Abstract
Preoperative induction therapy in stages II and III adenocarcinoma of the esophagogastric junction (AEG) and gastric cancer is now an accepted treatment choice in the Western world. Patients who respond to induction therapy have significantly improved survival compared to nonresponding patients. Until recently, however, no prospectively tested markers for predicting response and/or prognosis in this settingwere available. The MUNICON I study recently showed the utility of fluorodeoxyglucose-positron emission tomography (FDG-PET) in predicting response and prognosis in AEG and indicated the feasibility of a PET-guided treatment algorithm. These findings are an important step forward in tailoring multimodal treatment to tumor biology. In gastric cancer, the issue is more complicated, because approximately 30% of these cancers cannot be visualized with sufficient contrast for quantification. Insufficient FDG uptake is mostly associated with diffusetype gastric cancer with signet cells and mucinous content. In FDG avid patients, FDG-PET can be used for response evaluation. The prognosis of nonavid patients is similar to metabolic nonresponders. The addition of new tracers (eg, fluorothymidine) might increase the accuracy of these tests in the future. In AEG types I and II, PET-guided induction therapy is feasible and will undergo further evaluation in a randomized multicenter trial. In gastric cancer, there should be consideration of such treatment concepts as immediate resection after 2 weeks of induction therapy with or without adjuvant treatment in metabolic nonresponders or modified chemotherapy regimens possibly including biologically targeted drugs in FDG non-avid tumors.
After the publication of three randomized controlled trials showing benefit, neoadjuvant chemotherapy has become an accepted choice for the treatment of locally advanced adenocarcinomas of the esophagus and the esophagogastric junction (AEG) and gastric cancer.1–3 The use of neoadjuvant chemotherapy without the addition of radiotherapy is not generally accepted for AEG type I. In many institutions, concurrent or sequential radiotherapy is delivered, but a recent meta-analysis provides justification for both the neoadjuvant chemotherapy and chemoradiotherapy approach in the treatment of resectable adenocarcinomas of the esophagus.4 However, there is also some evidence that the addition of radiation therapy might increase the risk of postoperative morbidity and mortality compared to chemotherapy alone, which may be due to immunosuppression associated with radiation therapy.5,6 Due to these facts, neoadjuvant chemotherapy has been the treatment of choice for locally advanced esophageal adenocarcinomas in the authors’ and others’ institutions.
The potential benefits of giving chemotherapy before surgery are downsizing and downstaging of the primary tumor and lymph node metastases, early treatment of micrometastases, increased rates of curative resections, and improved tumor-related symptoms. A newer potential advantage is the possibility of testing in vivo the chemosensitivity of the primary tumor, which may influence choice of chemotherapy in the adjuvant setting.
The feasibility of neoadjuvant treatment in locally advanced gastric cancer has been shown in numerous phase II studies with different regimens.7–10 Compared to historical controls, prognosis of patients receiving neoadjuvant treatment seems to be improved and toxicity has been moderate in most studies.8,11 Treatment acceptance and compliance have been high and treatment has been well tolerated, with nearly all patients being able to receive the complete neoadjuvant dose.
In adenocarcinomas of the distal esophagus, an abdominothoracic approach and reconstruction with a small gastric tube interposition in the posterior mediastinum with intrathoracic anastomosis (Ivor-Lewis operation) including a two-field lymphadenectomy has become the procedure of choice.12 In Europe, an abdominal D2 lymphadenectomy is performed at most centers with extensive experience with gastric cancer and (in contrast to US centers) postoperative chemoradiotherapy is not a standard of care.13–15
IMPORTANCE OF PRETHERAPEUTIC STAGING IN GASTROESOPHAGEAL CANCER
Current treatment options for gastroesophageal cancer range from endoscopic mucosal resection to preoperative chemotherapy or chemoradiotherapy followed by esophagectomy or gastrectomy.16–18 Most of these approaches are associated with substantial morbidity and mortality, as well as long-term compromise in quality of life. Accurate pretherapeutic staging by imaging techniques is therefore crucial to selecting the appropriate form of therapy.
Generally, the first step in this process is to distinguish between patients with locoregional disease and those with systemic disease. For patients presenting with distant metastases (M1), no curative treatment is available and palliative treatment is required. Palliative resections should be considered only on an individualized basis for relief of symptoms or after response to chemotherapy or chemoradiotherapy in metastatic disease. Flourodeoxyglucosepositron emission tomography (FDG-PET) reaches sufficient sensitivity (67%) and good specificity (97%) in the detection of metastatic disease and is superior in this regard to computed tomography (CT) and other available diagnostic tools.16,19–28 A pretherapeutic change of planned treatment based on conventional staging modalities is generated by PET in about 20% of patients.16,21,25,29–31
In patients with locoregional disease, assessment of local tumor infiltration (T category) and regional lymph node involvement (N category) is necessary to decide whether a complete tumor resection (R0) is feasible.32 Local tumor infiltration is also frequently used for therapy stratification. Whereas patients with T1b and T2 disease without lymph node metastases are treated by primary resection and lymph node dissection, patients with T3 and T4 tumors frequently are offered preoperative chemotherapy or chemoradiotherapy to improve the rate of curative resections and, potentially, overall survival.33,34 Pretherapeutic assessment of T status by imaging techniques, therefore, has important consequences for the selection of therapy. Not FDG-PET, but endoscopic endoluminal ultrasound and CT scans are the required examinations for exact staging of pretherapeutic T status. For resectable tumors, the removal of the primary tumor together with a systematic lymphadenectomy is necessary for histopathologic lymph node staging and is the accepted standard therapy. FDG-PET offers a low sensitivity of 51% and a sufficient specificity of 84% for locoregional lymph node staging.16,26–28,35,36
Approximately one third of patients with gastric cancer, including locally advanced tumors, initially have insufficient FDG uptake for quantification.37 Distal-third tumors with diffuse growth pattern are especially unlikely to be visualized by FDG-PET. Therefore, FDG-PET is not routinely used for pretherapeutic staging in gastric cancer outside of the clinical study setting.
RESPONSE EVALUATION
Since 1999, it generally has been accepted that patients who respond to induction chemotherapy have significantly improved survival compared to nonresponding patients. 38 However, no standardized measures for evaluating response have been established so far. Clinical response evaluation by morphologic imaging techniques has specific limitations in gastric cancer. According to strict World Health Organization criteria, gastroesophageal cancer is not bidimensionally measureable.39 Criteria from the Response Evaluation Criteria in Solid Tumor (RECIST) Group, which use one-dimensional measurements, are, in principle, applicable to gastroesophageal cancer.40 However, measurement of wall thickness is critically dependent on the distension of the stomach during the examination. Only a few phase II trials of induction therapy have used RECIST criteria so far.41–45 Careful clinical response evaluation by a combination of endoluminal ultrasound, endoscopy, and CT scan used for restaging after one cycle or before surgery is predictive of histopathologic regression and prognosis in experienced centers.7,38,46–49
Histopathologic regression often is used for response evaluation. Yet, including only patients who undergo resection would cause a significant bias; thus, clinical response evaluation has to be included, and patients with progression during chemotherapy have to be classified as nonresponding patients. Although similar criteria for histopathologic regression have been used in several studies, these criteria are not standardized yet and may be investigator dependent.
A modification of the regression score used by Mandard and colleagues,50 who first described histopathologic regression for esophageal cancer after chemoradiotherapy, was published by Becker et al for gastric cancer.51 In this scoring system, patients with less than 10% residual tumor cells after neoadjuvant treatment are classified as histopathologic responders (score 1a = complete response and score 1b = less than 10% residual tumor cells). In other publications, only patients with complete tumor regression are classified as histopathologic responders.52,53 In contrast, Shah et al defined even patients with less than 50% residual tumor cells as histopathologic responders.54
All types of response evaluation, whether clinical or histopathologic, are strongly correlated with prognosis in the literature. However, a homogenization of the scoring systems used for clinical and histopathologic response evaluation is desirable in order to permit easier comparison of results of studies of induction therapy.
Model of Metabolic Response Evaluation in AEG
Measurements of early changes in tumor glucose uptake after only 2 weeks of induction therapy via FDG-PET has yielded reproducible results indicating accuracy in prediction of clinical and histopathologic response to neoadjuvant treatment in adenocarcinomas of the distal esophagus types I and II.47,48 In a study by our group, the cutoff value of a decrease of more than 35% of the initial standardized uptake value (SUV) after 2 weeks of induction therapy predicted response and prognosis. 47 Our major interest was to identify nonresponding patients early in the course of therapy to avoid toxic, expensive, and ineffective treatment. The cutoff value was confirmed in an independent patient population. 48 Specifically, we have demonstrated that the 35% decrease in initial SUV after 2 weeks of chemotherapy is highly accurate in identifying nonresponding patients.
This finding was used to individualize treatment in the MUNICON trial (the Metabolic response evalUatioN for Individualisation of neoadjuvant Chemotherapy in oesOphageal and oesophagogastric adeNocarcinoma).55,56 Metabolic responders after 2 weeks of induction chemotherapy continued to receive chemotherapy for a maximum of 12 weeks before undergoing surgery, whereas metabolic nonresponders discontinued chemotherapy after 2 weeks and were immediately transferred to surgery. Overall, 110 patients were evaluable for metabolic response in this trial, and 49% were classified as metabolic responders; 104 patients underwent resection.
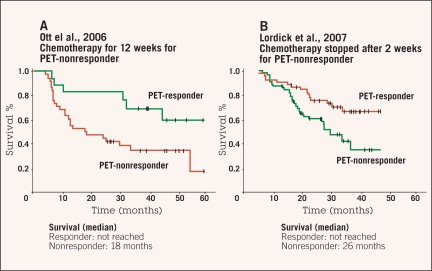
Histopathologic regression with less than 10% residual tumor cells was achieved in 58% of the metabolic responders, but no histopathologic regression 1a or 1b was achieved in metabolic nonresponders. The median overall survival for metabolic responders was not reached, whereas the median survival for metabolic nonresponders was 25.8 months (P = .015). Event-free survival was 29.7 months for metabolic responders and 14.1 months for nonresponders (P = .002).55,56 Interestingly, metabolic nonresponders, who underwent resection after only 2 weeks of induction therapy, had slightly better survival compared to historic controls consisting of metabolic nonresponders who completed two cycles of neoadjuvant treatment (Figure 1).48,55
Figure 1.
Comparison of overall survival of (A) patients with complete chemotherapy for 3 months48 and (B) patients with metabolic response-based neoadjuvant treatment.55 The median survival was 26 months in metabolic nonresponding patients with immediate resection after 2 weeks and 18 months in patients with complete chemotherapy in the historical control group. Stopping chemotherapy, thus, seems not to worsen prognosis of metabolic nonresponding patients.
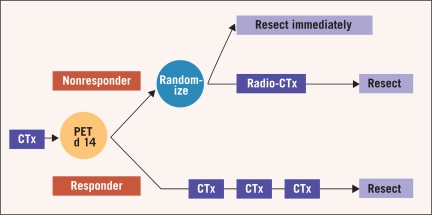
In summary, the MUNICON study prospectively confirmed the usefulness of metabolic response evaluation in AEG I and II and showed for the first time that a PET-guided treatment algorithm is feasible in the multidisciplinary treatment setting and leads to favorable treatment results. Based on these results, tailoring of multimodal treatment in accordance with individual tumor biology might be possible in future randomized trials; the EUROCON study is planned to randomize metabolic nonresponders after 2 weeks of chemotherapy to immediate resection or chemoradiation followed by surgery (Figure 2).
Figure 2.
Design of the planned EUROCON study with randomization of nonresponding patients after 2 weeks of chemotherapy (CTx) to immediate resection or chemoradiation therapy (Radio-CTx) followed by surgery. Abbreviation: d = day.
FDG-PET in Gastric Cancer
Current imaging modalities or molecular markers cannot reliably predict therapy response before or early in the course of treatment for gastric cancer.57–59 As noted above, approximately one third of gastric cancer patients initially have insufficient FDG uptake for quantification (Table 1).37,46,54,60–67 FDG non-avid tumors are associated with diffuse Lauren classification, small tumor size, good differentiation, mucinous content, and localization in the distal third.37,59–63,66
Table 1.
Proportion of FDG avid tumors in gastric cancer
We have shown that a decrease in tumor FDG uptake by more than 35% of the baseline value permitted prediction of response in patients with gastric cancer 2 weeks after initiation of cisplatin-based chemotherapy with an overall accuracy of 83% for 35 patients for whom image contrast was sufficient for quantitative analysis. Metabolic response in FDG-avid gastric cancer including AEG II is associated with histopathologic or clinical response, as shown in Table 2.46–48,54,55,67
Table 2.
Association between metabolic and clinical or histopathologic response
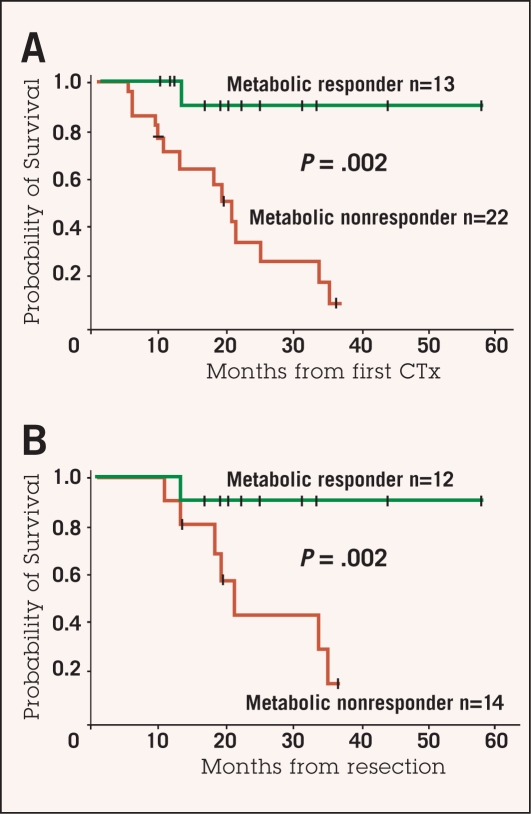
In our study, median survival for patients with a metabolic response was not reached (2-year survival rate 90%), whereas median survival was 18.9 months in nonresponders (2-year survival rate 25%, P = .002) (Figure 3).46 In a retrospective study by Shah et al in 41 patients with gastric cancer staged cT2-4NanycM0, a decrease of more than 45% in initial SUV after 35 days was identified as the best criterion for predicting response and prognosis; this cutoff value was significantly correlated with histopathologic response (less than 50% residual tumor, P = .007) and disease-free survival (P = .01).54 Further work must be done in defining cutoff values and standardizing test methodology before any of these findings can be translated to routine clinical practice.
Figure 3:
Overall survival of patients with locally advanced gastric cancer who had metabolic response or metabolic nonresponse calculated from the beginning of chemotherapy (A) and from time of complete resection (B). On both analyses, metabolic responders had significantly improved survival compared to metabolic nonresponders.
Adapted with permission from Ott et al.46
Another open question is whether early metabolic response evaluation is possible in patients with adenocarcinomas of the esophagogastric junction and the stomach treated with preoperative chemoradiotherapy. 10,68 No data providing guidance in this setting are available so far. Finally, a large proportion of gastric carcinomas are FDG non-avid and thus not suitable for response monitoring using the PET tracer 18F-FDG.
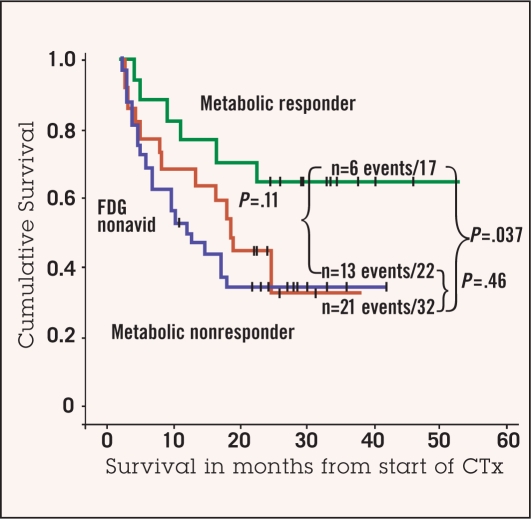
With regard to the latter finding, however, it is of interest that the response rate and prognosis of patients with FDG nonavid tumors seem to be similar to metabolic nonresponders, suggesting that non-avidity may define a subgroup of biologically unfavorable tumors (Figure 4).67 Our prospective in vivo testing of chemosensitivity by FDG-PET in 71 patients with locally advanced gastric cancer,67 including those in our earlier study,46 revealed three different metabolic response groups. Response and prognosis were predicted by FDG-PET in FDG-avid tumors. Metabolic responders showed a high histopathologic response rate (69%) and a favorable prognosis (median survival not reached), whereas metabolic nonresponders had a poorer prognosis (median survival 24.1 months) and a histopathologic response rate of only 17%. The histopathologic response rate of 24% in the third metabolic group, the nonavid tumors, was similar to FDG-avid nonresponders (P = .72). Survival of the non-avid patients (median 36.7 months) was not different from FDG-avid nonresponders (P = .46) (Figure 4).
Figure 4:
Overall survival of FDG-avid metabolic responding patients (green line), FDG-avid metabolic nonresponding patients (blue line), and FDG non-avid patients (red line). Prognosis of metabolic responding patients is significantly improved compared to metabolic nonresponding patients (P = .037). Prognosis of patients with non-avid tumors and FDG-avid nonresponding patients is not statistically different (P = .46). There is a trend for improved survival of metabolic responding patients compared to FDG non-avid patients (P = .11).
Adapted with permission from Ott et al.67
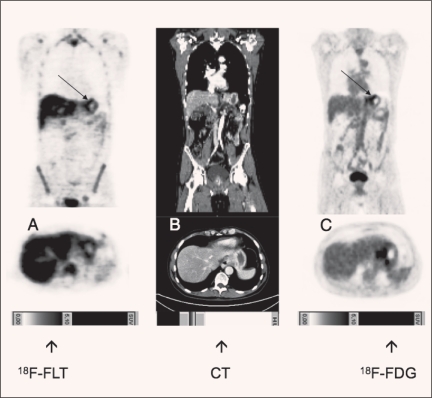
In a recent study in 45 patients, we compared fluorothymidine (FLT)-PET and FDG-PET for detection of locally advanced gastric cancer.69 FLT-PET had a higher sensitivity than FDG-PET and might serve as a useful diagnostic adjunct reflecting the quantitative assessment of proliferation (Figure 5). In the future, the addition of FLT-PET to FDG-PET could improve early evaluation of response to neoadjuvant treatment of gastric cancer.
Figure 5.
Visualization of locally advanced gastric cancer with FDG-PET and FLT-PET.
TREATMENT OF THE FUTURE: PET-GUIDED INDUCTION THERAPY
Recent randomized phase III studies have shown that induction therapy is effective in locally advanced gastroesophageal cancer.1,2 It is generally accepted that responders have improved survival compared to nonresponding patients.38 Thus far, however, no prospectively tested clinical, histopathologic, or molecular markers predicting response or prognosis prior to induction therapy are available for gastric cancer. Only metabolic response has predicted histologic response and survival with sufficient accuracy.46,47,54–56
There is ongoing discussion regarding whether responders or nonresponders after induction therapy in esophageal cancer are candidates for surgery. It is generally accepted that responding patients have a significantly improved prognosis compared to nonresponding patients after resection.38,52,70 However, in considering treatment outcomes, it is important to differentiate among treatment regimens (eg, chemoradiotherapy or chemotherapy) and histopathology (eg, AEG I or squamous cell cancer).71–73 Chemoradiotherapy is a local therapy targeting the primary tumor and the regional lymph nodes, with histopathologic response rates up to 50%.74,75 In contrast, chemotherapy is a systemic treatment, affecting both the primary tumor and potential distant micrometastases in all compartments. The histopathologic response rate of about 20% for the primary tumor in responding patients with adenocarcinomas of the esophagus after systemic chemotherapy is far less than after chemoradiotherapy in squamous cell esophageal cancer.7,46–48,76
It is of interest that chemoradiotherapy in patients with squamous cell carcinoma of the esophagus results in the suppression of T lymphocyte function. The proliferative defects of T cells after chemoradiotherapy may be linked to an impaired immune surveillance of cancer and to a higher risk of surgical complications associated with esophagectomy.5,6 In squamous cell cancer, nonresponding patients have increased postoperative mortality, morbidity, and complication rate in our department. 77 Based on such findings, we recommend resection for all responding patients after induction therapy (chemotherapy and chemoradiotherapy) who are fit for extended surgery.78,79 For the nonresponding patients with squamous cell esophageal cancer, definitive palliative treatment is indicated, with palliative surgery being reserved for individual cases due to the high postoperative complication rate and bad prognosis.77
Due to lower immunosuppression and lower complication rate in nonresponders with adenocarcinomas of the distal esophagus compared to squamous cell carcinomas, we suggest surgery even in nonresponding patients with the former.56 The MUNICON study has shown no increased complication rate or postoperative morbidity or mortality in metabolic nonresponding patients. 55,56 Intensive efforts should be made to determine whether the integration of chemoradiotherapy for metabolic nonresponding adenocarcinomas early in the course of therapy can increase response rates and improve survival in nonresponding patients.
DISCUSSION
In summary, accurate methods to predict response and prognosis are essential to establishing individualized treatment approaches in esophageal cancer. FDG-PET after 2 weeks of induction therapy offers accurate prediction of both response and prognosis. The early response evaluation by FDG-PET offers for the first time the possibility of a modification of treatment early in the course of therapy in esophageal cancer. The results of the MUNICON trial now have to be confirmed in a prospective, randomized multicenter trial (Figure 2).
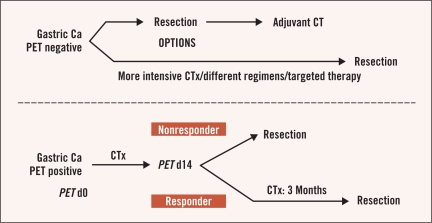
Given the large proportion of FDG-PET non-avid tumors, the situation is not so straightforward in gastric cancer. Response and survival for non-avid patients were not significantly better than metabolic nonresponders. Alternative treatment concepts that might be considered in these patients include immediate resection after 2 weeks of chemotherapy or adjustment of chemotherapy with or without adjuvant treatment for metabolic nonresponders, or modified or potentially more intensive perioperative chemotherapy regimens—possibly including biologically targeted drugs or intensity-modulated high precision radiotherapy — in initially FDG-PET non-avid tumors (Figure 6). Other techniques for early prediction of response/ prognosis, including use of FLT-PET or histopathologic or molecular markers, are likely to be of importance in individualizing therapy in gastric cancer.69
Figure 6:
Theoretical model of an individualized FDG-PET–based treatment strategy in locally advanced gastric cancer (ca).
Abbreviations: CTx = chemotherapy; d = day.
Footnotes
Disclosures of Potential Conflicts of Interest
The authors indicated no potential conflicts of interest.
REFERENCES
- 1.Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-Fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol. 2007;25(suppl) abstract 4510. [Google Scholar]
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 3.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med. 1998;339:1979–1984. doi: 10.1056/NEJM199812313392704. [DOI] [PubMed] [Google Scholar]
- 4.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–234. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 5.Heidecke CD, Weighardt H, Feith M, et al. Neoadjuvant treatment of esophageal cancer: immunosuppression following combined radiochemotherapy. Surgery. 2002;132:495–501. doi: 10.1067/msy.2002.127166. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds JV, Ravi N, Hollywood D, et al. Neoadjuvant chemoradiation may increase the risk of respiratory complications and sepsis after transthoracic esophagectomy. J Thorac Cardiovasc Surg. 2006;132:549–555. doi: 10.1016/j.jtcvs.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Ott K, Sendler A, Becker K, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer. 2003;6:159–167. doi: 10.1007/s10120-003-0245-4. [DOI] [PubMed] [Google Scholar]
- 8.Schumacher CP, Fink U, Becker K, et al. Neoadjuvant therapy for patients with locally advanced gastric carcinoma with etoposide, doxorubicin, and cisplatinum. Closing results after 5 years of follow-up. Cancer. 2001;91:918–927. [PubMed] [Google Scholar]
- 9.Ajani JA, Mansfield PF, Lynch PM, et al. Enhanced staging and all chemotherapy preoperatively in patients with potentially resectable gastric carcinoma. J Clin Oncol. 1999;17:2403–2411. doi: 10.1200/JCO.1999.17.8.2403. [DOI] [PubMed] [Google Scholar]
- 10.Ajani JA, Komaki R, Putnam JB, et al. A three-step strategy of induction chemotherapy then chemoradiation followed by surgery in patients with potentially resectable carcinoma of the esophagus or gastroesophageal junction. Cancer. 2001;92:279–286. doi: 10.1002/1097-0142(20010715)92:2<279::aid-cncr1320>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Ott K, Vogelsang H, Mueller J, et al. Chromosomal instability rather than p53 mutation is associated with response to neoadjuvant cisplatin-based chemotherapy in gastric carcinoma. Clin Cancer Res. 2003;9:2307–2315. [PubMed] [Google Scholar]
- 12.Siewert JR, Bartels H, Stein HJ. [Abdominoright-thoracic esophagectomy with intrathoracic anastomosis in Barrett's cancer] Chirurg. 2005;76:588–594. doi: 10.1007/s00104-005-1028-8. [DOI] [PubMed] [Google Scholar]
- 13.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 14.Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol. 2004;22:2069–2077. doi: 10.1200/JCO.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Siewert JR, Kestlmeier R, Busch R, et al. Benefits of D2 lymph node dissection for patients with gastric cancer and pN0 and pN1 lymph node metastases. Br J Surg. 1996;83:1144–1147. doi: 10.1002/bjs.1800830836. [DOI] [PubMed] [Google Scholar]
- 16.Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol. 2000;18:3202–3210. doi: 10.1200/JCO.2000.18.18.3202. [DOI] [PubMed] [Google Scholar]
- 17.Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 18.Ell C, May A, Gossner L, et al. Endoscopic mucosal resection of early cancer and highgrade dysplasia in Barrett's esophagus. Gastroenterology. 2000;118:670–677. doi: 10.1016/s0016-5085(00)70136-3. [DOI] [PubMed] [Google Scholar]
- 19.Block MI, Patterson GA, Sundaresan RS, et al. Improvement in staging of esophageal cancer with the addition of positron emission tomography. Ann Thorac Surg. 1997;64:770–776. doi: 10.1016/s0003-4975(97)00619-x. [DOI] [PubMed] [Google Scholar]
- 20.Kole AC, Plukker JT, Nieweg OE, et al. Positron emission tomography for staging of oesophageal and gastroesophageal malignancy. Br J Cancer. 1998;78:521–527. doi: 10.1038/bjc.1998.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rankin SC, Taylor H, Cook GJ, et al. Computed tomography and positron emission tomography in the pre-operative staging of oesophageal carcinoma. Clin Radiol. 1998;53:659–665. doi: 10.1016/s0009-9260(98)80292-4. [DOI] [PubMed] [Google Scholar]
- 22.Kobori O, Kirihara Y, Kosaka N, et al. Positron emission tomography of esophageal carcinoma using (11)C-choline and (18)F-fluorodeoxyglucose: a novel method of preoperative lymph node staging. Cancer. 1999;86:1638–1648. doi: 10.1002/(sici)1097-0142(19991101)86:9<1638::aid-cncr4>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 23.Choi JY, Lee KH, Shim YM, et al. Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by FDG PET. J Nucl Med. 2000;41:808–815. [PubMed] [Google Scholar]
- 24.Meltzer CC, Luketich JD, Friedman D, et al. Whole-body FDG positron emission tomographic imaging for staging esophageal cancer comparison with computed tomography. Clin Nucl Med. 2000;25:882–887. doi: 10.1097/00003072-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Kato H, Miyazaki T, Nakajima M, et al. The incremental effect of positron emission tomography on diagnostic accuracy in the initial staging of esophageal carcinoma. Cancer. 2005;103:148–156. doi: 10.1002/cncr.20724. [DOI] [PubMed] [Google Scholar]
- 26.Kato H, Kuwano H, Nakajima M, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer. 2002;94:921–928. [PubMed] [Google Scholar]
- 27.Wren SM, Stijns P, Srinivas S. Positron emission tomography in the initial staging of esophageal cancer. Arch Surg. 2002;137:1001–1006. doi: 10.1001/archsurg.137.9.1001. [DOI] [PubMed] [Google Scholar]
- 28.van Westreenen HL, Westerterp M, Bossuyt PM, et al. Systematic review of the staging performance of 18F-fluorodeoxyglucose positron emission tomography in esophageal cancer. J Clin Oncol. 2004;22:3805–3812. doi: 10.1200/JCO.2004.01.083. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan FL, Dehdashti F, Siegel BA, et al. Staging of esophageal cancer with 18F-fluorodeoxyglucose positron emission tomography. Am J Roentgenol. 1997;168:417–424. doi: 10.2214/ajr.168.2.9016218. [DOI] [PubMed] [Google Scholar]
- 30.Luketich JD, Friedman DM, Weigel TL, et al. Evaluation of distant metastases in esophageal cancer: 100 consecutive positron emission tomography scans. Ann Thorac Surg. 1999;68:1133–1136. doi: 10.1016/s0003-4975(99)00974-1. [DOI] [PubMed] [Google Scholar]
- 31.Heeren PA, Jager PL, Bongaerts F, et al. Detection of distant metastases in esophageal cancer with (18)F-FDG PET. J Nucl Med. 2004;45:980–987. [PubMed] [Google Scholar]
- 32.Stein HJ, Brucher BL, Sendler A, et al. Esophageal cancer: patient evaluation and pre-treatment staging. Surg Oncol. 2001;10:103–111. doi: 10.1016/s0960-7404(01)00023-8. [DOI] [PubMed] [Google Scholar]
- 33.Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–543. doi: 10.1016/s0002-9610(03)00066-7. [DOI] [PubMed] [Google Scholar]
- 34.Lordick F, Stein HJ, Peschel C, et al. Neoadjuvant therapy for oesophagogastric cancer. Br J Surg. 2004;91:540–551. doi: 10.1002/bjs.4575. [DOI] [PubMed] [Google Scholar]
- 35.Junginger T. [PET scan diagnosis of lymph node metastases of esophageal carcinoma] Chirurg. 2004;75:312–313. doi: 10.1007/s00104-004-0836-6. [DOI] [PubMed] [Google Scholar]
- 36.Yoon YC, Lee KS, Shim YM, et al. Metastasis to regional lymph nodes in patients with esophageal squamous cell carcinoma: CT versus FDG PET for presurgical detection prospective study. Radiology. 2003;227:764–770. doi: 10.1148/radiol.2281020423. [DOI] [PubMed] [Google Scholar]
- 37.Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging. 2003;30:288–295. doi: 10.1007/s00259-002-1029-5. [DOI] [PubMed] [Google Scholar]
- 38.Lowy AM, Mansfield PF, Leach SD, et al. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg. 1999;229:303–308. doi: 10.1097/00000658-199903000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 40.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 41.Park JO, Lee SI, Song SY, et al. Measuring response in solid tumors: comparison of RECIST and WHO response criteria. Jpn J Clin Oncol. 2003;33:533–537. doi: 10.1093/jjco/hyg093. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki T. [New guidelines to evaluate the response to treatment “RECIST”] Gan To Kagaku Ryoho. 2000;27:2179–2184. [PubMed] [Google Scholar]
- 43.Yoshida S, Miyata Y, Ohtsu A, et al. Significance of and problems in adopting response evaluation criteria in solid tumor RECIST for assessing anticancer effects of advanced gastric cancer. Gastric Cancer. 2000;3:128–133. doi: 10.1007/pl00011706. [DOI] [PubMed] [Google Scholar]
- 44.Jeung HC, Rha SY, Noh SH, et al. A phase II trial of weekly fractionated irinotecan and cisplatin for advanced gastric cancer. Cancer Chemother Pharmacol. 2007;59:313–320. doi: 10.1007/s00280-006-0272-z. [DOI] [PubMed] [Google Scholar]
- 45.Burge ME, Smith D, Topham C, et al. A phase I and II study of 2-weekly irinotecan with capecitabine in advanced gastroesophageal adenocarcinoma. Br J Cancer. 2006;94:1281–1286. doi: 10.1038/sj.bjc.6603084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol. 2003;21:4604–4610. doi: 10.1200/JCO.2003.06.574. [DOI] [PubMed] [Google Scholar]
- 47.Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol. 2001;19:3058–3065. doi: 10.1200/JCO.2001.19.12.3058. [DOI] [PubMed] [Google Scholar]
- 48.Ott K, Weber WA, Lordick F, et al. Metabolic imaging predicts response, survival, and recurrence in adenocarcinomas of the esophagogastric junction. J Clin Oncol. 2006;24:4692–4698. doi: 10.1200/JCO.2006.06.7801. [DOI] [PubMed] [Google Scholar]
- 49.Swisher SG, Maish M, Erasmus JJ, et al. Utility of PET, CT, and EUS to identify pathologic responders in esophageal cancer. Ann Thorac Surg. 2004;78:1152–1160. doi: 10.1016/j.athoracsur.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 50.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 51.Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521–1530. doi: 10.1002/cncr.11660. [DOI] [PubMed] [Google Scholar]
- 52.Rohatgi P, Swisher SG, Correa AM, et al. Characterization of pathologic complete response after preoperative chemoradiotherapy in carcinoma of the esophagus and outcome after pathologic complete response. Cancer. 2005;104:2365–2372. doi: 10.1002/cncr.21439. [DOI] [PubMed] [Google Scholar]
- 53.Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT) Ann Surg. 2005;241:810–817. doi: 10.1097/01.sla.0000161983.82345.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah MA, Yeung HW, Coit D, et al. A phase II study of preoperative chemotherapy with irinotecan (CPT) and cisplatin (CIS) for gastric cancer (NCI 5917): FDG-PET/CT predicts patient outcome. J Clin Oncol. 2007;25(suppl) abstract 4502. [Google Scholar]
- 55.Lordick F, Ott K, Krause BJ, et al. PET to assess early metabolic response and to guide treatment of adenocarcinoma of the oesophagogastric junction: the MUNICON phase II trial. Lancet Oncol. 2007;8:797–805. doi: 10.1016/S1470-2045(07)70244-9. [DOI] [PubMed] [Google Scholar]
- 56.Siewert JR, Lordick F, Ott K, et al. Induction chemotherapy in Barrett cancer: influence on surgical risk and outcome. Ann Surg. 2007;246:624–631. doi: 10.1097/SLA.0b013e318155a7d1. [DOI] [PubMed] [Google Scholar]
- 57.Beer AJ, Wieder HA, Lordick F, et al. Adenocarcinomas of esophagogastric junction: multidetector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology. 2006;239:472–480. doi: 10.1148/radiol.2391050043. [DOI] [PubMed] [Google Scholar]
- 58.Wieder HA, Beer AJ, Lordick F, et al. Comparison of changes in tumor metabolic activity and tumor size during chemotherapy of adenocarcinomas of the esophagogastric junction. J Nucl Med. 2005;46:2029–2034. [PubMed] [Google Scholar]
- 59.Mukai K, Ishida Y, Okajima K, et al. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer. 2006;9:192–196. doi: 10.1007/s10120-006-0374-7. [DOI] [PubMed] [Google Scholar]
- 60.Kim SK, Kang KW, Lee JS, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006;33:148–155. doi: 10.1007/s00259-005-1887-8. [DOI] [PubMed] [Google Scholar]
- 61.Chen J, Cheong JH, Yun MJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer. 2005;103:2383–2390. doi: 10.1002/cncr.21074. [DOI] [PubMed] [Google Scholar]
- 62.Tian J, Chen L, Wei B, et al. The value of vesicant 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in gastric malignancies. Nucl Med Commun. 2004;25:825–831. doi: 10.1097/01.mnm.0000135042.54461.f6. [DOI] [PubMed] [Google Scholar]
- 63.Mochiki E, Kuwano H, Katoh H, et al. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247–253. doi: 10.1007/s00268-003-7191-5. [DOI] [PubMed] [Google Scholar]
- 64.Wang T, Sun YE, Yu CH, et al. [Fluorine-18 fluorodeoxyglucose-positron emission tomography imaging of carcinoma of cardia or fundus of stomach] Zhonghua Wai Ke Za Zhi. 2006;44:661–664. [PubMed] [Google Scholar]
- 65.Yun M, Lim JS, Noh SH, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–1588. [PubMed] [Google Scholar]
- 66.Shah MA, Yeung HW, Tracola R, et al. The characteristics and utility of FDG-PET/CT scans in patients with localized gastric cancer (GC)American Society of Clinical Oncology 2007 Gastrointestinal Cancers Symposium; abstract 2
- 67.Ott K, Herrmann K, Lordick F, et al. Early metabolic response evaluation by fluorine-18 fluorodeoxyglucose positron emission tomography allows in vivo testing of chemosensitivity in gastric cancer: long term results of a prospective study. Clin Cancer Res. 2008;14:2012–2018. doi: 10.1158/1078-0432.CCR-07-0934. [DOI] [PubMed] [Google Scholar]
- 68.Lowy AM, Feig BW, Janjan N, et al. A pilot study of preoperative chemoradiotherapy for resectable gastric cancer. Ann Surg Oncol. 2001;8:519–524. doi: 10.1007/s10434-001-0519-1. [DOI] [PubMed] [Google Scholar]
- 69.Herrmann K, Ott K, Buck AK, et al. Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med. 2007;48:1945–1950. doi: 10.2967/jnumed.107.044867. [DOI] [PubMed] [Google Scholar]
- 70.Kelsen DP, Winter KA, Gunderson LL, et al. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719– 3725. doi: 10.1200/JCO.2006.10.4760. [DOI] [PubMed] [Google Scholar]
- 71.Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17:38–44. doi: 10.1016/j.semradonc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Rohatgi PR, Swisher SG, Correa AM, et al. Comparison of clinical stage, therapy response, and patient outcome between squamous cell carcinoma and adenocarcinoma of the esophagus. Int J Gastrointest Cancer. 2005;36:69–76. doi: 10.1385/IJGC:36:2:69. [DOI] [PubMed] [Google Scholar]
- 73.Rohatgi PR, Swisher SG, Correa AM, et al. Histologic subtypes as determinants of outcome in esophageal carcinoma patients with pathologic complete response after preoperative chemoradiotherapy. Cancer. 2006;106:552–558. doi: 10.1002/cncr.21601. [DOI] [PubMed] [Google Scholar]
- 74.Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–4337. doi: 10.1200/JCO.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 75.Brucher BL, Weber W, Bauer M, et al. Neoadjuvant therapy of esophageal squamous cell carcinoma: response evaluation by positron emission tomography. Ann Surg. 2001;233:300–309. doi: 10.1097/00000658-200103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer. 2001;91:2165–2174. [PubMed] [Google Scholar]
- 77.Brucher BL, Becker K, Lordick F, et al. The clinical impact of histopathologic response assessment by residual tumor cell quantification in esophageal squamous cell carcinomas. Cancer. 2006;106:2119–2127. doi: 10.1002/cncr.21850. [DOI] [PubMed] [Google Scholar]
- 78.Bartels H, Stein HJ, Siewert JR. Risk analysis in esophageal surgery. Recent Results Cancer Res. 2000;155:89–96. 89–96. doi: 10.1007/978-3-642-59600-1_8. [DOI] [PubMed] [Google Scholar]
- 79.Bartels H, Stein HJ, Siewert JR. Preoperative risk analysis and postoperative mortality of oesophagectomy for resectable oesophageal cancer. Br J Surg. 1998;85:840–844. doi: 10.1046/j.1365-2168.1998.00663.x. [DOI] [PubMed] [Google Scholar]