CASE REPORT
A 67-year-old otherwise-healthy woman was found on routine evaluation to be mildly anemic. Physical examination was nonfocal, except for guaiac-positive brown stool. Her hemoglobin level was mildly decreased at 10.9 gm/dL; bilirubin and creatinine were within normal limits at 0.8 mg/dL and 1.0 mg/dL, respectively. The patient’s ECOG (Eastern Cooperative Oncology Group) performance status was 0. She was on no chronic medications, had no known drug allergies, and was now retired. Her hobbies were cross-country skiing and knitting. A colonoscopy was performed, which revealed a non-obstructing mass at the hepatic flexure (Figure 1). Biopsy was positive for poorly differentiated adenocarcinoma (Figure 2). Computed tomography (CT) surveillance revealed multiple lung and liver metastases in a clearly unresectable pattern (Figures 3 and 4).
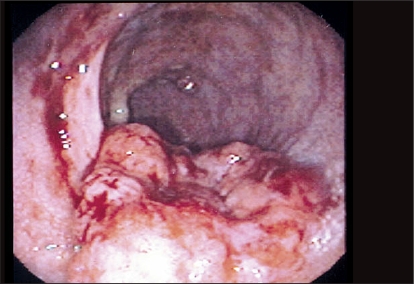
Figure 1.
Image obtained during colonoscopy reveals a mass at the hepatic flexure. The tumor is aggressive in appearance with a necrotic center. The lumen is not obstructed.
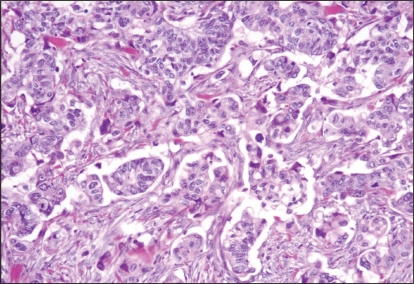
Figure 2.
Photomicrograph demonstrates poorly differentiated adenocarcinoma, with complete loss of glandular architecture.
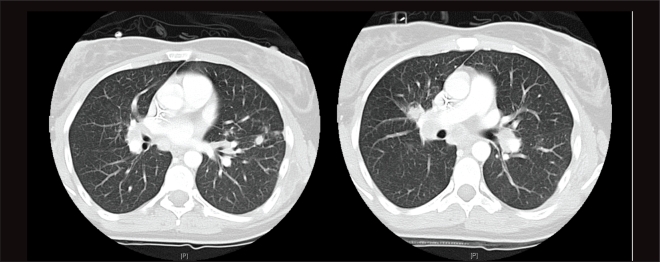
Figure 3.
Computed tomography images reveal multiple scattered lesions throughout the lungs.
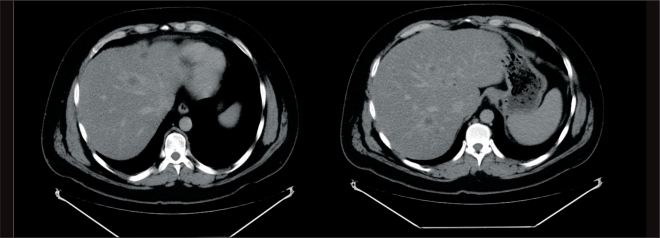
Figure 4.
Computed tomography scans of the liver show multiple, small-volume metastases scattered in a “buckshot type” throughout the organ.
Chemotherapy was initiated with FOLFIRI (infusional 5-fluorouracil [5-FU]/leucovorin/irinotecan) plus bevacizumab, and a good response was achieved. At 9 months, however, her disease was progressing, though she still enjoyed a relatively good quality of life, with an ECOG performance status of 1. It was decided to initiate second-line treatment. Tumor genotyping performed prior to starting chemotherapy had revealed that the patient’s tumor expressed wild-type KRAS; thus, second-line treatment with irinotecan (at the same dose and schedule that had last been given with her FOLFIRI) plus cetuximab was initiated. The patient achieved a second major response to this regimen, although the course was complicated by a persistent grade 2 skin rash and substantial skin dryness. After 8 months on this regimen, disease again progressed, and the patient was started on FOLFOX. Further tumor regression was noted, but after 6 months the tumor again progressed. The option of a phase I clinical trial of a novel agent vs. best supportive care was then discussed with the patient.
DISCUSSION
In this case, the patient presented with unequivocal, widespread metastatic disease that clearly precluded curative resection, and chemotherapy was the most appropriate choice for initial management. It is important to note that routine resection of the primary is not indicated in the absence of either severe bleeding (clearly not present in a patient with brown stool and a hemoglobin of 10.9) or impending obstruction (particularly uncommon in right-sided colonic lesions).
Prior to starting chemotherapy, KRAS genotyping of the patient’s tumor tissue was carried out. Patients with a mutated KRAS gene, for all practical purposes, do not benefit from treatment with anti-epidermal growth factor receptor (EGFR) agents such as cetuximab or panitumumab; thus, unnecessary exposure to toxicity and expense can be avoided in these cases. Moreover, understanding early whether or not anti-EGFR agents are going to be part of the process is much easier for the patient to prepare themselves for emotionally, rather than waiting until the remaining treatment options are either cetuximab or panitumumab and then finding out that, in fact, there are no options left. In this case, the patient’s tumor expressed wild-type KRAS, which provided useful information for developing a treatment strategy that did include, potentially, an EGFR inhibitor.
In the United States, FOLFOX (infusional 5-FU/folinic acid plus oxaliplatin) plus bevacizumab is the most common chemotherapy regimen used in front-line treatment of unresectable metastatic colon cancer, although multiple studies indicate that FOLFOX and FOLFIRI have very similar efficacy in the front-line setting. A common point of concern regarding chemotherapy in this setting is the perceived risk of bevacizumab-associated bowel perforation if the primary tumor is left intact. In fact, actual reported cases of perforation at the primary site are remarkably rare. Intestinal perforation tends to be more common in the small bowel than in the large bowel, and even when the large bowel is involved, perforation usually occurs somewhat distant to the known site of the primary. Of greater concern with bevacizumab is the potential risk of anastomotic breakdown in a patient status post resection.
It bears mentioning that recent data from a major phase III study of bevacizumab in combination with oxaliplatin-based chemotherapy indicated that the addition of bevacizumab to FOLFOX is far less beneficial than had been hoped.1 In this multicenter, randomized phase III study, 1,400 patients with metastatic colorectal cancer were randomly assigned in a 2×2 factorial design to either XELOX (capecitabine plus oxaliplatin) or FOLFOX-4 and then to bevacizumab vs. placebo. The primary end point was progression-free survival.
The addition of bevacizumab to oxaliplatin-based chemotherapy in this study yielded a modest, albeit statistically significant improvement in progression-free survival (by 1.4 months). Differences in overall survival, however, did not reach statistical significance, and response rate was not improved at all by the addition of bevacizumab. It was noted in this trial that due to toxicity, many patients discontinued chemotherapy, including bevacizumab, prior to progression, which may have blunted the contribution of bevacizumab to the outcome of this study. The authors concluded that treatment continuation until disease progression may be necessary to optimize the contribution of bevacizumab to therapy.
FOLFOX or FOLFIRI plus bevacizumab would be equally viable combinations for this patient. The choice of which regimen to use is influenced to a degree by the amount of neurotoxicity the patient can tolerate, and the relative toxicities of irinotecan vs. oxaliplatin should be explained. In addition, one of the major issues with irinotecan relative to oxaliplatin is alopecia, which is of greater concern to some patients than one might think. Thus, it is important to explore the options with individual patients to learn which of these treatment courses would be more acceptable to them.
Since this patient has the wild-type KRAS genotype, FOLFIRI plus cetuximab is also an option. Substantial skin toxicity, however, is essentially a sine qua non of cetuximab activity, and it can be a very socially debilitating side effect. As such, in my practice, cetuximab would usually be reserved for later-line therapy. Interestingly, two large randomized trials showed that combination chemotherapy with bevacizumab plus an anti-EGFR agent was actually detrimental to patients. Results from the CAIRO-2 study2 and PACCE trial3,4 showed that adding cetuximab or panitumumab, respectively, to combination chemotherapy with bevacizumab resulted in shorter progression-free survival and greater toxicity.
The decision in this case was to initiate treatment with FOLFIRI plus bevacizumab. The patient initially responded well, but then progressed after 7 months of treatment. Since she still enjoyed a good performance status, it was decided to continue with secondline chemotherapy. Having progressed on FOLFIRI and bevacizumab as her only chemotherapy thus far, second-line regimens under consideration included FOLFOX, irinotecan plus cetuximab, or irinotecan plus panitumumab. At present, no randomized data support the use of bevacizumab in a second-line regimen after progression on a first-line bevacizumab-containing regimen; such continuation of bevacizumab beyond progression is technically an off-label use, and would not be part of my routine practice. Studies investigating this question are currently in progress.
Regarding anti-EGFR combinations, the choice of panitumumab vs. cetuximab might, in part, be regionally driven. Interestingly, hypersensitivity reactions to cetuximab seem to cluster in certain regions.5 In the United States, for example, these hypersensitivity reactions to cetuximab seem to occur relatively frequently in and around the regions of North Carolina, Tennessee, and Kentucky, where serious allergic reactions to a first dose of cetuximab develop in up to one in five patients. Accordingly, in that area, panitumumab might be the EGFR agent of choice. In the northeastern United States, on the other hand, serious allergic reactions to cetuximab are seen in less than 1% of patients. To date, no patient has been hospitalized overnight at Memorial Sloan-Kettering due to a cetuximab reaction.
If EGFR therapy is indicated, cetuximab would be my usual preferred drug, since severe allergic reaction is not a major concern in this region, and data suggest that continued treatment with cetuximab in combination with irinotecan—even after failure with initial irinotecan—offers higher activity than with cetuximab alone. That might be the case with irinotecan plus panitumumab as well, but specific data with that combination are lacking. FOLFOX would be the other preferred regimen, and it remains an option for third-line therapy. Often, if the choice is made to avoid FOLFOX as front-line treatment, the desire to avoid neurotoxicity may persist to the extent that FOLFOX is delayed until other options have been exhausted.
The decision to use FOLFIRI and bevacizumab over FOLFOX and bevacizumab may well be driven by the fact that a patient has specific concerns about neurotoxicity. For example, a person who depends on fine motor skills (such as a musician, or someone who spends a lot of time typing) or perhaps a person who works in cold environments (such as a construction worker or fisherman in the North East) might be somebody who would have relatively greater difficulty coping with oxaliplatin-associated neurotoxicity. In such cases, irinotecan plus cetuximab might be the preferred second-line choice, with FOLFOX held in reserve as salvage therapy. Ultimately, when making treatment decisions in this setting, it is important to communicate effectively with the patient, who is going to be looking at an extended period of anticancer therapy—arguably lifelong chemotherapy in this case—relieved, it is hoped, by occasional holidays.
Footnotes
Disclosure of Potential Conflicts of Interest
Dr. Saltz indicated no potential conflicts of interest.
REFERENCES
- 1.Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 2.Punt CJ, Tol J, Rodenburg CJ, et al. Randomized phase III study of capecitabine, oxaliplatin, and bevacizumab with or without cetuximab in advanced colorectal cancer (ACC), the CAIRO2 study of the Dutch Colorectal Cancer Group (DCCG) J Clin Oncol. 2008;26 doi: 10.1093/annonc/mdm607. (abstr LBA4011) [DOI] [PubMed] [Google Scholar]
- 3.Hecht JR, Mitchell E, Chidiac T, et al. An updated analysis of safety and efficacy of oxaliplatin/bevacizumab +/− panitumumab for first-line treatment of metastatic colorectal cancer from a randomized, controlled trial (PACCE) Program and abstracts of the 2008 Gastrointestinal Cancers Symposium (GCS)January 25–27, 2008Orlando, Florida(abstr 273) [Google Scholar]
- 4.Hecht JR, Mitchell E, Chidiac T, et al. Interim results from PACCE: irinotecan (Iri)/bevacizumab (bev) +/− panitumumab (pmab) as first-line treatment (tx) for metastatic colorectal cancer (mCRC) Program and abstracts of the 2008 Gastrointestinal Cancers Symposium (GCS)January 25–27, 2008Orlando, Florida(abstr 279) [Google Scholar]
- 5.O’Neil BH, Allen R, Spigel DR, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]