Abstract
Eye movements were measured during the performance of a computerized Tower of London task to specify the source of planning abnormalities in patients with 1st-episode schizophrenia or schizoaffective disorder. Subjects viewed 2 arrays of colored balls in the upper and lower parts of the screen. They were asked to plan the shortest sequence of moves required to rearrange the balls in the lower screen to match the upper arrangement. Compared with healthy controls, patients made more planning errors, and decision times were longer. However, the patients showed the same gaze biases as controls prior to making a response, indicating that they understood the requirements of the task, approached the task in a strategic manner by identifying the nature of the problem, and used appropriate fixation strategies to plan and elaborate solutions. The patients showed increased duration of long-gaze periods toward both parts of the screen. This suggests that the patients had difficulty in encoding the essential features of the stimulus array. This finding is compatible with slowing of working memory consolidation.
Keywords: schizophrenia, eye movements, Tower of London planning task, cognitive impairments, working memory
Patients with schizophrenia show pronounced deficits on tests of executive function at all stages of the illness (e.g., Elliott, McKenna, Robbins, & Sahakian, 1998; Hutton et al., 1998; Owen, Roberts, Polkey, Sahakian, & Robbins, 1991; Pantelis et al., 1997). The term executive function encompasses several discrete cognitive processes, such as working memory, response inhibition, and attentional set shifting, which interact to optimize performance under changing or novel conditions. Such impairments have clinical relevance, as they have been shown to impact on everyday community function (Green, Kern, Braff, & Mintz, 2000). However, it is often not clear whether executive performance decrements reflect disturbances of specific executive processes or more general impairments (see Joyce & Huddy, 2004; MacDonald & Carter 2002). Patients with schizophrenia tend to perform poorly whatever the cognitive domain being tested (e.g., Bilder et al., 2000; Mohamed, Paulsen, O'Leary, Arndt, & Andreasen, 1999), and possible influences on performance range from problems with early information processing to the inability to understand task requirements. Residual symptoms, causing poor motivation or distractibility, might also contribute to apparent executive dysfunction because of the failure to engage with the task.
The study of natural eye movements during the execution of complex tasks is a promising means of specifying the cognitive operations used and how these relate to performance proficiency. Evidence suggests that when a task is structured so that performance requirements are explicit, the eye movements generated can be interpreted as purposeful shifts of attention (Hayhoe & Ballard, 2005; Land & Furneaux, 1997; Liversedge & Finlay, 2000). For example, Ballard, Hayhoe, and Pelz (1995; Hayhoe, Bensinger, & Ballard, 1998) found that the location and duration of fixations during the performance of visuospatial matching tasks reflected different cognitive operations depending on when they occurred during task solution. That is, subjects appeared to use fixations to acquire relevant information about the visual problem—for example, color or location—at the time that they needed it rather than rely on an internal memory of the global visual scene to guide responses. The researchers concluded that fixations can be used strategically to relieve the burden on visuospatial working memory. In support of this, Epelboim et al. (1995), using a task in which subjects were required to look at an array of targets in a specified order, found that when visual search was used to locate targets on each trial, performance was faster than when subjects relied on memory for target locations. Other task-specific gaze biases have been shown to correlate with cognitive operations that distinguish efficient from inefficient problem solvers. For example, Suppes, Cohen, Laddage, and Floyd (1983) found that during the solution of arithmetic problems, success was related to the use of certain sequences of eye movements and that within the subject group, the less able subjects made more out-of-sequence eye movements.
These studies demonstrate that when the requirements of a task are highly specified, the study of eye movements during performance can reveal the cognitive strategies used by subjects to aid performance. Hodgson, Bajwa, Owen, and Kennard (2000; Hodgson, Tiesman, Owen, & Kennard, 2002) used this paradigm to examine the strategic control of gaze during planning using a computerized version of the Tower of London task (Owen et al., 1995; Shallice, 1982). In the original Cambridge Automated Neuropsychological Test Battery (CANTAB) version of this task (Owen, Downes, Sahakian, Polkey, & Robbins. 1990), subjects are shown two different arrays of balls on a computer screen, one above the other, and are asked to solve the problem by planning and executing the shortest sequence of moves so that the balls in the bottom array (the work space) match the pattern of balls in the top array (the goal space). The problems are graded in difficulty in that they require between one and five moves. Because of the constrained arrangement of the balls into three “pockets,” some of the more difficult four- and five-move problems require certain critical balls to be shunted into temporary locations to free up space for the movement of other balls (see Figure 1). Therefore, this is more than a visuospatial matching task, because to reach a perfect solution, one needs to plan the sequence of moves ahead before response initiation. In the “one touch” version (Owen et al. 1995) used by Hodgson et al. (2000, 2002), the subjects are not required to execute the task manually but must indicate the number of moves required to solve the problem by a single touch of a key. In this case, it can be inferred that eye movements prior to the response reflect the planning process.
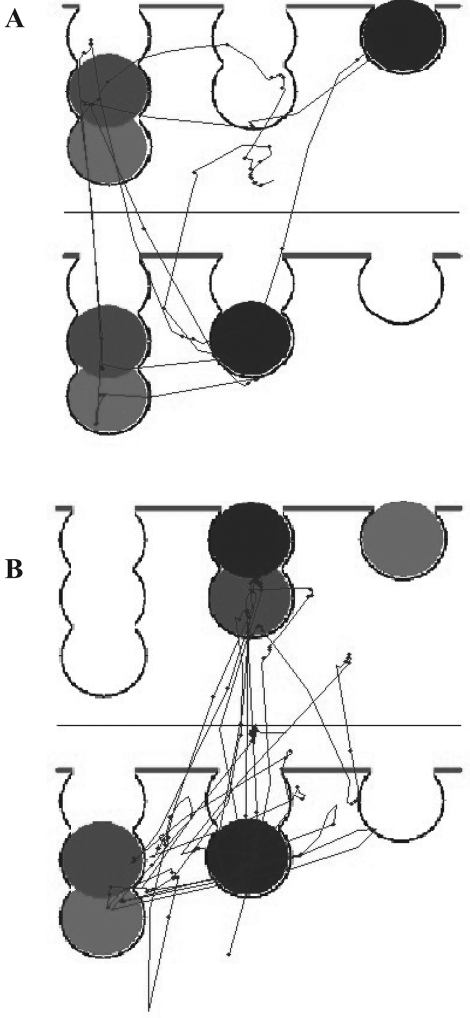
Figure 1. Example X–Y plots of subjects planning solutions to one-move (A) or four-move (B) one touch Tower of London problems. The upper panel is the goal space, and the lower panel is the work space. The more complex, four-move problem is associated with more saccades and fixations, particularly in the work space.
The gaze shifts performed by healthy subjects on this task correlated with discrete phases of problem solving. There was a strong bias for fixations to occur in the goal space at the beginning of the trial, no matter how difficult the problem. During the remainder of the trial, the bias was for fixations to occur mostly in the work space. As problem complexity increased, progressively more time was spent fixating the work space, and sampling of the goal space occurred throughout the trial as well as at the beginning. This suggests that on easier problems, the initial fixations directed toward the goal space reflected the acquisition of task-relevant information, which was held in working memory while possible solutions were worked out or elaborated through fixations in the work space. The increasing time spent in the work space as problems' solutions require more moves can be seen as reflecting the elaboration of more complex solutions. Furthermore, because the number of possible alternative moves increase at the harder levels, subjects appeared to refixate the goal space during solution elaboration as a strategy to relieve the increasing burden on working memory (Hodgson et al., 2000, 2002). In addition, the planners who made very few errors selectively biased their gaze in the work space to the balls that were critical to the problem in hand, whereas the error makers directed their gaze to irrelevant balls or to the location of balls that were relevant in the previous problem (Hodgson et al., 2000). The finding that planning skill was related to the locus of fixations in the work space supports the view that work space fixations reflect the planning of solutions.
Patients with schizophrenia are impaired on manual and one touch computerized versions of the Tower of London task (Elliott et al., 1998; Hutton et al., 1998; Morris, Rushe, Woodruffe, & Murray, 1995; Pantelis et al., 1997). Patients with schizoaffective disorder are equally as impaired as patients with schizophrenia (Stip et al., 2005). In general, patients solve fewer problems than healthy control subjects and take more moves to solve problems correctly. The aim of the current study is to elucidate whether specific executive or more general abnormalities underlie the planning impairment in schizophrenia and schizoaffective disorder by examining gaze strategies during performance of the one touch Tower of London task.
On the basis of the previous studies using this paradigm, several predictions can be made that implicate specific executive deficits of working memory or attentional set shifting as underlying planning impairments. Hodgson et al. (2002) found that patients with Parkinson's disease made more errors and spent equivalent amounts of time in the goal space and work space across all levels of problem difficulty, in contrast to healthy controls, who increasingly biased their gaze toward the work space as problem complexity increased. This absence of gaze bias to the work space was considered to represent a transsaccadic failure of working memory. In other words, the Parkinson's disease patients were unable to maintain the goal position of balls in working memory sufficiently to elaborate the problems in the work space, and this resulted in the need to spend more time fixating the goal space than controls. One of the most consistently reported cognitive abnormalities in schizophrenia is impaired working memory (see Lee & Park, 2005). This can be present in the absence of other cognitive impairments, and working memory performance has been found to correlate with planning impairments on the CANTAB version of the Tower of London task (Badcock, Michie, & Rock, 2005; Joyce, Hutton, Mutsatsa, & Barnes, 2005). One prediction that follows from the observation of patients with Parkinson's disease is that patients with schizophrenia will also fail to show a gaze bias toward the work space as problems become more complex.
A second possibility is that patients will be unable to shift between optimum gaze strategies for the solution of different problems. Hodgson et al. (2000) found that the healthy subjects who made errors tended to bias their gaze in the same way for each trial irrespective of the location of task-critical balls. In addition, when the optimum gaze strategy changed between two successive trials, response times were significantly longer on the second of the trials, indicative of an interference effect from the previous trial in the error makers. Such an abnormality would be expected if subjects have difficulty in disengaging from one response set and shifting to another. Problems with response inhibition and set shifting have been well demonstrated in schizophrenia, especially during the performance of the Wisconsin Card Sorting Task (Grant & Berg, 1948), when patients characteristically perseverate with a previously successful response when this is no longer correct. In the current study, this impairment would be shown as a failure to bias gaze toward the problem-critical balls across trials.
A third possible pattern of impairment is of abnormal or restricted visual scanning of the stimulus array. This would be predicted if more nonspecific impairments, such as poor motivation or distractibility, impacted on performance. This pattern has been previously observed when patients with schizophrenia perform cognitive tasks such as feature detection paradigms with simple or complex targets (Kojima et al., 2001; Kurachi et al., 1994) or Rorschach inkblot interpretation (Minassian, Granholm, Verney, & Perry, 2005).
Method
Subjects
The patients were recruited as part of a prospective, longitudinal study of first-episode psychosis in West London. Patients eligible for the study were screened with the World Health Organization Psychosis Screen (Jablensky et al., 1992) and were recruited if they were between 16 and 50 years old, were presenting with a psychotic illness for the first time, and had received no more than 12 weeks of antipsychotic medication. The diagnosis was ascertained by means of a structured interview, the diagnostic module of the Diagnostic Interview for Psychosis (Jablensky et al., 2000), which includes items from the Operational Criteria Checklist for Psychosis (OPCRIT; McGuffin, Farmer, & Harvey, 1991) and the World Health Organization Schedules for Clinical Assessment in Neuropsychiatry (Wing et al., 1990). Two psychiatric research nurses were trained to a consistent level in the use of the diagnostic instrument by an experienced psychiatrist (Thomas R. E. Barnes). A computerized algorithm generates diagnoses under several classification systems, including the Diagnostic and Statistical Manual of Mental Disorders (3rd ed., rev.; American Psychiatric Association, 1987) and International Statistical Classification of Diseases and Related Health Problems (10th ed.; World Health Organization, 2006), and these diagnoses were verified against DSM–IV (American Psychiatric Association, 1994) criteria via OPCRIT for Windows (http://sgdp.iop.kcl.ac.uk/opcrit/). The subjects participating in the present study received DSM–IV diagnoses of schizophrenia (n = 10), schizophreniform disorder (n = 6), or schizoaffective disorder (n = 4) at the time of testing.
As part of our longitudinal study, all participants are routinely contacted 1 year later for repeat assessments. Fourteen patients agreed to undergo a repeat diagnostic interview. The diagnostic outcome of the remaining 6 patients was established by two psychiatrists (Thomas R. E. Barnes and Eileen M. Joyce) using the OPCRIT checklist to compile information from the psychiatrists and community psychiatric nurses of the responsible clinical team and from the clinical notes. The follow-up diagnoses were verified against DSM–IV criteria via OPCRIT for Windows. Nineteen patients were still under the care of their community team and taking medication 1 year after study participation. One patient was nonadherent with treatment but agreed to a face-to-face diagnostic interview with our research team. The DSM–IV diagnoses remained the same for the patients with an initial diagnosis of schizophrenia or schizoaffective disorder. All patients with an initial diagnosis of schizophreniform disorder subsequently fulfilled the DSM–IV criteria for schizophrenia at 1 year because they had endured either psychotic symptoms or a psychotic relapse. As part of the structured interview, patients are asked about drug and alcohol intake, and this is factored into the algorithm that generates diagnoses.
These patients were compared with 20 healthy volunteers recruited from the same catchment area as patients through advertisements in local colleges and hospitals. Exclusion criteria were a history of psychiatric illness in subjects or their first-degree relatives; previous head injury, neurological illness, or endocrine disorder affecting brain function, such as epilepsy and thyroid disease; and drug or alcohol abuse. Permission to conduct the study was obtained from Merton, Sutton and Wandsworth, Riverside, and Ealing research ethics committees. All subjects gave written informed consent and were paid an honorarium for their time.
Symptom type and severity were assessed in patients at the time of recruitment via the Scales for the Assessment of Positive Symptoms (Andreasen, 1983) and Negative Symptoms (Andreasen, 1981). Scores for the three syndromes of schizophrenia (Liddle & Barnes, 1990) were calculated (positive: sum of Scale for the Assessment of Positive Symptoms Hallucinations and Delusions global subscale scores; disorganization: sum of Scale for the Assessment of Positive Symptoms Bizarre Behaviour and Positive Thought Disorder global subscale scores; negative: sum of all Scale for the Assessment of Negative Symptoms global subscale scores) and expressed as the ratio of the maximum possible score. Cognitive assessments were performed when the patients were clinically stable and able to cooperate with the procedure, as judged by the clinical team and research nurses, a median of 30 days after clinical assessment. All patients were being prescribed antipsychotic medication: Eighteen patients were receiving second-generation and 2 were receiving first-generation antipsychotics.
Neuropsychological Assessments
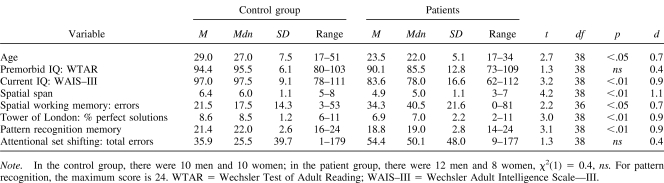
Premorbid IQ was assessed with the Wechsler Test of Adult Reading (Wechsler, 2001). Current IQ was estimated from the four subtest forms of the Wechsler Adult Intelligence Scale III (Wechsler, 1999), validated for use in schizophrenia (Blyler, Gold, Iannone, & Buchanan, 2000). Executive and memory tests were taken from the CANTAB as follows.
Spatial span (Owen et al., 1990)
This test measures the ability to remember the order of sequences of squares presented on the screen in increasing number.
Pattern recognition memory (Sahakian et al., 1988)
Twelve abstract visual stimuli are presented sequentially on the screen. Each stimulus is then presented along with a novel stimulus, and patients are asked to touch the familiar stimulus. This is repeated with 12 different stimuli, giving a maximum possible score of 24.
Spatial working memory (Owen et al., 1990)
Patients are required to open sets of boxes, varying between three and eight in number, to find tokens. Errors are recorded when boxes in which tokens have been found are reopened.
Planning (Owen et al., 1990)
Subjects move colored balls in an arrangement displayed on the screen to match a goal arrangement. Subjects are asked to attempt the solution in the minimum number of moves, which could be two, three, four, or five. Accuracy is measured by the proportion of problems solved in the minimum number of moves. Response times are recorded during execution of the problems and also during execution of a yoked control task that mimics the exact sequence of moves generated by the subject during problem solution. Subtraction of the copying times from the execution times gives a measure of thinking time, calculated both before the solution is attempted (initial thinking time) and during the subsequent period (subsequent thinking time).
Attentional set shifting (Owen et al., 1991)
Subjects are required to learn a series of visual discriminations. One of two stimulus dimensions (shape or line) is relevant. Once correct responding is established, subjects are introduced to different exemplars of the same dimension for correct responding, which tests their ability to generalize the rule they have just learned (intradimensional shift). At the later, extradimensional shift stage, the rule is reversed, so that a previously irrelevant dimension now becomes relevant. This assesses the ability to inhibit the previously correct response set by shifting attention from one dimension to another.
Planning With Eye Movement Recording
This was adapted from the one touch Tower of London task (Owen et al., 1995). The stimuli were identical in configuration to those presented in the CANTAB planning task described above. Each subject viewed 20 pictures in which the arrangement of balls in the lower field was fixed and the upper ball configuration varied from trial to trial (see Figure 1). Subjects viewed each picture twice, for a total of 40 trials. Problems differed in difficulty in that they required a minimum of one move to a maximum of five.
Subjects were instructed to plan the movements of the colored balls in the imaginary pockets so that the pattern of balls in the upper array matched the pattern of those in the lower array. Special effort was made to ensure that subjects understood that they would now be planning the moves in their head rather than touching the screen and moving balls by hand. For each trial, a central fixation dot was first displayed. This was extinguished when the experimenter was satisfied that fixation had occurred, and the problem picture was then displayed. Once the subjects thought they had worked out the correct solution to each problem, they pressed the mouse key and gave a verbal response to indicate the minimum number of moves required. Error trials occurred when subjects indicated the wrong number of moves.
Eye movements were recorded with the Eyelink system (Sensorimotoric Systems GMbH, Berlin, Germany), a video-based pupil tracker, with head movement compensation system sampling at 250 Hz. Subjects were seated in front of the display monitor approximately 60 cm from the screen. Pupil position was monitored via two miniature infrared charge-coupled device video cameras mounted on an adjustable headband. Subjects were instructed to keep head movements to a minimum, and no active restraint of head movements was required to obtain sufficiently accurate gaze position recordings. Online parsing of saccades, fixations, and blinks was performed by Eyelink parser software. Individual saccades were defined as positions in the eye position signal where absolute velocity information rose above 30° per second for more than two consecutive samples. Eye movement data for which the pupil was very small or missing were detected by the parsing software and removed from analysis. The Eyelink parsing software identified fixations as pauses between saccades. To exclude short fixations preceding corrective saccades, we included only fixations of over 50 ms in analysis.
Analysis of Eye Movements
Visual scanning
This was considered separately for the goal space and work space. Visual scanning measures were the number and duration of fixations, the number and amplitude of saccades, and the total scan path length in degrees of visual angle. In addition, the number of shifts of gaze that occurred from fixations in the work space to fixations in the goal space, and vice versa, was determined.
Analysis of gaze periods
Hodgson et al. (2000) demonstrated that whereas gaze is largely directed toward the work space on complex trials, throughout these trials the subject regularly makes shifts in gaze to the goal space. Thus, gaze directed toward the goal space or work space can be divided into short periods during which only a single fixation occurs or longer periods of many saccades and fixations. To determine the number and duration of these periods, we identified three types of gaze period: (a) check periods, when the particular spell in either the work space or the goal space was characterized by a single fixation (e.g., the subject may be fixating in the work space, then look up to the goal space to check the location of a particular ball and, without making another saccade within the goal space, immediately return to the work space); (b) short-gaze periods, when a single horizontal saccade within the work space or goal space occurred; and (c) long-gaze periods, when a succession of more than one saccade was made within the goal space or work space. The boundaries of each gaze period were defined as the period between the start of the first fixation and the end of the last fixation.1 Hence, in addition to the total number of check, short-gaze, and long-gaze periods during the trial, it was also possible to measure the total duration of each type of gaze period. Furthermore, the total number of saccades that occurred during long-gaze periods within a trial was established.
Problem-dependent gaze shifts
Hodgson et al. (2000) found that gaze was not distributed evenly across all locations within the work space region but was biased to certain problem-relevant items. To examine this bias, we categorized fixations according to where they landed on a 3 × 2 grid, which divided pictures into sectors of equal area (Figure 1). For four-move problems, blue ball trials always require a shunting maneuver in which the central blue ball is moved to a temporary location while other, intervening moves are performed. Hence, analysis of the left and central regions of the lower portion of the screen allows any selectivity of gaze to be determined.
Statistical Methods
Comparison between patient and control groups on behavioral and eye movement measures were analyzed with separate analyses of variance, t tests, and chi-square tests when appropriate, via SPSS Version 13.0. Effect sizes (ηp2) are given for group comparisons.
Results
The means (and standard deviations) for the schizophrenia syndrome scores were as follows: positive syndrome, M = 0.78 (SD = 0.27); disorganization syndrome, M = 0.42 (SD = 0.36); and negative syndrome, M = 0.38 (SD = 0.25). Patients and control subjects were matched for sex ratio, but controls were significantly older than patients (see Table 1).
Table 1. Comparison of Patient and Control Groups on Demographic and Neuropsychological Variables.
Neuropsychology
The pattern of impairment on the neuropsychological tasks was similar to that previously reported in a different, larger group of first-episode patients (Joyce et al., 2002, 2005). There was no significant difference in the estimated premorbid IQ between the groups, but the controls had a significantly higher current IQ (Table 1). Further analysis revealed that current IQ had fallen from estimated premorbid values in patients, t(19) = 2.92, p < .01, but not controls, t(19) = −1.26, ns. Table 1 also shows that the patients were significantly impaired on the CANTAB pattern recognition memory, spatial working memory, spatial span, and planning tasks and made more errors on the attentional set shifting task, although this failed to reach significance.
Planning With Eye Movement Recordings
General performance
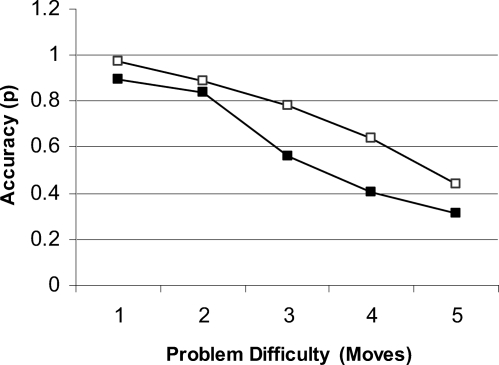
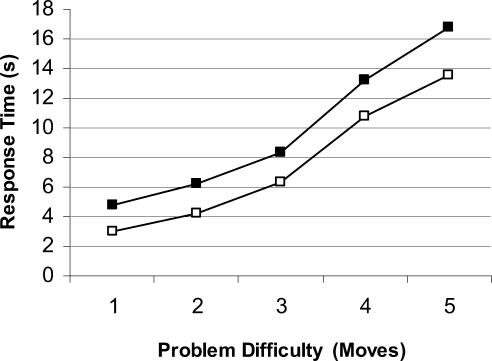
Accuracy and response times are shown in Figures 2 and 3. Accuracy decreased significantly as problem difficultly increased, F(4, 152) = 80.63, p < .001. Patients made more errors than controls, F(1, 38) = 5.70, p < .05 (ηp2 = .13), and the effect of problem difficulty tended to be greater in patients, F(4, 152) = 2.16, p = .08 (ηp2 = .05). Response times increased significantly with problem length, F(4, 152) = 82.50, p < .01. The patients took significantly longer to execute a response overall, F(1, 38) = 4.13, p < .05 (ηp2 = .10), with no interaction effect between subject group and problem difficulty, F(4, 152) = 0.22 (ηp2 = .006).
Figure 2. Accuracy for both patients (black squares) and healthy controls (white squares) on the one touch Tower of London task for each level of problem difficulty.
Figure 3. Response time for both patients (black squares) and healthy controls (white squares) on the one touch Tower of London task for each level of problem difficulty.
Scanning and eye movements
There were no significant group differences on any of the measures of visual scanning, including scan path length, F(1, 38) = 0.43 (ηp2 = .01); amplitude of saccades, F(1, 38) = 0.01 (ηp2 = .00); and number of saccadic shifts between the goal space and work space, F(1, 38) = 0.08 (ηp2 = .00). Taken together, these results indicate that the scan paths of the patient group were not abnormal or restricted compared with those of the controls. For both groups, scan path length significantly increased with problem difficulty, F(4, 152) = 55.39, p < .01, and these were longer in the work space than in the goal space, F(1, 152) = 46.21, p < .01.
Number of gaze periods
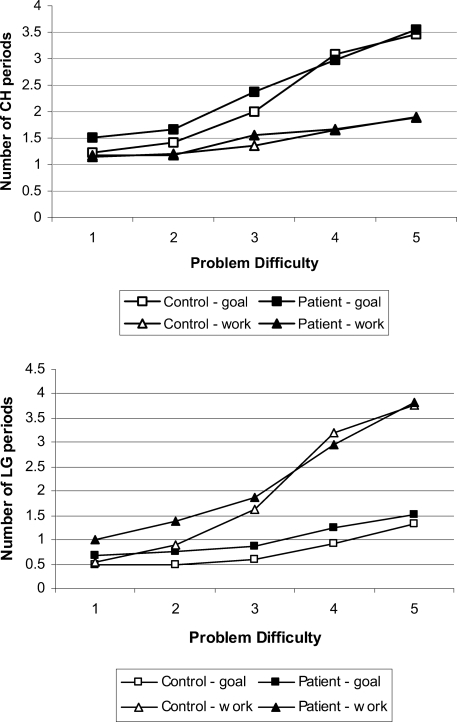
There was a significant increase in the numbers of check periods in the goal space compared with the work space as problem difficulty increased (Figure 4, top panel), F(4, 152) = 25.93, p < .01. This effect did not interact with subject group, F(4, 152) = 0.43 (ηp2 = .01), which suggests that this gaze bias was intact in patients. The opposite pattern occurred for the number of long-gaze periods (see Figure 4, bottom panel), with an increase in the work space compared with the goal space, F(4, 152) = 48.55, p < .01. Again, this did not interact with subject group, F(4, 152) = 1.46 (ηp2 = .04). To clarify this effect further, we performed separate analyses for patient and control groups. This revealed that patients directed more long-gaze periods to the work space compared with the goal space with increasing problem difficulty, F(4, 76) = 18.12, p < .01 (ηp2 = .49), with the control group showing a slightly stronger effect, F(4, 76) = 32.03, p < .01 (ηp2 = .63). The short-gaze periods showed a pattern similar to that for the check periods, as these were directed more to the goal space than the work space as the problems increased in difficulty, F(4, 152) = 11.92, p < .01.
Figure 4. Total number of check periods (CH; top panel) and longer periods of gaze (LG; bottom panel) for patients and controls. The periods directed to the goal space are marked as squares, and periods directed to the work space are marked as triangles.
Duration of gaze periods
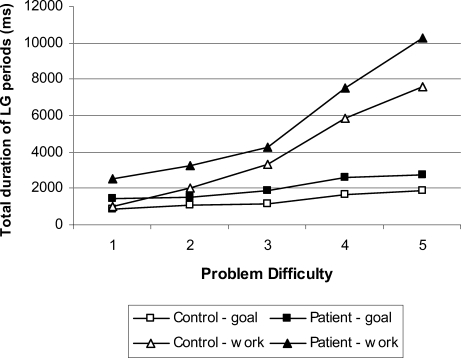
The total duration of long-gaze periods increased with problem difficulty, F(4, 152) = 61.20, p < .01, and was significantly longer when directed to the work space compared with the goal space, F(1, 152) = 63.10, p < .01. The absence of a three-way interaction of goal space versus workspace, problem difficulty, and group, F(4, 152) = 0.50, ns (ηp2 = .01), indicates that these effects were equivalent in both groups. When correct and error trials were separated out in the analysis, the patient group showed the same gaze bias on correct, F(1, 19) = 16.53, p < .05 (ηp2 = .47), and error trials, F(1, 19) = 9.26, p < .05 (ηp2 = .33). This pattern was also found in the control group on correct, F(1, 19) = 14.31, p < .05 (ηp2 = .43), and error trials, F(1, 19) = 9.27, p < .05 (ηp2 = .33). The patients showed significantly increased total duration of long-gaze periods compared with controls, F(1, 38) = 6.93, p < .05 (ηp2 = .11; see Figure 5), but not for the check, F(1, 38) = 0.77, ns (ηp2 = .02), or short-gaze periods, F(1, 38) = 1.54, ns (ηp2 = .04). Within the long-gaze periods, there was a tendency for the increase in duration to be accompanied by an increase in the number of fixations in the patient group, F(1, 38) = 2.96, p = .09 (ηp2 = .07).
Figure 5. Total duration of long periods of gaze (LG) for each level of problem difficulty for both patients and controls. Duration of periods of gaze directed to the goal space are marked as squares, and those directed to the work space are marked as triangles.
Problem-dependent gaze shifts
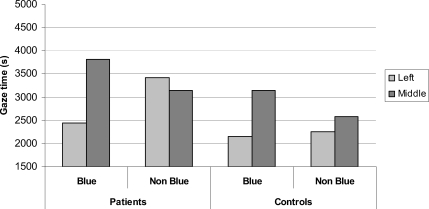
Examining the total duration of fixations in the left and middle regions of the work space on blue ball and nonblue ball problems revealed a significant gaze bias to the central area on blue ball trials that was not present on nonblue ball trials (see Figure 6), evidenced by an interaction between problem type (blue ball vs. nonblue ball) and location (left or middle), F(1, 38) = 13.98, p < .01. There was no group interaction, indicating that the gaze bias to the central location on blue ball trials was found equally in both patient and control groups, F(1, 38) = 2.63, ns (ηp2 = .07). To clarify this effect further, individual group analyses confirmed intact gaze biases in both control subjects, F(1, 38) = 6.21, p < .05 (ηp2 = .23), and patients, F(1, 38) = 8.76, p < .01 (ηp2 = .32).
Figure 6. Mean total gaze time spent by patients and controls in left and middle locations for blue and nonblue problem types.
Subgroup analysis of schizophrenia patients
Analysis of the 16 subjects in the sample who received a diagnosis of schizophrenia 1 year after presentation revealed the same pattern of effects as the larger group that included patients with schizoaffective disorder. There were no significant differences between the patient group and the controls on scan path length, F(1, 34) = 0.27 (ηp2 = .01); number of shifts, F(1, 34) = 0.01 (ηp2 = .00); or amplitude of saccades, F(1, 34) = 0.01, (ηp2 = .00). In addition, the schizophrenia subgroup showed the same increase as did controls in the number of check periods directed to the goal space with increasing difficulty, F(4, 136) = 0.01, ns (ηp2 = .00). Similarly, there was an increased number of long-gaze periods directed to the work space compared with the goal space, F(4, 136) = 48.99, p < .001, and this did not interact with group, F(4, 136) = 0.64, (ηp2 = .03). As before, analysis of this effect in each group confirmed this gaze bias in both control subjects, F(4, 76) = 21.11, p < .001 (ηp2 = .53), and patients, F(4, 60) = 18.10, p < .001 (ηp2 = .55). The increased duration of longer gaze periods overall was also significant in this subgroup, F(1, 34) = 4.96, p < .05 (ηp2 = .13). These patients also directed their gaze to problem-critical balls in the same manner as did the control group, F(1, 34) = 1.43, ns (ηp2 = .04). These findings indicate a preserved pattern of gaze biases in patients with a diagnosis of schizophrenia.
Discussion
In this study we have measured eye movements during the performance of a computerized version of the Tower of London task to specify the source of planning abnormalities in patients with schizophrenia or schizoaffective disorder (Hodgson et al., 2000, 2002; Owen et al., 1995). In comparison to healthy control subjects, the patients were impaired on the task, as demonstrated by significantly longer response times and more decision errors. However, the patients showed the same gaze biases as the controls prior to making a response, which indicates that they understood the requirements of the task, approached the task in a strategic manner by identifying the nature of the problem, and used appropriate fixation strategies to plan and elaborate solutions. Thus, when gaze periods were parsed into single glances between the goal space and the work space (check periods), single shifts within the work space or goal space (short-gaze periods) and long-gaze periods during which a number of successive eye movements were made within the goal space or work space, we found both groups directed their gaze in the same manner and that this corresponded to similar planning strategies identified in previous studies of healthy subjects (Hodgson et al., 2000, 2002).
As the problems became more difficult, both groups showed a progressive increase in the number of long-gaze periods in the work space and more short-gaze and check periods directed at the goal space. The increased number of long sequences of eye movements directed at the work space on more difficult problems is compatible with the idea that both groups used this space to plan the sequence of moves needed to solve the task. Mirroring this, the increasing number of single glances and single eye movements directed at the goal space suggests that both groups were checking the goal array more frequently as problems became more difficult. When planning proficiency was examined, patients were no different from controls in the way they directed their gaze during the solution of different problems. During some problems requiring four or five moves, a specific ball needs initially to be shunted to an intermediate position to enable the execution of other, intermediate moves (see Figure 1). It has previously been shown that planners who make very few errors locate and focus on these problem-critical balls in the work space, whereas error makers tend to direct their gaze in the same way for each problem irrespective of where the problem-critical ball is located (Hodgson et al., 2000). In the current study, patients correctly directed their gaze at problem-critical balls to the same degree as healthy controls. This suggests that the patients were able to shift between optimum gaze strategies for the solution of different problems and excludes difficulties with response inhibition and attentional set shifting as an explanation of patients' planning impairment.
The general pattern of gaze biases during planning was remarkably similar in patients and healthy controls, but the use of these cognitive strategies was not sufficient to match control performance on the one touch Tower of London task. Patients took longer to make decisions and were less accurate. We were able to specify further the prolonged response times in the patients by analyzing the gaze periods, which revealed a specific increase in the duration of the long-gaze periods in the goal space and work space. Although this pattern of abnormality was not predicted, it may allow for a resolution of the apparent contradiction that patients show performance abnormalities yet show intact gaze biases.
A recent study by Fuller, Luck, McMahon, and Gold (2005), using a change detection paradigm, provided evidence relevant to the interpretation of this finding. The authors found that when schizophrenia patients were required to search and make decisions about a simple, three-item array of squares, they showed slowing of postperceptual consolidation processes in working memory. That is, in a pattern-masking working memory paradigm that included a perceptual control condition, the authors found that patients with schizophrenia never reached control performance even when the interval between the target and the mask was twice that needed for controls to perform at ceiling. This finding demonstrates that patients with schizophrenia are impaired even at the very early encoding stage of working memory, in which perceptions are consolidated for better maintenance in working memory. On the basis of their finding, Fuller et al. (2005) predicted that on visual tasks, schizophrenia patients will try to compensate by increasing the amount of time they spend inspecting an array of targets. Consistent with this, our patients showed increased duration of longer periods of gaze directed to both the goal space and the work space. The lack of an interaction effect with trial type can be explained by the fact that the visual complexity of the stimuli does not vary across trials—the requirement for internal manipulation of the balls determines task difficulty. Fuller et al. (2005) also predicted that under conditions of high working memory load, when there is a need for rapid updating of information in working memory, slow consolidation will render perceptual representations vulnerable to interference. In our paradigm, it might be predicted that patients will check the goal space more often than controls as the problems become more complex. The fact that we did not find this pattern suggests the possibility that the patients failed to adopt this compensatory strategy and thus made more errors. One could explore this explanation further with this task by examining the effect of direct instruction and cuing (see Unterrainer, Rahm, Leonhart, Ruff, & Halsband, 2003) on the use of compensatory gaze strategies and subsequent performance.
It is important to note that the increase in duration of long-gaze periods was not indicative of generalized slowing. The duration of check and short periods of gaze did not differ between groups. Furthermore, on the CANTAB version of the Tower of London task, in which subjects were actually required to move the balls on a touch screen, response latencies were not increased. Thus, the increased duration of long-gaze periods appears to represent a response to slowed working memory consolidation specifically rather than generalized slowing. Two further observations support this conclusion. First, the extended long-gaze periods were a consistent facet of the way the patients performed the Tower of London task in that they were present irrespective of whether the problems were solved correctly. Second, extended long-gaze periods were present at all levels of problem difficulty, whereas increased decision errors only became apparent from the three-move level of difficulty upward.
This interpretation of our findings is also compatible with a study by Hartman, Steketee, Silva, Lanning, and McCann (2003), who found that slowing of the speed of encoding in working memory was the strongest explanatory factor for poor performance on another executive task, the Wisconsin Card Sorting Test. Also, a recent meta-analysis of working memory studies found that working memory impairment was consistently reported even at very short delays, suggesting that impaired encoding of visual targets in working memory is a fundamental abnormality in patients with schizophrenia (Lee & Park, 2005). Our findings might also be analogous to those of Minassian et al. (2005), who found that patients with schizophrenia displayed staring episodes when inspecting Rorschach inkblots, and those of Wolwer and Gaebel (2002), who found increased planning fixations during the performance of the Trail Making Test.
However, other abnormalities of working memory processes, such as the maintenance of task-relevant information, are also present in patients with schizophrenia (Barch et al., 2001; Braver, Barch, & Cohen, 1999). In fact, we originally predicted a pattern of abnormality that would be more compatible with this hypothesis on the basis of previous findings with Parkinson's disease patients on this task. Hodgson et al. (2002) found that patients with Parkinson's disease failed to bias their gaze toward the work space as problem complexity increased and spent equivalent amounts of time in the goal space and the work space across all levels of problem difficulty. The favored explanation was that this was an exaggeration of the finding that healthy subjects use fixations in the goal space to relieve the burden of having to hold the goal array in working memory during planning in the work space. In other words, Parkinson's disease patients “keep forgetting the arrangement of the balls in the Goalspace every time they look away” (Hodgson et al., 2002, p. 419) and consequently look at the goal space more often. This abnormality was considered to reflect reduced dopamine neurotransmission in the frontostriatal processes mediating working memory (Hodgson et al., 2002), a conclusion compatible with accumulating evidence from animal studies suggesting that dopamine acts to determine how strongly representations are maintained in working memory (see Seamans & Yang, 2004). Our data do not allow us to understand the differences between the performance of the schizophrenia and Parkinson's disease patients on this task with respect to working memory processing but presumably reflect the task requirements and the degree of dopamine dysfunction in frontostriatal processes in the two disorders.
These findings of generally intact gaze biases exclude an explanation of the planning impairment in terms of more general impairments, such as poor motivation or distractibility. Furthermore, more basic impairments of oculomotor function seen in the disorder, such as impaired smooth pursuit and saccadic eye movements (e.g., Hutton et al., 2004) or restricted visual scanning of objects (e.g., Koijma et al., 2001), also do not account for planning errors, as we found no differences between patients and controls on global measures of scanning, such as scan path length, and there was no evidence of a reduced number of saccades or saccadic hypometria.
A possible explanation of the intact aspects of performance is that the patients were tested following their first presentation of psychosis and may be a high-functioning group, especially as a proportion of the patients received a diagnosis of schizophreniform disorder, not schizophrenia. However, when we followed up with the patients 1 year later and reassessed the diagnoses, we found that all patients had an enduring mental illness corresponding to DSM–IV schizophrenia or schizoaffective disorder and that all but 1 were actively under the care of community mental health teams; the remaining patient, having disengaged, was psychotic when reassessed. Furthermore, the background neuropsychological profile of the patients at the time of the current study indicated that this group was impaired on a wide range of cognitive functions, including spatial span, spatial working memory, and recognition memory, to a similar degree as other first-episode groups with confirmed schizophrenia that we have tested (Joyce et al., 2002). Furthermore, although the patient and control groups were matched for premorbid IQ, the patients showed a small but significant fall in current IQ from premorbid levels, another finding previously reported by us in a different group (Joyce et al., 2005), which again suggests that we were not testing an atypical group of patients. We also examined whether the inclusion of patients with schizoaffective disorder might have biased the results. When we excluded these cases, the profile of results remained the same for the subgroup with a diagnosis of schizophrenia. The similarity in planning performance by patients with schizophrenia and schizoaffective disorder is supported by a recent study that compared the two diagnostic groups on the CANTAB version of the planning task and found no differences in performance (Stip et al., 2005). Furthermore, Gooding and Tallent (2002) found no differences in the executive performance of these two diagnostic groups on a spatial working memory task and the Wisconsin Card Sorting Test.
Our finding that impaired planning in schizophrenia could not be accounted for by abnormal gaze strategies or other, more general impairments does not preclude such explanations being relevant for other patient groups and settings. However, our findings suggest that patients with schizophrenia, despite having generalized cognitive impairments, can approach complex tasks and develop strategies in a perfectly normal manner but are compromised by slowing of information processing in working memory. This finding adds to the growing body of evidence suggesting that, whatever other cognitive impairments are present in individuals with schizophrenia, working memory impairment is a central feature of the disorder.
Acknowledgments
Portions of this study were presented at the International Congress on Schizophrenia Research, Savannah, Georgia, April 2005. This study was supported by a Wellcome Trust program grant. We are grateful to the consultants and nurses of West London and South West London and St. George's Mental Health National Health Service Trusts for greatly facilitating the study. We thank Chris Kennard for use of the oculomotor laboratory and Clare Foster for administrative support.
Footnotes
For check periods, this would correspond to the fixation duration.
References
- American Psychiatric Association (1987). Diagnostic and statistical manual of mental disorders (3rd ed., text rev.). Washington, DC: Author. [Google Scholar]
- American Psychiatric Association (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Andreasen N. C. (1981). Schedule for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa Press. [Google Scholar]
- Andreasen N. C. (1983). Schedule for the Assessment of Positive Symptoms (SAPS). Iowa City: University of Iowa Press. [Google Scholar]
- Badcock J. C., Michie P. T., & Rock D. (2005). Spatial working memory and planning ability: Contrasts between schizophrenia and bipolar disorder. Cortex, 41, 753–763. [DOI] [PubMed] [Google Scholar]
- Ballard D., Hayhoe M., & Pelz J. (1995). Memory representations in natural tasks. Journal of Cognitive Neuroscience, 7, 66–80. [DOI] [PubMed] [Google Scholar]
- Barch D. M., Cartet C. S., Braver T. S., Macdonald A., Sabb F. W., Noll D. C., & Cohen J. D. (2001). Selective deficits in prefrontal regions in medication naive schizophrenia patients. Archives of General Psychiatry, 50, 280–288. [DOI] [PubMed] [Google Scholar]
- Bilder R. M., Goldman R. S., Robinson D., Reiter G., Bell L., Bates J. A., et al. (2000). Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. American Journal of Psychiatry, 157, 549–559. [DOI] [PubMed] [Google Scholar]
- Blyler C. R., Gold J. M., Iannone V. N., & Buchanan R. W. (2000). Short form of the WAIS-III for use with patients with schizophrenia. Schizophrenia Research, 46, 209–215. [DOI] [PubMed] [Google Scholar]
- Braver T. S., Barch D. M., & Cohen J. D. (1999). Cognition and control in schizophrenia: A computational model of dopamine and prefrontal function. Biological Psychiatry, 46, 312–328. [DOI] [PubMed] [Google Scholar]
- Elliott R., McKenna P. J., Robbins T. W., & Sahakian B. J. (1998). Specific neuropsychological deficits in schizophrenic patients with preserved intellectual function. Cognitive Neuropsychiatry, 3, 45–70. [Google Scholar]
- Epelboim J., Steinman R. M., Kowler E., Edwards M., Pizlo Z., Erkelens C. J., & Collewijn H. (1995). The function of visual search and memory in sequential looking tasks. Vision Research, 35, 3401–3422. [DOI] [PubMed] [Google Scholar]
- Fuller R. L., Luck S. J., McMahon R. P., & Gold J. M. (2005). Working memory consolidation is abnormally slow in schizophrenia. Journal of Abnormal Psychology, 114, 279–290. [DOI] [PubMed] [Google Scholar]
- Gooding D. C., & Tallent K. A. (2002). Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: A tale of two disorders? Schizophrenia Research, 53, 209–218. [DOI] [PubMed] [Google Scholar]
- Grant D. A., & Berg E. A. (1948). A behavioral analysis of the degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem Journal of Experimental Psychology, 38, 404–411. [DOI] [PubMed] [Google Scholar]
- Green M. F., Kern R. S., Braff D. L., & Mintz J. (2000). Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”?. Schizophrenia Bulletin, 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Hartman M., Steketee M. C., Silva S., Lanning K., & McCann H. (2003). Working memory and schizophrenia: Evidence for slowed encoding. Schizophrenia Research, 59, 99–113. [DOI] [PubMed] [Google Scholar]
- Hayhoe M., & Ballard D. (2005). Eye movements in natural behaviour. Trends in Cognitive Science, 9, 188–194. [DOI] [PubMed] [Google Scholar]
- Hayhoe M. M., Bensinger D. G., & Ballard D. H. (1998). Task constraints in visual working memory. Vision Research, 38, 125–137. [DOI] [PubMed] [Google Scholar]
- Hodgson T. L., Bajwa A., Owen A. M., & Kennard C. (2000). The strategic control of gaze direction in the Tower of London task. Journal of Cognitive Neuroscience, 12, 894–907. [DOI] [PubMed] [Google Scholar]
- Hodgson T. L., Tiesman B., Owen A. M., & Kennard C. (2002). Abnormal gaze strategies during problem solving in Parkinson's disease. Neuropsychologia, 40, 411–422. [DOI] [PubMed] [Google Scholar]
- Hutton S. B., Huddy V., Barnes T. R. E., Robbins T. W., Crawford T. J., Kennard C., et al. (2004). The relationship between antisaccades, smooth pursuit, and executive dysfunction in first-episode schizophrenia. Biological Psychiatry, 56, 553–559. [DOI] [PubMed] [Google Scholar]
- Hutton S. B., Puri B. K., Duncan L. J., Robbins T. W., Barnes T. R. E., & Joyce E. M. (1998). Executive function in first-episode schizophrenia. Psychological Medicine, 28, 463–473. [DOI] [PubMed] [Google Scholar]
- Jablensky A., McGrath J., Herrman H., Castle D., Gureje O., Evans M., et al. (2000). Psychotic disorders in urban areas: An overview of the Study on Low Prevalence Disorders. Australian and New Zealand Journal of Psychiatry, 34, 221–236. [DOI] [PubMed] [Google Scholar]
- Jablensky A., Sartorius N., Ernberg G., Anker M., Korten A., Cooper J. E., et al. (1992). Schizophrenia—manifestations, incidence and course in different cultures—A World Health Organization ten-country study. Psychological Medicine, Suppl. 20. [DOI] [PubMed] [Google Scholar]
- Joyce E. M., & Huddy V. (2004). Defining the cognitive impairment in schizophrenia. Psychological Medicine, 34, 1151–1155. [DOI] [PubMed] [Google Scholar]
- Joyce E. M., Hutton S. B., Mutsatsa S., & Barnes T. (2005). Cognitive heterogeneity in first-episode schizophrenia. British Journal of Psychiatry, 187, 516–522. [DOI] [PubMed] [Google Scholar]
- Joyce E., Hutton S., Mutsatsa S., Gibbins H., Webb E., Paul S., et al. (2002). Executive dysfunction in first-episode schizophrenia and relationship to duration of untreated psychosis: The West London Study. British Journal of Psychiatry, 181, S38–S44. [DOI] [PubMed] [Google Scholar]
- Kojima T., Matsushima E., Ohta K., Toru M., Han Y. H., Shen Y. C., et al. (2001). Stability of exploratory eye movements as a marker of schizophrenia—a WHO multi-center study. Schizophrenia Research, 52, 203–213. [DOI] [PubMed] [Google Scholar]
- Kurachi M., Matsui M., Kiba K., Suzuki M., Tsunoda M., & Yamaguchi N. (1994). Limited visual-search on the WAIS Picture Completion test in patients with schizophrenia. Schizophrenia Research, 12, 75–80. [DOI] [PubMed] [Google Scholar]
- Land M., & Furneaux S. (1997). The knowledge base of the oculomotor system. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 352, 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., & Park S. (2005). Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology, 114, 599–611. [DOI] [PubMed] [Google Scholar]
- Liddle P. F., & Barnes T. R. (1990). Syndromes of chronic schizophrenia. British Journal of Psychiatry, 157, 558–561. [DOI] [PubMed] [Google Scholar]
- Liversedge S. P., & Finlay J. M. (2000). Saccadic eye movement and cognition. Trends in Cognitive Science, 4, 6–14. [DOI] [PubMed] [Google Scholar]
- MacDonald A. W., & Carter C. S. (2002). Cognitive experimental approaches to investigating impaired cognition in schizophrenia: A paradigm shift. Journal of Clinical and Experimental Neuropsychology, 24, 873–882. [DOI] [PubMed] [Google Scholar]
- McGuffin P., Farmer A., & Harvey I. (1991). A polydiagnostic application of operational criteria in studies of psychotic illness: Development and reliability of the Opcrit System. Archives of General Psychiatry, 48, 764–770. [DOI] [PubMed] [Google Scholar]
- Minassian A., Granholm E., Verney S., & Perry W. (2005). Visual scanning deficits in schizophrenia and their relationship to executive functioning impairment. Schizophrenia Research, 74, 69–79. [DOI] [PubMed] [Google Scholar]
- Mohamed S., Paulsen J. S., O'Leary D., Arndt S., & Andreasen N. (1999). Generalized cognitive deficits in schizophrenia: A study of first-episode patients. Archives of General Psychiatry, 56, 749–754. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Rushe T., Woodruffe P. W. R., & Murray R. M. (1995). Problem solving in schizophrenia: A specific deficit in planning ability. Schizophrenia Research, 14, 235–246. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Downes J., Sahakian B. J., Polkey C. E., & Robbins T. W. (1990). Planning and spatial working memory following frontal lobe lesions in man. Neuropsychologia, 28, 1021–1034. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Roberts A. C., Polkey C. E., Sahakian B. J., & Robbins T. W. (1991). Extra-dimensional versus intra-dimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia, 29, 993–1006. [DOI] [PubMed] [Google Scholar]
- Owen A. M., Sahakian B. J., Hodges J. R., Summers B. A., Polkey C. E., & Robbins T. W. (1995). Dopamine-dependent frontostriatal planning deficits in early Parkinson's disease. Neuropsychology, 9, 126–140. [Google Scholar]
- Pantelis C., Barnes T. R. E., Nelson H. E., Tanner S., Weatherley L., Owen A. M., et al. (1997). Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain, 120, 1823–1843. [DOI] [PubMed] [Google Scholar]
- Sahakian B. J., Morris R. G., Evenden J. L., Heald A., Levy R., Philpot M., et al. (1988). A comparative-study of visuospatial memory and learning in Alzheimer-type dementia and Parkinson's disease. Brain, 111, 695–718. [DOI] [PubMed] [Google Scholar]
- Seamans J. K., & Yang C. R. (2004). The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Progress in Neurobiology, 74, 1–57. [DOI] [PubMed] [Google Scholar]
- Shallice T. (1982). Specific impairments of planning. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences, 298, 199–209. [DOI] [PubMed] [Google Scholar]
- Stip E., Sepehry A. A., Prouteau A., Briand L. N., Lalonde P., & Lesage A. (2005). Cognitive discernible factors between schizophrenia and schizoaffective disorder. Brain and Cognition, 59, 292–296. [DOI] [PubMed] [Google Scholar]
- Suppes P., Cohen M., Laddage R., & Floyd H. (1983). A procedural theory of eye movements in doing arithmetic. Journal of Mathematical Psychology, 27, 341–369. [Google Scholar]
- Unterrainer J. M., Rahm B., Leonhart J., Ruff C. C., & Halsband U. (2003). The Tower of London: The impact of instructions, cueing, and learning on planning abilities. Cognitive Brain Research, 17, 675–683. [DOI] [PubMed] [Google Scholar]
- Wechsler D. (1999). Wechsler Adult Intelligence Scale—Third Edition. London: Psychological Corporation. [Google Scholar]
- Wechsler D. (2001). Wechsler Test of Adult Reading. London: Psychological Corporation. [Google Scholar]
- Wing J. K., Babor T., Brugha T., Burke J., Cooper J. E., Giel R., et al. (1990). SCAN: Schedules for Clinical Assessment in Neuropsychiatry. Archives of General Psychiatry, 47, 589–593. [DOI] [PubMed] [Google Scholar]
- Wolwer W., & Gaebel W. (2002). Impaired Trail-Making Test-B performance in patients with acute schizophrenia is related to inefficient sequencing of planning and acting. Journal of Psychiatric Research, 36, 407–416. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2006). International statistical classification of diseases and related health problems (10th ed.). Geneva, Switzerland: Author. [Google Scholar]