Abstract
Background and Purpose:
Unresectable cholangiocarcinoma is an intractable disease marked by recurrent bouts of biliary obstruction and infection. Traditional treatment methods provide only symptomatic relief and no proven survival advantage. We assessed the tolerability of helical tomotherapy intensity modulated radiotherapy (IMRT) with concurrent capecitabine and photodynamic therapy (PDT) in patients with unresectable hilar cholangiocarcinoma.
Methods:
Ten patients with unresectable hilar cholangiocarcinoma were treated with helical tomotherapy IMRT. An accelerated dose of 50 Gy in 20 fractions (2.5 Gy/fraction) was used. Planning target volume (PTV) consisted of a 1.5 cm radial expansion and a 2 cm craniocaudal expansion of the magnetic resonance imaging and/or contrast enhanced computed tomography-defined gross target volume. PTV ranged from 123 cc to 693 cc (mean 349 cc). Concurrent chronomodulated capecitabine was administered on days of irradiation. Six patients received PDT.
Results:
All patients developed side effects, including grade 2 nausea, and 9 of 10 experienced mild fatigue. Patients lost 3% of their body weight on average. Three patients required brief hospital admission and stent revision for cholangitis during chemoradiotherapy. Capecitabine was discontinued in one patient and decreased in dose for another due to increasing liver enzymes. Median overall survival was 13 months, and median disease-free survival was 10 to 11 months. One patient underwent successful cadaveric liver transplant after chemoradiotherapy and remains disease free 2 years later.
Conclusions:
Concurrent chemoradiotherapy with helical tomotherapy IMRT and capecitabine in conjunction with PDT is well tolerated in patients with hilar cholangiocarcinoma.
Approximately 4,000 new cases of cholangiocarcinoma are diagnosed each year in the United States, with nearly the same number of patients dying annually of the disease.1,2 The incidence of cholangiocarcinoma appears to be on the rise,3 and there remains a lack of level I evidence regarding treatment. Due to poor cure rates in patients who cannot undergo surgery, current treatment approaches consist largely of combined-modality therapy (radiation combined with chemotherapy) that is aimed mainly at providing palliation of symptoms.
Evidence indicating that cholangiocarcinoma is chemosensitive and radiosensitive has emerged from several recent studies. A review of 104 chemotherapy trials reported between 1985 and 2006 indicated a response rate of 23% and a tumor control rate of around 57% for unresected cholangiocarcinoma.4 Results with radiotherapy alone indicate that adjuvant radiotherapy improves survival,5–7 and several reports describe neoadjuvant chemoradiotherapy and liver transplantation with 5-year survival rates greater than 75% and significant tumor responses noted in resected specimens.8–10 Other reports have indicated the tolerability of concurrent chemoradiotherapy substituting capecitabine for other agents, though data on clinical outcomes are scarce.11
Here, we describe a multimodality approach to unresectable cholangiocarcinoma used at the University of Virginia, consisting of helical tomotherapy intensity modulated radiotherapy (IMRT) with concurrent capecitabine and photodynamic therapy (PDT). Details on the patients treated are included to substantiate the feasibility and tolerability of this treatment method. The treatment approach involves a coordinated effort from interventional endoscopists (for diagnosis, biliary system stenting, and PDT), diagnostic radiology (for determining extent of disease), surgical pathology (for establishing diagnosis), radiation oncology (for planning of radiotherapy), and hematology/oncology (for chemotherapy and surgery).
PATIENTS AND METHODS
This report includes data from 10 patients with unresectable cholangiocarcinoma undergoing multimodality treatment including helical tomotherapy IMRT and capecitabine chemotherapy. All patients provided institutional informed consent.
Initial workup generally included chest x-ray, lab studies (complete blood cell [CBC] count, comprehensive metabolic panel), abdominal computed tomography (CT) and/or magnetic resonance imaging (MRI). Diagnostic biopsy procedures usually involved endoscopic retrograde cholangiopancreatography (ERCP). Nine patients had biopsy proven cholangiocarcinomas. One patient was treated with a presumptive diagnosis of cholangiocarcinoma after six separate biopsy attempts over a period of 8 months and imaging compatible with malignancy. All cases were presented at the University of Virginia Multi-Disciplinary Gastro-Intestinal Tumor Board after initial biopsy and workup. All of the patients had unresectable hilar cholangiocarcinoma (Bismuth III or IV). In those with biliary obstruction, biliary stenting was performed to relieve symptoms.
Photodynamic Therapy
PDT was offered to all patients without contraindications to porfimer sodium, such as compromised kidney or hepatic function, leukopenia or thrombocytopenia, or evidence of cancer of another organ. PDT was performed before chemoradiotherapy in obstructed patients or after chemoradiotherapy if there was no sign of biliary obstruction. Porfimer sodium (Photofrin, Axcan Pharma Inc, Quebec, Canada) was used as a photosensitizing agent, administered intravenously at a dose of 2 mg/kg 48 hours prior to illumination. A diode laser system (InGaAIP Laser Diode, Diomed Inc, Andover, MA) with a maximum power output of 2000 mW and a wavelength of 633 ± 3 nm was used as a light source, delivered through a 3.0 m length fiber having a 2.5 cm long cylindrical diffuser at its distal end (Pioneer Optics, Windsor Locks, CT). During ERCP, photoactivation was performed at 620 nm using a light dose of 180 J/cm2, fluence of 0.250 W/cm2, and irradiation time of 750 seconds. One or two segments were treated at the discretion of the endoscopist. Following photoactivation, only plastic stents were inserted to decompress opacified radicals proximal to the treated lesion. PDT was repeated at 3-month intervals, at which time stents were replaced; stent exchanges were performed earlier in the case of premature occlusion or migration to maintain optimal decompression. All patients received periprocedure antibiotic prophylaxis.
Chemoradiotherapy
For radiotherapy planning, patients underwent CT simulation in the supine treatment position with a custom immobilization device. Diagnostic CT or MRI images were fused with the planning CT for target delineation. The gross tumor volume (GTV) was identified from these images after review with the interventional and diagnostic radiologists. The planning target volume (PTV) consisted of a 10 to 15 mm radial expansion on the GTV and a 10 to 20 mm craniocaudal expansion. These expansions were used to account for microscopic disease extension as well as patient setup error and intrafraction motion. Regional lymph nodes were not intentionally covered unless pathologically enlarged.
Inverse treatment planning was performed with the goal of minimizing the dose to the normal liver while also sparing the kidneys. All patients received a prescription of 50 Gy delivered in 20 fractions. This was prescribed to 95% of the PTV and delivered with helical tomotherapy IMRT. Treatments were delivered 5 days per week, Monday through Friday. Capecitabine was started on the first day of irradiation in a dosage of 1 g in the morning and 2 g in the evening, taken 1 hour after meals.
Patients were seen weekly to monitor their weight, address side effects, and monitor laboratory values including CBC count, chemistries, liver enzymes, bilirubin, and INR in those taking warfarin. After completing chemoradiotherapy, follow up was performed at 1 to 2 weeks post-treatment and at 1-month intervals thereafter. Repeat diagnostic images were obtained to evaluate response to treatment at regular follow-up visits, generally every 4 months.
Statistical Analysis
Survival analysis was performed using WINKS statistical software (TexaSoft, Cedar Hill, TX). Kaplan-Meier survival plots were obtained from the entered patient data, also providing mean and median survival statistics.
RESULTS
Ten patients received the chemoradiotherapy portion of the combined-modality regimen, with six receiving PDT. Patient, disease, and treatment characteristics, including duration of chemoradiation, PTV, and volume of liver receiving 30 Gy, are summarized in Table 1.
Table 1.
Patient, disease, and treatment characteristics
| Patient | Age | Bismuth type | Enlarged nodes | CRT duration (days) | PDT sessions | Liver volume (cc) | PTV (cc) | Liver V30 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 71 | IIIA | Yes | 28 | 2 | 1486 | 182 | 27 |
| 2 | 43 | IV | No | 31 | 1 | 2293 | 131 | 13 |
| 3 | 64 | III | No | 34 | 1 | 2069 | 261 | 13 |
| 4 | 53 | III | No | 33 | 0 | 2813 | 693 | 37 |
| 5 | 66 | IV | Yes | 27 | 0 | 1109 | 372 | 27 |
| 6 | 50 | IV | No | 38 | 1 | 3503 | 560 | 25 |
| 7 | 59 | IV | No | 32 | 0 | 2572 | 448 | 25 |
| 8 | 72 | IV | No | 34 | 1 | 2062 | 369 | 19 |
| 9 | 75 | IV | No | 29 | 1 | 2181 | 123 | 10 |
| 10 | 80 | III | No | 26 | 0 | 1090 | 346 | 45 |
Abbreviations: CRT = chemoradiotherapy; liver V30 = volume of normal liver receiving 30 Gy; PDT = photodynamic therapy; PTV = planning target volume.
PDT was delivered both prior to and following chemoradiotherapy, depending on the patient. PDT was well tolerated, with no adverse reactions being observed. Chemoradiotherapy was also well tolerated, as shown in Table 2. All patients were able to complete the irradiation course, and all patients experienced grade 2 nausea. Nausea was transient and well managed with antiemetics. One patient (patient 2) discontinued capecitabine and one (patient 5) had the capecitabine dose reduced due to asymptomatic increases in liver enzymes during chemoradiotherapy. Neither of these patients had evidence of stent occlusion.
Table 2.
Adverse events by Radiation Therapy Oncology Group grade and weight loss
| Patient | Nausea | Pain | Diarrhea | Fatigue | Weight loss (%) |
|---|---|---|---|---|---|
| 1 | 2 | 0.9 | |||
| 2 | 2 | 2 | 1 | 3.5 | |
| 3 | 2 | 1 | 4.4 | ||
| 4 | 2 | 2 | 2 | 9.1 | |
| 5 | 2 | 2 | 1 | 0 | |
| 6 | 2 | 1 | 5.4 | ||
| 7 | 2 | 2 | 1 | 0 | |
| 8 | 2 | 2 | 1 | 3.4 | |
| 9 | 2 | 1 | 8.6 | ||
| 10 | 2 | 1 | 0.7 |
The average loss of body weight was 5 pounds, or 3.2% of body mass. Two patients lost 15 pounds, representing 8% to 9% of their body mass. Three patients were admitted to the hospital during their course of radiotherapy for cholangitis, which was resolved with intravenous antibiotics and stent replacement. These three patients missed 0, 3, and 4 days of chemoradiotherapy, respectively. Four patients required narcotics for analgesia.
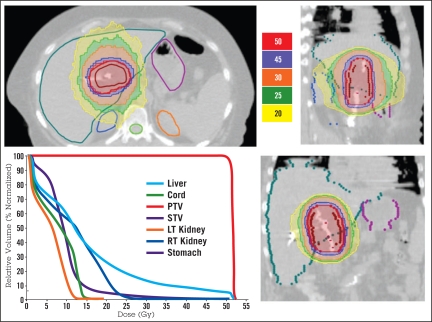
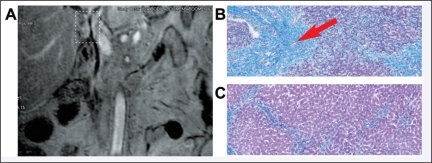
Analysis of the helical tomotherapy IMRT dosimetry revealed excellent homogenous coverage of the PTV with adequate sparing of normal tissues. The average PTV was 349 cc; the average liver volume was 2118 cc (Table 1); the average volume of normal liver receiving 30 Gy (V30) was 24.1% (range 10% to 45%). Figure 1 is an example of a patient’s (patient 2) dosimetry illustrating the excellent conformality of the dose delivered to the PTV, as well as the sparing of normal tissue. Images of the same patient’s tumor prior to treatment are shown in Figure 2, panel A. The patient underwent complete hepatectomy and cadaveric liver transplantation after chemoradiotherapy. Figure 2, panel B, depicts a histologic specimen showing the effect of chemoradiotherapy on this tumor; the area that received the entire prescription shows no evidence of viable tumor cells and replacement of the normal hepatic architecture with scar tissue. Figure 2, panel C, shows an area outside the target; only chronic scarring is evident, consistent with the patient’s history of nonalcoholic cirrhosis.
Figure 1.
An example of dosimetry for a patient in this study. This patient later underwent cadaveric liver transplantation.
Figure 2.
Panel A shows MRI images of a patient who received cadaveric liver transplantation after chemoradiotherapy. Tumor is highlighted by dotted white rectangle. Refer to Figure 1 for dosimetric coverage of this tumor. Panel B shows histology of the postresection tumor specimen, as evaluated by hematoxylin and eosin staining. Panel C shows histology of the nonirradiated liver from the same specimen.
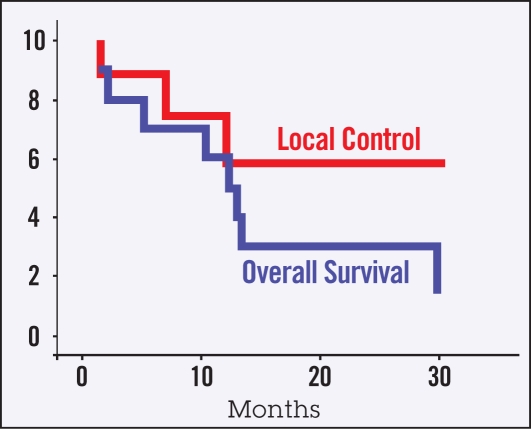
Of the 10 patients treated, three remain alive. One of the surviving patients has had regular follow-up for 2 years with no evidence of disease progression after receiving a cadaveric liver transplant. The remaining survivors suffered failure in the liver outside the primary tumor site. Of the 7 non-survivors, one patient died of hepatic abscess with no evidence of tumor recurrence. Follow-up CT scans demonstrated clear disease progression for the remaining patients. Of the patients who progressed, four experienced local progression of their primary disease, three experienced failure elsewhere in the liver and one had a distant failure. No patients were noted to have regional nodal failure. Five patients experienced recurrent cholangitis after chemoradiotherapy; these were adequately addressed with stent changes and intravenous antibiotics. Survival and local control is assessed by Kaplan-Meier analysis in Figure 3. The median overall survival time was 13 months (range 3.4 to 24.2 months). The median disease-free survival time was between 10 and 11 months (range 1.4 to 22.2 months).
Figure 3.
Kaplan-Meier curves for overall survival and local disease control.
DISCUSSION
Recent advances in the treatment of cholangiocarcinoma include use of PDT, ortho-topic liver transplantation, and conformal radiotherapy, such as IMRT. The most promising results have been achieved with combinations of these techniques, including achievement of 5-year survival of greater than 75% with use of neoadjuvant chemoradiotherapy prior to orthotopic liver transplantation at the Mayo Clinic.10 This approach8,10 has provided improved survival rates compared with rates reported with use of resection alone or resection followed by adjuvant therapy—eg, generally 5-year survival of 20% to 40%.10,12,13
The Mayo Clinic experience, including a report of no residual identifiable tumor in 16 of 38 liver specimens from patients receiving neoadjuvant chemoradiotherapy,10 suggests that cholangiocarcinoma is a chemoradiosensitive disease. The Mayo Clinic protocol requires collaboration of surgeons, medical oncologists, radiation oncologists, gastroenterologists, radiologists, and pathologists to achieve this success. The findings in our own cohort confirm that the multidisciplinary, multimodality treatment of unresectable cholangiocarcinoma is a tolerable treatment approach and may lead to significant improvements in patient outcome.
All 10 patients in our cohort tolerated the radiotherapy portions of their treatment, and the majority tolerated the complete course of capecitabine. In part, the tolerability reflects the close attention given this cohort by the gastroenterology team, with three patients undergoing stent revision for cholangitis during their chemoradiotherapy, with minimal breaks in treatment. The potential for this treatment approach to improve patient outcomes is highlighted by the course of patient 2 (Figures 1 and 2).
Patient 2, a 41-year-old white woman, was diagnosed with a Bismuth IV cholangiocarcinoma after presenting with obstructive jaundice. After her diagnosis in June 2005, she was noted to have peri-portal lymphadenopathy. She underwent a hilar lymphadenectomy in August 2005, revealing no evidence of tumor within these enlarged nodes. After a period of healing, she received a course of chemoradiotherapy in October 2005. Between June 2005 and February 2006, she suffered recurrent obstructive jaundice and underwent seven total stent placements/revisions, including one stent revision during her chemoradiotherapy that resulted in a 2-day break from treatment. Additionally, her capecitabine was discontinued for the last week of treatment due to increased transaminases. This elevation was likely from her obstruction, but capecitabine was held as a precaution. In December 2005, 6 weeks after completing her chemoradiotherapy, she received PDT treatment along with a stent revision. On her follow-up scans, she had substantial changes interpreted as postradiation changes or tumor progression. After a 3-month period with no stent revisions and relatively few symptoms, she underwent a cadaveric liver transplantation in May 2006.
As shown in Figure 2, histologic specimens from her liver showed no evidence of viable tumor after chemoradiotherapy. Her postoperative course was complicated with several hospital admissions for upper gastrointestinal bleeding due to a duodenal ulcer as well as pseudomembranous colitis and portal vein thrombosis. After healing of these, follow-up scans have shown no evidence of disease, and repeat endoscopy has shown healing of her damaged duodenum. She remains with no evidence of disease 2 years after her initial diagnosis. This patient was the only one in this series who met our institution’s very strict transplantation criteria, including no metastatic disease on laparoscopy before and after chemoradiotherapy and excellent clinical condition predicting the ability to tolerate the operation. The clinical course of this patient highlights the interplay among the treatment modalities and the key role of stents and PDT in providing successful short-term symptom management that increases the ability to provide definitive treatment.
The treatment approach in the Mayo Clinic reports included a brachytherapy boost to the biliary tree, an effort to temporize patient course and decrease the likelihood of cholangitis prior to surgery. In our cohort, we used PDT in a similar manner, allowing us to successfully bridge a patient for a period of 3 months after chemoradiotherapy and PDT. During this time, the decision to attempt a transplant was strengthened by the patient’s continued smooth clinical course and lack of disease progression.
One constant concern regarding patients with cholangiocarcinoma is recurrent cholangitis. This can prove rapidly fatal if not addressed. None of the patients in our cohort is known to have died of cholangitis, and those who developed recurrent cholangitis were effectively treated with antibiotics and stent revision with little difficulty. This is in agreement with other reports.14 PDT involves the intravenous administration of a photosensitizing agent followed by its activation using light illumination of a specific wavelength, resulting in ischemic necrosis15–17 proportional to tissue oxygenation.18,19 The technique has shown benefit in the treatment of cholangiocarcinoma in both preclinical and clinical studies.
PDT was demonstrated to reduce human cholangiocarcinoma xenograft tumor volume by 60% in a mouse model.20 In uncontrolled human studies in which porfimer sodium-based PDT was combined with stenting, there was improvement in cholestasis and survival, and few complications related to porfimer sodium were observed.21–24 A prospective, randomized, controlled trial confirmed a significant advantage attributable to PDT in relief of jaundice, quality of life, and survival.25
Other reports have indicated improvements in patient outcomes with multimodality treatment of cholangiocarcinoma using fluoropyrimidines as radiosensitizing chemotherapy.26,27 The responses noted in surgical specimens have prompted evaluation of dose escalation. One group reported dose-escalation studies for hepatobiliary cancer with either systemic 5-fluorouracil (5-FU) or 5-FU infused via the hepatic vein that included 81 patients with unresectable cholangiocarcinoma.28 These patients received radiation doses between 23 and 88.2 Gy, with no association between dose and survival being found. Another study analyzed outcome by radiotherapy dose and found no statistically significant difference in patient survival or freedom from local progression with dose escalation.29 These studies were limited by small patient numbers, and there is little additional data available on dose escalation. Thus, it seems that dose escalation with chemoradiotherapy may offer control rates and potentially survival rates on par with the Mayo Clinic reports of liver transplant following chemoradiotherapy.
The use of helical tomotherapy IMRT in the treatment of the biliary disease presents some technical challenges, considering the high degree of conformality as well as the sliced-based nature of delivery. The greatest concern is that organ motion could result in both decreased tumor coverage and increased normal tissue dose. Our experience with target delineation with MRI and magnetic resonance cholangiopancreatography including MRI fusion suggests that this process is relatively accurate, in accordance with prior work.30 PTV expansions on the target included accommodation for respiratory excursion, though we were unable to directly measure this and relied on previously established conventions.31,32
Several reports have concluded that respiratory motion results in differences between the planned dose and the delivered dose when employing IMRT, though these differences appear to be clinically insignificant in magnitude, particularly when averaged over a treatment course.33,34 This was found to be true in the treatment of liver tumors, as well.35 The potential for these differences was specifically addressed with the helical tomotherapy device using a motion phantom, and despite the sliced-based delivery, this approach was found to result in clinically insignificant errors in dose delivered for treatment regimens greater than three fractions.36 In addition, helical tomotherapy daily image guidance permits accurate patient alignment, decreasing errors in delivery. Therefore, the coverage of the PTV using helical tomotherapy IMRT appears to be acceptable, despite respiratory motion. In addition, all hot spots are within the PTV, indicating that there is very little chance of delivering significantly increased dose to nearby normal organs, given the above considerations.
The experience reported here, particularly of the patient who underwent ortho-topic transplant, confirms that PTV coverage is adequate despite relatively tight margins, since that patient was noted to have a complete pathologic response. Other forms of nontomotherapy-based IMRT may provide the ability to further reduce the PTV for patients with cholangiocarcinoma with respiratory-gating or breath-hold techniques.
In summary, the multimodality team approach to unresectable cholangiocarcinoma may represent optimal care. This treatment strategy is tolerable and provides flexibility to address symptoms as required on a patient-specific basis. It also allows for the reassessment of patients on a regular basis and thus facilitates easier adjustments in patient care as needed. Our findings add some evidence to the growing body of literature indicating that cholangiocarcinoma is a chemoradiosensitive disease.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors report no potential conflicts of interest.
REFERENCES
- 1.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1 Suppl):171–190. doi: 10.1002/1097-0142(19950101)75:1+<171::aid-cncr2820751306>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics. CA Cancer J Clin. 2003;2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Rajagopalan V, Daines WP, Grossbard ML, Kozuch P. Gallbladder and biliary tract carcinoma: a comprehensive update, Part 1. Oncology (Williston Park) 2004;18:889–896. [PubMed] [Google Scholar]
- 4.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerhards MF, van Gulik TM, Gonzalez Gonzalez D, Rauws EA, Gouma DJ. Results of postoperative radiotherapy for resectable hilar cholangiocarcinoma. World J Surg. 2003;27:173–179. doi: 10.1007/s00268-002-6434-1. [DOI] [PubMed] [Google Scholar]
- 6.Todoroki T. Chemotherapy for bile duct carcinoma in the light of adjuvant chemotherapy to surgery. Hepatogastroenterology. 2000;47:644–649. [PubMed] [Google Scholar]
- 7.Itoh H, Nishijima K, Kurosaka Y, et al. Magnitude of combination therapy of radical resection and external beam radiotherapy for patients with carcinomas of the extrahepatic bile duct and gallbladder. Dig Dis Sci. 2005;50:2231–2242. doi: 10.1007/s10620-005-3040-8. [DOI] [PubMed] [Google Scholar]
- 8.Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006;82:1703–1707. doi: 10.1097/01.tp.0000253551.43583.d1. [DOI] [PubMed] [Google Scholar]
- 9.Heimbach JK, Haddock MG, Alberts SR, et al. Transplantation for hilar cholangiocarcinoma. Liver Transpl. 2004;10(10 Suppl 2):S65–S68. doi: 10.1002/lt.20266. [DOI] [PubMed] [Google Scholar]
- 10.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. discussion 458–461, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das P, Wolff RA, Abbruzzese JL, et al. Concurrent capecitabine and upper abdominal radiation therapy is well tolerated. Radiat Oncol. 2006;1:41. doi: 10.1186/1748-717X-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rea DJ, Munoz-Juarez M, Farnell MB, et al. Major hepatic resection for hilar cholangiocarcinoma: analysis of 46 patients. Arch Surg. 139:514–523. doi: 10.1001/archsurg.139.5.514. discussion 523–515, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Kosuge T, Yamamoto J, Shimada K, Yamasaki S, Makuuchi M. Improved surgical results for hilar cholangiocarcinoma with procedures including major hepatic resection. Ann Surg. 1999;230:663–671. doi: 10.1097/00000658-199911000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahaleh M, Mishra R, Shami VM, et al. Unresectable cholangiocarcinoma: comparison of survival in biliary stenting alone versus stenting with photodynamic therapy. Clin Gastroenterol Hepatol. 2008;6:290–297. doi: 10.1016/j.cgh.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Hsi RA, Rosenthal DI, Glatstein E. Photodynamic therapy in the treatment of cancer: current state of the art. Drugs. 1999;57:725–734. doi: 10.2165/00003495-199957050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Webber J, Herman M, Kessel D, et al. Current concepts in gastrointestinal photodynamic therapy. Ann Surg. 1999;230:12–23. doi: 10.1097/00000658-199907000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oleinick NL, Evans HH. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:S146–S156. [PubMed] [Google Scholar]
- 18.Nelson JS, Liaw LH, Orenstein A, et al. Mechanism of tumor destruction following photodynamic therapy with hematoporphyrin derivative, chlorin, and phthalocyanine. J Natl Cancer Inst. 1988;80:1599–1605. doi: 10.1093/jnci/80.20.1599. [DOI] [PubMed] [Google Scholar]
- 19.Pass HI. Photodynamic therapy in oncology: mechanisms and clinical use. J Natl Cancer Inst. 1993;85:443–456. doi: 10.1093/jnci/85.6.443. [DOI] [PubMed] [Google Scholar]
- 20.Wong Kee Song LM, Wang KK, Zinsmeister AR. Mono-L-aspartyl chlorin e6 (NPe6) and hematoporphyrin derivative (HpD) in photodynamic therapy administered to a human cholangiocarcinoma model. Cancer. 1998;82:421–427. [PubMed] [Google Scholar]
- 21.McCaughan JS, Jr, Mertens BF, Cho C, et al. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts: a case report. Arch Surg. 1991;126:111–113. doi: 10.1001/archsurg.1991.01410250119022. [DOI] [PubMed] [Google Scholar]
- 22.Rumalla A, Baron TH, Wang KK, et al. Endoscopic application of photodynamic therapy for cholangiocarcinoma. Gastrointest Endosc. 2001;53:500–504. doi: 10.1067/mge.2001.113386. [DOI] [PubMed] [Google Scholar]
- 23.Ortner MA, Liebetruth J, Schreiber S, et al. Photodynamic therapy of nonresectable cholangiocarcinoma. Gastroenterology. 1998;114:536–542. doi: 10.1016/s0016-5085(98)70537-2. [DOI] [PubMed] [Google Scholar]
- 24.Berr F, Wiedmann M, Tannapfel A, et al. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology. 2000;31:291–298. doi: 10.1002/hep.510310205. [DOI] [PubMed] [Google Scholar]
- 25.Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology. 2003;125:1355–1363. doi: 10.1016/j.gastro.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004;24:201–207. doi: 10.1055/s-2004-828896. [DOI] [PubMed] [Google Scholar]
- 27.Rich TA, Evans DB, Curley SA, Ajani JA. Adjuvant radiotherapy and chemotherapy for biliary and pancreatic cancer. Ann Oncol. 1994;5(Suppl 3):75–80. doi: 10.1093/annonc/5.suppl_3.s75. [DOI] [PubMed] [Google Scholar]
- 28.Ben-David MA, Griffith KA, Abu-Isa E, et al. External-beam radiotherapy for localized extra-hepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2006;66:772–779. doi: 10.1016/j.ijrobp.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 29.Crane CH, Macdonald KO, Vauthey JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969–974. doi: 10.1016/s0360-3016(02)02845-6. [DOI] [PubMed] [Google Scholar]
- 30.Khoo VS, Joon DL. New developments in MRI for target volume delineation in radiotherapy. Br J Radiol. 2006;79(Spec1):S2–S15. doi: 10.1259/bjr/41321492. [DOI] [PubMed] [Google Scholar]
- 31.Herfarth KK, Debus J, Lohr F, et al. Extracranial stereotactic radiation therapy: set-up accuracy of patients treated for liver metastases. Int J Radiat Oncol Biol Phys. 2000;46:329–335. doi: 10.1016/s0360-3016(99)00413-7. [DOI] [PubMed] [Google Scholar]
- 32.Song DY, Kavanagh BD, Benedict SH, Schefter T. Stereotactic body radiation therapy. Rationale, techniques, applications, and optimization. Oncology (Williston Park) 18:1419–1430. discussion 1430,1432, 1435–1416, 2004. [PubMed] [Google Scholar]
- 33.Duan J, Shen S, Fiveash JB, Popple RA, Brezovich IA. Dosimetric and radiobiological impact of dose fractionation on respiratory motion induced IMRT delivery errors: a volumetric dose measurement study. Med Phys. 2006;2006;33:1380–1387. doi: 10.1118/1.2192908. [DOI] [PubMed] [Google Scholar]
- 34.Chui CS, Yorke E, Hong L. The effects of intra-fraction organ motion on the delivery of intensity-modulated field with a multileaf collimator. Med Phys. 2003;30:1736–1746. doi: 10.1118/1.1578771. [DOI] [PubMed] [Google Scholar]
- 35.Kuo HC, Chuang KS, Liu WS, Wu A, Lalonde R. Analysis of organ motion effects on the effective fluences for liver IMRT. Phys Med Biol. 2007;52:4227–4244. doi: 10.1088/0031-9155/52/14/014. [DOI] [PubMed] [Google Scholar]
- 36.Kanagaki B, Read PW, Molloy JA, Larner JM, Sheng K. A motion phantom study on helical tomotherapy: the dosimetric impacts of delivery technique and motion. Phys Med Biol. 2007;52:243–255. doi: 10.1088/0031-9155/52/1/016. [DOI] [PubMed] [Google Scholar]