmental vulnerability that is partly of an hereditary nature and partly result of early environmental influences. Social adversities later in life such as migration or cannabis abuse, propel the individual into a state of dopamine-induced perceptual aberrations. Thereafter, cognitive biases result into delusional interpretation of these abnormal perceptual experiences.2–4 The model of psychosis needs to be a neuropsychiatric model. Although there are cognitive models and biological models of psychosis, an integrated neuropsychiatric model is still lacking. This article reviews the dominant models that connect cognitive models of psychosis with biological models. The proposed neuropsychiatric model also has clinical significance because the model links pharmacological and cognitive-behavioral treatments. The proposed model can be considered as an extension of cognitive neuroscience into the domain of psychiatry.5 The model discriminates biological remission from psychological recovery of psychotic symptoms and explains the (partly) different effects of pharmacotherapy and cognitive-behavior therapy (CBT) on psychosis.
Antipsychotic medication and CBT have an additive effect in recovering from psychosis. When antipsychotic medication is insufficiently helpful, CBT can be an effective add-on therapy in psychosis. CBT is a psychotherapeutic approach that has proven to be effective in ameliorating psychotic symptoms in medicated patients.6–9
This selective review of delusions and auditory verbal hallucinations (AVHs) searched Medline and Psychinfo on schizophrenia and other psychosis (“schizophrenia” OR “psychosis” OR “delusion” OR “hallucinations” OR “paranoia”) and different models (“cognitive model” OR “neuropsychiatry” OR “neurocognition” OR “neurobiology” OR “attribution” OR “theory of mind” OR “self-serving bias” OR “selective attention” OR “source monitoring” OR “confirmatory bias” OR “stigma”). This selection was further limited to articles on human subjects and review articles. This resulted in 394 articles. The selection of reviewed articles stresses the links between psychosis and a cognitive model and the effects on the mind of neurotransmitter dysregulation. Articles that do not address one of the main models are not discussed in this article.
Biological Processes in Psychosis
Dopamine and Psychosis
Dopamine is a neurotransmitter that is central to psychosis. Although other neurotransmitter systems do play a role in psychoses, dopamine is associated with the primary perceptual aberrations, delusions, and negative symptoms, while for instance, serotonin is associated with depression in psychosis.10 Recent research has been able to pinpoint some of the brain areas involved in psychosis with modern imaging techniques such as positron emission tomography, single positron emission tomography, and magnetic resonance imaging.11,12 Cognitive dysfunctions are associated to hypodopaminergic function of the dorsolateral prefrontal cortex (DLPFC). The mood symptoms are like depression associated to medial prefrontal cortex (MPFC), the anterior cingulate cortex (ACC), and the orbitofrontal cortex. Positive symptoms are associated with excessive dopamine in the mesolimbic dopamine pathways.13
General Models
Several general models have been proposed for schizophrenia that try to explain schizophrenia symptoms. One of the earliest models suggests that schizophrenia is characterized by “abnormal lateralization.”14,15 Another model is the “disconnection model.” This model does not just localize the problems in information flow in the interhemispheric connections but expects a widespread failure of corticocortical and corticosubcortical connections to be involved in psychosis.16 The “imbalanced brain model” stresses the linkage between emotion processing in the amygdala and cognitive conscious processing in prefrontal areas.17 And finally, the “cognitive dysmetria model” stresses the widespread cognitive failures in attention, emotion, memory, and executive functions.18 Although there is evidence for all these models, no conclusive evidence has been found for any of the comprehensive models.19 Groups of symptoms on a syndrome level cannot be put into models at this moment. Therefore, in this review, we limit ourselves to models on a symptom level that explain the positive symptoms of psychosis: delusions and AVHs.
Symptom Models
Delusions
A central role of dopamine is to mediate the “salience” of environmental events and internal representations. Not all new stimuli that enter the perceptual field attract conscious attention. This only happens to stimuli that are novel and that demand a response. Dopamine gives salience and focuses attention on the stimulus, attaches an emotional value, and prepares a response. In psychosis, dopamine is not only released in reaction to a stimulus but also at random moments. Random increases in dopamine release in the mesolimbic pathways lead to abnormal “gating” of information into the prefrontal cortex.20 In this way, irrelevant information can get access to conscious processing as if it concerns important information.21
“I noticed things that I had never paid attention to before …” The person tries to make sense of these salient experiences, and in this way, delusions can result: “I felt enormous significance, and suddenly it was clear to me. All the pieces of the puzzle fell into their place.” While delusions are a cognitive effort by the patient to make sense of these aberrantly salient experiences, hallucinations reflect a direct experience of the aberrant salience of internal representations.3 The explanations the patient gives for the appearance of voices can easily become secondary delusions on the origin, power, and malevolence of the voices.
Paranoid patients experience many trivial stimuli as meaningful: eg, seeing an unknown car with an antenna in the street may trigger the thought: “they must be spying on me through this antenna.” This heightened selective attention for “threatening” stimuli is reflected in reduced activation of rostral-ventral ACC which is involved in self-monitoring and increased posterior cingulate cortex activation which results in impaired self-reflection.22
There is also evidence that a functional disconnection characterizes paranoia in autonomic and central systems for processing threat-related signals. The MPFC regulates the response of the amygdala to acute stressful stimuli via the nucleus accumbens (NAcc). This overactivation in the amygdala and underactivation of the MPFC locks the visceral circuit. Inappropriate emotional reactions continue, and inhibition is impossible. Paranoid cognition may reflect an internally generated cycle of misattribution regarding incoming fear signals due to a breakdown in the regulation of these systems.23
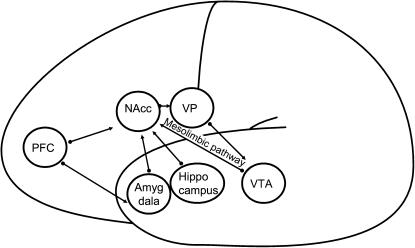
When the mesolimbic pathway that originates in the ventral tegmental area (VTA) stimulates the NAcc with phasic dopamine, the hippocampus inputs are facilitated via D1 receptor activation, which in turn enhances NAcc activity. As a consequence, ventral pallidus (VP) activity is suppressed, causing the prefontal cortex (PFC) inputs to be attenuated. In this way, learning can take place. Perseveration of learned responses results from mesolimbic overstimulation. The PFC is dominant in set switching, which is necessary to unlearn old responses. The PFC can only reset the system by a downregulation of tonic dopamine in the NAcc. In this case, PFC inputs are facilitated. As a result, VP activity is further elevated, suppressing tonic firing in VTA dopamine neurons.24,25
Figure 1 shows the networks involved in the paranoid delusional state. Phasic dopamine and high tonic dopamine result in underactivation of the PFC. In the amygdala network, this results in persistent inappropriate emotional reactions to irrelevant stimuli and the inability to secondary appraisal of emotional stimuli. The hippocampus network is locked as well. Evaluation of learned responses lacks, and the patient perseveres in inadequate responses.
Fig. 1.
The Amygdala-Visceral and Hippocampus-Contextual Networks in Delusions.
Hallucinations
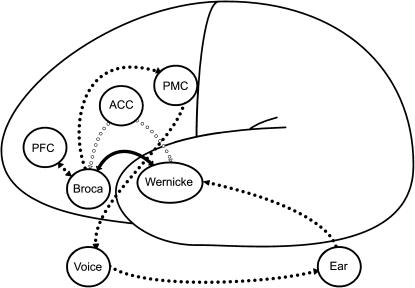
A meta-analysis has shown that hallucinations are in general associated with sensory modality-specific activation in cerebral areas involved in normal sensory processing.26 AVHs are associated with activation in the middle and superior temporal cortex (Wernicke).12,27 Wernicke is involved in processing speech. The temporal regions are central in AVHs, but other regions are involved in a network including the inferior frontal (Broca), PFC, ACC, and primary motor cortex (PMC). AVHs are considered misattributions of self-generated speech. Accurate identification of one's own speech appears to depend on ACC and PFC activity.28 One explanation of AVHs is that alterations of white matter fiber tracts lead to abnormal coactivation in regions related to the acoustical processing of external stimuli. This abnormal activation may account for the inability to distinguish self-generated thoughts from external stimulation.29 A recent study into the temporal course of AVHs showed activation of left inferior frontal and right middle temporal gyri (Broca: generation of inner speech) that preceded the self-report of an AVH. After 6–9 seconds, the bilateral temporal area and the left insula (Wernicke: perception of auditory verbal material) were activated.30 Voices that are heard from outside in contrast to voices heard from inside are characterized by activation of the plenum temporale.31
Figure 2 shows the most important parts of the AVH network. Broca is involved to produce the inner speech that is misperceived as not self-generated. Normally, Wernicke can decide whether the perception is outer generated (coming from the ear) or inner generated (coming from Broca) by a corollary discharge to the ACC that functions as a central monitor. The ACC prepares Wernicke for the perception of self-generated inner speech. In AVHs, this monitor function fails. The inner speech reaches the PMC which in turn activates the vocal chords.32,33 AVHs can be reduced by auditory input or by activating the speech-generating areas.34 Reducing the input by wearing an ear plug has also been found to reduce AVHs.35 Failure in the monitor loop seems to be a prerequisite for AVH, but the increased connectivity between Broca and Wernicke29 can easily enhance AVH (H. Smid and A. Aleman, unpublished data, 2006).
Fig. 2.
The Network in AVHs.
As sporadic instances of AVHs are quite common among the general population,36–38 especially in conditions of sensory deprivation,39 the auditory system might be prone to hallucinatory experiences. Research showed that during silence, the speech-sensitive auditory cortex is characterized by intermittent episodes of significantly increased activity in a large proportion of people. Some cases showed more than 30% increase. Bilateral increases in activity were associated with spontaneous activation in the left primary and association auditory cortices and ACC. This suggests that the endogenous activity is modulated by the ACC, resulting in spontaneous activation during silence. This also explains why the auditory system is prone to AVHs during silence.40
Cognitive Processes in Psychosis
Early Models of Psychosis
Weakening of Stored Memories
Hemsley and coworkers41,42 presented an experimental psychological model for psychosis. They considered psychosis to be a weakening of the influence of stored memories of regularities of previous input on current perception. Details against a meaningless background then characterize perception. Continuity in perception over space and time is disrupted, and also, the sense of self is afflicted. The model predicts that schizophrenic patients will perform differently on specific cognitive tasks, and this has been found for,eg, prepulse inhibition.43 Deficits in prepulse inhibition result in sensory flooding and can be countered by antipsychotic medication.44 Hemsley and coworkers3 never speculated about the brain areas involved. Yet, the model resembles the disruption of the perceptual process that is described by Kapur in biological concepts and is the result of a hyperdopaminergic state in the mesolimbic pathways.
Monitoring of Willed Intentions
Another important model has been proposed by Frith.45 This model can explain delusions of control and AVHs. A central monitor compares actions to the expected results of that action. In psychotic patients, intentions of will lead to actions but these willed intentions are not monitored correctly. This apparent discrepancy between will and action gives rise to delusions of control and passivity. During delusions of control, there is overactivation of the parietal cortex because the movements are discrepant from expectations. This might be due to lesions in corticocortical connections.46
Theory of Mind
Patients with schizophrenia also have trouble to mentalize the knowledge of another person.47 The mentalizing deficit can lead to many misunderstandings in social functioning. The deficits of theory of mind is documented by evidence in schizophrenia, although the extent of the deficits is relatively small and not always present in schizophrenic patients.48–50 The brain regions involved are the amygdala and the medial and inferior prefrontal cortex (representation of the self),49 lateral inferior frontal cortex (representation of actions and goals of self and others),51 and superior temporal sulcus (representation of the behavior of others).51
Perceptual Aberrations
Maher was the first to point to perceptual aberrations in the development of psychosis.52 The patient tries to make sense of these aberrations by reasoning, and in this way, delusions would develop as explanations of the perceptual aberrations. Recently, the importance of anomalous internal experiences in the development of delusions was found once more.53 About 80% of the patients had delusions based on anomalous experiences. Only 16% of these patients could think of an alternative explanation, while 30% of the patients with delusions based on external events could do so. Although normal reasoning processes play a role in the development of the delusional explanation, there are also reasoning biases. It is not a 1-stage process as Maher suggested but a 2-stage process: perceptual aberrations and reasoning biases lead to delusions.50
Cognitive Biases
Data-Gathering Bias
Jumping to conclusions characterizes delusional subjects. This data-gathering bias was found during a probabilistic reasoning task.54–56 Delusional subjects tend to jump to a conclusion and then feel quite confident about the conclusion. The same results have been found in normal people that score high on delusional ideation.57,58 When the emotional arousal is increased, the jumping to conclusions bias is further exaggerated in schizophrenic patients.59
Attentional Bias
Selective attention and confirmatory bias characterize paranoid patients who have a selective attention for potentially threatening events. They continuously scan the world for potential dangers and of course will find many stimuli that signal potential threat.60 This constant preoccupation with threat and threat-related memories reinforces the paranoid convictions about the world and conspiracy.51 Attention to threatening, stimuli activates a network between the lateral inferior frontal cortex (“deep” levels of semantic meaning), ventral striatum (egocentric memory), and ACC (motivational content of stimuli).51
Although potential threats are quickly spotted, the paranoid patient spends less time on a secondary more conscious appraisal.61 Jumping to conclusions relates to a confirmatory bias. The patient is just collecting confirmatory evidence for the danger but does not challenge the evidence because this is considered as too dangerous. The policy is, Better safe than sorry. Activation of the amygdala biases attention to the perception of threat. This is a fast preconscious process involved in the evaluation of social meaning and the trustworthiness of another person based on facial mimic. Overactivation of this area will result in the perception of threat where none actually exists based on the misinterpretation of the body state. The dopamine excess increases the signal-to-noise ratio, leading to heightened salience of threatening stimuli and reduces the generation of alternative hypotheses taking other environmental cues into account.62
Meta-Cognitive Bias
Recent research has demonstrated the importance of meta-cognition in AVHs.63–65 Patients have an externalizing attributional style and strong beliefs in the uncontrollability and danger of thoughts and positive beliefs on worrying. This bias is also found in hallucination-prone normal subjects.66
Source-Monitoring Bias
Patients with AVHs have a source-monitoring bias. Hallucinating patients make more mistakes in identifying their own written thoughts.67 A comparison between hallucinating and delusional patients was made 1 week after a learning session. They were confronted with answers to questions that had been posed the week before. The patients had to decide whether the answer was an answer of their own, of the experimenter, or a new answer. Hallucinating patients tended to attribute their own answers to the experimenter.68 A replication of the study confirmed the attribution bias and also showed that hallucinating patients have more confidence that their mistakes are actually correct answers.69 The bias is stronger for emotional words63 and when the attention is directed to the self.70 Recent research compared several conditions: (1) saying words aloud vs imagining speaking words, (2) listening to experimenter vs imagining experimenter speaking, (3) imagining self speech vs imagining other speech, and (4) listening to male voices vs listening to female voices. The study reconfirmed the source-monitoring problem, but the bias is not specific for internal and external sources. Also the gender of the voice (both external) or imaging self or other (both internal) showed mistakes.71 There is also a bias in reading. Afterward, hallucinating patients tend to remember silently read words as words that were read aloud.72 People that score high on hallucination proneness show the same source-monitoring bias as hallucinating patients.73
These early models and cognitive biases have been integrated in a series of more multifaceted cognitive models by Freeman, Garety, Beck, and others.54,74–80 These models are now the leading models that guide therapists in CBT with psychosis. We will continue with the effects of both pharmacotherapy and CBT on symptoms.
Effects of Pharmacotherapy and Cognitive Therapy
Pharmacotherapy
Antipsychotic medications reduce the dopamine levels. They do not eradicate symptoms but create a state of “detachment” from them. This means that the patient is still deluded but less preoccupied with the delusions and less motivated to act on his delusions. These findings were already described in the first years of antipsychotic pharmacotherapy. Chlorpromazine results in an apparent indifference and affective neutrality, a decrease in initiative and preoccupation without alteration in conscious awareness or intellectual faculties.81 The dopamine antagonists dampen aberrant as well as normal motivational salience. The effect of abolishing salience is very rapid, but the recovery from delusions is slow because psychological reappraisal is needed.4 Antipsychotic medication does not affect the narrative delusional explanations of the psychotic experiences in the past of the patient. A paranoid patient was obsessed by noises in the heating radiator (“They move microphones through the pipes to spy on me”), salient objects in his house (“They must have moved it when they were in my house”), and noise in the television signal (“They have now switched to camera mode and look at me”). After taking antipsychotic medication, he reported after a few weeks: “I have no longer heard noises, objects were not moved, and I was not watched by the television. I guess they have a short holiday.” The salient experiences were now gone, but the secondary delusional explanation of conspiracy was still present.
Cognitive Remediation
Only one study describes the effects of cognitive remediation training on the brain. Functional magnetic resonance imaging (fMRI) was done during the 2-back test that measures working memory.82 The authors found decreased activation in control group and increased activation in DLPFC in the trained group. This reflects an improved working memory.
CBT and Pharmacotherapy
Similar Effects on the Brain
There are no research publications in psychosis, but we can learn from research in anxiety disorders. CBT and drug treatment resulted in similar changes in brain functioning in anxiety patients. CBT and imipramine both showed reductions in overactivation of rostral caudate nucleus in obsessive-compulsive disorder patients.83 CBT and citalopram both normalized frontal metabolism in social phobia.84 Also in spider phobia, CBT normalized the frontal metabolism.85 These findings suggest that a psychotherapeutic approach, such as CBT, has the potential to modify the dysfunctional neural circuitry associated with anxiety disorders. They further indicate that the changes made at the mind level, within a psychotherapeutic context, are able to functionally “rewire” the brain.
Differential Effects
In depression, CBT affects MPFC, ACC, and hippocampus, while pharmacotherapy affects limbic subcortical areas.86 Treatment response was associated with significant metabolic changes in both treatments. CBT starts at cognition and meaning and works down to inhibit emotional circuits. Pharmacotherapy directly influences neurotransmission that leads to a secondary indifference toward the original delusions. The direction of CBT is top-down, while pharmacotherapy works bottom-up.
In a review, the authors conclude that this might also be true for panic disorder. CBT affects PFC and hippocampus (top-down), while pharmacotherapy affects amygdala, hypothalamus, and brain stem (bottom-up).87 Medications, particularly those that influence the serotonin system, are hypothesized to desensitize the fear network from the level of the amygdala through its projects to the hypothalamus and the brain stem. Effective psychosocial treatments may also reduce contextual fear and cognitive misattributions at the level of the PFC and hippocampus.
Pharmacotherapy and CBT are both able to rewire and influence brain processes. However, they both have different points of action in the brain. Pharmacotherapy operates from the deeper and more central parts of the brain that are involved in emotional processes, while CBT operates at the level of the cortex and processing of meaning. Pharmacotherapy has a bottom-up direction, while CBT has a top-down direction. Pharmacotherapy restores neurotransmitter imbalance and indirectly affects the strong emotions that accompany psychotic symptoms. CBT helps to reinterpret symptoms in a less harmful way and indirectly affects the strong emotions that accompany psychotic symptoms.
CBT Changes Appraisal
CBT does not target neurotransmission. CBT in psychosis acts directly on psychological processes. In particular, it is most effective when the key appraisal, of inner mental disturbance as externally caused, is reappraised as inner. Both symptoms and the risk of relapse are thereby reduced.74 Appraisal of stimuli is done in 2 circuits.88 The first one is extremely fast and runs from the eyes to emotional appraisal and behavioral preparation to fight or flight bypassing the visual cortex.89 The secondary appraisal is slow and more conscious and reappraises the situation. When the appraisals differ, the slow prefrontal process is capable of inhibiting the fast subcortical process.90–92
CBT helps to reappraise the anxiety-provoking stimuli and can inhibit the visceral appraisal of anxiety. CBT uses a top-down strategy in symptom control by targeting the secondary delusions in AVHs. These secondary delusions are about the identity and power of the voices. When the patient can understand and acknowledge that nobody else is involved in his voices and that he does not have to obey the voices because their threats cannot harm him, he can start to feel indifferent to his voices. The involvement with the voices can then stop. The patient does not fight nor does he pay attention to the voices anymore. The patient can then become involved in more meaningful and fulfilling daily activities and regain purpose in life again. In paranoia, CBT targets the cognitive biases such as selective attention and confirmatory bias and the style of jumping to conclusions and tries to generate alternative explanations.
While pharmacotherapy reduces symptoms into remission, CBT fosters psychic recovery. This is an individual process in which an individual patient overcomes the personal disaster of his psychosis.93 Although symptoms may stay, the patient finds a personal adaptation to the symptoms and learns to compensate for restrictions imposed by the disease, and at the same time, the patient builds hope for a future. When recovery progresses, the patient can again reintegrate in social life, find new meanings and satisfying roles in society, even when symptoms are still bothering every now and then.
fMRI can be used to evaluate therapy effects in future. In paranoid patients, belief often overrules logical and rational thinking. This “Better safe than sorry” policy is characterized by activation of the ventral-medial PFC.94 In successful therapy, this activation will be replaced by activation of the right lateral PFC to inhibit responses associated with belief in order to reach the correct solution to a logical reasoning task.
A 4-Component Neuropsychiatric Model
This model contains the comprehensive cognitive model of psychosis as described before and is taken one step further by connecting the model to the biology of psychosis. We have shown that many brain regions are involved in psychosis and in anxiety in paranoid states. The basic biological dysregulation in psychosis is the hyperdopaminergic state in the mesolimbic pathways. This first component in the model alters the salience of percepts, weakens the influence of memories, and prompts the process of anomalous perceptions and hallucinations. This bottom-up biological process also has repercussions on a mind level. Experiences become extremely salient and also thoughts can be racing, intrusions occur more often, and thoughts are experienced with high salience and feelings of personal significance. The process that produces primary psychotic experiences is followed by an attempt to understand what is going on.
The second component is the top-down process of normal thinking, reasoning and testing, to explain the aberrant experiences in a meaningful way. A number of cognitive biases disturb reasoning.
The third component is a mediating component constructed of cognitive biases. Patients in a delusional mood tend to jump to conclusions without developing and testing alternative explanations. The deficits in theory of mind contribute to the social misunderstandings. In patients with hallucinations, the source-monitoring bias makes them attribute aberrant cognitive processes and intrusive thoughts to an external origin. Selective attention is important in the development of paranoia but also in its consolidation. Once psychosis has started and secondary delusions have been developed in an attempt to understand the salient experiences and the aberrant perceptions, partly due to cognitive and reasoning biases, the consolidating processes set in.
The fourth component is formed by the processes that consolidate the delusions and secondary delusional beliefs on the origin and meaning of voices. First, there is the selective attention and confirmatory bias51,76 as a result of overactivity in the amygdala and hippocampus and underactivation in the PFC. As in anxiety disorders, the expectation of threat leads to an alarmed reaction and safety behaviors in many instances. The avoidance of possible threat prevents corrective experiences, where patients can learn that the alarm was false. The overinvolvement in voices by indulging or fighting voices further strengthens the thoughts and circuits involved in psychosis, and a separate world and identity will develop. Self-stigmatization and perceived stigmatization harms self-esteem.95 Patients do not talk much about their experiences and beliefs about their weakness. Sometimes, the conviction of implicit badness make them helpless and depressed.96 Another factor attributing to the entrapment in psychosis is the negative appraisal of cognitive functioning. Meta-cognitive beliefs about the danger and uncontrollability of ones' thought processes set up to eagerly wanting to control ones own mind, an impossible task that will lead to more thoughts that are intrusive. A lack of cognitive confidence and superstitious beliefs lead to the expectations of punishment and responsibility.64 In general, detrimental meta-cognition within the narratives of persons with schizophrenia are linked with symptoms, quality of life, neurocognition, and poorer awareness of illness.97
A New Look on Symptom Remission and Psychic Recovery
The 4-component model brings a new look on remission and recovery. Many patients reach remission of florid symptoms with the aid of antipsychotic medications. The salient experiences are dampened, and the world becomes more predictable and less harmful. The content of the cognitive secondary delusional explanations of past incidents are not affected by the antipsychotic agent itself and have to be corrected by reflecting on the nature of their experiences, conversations with others, and reconsidering alternative explanations. The dampening of salience facilitates reappraisal of symptoms and past events, but not all patients resolve their delusional explanation (“Nobody is following me anymore. I guess that all secret agents have been called back to Israel because of the intifada.”). A future dysregulation of dopamine will result in an easy relapse in these cases because the delusional schemes and explanations need little reinforcement to be reinstalled again. In these patients, CBT could change the delusional explanations and prevent a future relapse in the identical delusional psychosis.
In other patients who continue to hear voices in spite of adequate antipsychotic therapy, psychic recovery is still possible in some cases. For instance, when a patient can change the appraisals of voices from powerful and dangerous external intelligences to annoying but innocent psychic experiences of internal origin that have no power over him, then that patient can control emotional arousal and spend time and attention to other more useful daily activities. As in the movie “A beautiful mind,” the patient is no longer terrorized by his hallucinations and can involve in more social and vocational activities. In these patients, one could speak of psychic recovery without symptom remission.
Full remission and recovery by the combination of pharmacotherapy and CBT has not been subject to research now but could potentially affect the chances of relapse in a favorable way. A prospective trial can investigate the level of symptom remission and psychological recovery in patients and their rates of relapse. Imaging techniques can be applied to trials in which pharmacotherapy is compared with pharmacotherapy and CBT to gain greater understanding of the mutual reinforcement of both therapies.
Strength, Limitation, and Testability of the Model
An implicit weakness of the model is that it tries to incorporate 2 different paradigms into one model. Cognitive processes are described in very different concepts and levels of abstraction than are neurotransmitter dynamics and brain areas. Although this endeavor might be impossible, there is a growing need in the psychiatric field to understand how these 2 approaches can reinforce each other in the pursuit of recovering from psychosis.
Another drawback is that schizophrenia and psychosis are such complex conditions. The cognitive models are beginning to emerge, but the neurobiological models still compete for more evidence. For this reason, the proposed neuropsychiatric model is not a model for schizophrenia but can only partly represent delusions and AVHs.
The model is largely overlapping with the psychological models that have been published in recent years but emphasizes the neurobiological component more pronounced. This is the strength of the current model as it can have a clinical and heuristic value in psychiatric practice. Patients accept to a certain degree that some experiences just happen to them because of biological causes but also consider their personal reactions to events as an important factor. The clinician is helped by the model to distinguish aspects of psychosis that are open to CBT and those that are not. The same is true for the effects of antipsychotic medications that target emotions and aberrant perceptions but do not correct the narrative explanations that patients have constructed cognitively over a period of time to understand what is happening to them. Sensitivity to both aspects of psychosis can help the patient and therapist to overcome psychosis more fully.
The evidence for the 4 components is quite extensive. The dysregulation of dopamine has been central to psychosis for decades. The psychological aspect of salient perceptions of personal importance with high levels of dopamine in the mesolimbic tract is connecting biology with psychology. There is quite some evidence that the process of explaining what is going on is influenced by reasoning biases found in psychotic patients. The consolidation process is equal to the consolidation in many other disorders. Avoidance behavior plays such a role in paranoia and in anxiety disorders. The avoidance behavior prevents corrective experiences and preserves dysfunctional appraisals. The proposed model connects all these processes and also presumes a partial independence of biological and psychological processes. This is still controversial and needs more research. It is clear that antipsychotic medications take away the salience of perceptions and reduce the drive to act on delusions and hallucinations. It is unclear to what extend cognitive biases are corrected by antipsychotic medications. The effects of antipsychotic medications on cognition are positive, but the effect sizes are small.98 The cognitive processes in that meta-analysis were attention, memory, and executive functions but not the specific biases reported to be involved in psychosis. The data-gathering bias is most profound in delusional subjects but does not stop with the resolution of psychosis and is also found in some normal subjects.58 The monitoring bias in hallucinating patients is improved with antipsychotic medications,99 but the monitoring bias is also present in psychosis-prone healthy subjects.100 The cognitive biases seem exaggerated by psychosis but also persist independent of psychosis. Although CBT can be effective in patients with antipsychotic medications who still experience persistent AVHs, the question remains whether CBT can be effective even without antipsychotic medications. In all, 2 lines of research can answer this question: experimental studies and clinical studies. The experimental studies should further investigate the extend in which cognitive biases are the result of dopamine dysregulation and to what extend they operate independently. The clinical studies have compared CBT as an add-on therapy to antipsychotic medication. A clinical design would be a 2 × 2 design comparing antipsychotic medications or placebo with CBT or standard care. While randomization over placebo might raise ethical questions, researchers could start with a trial comparing CBT to standard treatment in patients that refuse to take antipsychotic medications at all. The trials should not only look into therapeutics success but also to the impact on cognitive biases.
References
- 1.Liddle, PF, Barnes, TR, Morris, D, Haque, S. Three syndromes in chronic schizophrenia. Br J Psychiatry Suppl 1989. ;7119–122. [PubMed] [Google Scholar]
- 2.Broome, MR, Woolley, JB, Tabraham, P, et al. What causes the onset of psychosis? Schizophr Res 2005. ;7923–34. [DOI] [PubMed] [Google Scholar]
- 3.Kapur, S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003. ;16013–23. [DOI] [PubMed] [Google Scholar]
- 4.Kapur, S, Mizrahi, R, Li, M. From dopamine to salience to psychosis-linking biology, pharmacology and phenomenology of psychosis. Schizophr Res 2005. ;7959–68. [DOI] [PubMed] [Google Scholar]
- 5.Hohwy, J and Rosenberg, R. Cognitive neuropsychiatry: conceptual, methodological and philosophical perspectives. World J Biol Psychiatry 2005. ;6192–197. [DOI] [PubMed] [Google Scholar]
- 6.Tarrier, N. Cognitive behaviour therapy for schizophrenia—a review of development, evidence and implementation. Psychother Psychosom 2005. ;74136–144. [DOI] [PubMed] [Google Scholar]
- 7.Pilling, S, Bebbington, P, Kuipers, E, et al. Psychological treatments in schizophrenia: I. Meta-analysis of family intervention and cognitive behaviour therapy. Psychol Med 2002. ;32763–782. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Clinical Excellence. Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care 2002. London: National Collaborating Centre for Mental Health Retrieved August 9, 2006, from http://www.nice.org.uk/page.aspx?o=CG001.
- 9.Jones, C, Cormac, I, Silveira da Mota Neto, JI, Campbell, C. Cognitive behaviour therapy for schizophrenia. Cochrane Database Syst Rev 2004. ;4CD000524. [DOI] [PubMed] [Google Scholar]
- 10.Kasper, S, Tauscher, J, Kufferle, B, Barnas, C, Pezawas, L, Quiner, S. Dopamine- and serotonin-receptors in schizophrenia: results of imaging- studies and implications for pharmacotherapy in schizophrenia. Eur Arch Psychiatry Clin Neurosci 1999. ;249suppl 4, 83–89. [DOI] [PubMed] [Google Scholar]
- 11.David, AS. Auditory hallucinations: phenomenology, neuropsychology and neuroimaging update. Acta Psychiatr Scand Suppl 1999. ;39595–104. [DOI] [PubMed] [Google Scholar]
- 12.Kircher, TT and Thienel, R. Functional brain imaging of symptoms and cognition in schizophrenia. Prog Brain Res 2005. ;150299–308. [DOI] [PubMed] [Google Scholar]
- 13.Stahl, SM. Symptoms and circuits, part 3: schizophrenia. J Clin Psychiatry 2004. ;658–9. [DOI] [PubMed] [Google Scholar]
- 14.Gruzelier, JH. Hemispheric imbalances in schizophrenia. Int J Psychophysiol 1984. ;1227–240. [DOI] [PubMed] [Google Scholar]
- 15.Crow, TJ. Temporal lobe asymmetries as the key to the etiology of schizophrenia. Schizophr Bull 1990. ;16433–443. [DOI] [PubMed] [Google Scholar]
- 16.Friston, KJ and Frith, CD. Schizophrenia: a disconnection syndrome? Clin Neurosci 1995. ;389–97. [PubMed] [Google Scholar]
- 17.Grossberg, S. The imbalanced brain: from normal behavior to schizophrenia. Biol Psychiatry 2000. ;4881–98. [DOI] [PubMed] [Google Scholar]
- 18.Andreasen, NC, Paradiso, S, O'Leary, DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998. ;24203–218. [DOI] [PubMed] [Google Scholar]
- 19.Williamson, P. Mind, Brain, and Schizophrenia 2006. Oxford, NY: University Press.
- 20.Braver, TS, Barch, DM, Cohen, JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biol Psychiatry 1999. ;46312–328. [DOI] [PubMed] [Google Scholar]
- 21.Shaner, A. Delusions, superstitious conditioning and chaotic dopamine neurodynamics. Med Hypotheses 1999. ;52119–123. [DOI] [PubMed] [Google Scholar]
- 22.Blackwood, NJ, Bentall, RP, Ffytche, DH, Simmons, A, Murray, RM, Howard, RJ. Persecutory delusions and the determination of self-relevance: an fMRI investigation. Psychol Med 2004. ;34591–596. [DOI] [PubMed] [Google Scholar]
- 23.Williams, LM, Das, P, Harris, AW, et al. Dysregulation of arousal and amygdala-prefrontal systems in paranoid schizophrenia. Am J Psychiatry 2004. ;161480–489. [DOI] [PubMed] [Google Scholar]
- 24.Goto, Y and Grace, AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron 2005. ;47255–266. [DOI] [PubMed] [Google Scholar]
- 25.Goto, Y and Grace, AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 2005. ;8805–812. [DOI] [PubMed] [Google Scholar]
- 26.Weiss, AP and Heckers, S. Neuroimaging of hallucinations: a review of the literature. Psychiatry Res 1999. ;9261–74. [DOI] [PubMed] [Google Scholar]
- 27.Shergill, SS, Cameron, LA, Brammer, MJ, Williams, SC, Murray, RM, McGuire, PK. Modality specific neural correlates of auditory and somatic hallucinations. J Neurol Neurosurg Psychiatry 2001. ;71688–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Allen, PP, Amaro, E, Fu, CH, Williams, SC, Brammer, M, Johns, LC, McGuire, PK. Neural correlates of the misattribution of self-generated speech. Hum Brain Mapp 2005. ;2644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubl, D, Koenig, T, Strik, W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry 2004. ;61658–668. [DOI] [PubMed] [Google Scholar]
- 30.Shergill, SS, Brammer, MJ, Amaro, E, Williams, SC, Murray, RM, McGuire, PK. Temporal course of auditory hallucinations. Br J Psychiatry 2004. ;185516–517. [DOI] [PubMed] [Google Scholar]
- 31.Hunter, MD, Griffiths, TD, Farrow, TF, et al. A neural basis for the perception of voices in external auditory space. Brain 2003. ;126pt 1161–169. [DOI] [PubMed] [Google Scholar]
- 32.Green, MF and Kinsbourne, M. Subvocal activity and auditory hallucinations: clues for behavioral treatments? Schizophr Bull 1990. ;16617–625. [DOI] [PubMed] [Google Scholar]
- 33.Gould, LN. Verbal hallucinations and activity of vocal musculature. Am J Psychiatry 1948. ;105169–177. [DOI] [PubMed] [Google Scholar]
- 34.Margo, A, Hemsley, DR, Slade, PD. The effects of varying auditory input on schizophrenic hallucinations. Br J Psychiatry 1981. ;139122–127. [DOI] [PubMed] [Google Scholar]
- 35.Done, DJ, Frith, CD, Owens, DC. Reducing persistent auditory hallucinations by wearing an ear-plug. Br J Clin Psychol 1986. ;25pt 2151–152. [DOI] [PubMed] [Google Scholar]
- 36.van Os, J, Hanssen, M, Bijl, RV, Vollebergh, W. Prevalence of psychotic disorder and community level of psychotic symptoms: an urban-rural comparison. Arch Gen Psychiatry 2001. ;58663–668. [DOI] [PubMed] [Google Scholar]
- 37.Barrett, TR and Etheridge, JB. Verbal hallucinations in normals: I. People who hear “voices.”. Appl Cognit Psychol 1992. ;6379–387. [Google Scholar]
- 38.Ohayon, MM. Prevalence of hallucinations and their pathological associations in the general population. Psychiatry Res 2000. ;97153–164. [DOI] [PubMed] [Google Scholar]
- 39.Schulman, CA, Richlin, M, Weinstein, S. Hallucinations and disturbances of affect, cognition, and physical state as a function of sensory deprivation. Percept Mot Skills 1967. ;251001–1024. [DOI] [PubMed] [Google Scholar]
- 40.Hunter, MD, Eickhoff, SB, Miller, TW, Farrow, TF, Wilkinson, ID, Woodruff, PW. Neural activity in speech-sensitive auditory cortex during silence. Proc Natl Acad Sci U S A 2005. ;103189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hemsley, DR. An experimental psychological model for schizophrenia. In Hafner, H, Gattaz, WF, Janzarik, W (Eds.). Search for the Causes of Schizophrenia 1987. New York, NY: Springer Vol ;1 pp. 179–188. [Google Scholar]
- 42.Hemsley, DR. A simple (or simplistic?) cognitive model for schizophrenia. Behav Res Ther 1993. ;31633–645. [DOI] [PubMed] [Google Scholar]
- 43.Hemsley, DR. The development of a cognitive model of schizophrenia: placing it in context. Neurosci Biobehav Rev 2005. ;29977–988. [DOI] [PubMed] [Google Scholar]
- 44.Swerdlow, NR, Braff, DL, Taaid, N, Geyer, MA. Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 1994. ;51139–154. [DOI] [PubMed] [Google Scholar]
- 45.Frith, CD. The positive and negative symptoms of schizophrenia reflect impairments in the perception and initiation of action. Psychol Med 1987. ;17631–648. [DOI] [PubMed] [Google Scholar]
- 46.Frith, CD, Blakemore, S, Wolpert, DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev 2000. ;312–3357–363. [DOI] [PubMed] [Google Scholar]
- 47.Corcoran, R, Mercer, G, Frith, CD. Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res 1995. ;175–13. [DOI] [PubMed] [Google Scholar]
- 48.Frith, CD. Schizophrenia and theory of mind. Psychol Med 2004. ;34385–389. [DOI] [PubMed] [Google Scholar]
- 49.Brune, M. “Theory of mind” in schizophrenia: a review of the literature. Schizophr Bull 2005. ;3121–42. [DOI] [PubMed] [Google Scholar]
- 50.Gilleen, J and David, AS. The cognitive neuropsychiatry of delusions: from psychopathology to neuropsychology and back again. Psychol Med 2005. ;355–12. [DOI] [PubMed] [Google Scholar]
- 51.Blackwood, NJ, Howard, RJ, Bentall, RP, Murray, RM. Cognitive neuropsychiatric models of persecutory delusions. Am J Psychiatry 2001. ;158527–539. [DOI] [PubMed] [Google Scholar]
- 52.Maher, BA. Anomalous experience and delusional thinking: the logic of explanations. In Oltmanns, TF and Maher, BA (Eds.). Delusional Beliefs 1988. New York, NY: John Wiley pp. 15–33.
- 53.Freeman, D, Garety, P, Fowler, D, Kuipers, E, Bebbington, P, Dunn, G. Why do people with delusions fail to choose more realistic explanations for their experiences? An empirical investigation. J Consult Clin Psychol 2004. ;724671–680. [DOI] [PubMed] [Google Scholar]
- 54.Garety, PA, Hemsley, DR, Wessely, S. Reasoning in deluded schizophrenic and paranoid patients. Biases in performance on a probabilistic inference task. J Nerv Ment Dis 1991. ;179194–201. [DOI] [PubMed] [Google Scholar]
- 55.Garety, P. Reasoning and delusions. Br J Psychiatry Suppl November1991. suppl 14, 14–18. [PubMed]
- 56.Huq, SF, Garety, PA, Hemsley, DR. Probabilistic judgements in deluded and non-deluded subjects. Q J Exp Psychol A 1988. ;40801–812. [DOI] [PubMed] [Google Scholar]
- 57.Colbert, SM and Peters, ER. Need for closure and jumping-to-conclusions in delusion-prone individuals. J Nerv Ment Dis 2002. ;19027–31. [DOI] [PubMed] [Google Scholar]
- 58.Linney, YM, Peters, ER, Ayton, P. Reasoning biases in delusion-prone individuals. Br J Clin Psychol 1998. ;37pt 3285–302. [DOI] [PubMed] [Google Scholar]
- 59.Mujica-Parodi, LR, Corcoran, C, Greenberg, T, Sackeim, HA, Malaspina, D. Are cognitive symptoms of schizophrenia mediated by abnormalities in emotional arousal? CNS Spectr 2002. ;7158–69. [DOI] [PubMed] [Google Scholar]
- 60.Fear, C, Sharp, H, Healy, D. Cognitive processes in delusional disorders. Br J Psychiatry 1996. ;16861–67. [DOI] [PubMed] [Google Scholar]
- 61.Phillips, ML, Senior, C, David, AS. Perception of threat in schizophrenics with persecutory delusions: an investigation using visual scan paths. Psychol Med 2000. ;30157–167. [DOI] [PubMed] [Google Scholar]
- 62.Spitzer, M. A neurocomputational approach to delusions. Compr Psychiatry 1995. ;3683–105. [DOI] [PubMed] [Google Scholar]
- 63.Baker, CA and Morrison, AP. Cognitive processes in auditory hallucinations: attributional biases and metacognition. Psychol Med 1998. ;281199–1208. [DOI] [PubMed] [Google Scholar]
- 64.Morrison, AP and Wells, A. A comparison of metacognitions in patients with hallucinations, delusions, panic disorder, and non-patient controls. Behav Res Ther 2003. ;41251–256. [DOI] [PubMed] [Google Scholar]
- 65.Garcia-Montes, JM, Perez-Alvarez, M, Soto Balbuena, C, Perona Garcelan, S, Cangas, AJ. Metacognitions in patients with hallucinations and obsessive-compulsive disorder: The superstition factor. Behav Res Ther 2005. ;441091–1104. [DOI] [PubMed] [Google Scholar]
- 66.Laroi, F and Van der Linden, M. Metacognitions in proneness towards hallucinations and delusions. Behav Res Ther 2005. ;431425–1441. [DOI] [PubMed] [Google Scholar]
- 67.Heilbrun, AB Jr. Impaired recognition of self-expressed thought in patients with auditory hallucinations. J Abnorm Psychol 1980. ;89728–736. [DOI] [PubMed] [Google Scholar]
- 68.Bentall, RP, Kaney, S, Dewey, ME. Paranoia and social reasoning: an attribution theory analysis. Br J Clin Psychol 1991. ;30pt 113–23. [DOI] [PubMed] [Google Scholar]
- 69.Moritz, S, Woodward, TS, Ruff, CC. Source monitoring and memory confidence in schizophrenia. Psychol Med 2003. ;33131–139. [DOI] [PubMed] [Google Scholar]
- 70.Ensum, I and Morrison, AP. The effects of focus of attention on attributional bias in patients experiencing auditory hallucinations. Behav Res Ther 2003. ;41895–907. [DOI] [PubMed] [Google Scholar]
- 71.Keefe, RS, Arnold, MC, Bayen, UJ, McEvoy, JP, Wilson, WH. Source-monitoring deficits for self-generated stimuli in schizophrenia: multinomial modeling of data from three sources. Schizophr Res 2002. ;5751–67. [DOI] [PubMed] [Google Scholar]
- 72.Franck, N, Rouby, P, Daprati, E, Dalery, J, Marie-Cardine, M, Georgieff, N. Confusion between silent and overt reading in schizophrenia. Schizophr Res 2000. ;41357–364. [DOI] [PubMed] [Google Scholar]
- 73.Laroi, F, Van der Linden, M, Marczewski, P. The effects of emotional salience, cognitive effort and meta-cognitive beliefs on a reality monitoring task in hallucination-prone subjects. Br J Clin Psychol 2004. ;43pt 3221–233. [DOI] [PubMed] [Google Scholar]
- 74.Garety, P, Kuipers, E, Fowler, D, Freeman, D, Bebbington, P. A cognitive model of the positive symptoms of psychosis. Psychol Med 2001. ;31189–195. [DOI] [PubMed] [Google Scholar]
- 75.Freeman, D, Garety, PA, Kuipers, E, Fowler, D, Bebbington, PE. A cognitive model of persecutory delusions. Br J Clin Psychol 2002. ;41pt 4331–347. [DOI] [PubMed] [Google Scholar]
- 76.Beck, AT and Rector, NA. A cognitive model of hallucinations. Cogn Ther Res 2003. ;2719–52. [Google Scholar]
- 77.Beck, AT and Rector, NA. Delusions: a cognitive perspective. J Cogn Psychother 2002. ;16455–468. [Google Scholar]
- 78.Freeman, D and Garety, PA. Connecting neurosis and psychosis: the direct influence of emotion on delusions and hallucinations. Behav Res Ther 2003. ;4192–947. [DOI] [PubMed] [Google Scholar]
- 79.Freeman, D, Garety, PA, Kuipers, E. Persecutory delusions: developing the understanding of belief maintenance and emotional distress. Psychol Med 2001. ;311293–1306. [DOI] [PubMed] [Google Scholar]
- 80.Garety, PA, Freeman, D, Jolley, S, et al. Reasoning, emotions, and delusional conviction in psychosis. J Abnorm Psychol 2005. ;114373–384. [DOI] [PubMed] [Google Scholar]
- 81.Swazey, J. Chlorpromazine in Psychiatry 1974. Cambridge, MA: Massachusetts Institute of Technology Press.
- 82.Wykes, T, Brammer, M, Mellers, J, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy: functional magnetic resonance imaging in schizophrenia. Br J Psychiatry 2002. ;181144–152. [DOI] [PubMed] [Google Scholar]
- 83.Baxter, LR Jr, Schwartz, JM, Bergman, KS, et al. Caudate glucose metabolic rate changes with both drug and behavior therapy for obsessive-compulsive disorder. Arch Gen Psychiatry 1992. ;49681–689. [DOI] [PubMed] [Google Scholar]
- 84.Furmark, T, Tillfors, M, Marteinsdottir, I, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 2002. ;59425–433. [DOI] [PubMed] [Google Scholar]
- 85.Paquette, V, Levesque, J, Mensour, B, et al. “Change the mind and you change the brain”: effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage 2003. ;18401–409. [DOI] [PubMed] [Google Scholar]
- 86.Goldapple, K, Segal, Z, Garson, C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004. ;6134–41. [DOI] [PubMed] [Google Scholar]
- 87.Gorman, JM, Kent, JM, Sullivan, GM, Coplan, JD. Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 2000. ;157493–505. [DOI] [PubMed] [Google Scholar]
- 88.Lang, PJ, Davis, M, Ohman, A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord 2000. ;61137–159. [DOI] [PubMed] [Google Scholar]
- 89.Liddell, BJ, Brown, KJ, Kemp, AH, et al. A direct brainstem-amygdala-cortical ‘alarm’ system for subliminal signals of fear. Neuroimage 2005. ;24235–243. [DOI] [PubMed] [Google Scholar]
- 90.Hariri, AR, Mattay, VS, Tessitore, A, Fera, F, Weinberger, DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry 2003. ;53494–501. [DOI] [PubMed] [Google Scholar]
- 91.Quirk, GJ and Gehlert, DR. Inhibition of the amygdala: key to pathological states? Ann N Y Acad Sci 2003. ;985263–272. [DOI] [PubMed] [Google Scholar]
- 92.Sotres-Bayon, F, Cain, CK, Ledoux, JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biol Psychiatry In press. [DOI] [PubMed]
- 93.Anthony, W. Recovery from mental illness: The guiding vision of the mental health services in the 1990s. Psychosoc Rehab J 1993. ;1611–23. [Google Scholar]
- 94.Goel, V and Dolan, RJ. Explaining modulation of reasoning by belief. Cognition 2003. ;871B11–22. [DOI] [PubMed] [Google Scholar]
- 95.Link, BG, Struening, EL, Neese-Todd, S, Asmussen, S, Phelan, JC. Stigma as a barrier to recovery: the consequences of stigma for the self-esteem of people with mental illnesses. Psychiatr Serv 2001. ;521621–1626. [DOI] [PubMed] [Google Scholar]
- 96.Peters, E and Garety, P. Cognitive functioning in delusions: a longitudinal analysis. Behav Res Ther 2006. ;44481–514. [DOI] [PubMed] [Google Scholar]
- 97.Lysaker, PH, Carcione, A, Dimaggio, G, et al. Metacognition amidst narratives of self and illness in schizophrenia: associations with neurocognition, symptoms, insight and quality of life. Acta Psychiatr Scand 2005. ;11264–71. [DOI] [PubMed] [Google Scholar]
- 98.Keefe, RS, Silva, SG, Perkins, DO, Lieberman, JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull 1999. ;25201–222. [DOI] [PubMed] [Google Scholar]
- 99.Keefe, RS, Poe, MP, McEvoy, JP, Vaughan, A. Source monitoring improvement in patients with schizophrenia receiving antipsychotic medications. Psychopharmacology (Berl) 2003. ;169383–389. [DOI] [PubMed] [Google Scholar]
- 100.Allen, P, Freeman, D, Johns, L, McGuire, P. Misattribution of self-generated speech in relation to hallucinatory proneness and delusional ideation in healthy volunteers. Schizophr Res 2006. ;84281–288. [DOI] [PubMed] [Google Scholar]