Abstract
Ethanol exerts effects on the brain noradrenergic system, and these are thought to contribute to the sedative/hypnotic (depressant) effects of ethanol. Recent studies suggest that the norepinephrine transporter (NET) plays an important role in modulating ethanol's depressant effects. The aim of the present study was to further characterize this role. Transporter blockers with varying affinity for NET versus the serotonin transporter (desipramine > fluoxetine > citalopram) were tested for their ability to alter ethanol's depressant effects, and for comparison, hypothermic effects. Effects of desipramine on another depressant, pentobarbital, were examined. Desipramine potentiation of ethanol's depressant effects was assessed following depletion of brain norepinephrine via N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4) treatment, or depletion of brain 5-HT via para-chlorophenylalanine methyl ester hydrochloride (PCPA) treatment. The effects of co-administration of either the selective α2-adrenoreceptor agonist (dexmedetomidine) or the selective α2-adrenoreceptor antagonist (atipamezole) on desipramine's effect on ethanol's depressant effects were examined. Given the close link between stress, ethanol and norepinephrine, desipramine potentiation of ethanol's depressant effects was tested following repeated forced swim stress. Results showed that desipramine, but not SERT-selective doses of citalopram or fluoxetine, strongly potentiated the depressant (not hypothermic) effects of ethanol. These effects were mimicked by dexmedetomidine and blocked by atipamezole, but not by depletion of either norepinephrine or 5-HT. Desipramine potentiation of ethanol's depressant effects was abolished following repeated stress. Present findings further support a major role for NET and the α2-adrenoreceptor in modulating the depressant effects of ethanol, with possible implications for understanding the role of noradrenergic dysfunction in stress-related alcoholism.
Keywords: Ethanol, Norepinephrine, Norepinephrine transporter, Sedation, Stress, Serotonin
1. Introduction
The activity of noradrenergic neurons in the locus coeruleus (LC) is associated with the maintenance of arousal and consciousness (Aston-Jones and Bloom, 1981; Foote et al., 1980; Jouvet, 1969; Robbins, 1984). In rodents, administration of high doses of ethanol inhibits stimulated LC neuronal firing, and reduces norepinephrine release in vitro and extracellular forebrain levels of norepinephrine (NE) in vivo (Aston-Jones et al., 1982; Corrodi et al., 1966; Gursey and Olson, 1960; Pohorecky and Brick, 1977; Verbanck et al., 1990). The profound effects of ethanol on noradrenergic activity are believed to contribute to the sedative/hypnotic (depressant) effects that are produced by high dose ethanol (Aston-Jones et al., 1982; Howerton and Collins, 1984; Rossetti et al., 1992; Shefner and Tabakoff, 1985).
The available evidence suggests that the role of norepinephrine in mediating ethanol's depressant effects is complex. Depleting brain norepinephrine by pharmacological or genetic disruption of noradrenergic synthesis has been shown to increase the magnitude of ethanol-induced sedation/hypnosis in various mouse strains, while augmenting tyrosine hydroxylase levels had the effect of reducing these to actions (Blum et al., 1972; Erwin and Cornell, 1986; French et al.,1988; Masserano and Weiner,1982; Weinshenker et al., 2000). On the other hand, 6-hydroxydopamine ablation of the LC, but not the dorsal noradrenergic bundle, blocked the development of chronic tolerance to ethanol's depressant effects in rats and mice without affecting initial sensitivity (Ritzmann and Tabakoff, 1976; Steketee et al., 1989; Tabakoff and Ritzmann, 1977). Consistent with the contribution of the α-adrenoreceptor class of NE receptors to the interaction between norepinephrine and ethanol, treatment with α-adrenoreceptor agonists such as clonidine and dexmedetomidine has been shown to potentiate ethanol's depressant effects while, conversely, administration of α-adrenoreceptor antagonists such as atipamezole and phentolamine attenuated ethanol-induced sedation/hypnosis (Idanpaan-Heikkila et al., 1995; Lister et al., 1989; Masserano and Weiner, 1982; Seppala et al., 1994).
By removing norepinephrine from the extracellular space, the NE transporter (NET) provides a major mechanism for the regulation of synaptic levels of norepinephrine (Pacholczyk et al., 1991). In vivo voltametric studies have demonstrated that ethanol can inhibit clearance of norepinephrine in rat brain (Lin et al., 1997, 1993), although data on ethanol effects on norepinephrine reuptake in synaptosomal preparations have been equivocal (Haughey et al., 2005; Sun et al., 1977). Recent studies demonstrate that genetic and pharmacological alterations in NET affect ethanol's depressant effects. For example, quantitative trait locus analysis of differences in ethanol-induced sedation/hypnosis between ‘inbred long sleep’ (ILS) and ‘inbred short sleep’ (ISS) mice has identified a chromosomal region harboring the NET gene (Slc6a2) (Bennett and Johnson, 1998; Haughey et al., 2005; Markel et al., 1997). Interestingly, ILS mice also exhibit less forebrain NET binding and mRNA and relatively less synaptosomal norepinephrine reuptake as compared to ISS mice (Haughey et al., 2005)(c.f. Howerton et al., 1982). Moreover, treatment with the NET blockers desipramine or nisoxetine potentiates the depressant effects of ethanol, and these effects were found to be greater in ILS than ISS mice (Cott and Ogren, 1980; Haughey et al., 2005). One possible interpretation of these data is that NET deficiency promotes ethanol's depressant effects. However, constitutive gene knockout of NET in mice does not alter these effects (Haughey et al., 2005). Also, rats bred for high ethanol consumption that have lesser LC NET binding are less, rather than more, sensitive to ethanol's depressant effects (Hwang et al., 2000; Kurtz et al., 1996).
The aim of the present study was to further elucidate the role for NET in ethanol's depressant effects. We first compared the effects of three compounds with varying affinity for NET versus the serotonin transporter (desipramine > fluoxetine > citalopram) (Frazer, 1997; Hyttel, 1994; Sanchez and Hyttel, 1999). Next, we tested whether the effects of these compounds generalized to another measure of ethanol's acute effects (hypothermia), and whether desipramine's effects generalized to another major depressant (pentobarbital). Results showed a strong and selective potentiation of ethanol's depressant effects by desipramine. We examined whether this effect of DMI was modified by depletion of brain norepinephrine or 5-hydroxytryptamine (5-HT). The ability of a selective α2-adrenoreceptor agonist (dexmedetomidine) and antagonist (atipamezole) (Haapalinna et al., 1997) on ethanol's depressant effects, either alone or in combination with desipramine was assessed. Given the known effects of stress on both the norepinephrine system and ethanol's depressant effects (Aston-Jones and Cohen, 2005; Boyce-Rustay et al., 2007, 2008; Pohorecky, 1990; Stanford, 1995; Valentino and Van Bockstaele, 2008) and recent evidence that repeated cold stress alters NET expression in rats (Miner et al., 2006), we tested whether repeated swim stress altered the ability of desipramine to potentiate ethanol's depressant effects.
2. Methods
2.1. Subjects
Subjects were adult male C57BL/6J mice obtained from the Jackson Laboratory (Bar Harbor, ME), housed in groups of 2−4/cage in a temperature- and humidity-controlled vivarium, under a 12 h light/dark cycle (lights on 06:00 h). Separate cohorts of ethanol-naïve mice were used for each experiment. The number of mice used in each experiment is given in the figure legends. Testing was conducted during the light phase of the light/dark cycle (06:00−18:00 h) after mice had been acclimated to the test room for 1 h. All experimental procedures were approved by the National Institute on Alcohol Abuse and Alcoholism Animal Care and Use Committee, and followed the National Institute of Health guidelines outlined in ‘Using Animals in Intramural Research.’
2.2. Effects of desipramine, fluoxetine and citalopram on ethanol-induced sedation/hypnosis
Mice were injected intraperitoneally (i.p.) with 0 or 30 mg/kg of fluoxetine (FLX) or citalopram (CIT) and, 30 min later, injected i.p. with 3.0 g/kg ethanol. Immediately following ethanol injection, mice were placed in the supine position in ‘V’-shaped chambers and sleep time was measured as the time between ethanol injection and recovery of the righting reflex (LORR) (defined as turning onto all 4 paws 2 times in 30 s after the first instance of self-righting) (Boyce-Rustay and Holmes, 2006; Palmer et al., 2004).
A separate experiment assessed the dose-related effects of desipramine (DMI). Mice were injected with 0, 10, 20, or 30 mg/kg DMI and, 30 min later, tested for 3.0 g/ kg ethanol-induced sleep time as above. We have previously shown that neither FLX nor CIT treatment affects ethanol metabolism (Daws et al., 2006). To confirm that DMI treatment did not alter ethanol metabolism, mice were sacrificed upon awakening via cervical dislocation and rapid decapitation, and trunk blood was taken for analysis of blood ethanol concentration using the Analox AM1 Alcohol Analyzer (Analox Instruments USA Inc, Lunenburg, MA).
2.3. Effects of desipramine on pentobarbital-induced sedation/hypnosis
Mice were injected i.p. with 0, 10, 20, or 30 mg/kg DMI and, 30 min later, injected i.p. with 30 mg/kg pentobarbital and tested for sleep time. This dose of pentobarbital typically produces a similar duration of sleep time as 3 g/kg ethanol in mice (Blednov et al., 2003; Hefner and Holmes, 2007).
2.4. Effects of desipramine, fluoxetine and citalopram on ethanol-induced hypothermia
Baseline core body temperature was first measured by inserting a Thermalert TH-5 thermometer (Physitemp, Clifton, NJ) 2 cm into the rectum to obtain a stable reading, as previously described (Boyce-Rustay and Holmes, 2006). Thirty minutes later, mice were treated with 30 mg/kg of CIT, FLX or DMI. Body temperature was measured 30 min later and mice were then immediately injected i.p. with 3.0 g/kg ethanol. Body temperature was taken 30, 60, 90, 120 and 150 min later.
2.5. Effects of NE and 5-HT depletion on desipramine potentiation of ethanol-induced sedation/hypnosis
Mice were injected i.p. with 0 or 40 mg/kg of the selective norepinephrine neurotoxin N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4) (Fritschy and Grzanna, 1989, 1991; Jonsson et al., 1981; Magnuson et al., 1993). Eight days later, mice were injected i.p. with 0 or 20 mg/kg DMI and, 30 min later, injected with 3.0 g/kg ethanol and tested for sleep time. A separate cohort was injected i.p. daily for 3 days with 0 or 250 mg/kg of the 5-HT synthesis inhibitor para-chlorophenylalanine methyl ester hydrochloride (PCPA) (Fratta et al., 1973). Twenty-four hours after the final treatment, mice were injected i.p. with 0 or 20 mg/kg DMI and, 30 min later, injected i.p. with 3.0 g/kg ethanol and tested for sleep time. The dose and treatment regimen for DSP-4 and PCPA were based on studies showing largely selective depletion of brain NE and 5-HT, respectively, in mice (Dailly et al., 2006).
To confirm NE and 5-HT depletion following DSP-4 and PCPA treatment, respectively, mice were sacrificed via cervical dislocation and decapitation on awakening. Neurochemical analysis was performed on cortical and hippocampus tissue via high performance liquid chromatography (HPLC Norcross et al., in press). Frozen samples were homogenized by ultrasonic processing in 800 mL of 0.1 M perchloric acid containing 1% ethanol and 0.02% EDTA and centrifuged for 20 min at 3000g. Thirty microliters of the homogenate was used for catecholamine analysis by HPLC using a Luna 5μ C18(2), 250 × 2.0 mm column (Phenomenex 00G-4252-B0, Torrance, CA) held at 30 °C, a Waters Corporation (Milford, MA) 717plus autosampler at 4 °C, 510 pump at 0.4 ml/min and amperometric electrochemical detector (EiCOM CB100) set at Eox. 0.82 V. The mobile phase contained 2.8 g 1-heptanesulfonic acid sodium salt, 0.17 g EDTA, 20 mL triethylamine, dissolved in 2.2 L water, pH adjusted to 2.5 with 13 mL 85% phosphoric acid, plus 90 mL acetonitrile. The detector output was recorded and analyzed with Waters Empower 2 Chromatography Data Software.
2.6. Effects of α2-adrenoreceptor agonist and antagonist on desipramine potentiation of ethanol-induced sedation/hypnosis
Mice were either injected subcutaneously (s.c.) with 0, 0.003, 0.01, or 0.03 mg/kg of the selective α2-adrenoreceptor agonist, dexmedetomidine hydrochloride (MED), or 0, 0.3, 1.0, or 3.0 mg/kg of the selective α2-adrenoreceptor antagonist, atipamezole hydrochloride (ATI) and, 30 min later, injected i.p. with 3.0 g/kg ethanol and tested for sleep time. Doses of MED and ATI were based on previous behavioral studies in rats and mice (Millan et al., 2000; Newman-Tancredi et al., 1998; Powell et al., 2005; Seppala et al., 1994).
A separate cohort was injected s.c. with 0 or a sub-threshold dose of ATI (1.0 mg/ kg). Thirty minutes later mice were injected i.p. with 0 or 20 mg/kg DMI, and 30 min after injected i.p with 3.0 g/kg ethanol and tested for sleep time. Another cohort was tested in the same manner with the exception that mice were treated with 20 mg/kg FLX instead of DMI.
2.7. Effects of H1 histamine receptor antagonist on desipramine potentiation of ethanol-induced sedation/hypnosis
The histamine system is a mediator of behavioral arousal, probably via actions at the H1 receptor subtype (Tasaka et al., 1989). Histamine is known to increase norepinephrine release in vivo (Bealer, 1993), while desipramine and fluoxetine have affinity for this receptor (Taylor and Richelson, 1980). This raises the possibility that the ability of these drugs to promote ethanol-induced sedation/hypnosis may be in part mediated via effects at the H1 receptor. To test for this, we occupied the H1 receptor prior to desipramine treatment. Mice were either injected i.p. with 0 or 6.0 mg/kg of the selective H1 receptor antagonist, pyrilamine (mepyramine) maleate. Thirty minutes later mice were injected i.p. with 0 or 20 mg/kg DMI, and 30 min after injected i.p with 3.0 g/kg ethanol and tested for sleep time. The dose was based on pilot studies showing that the drug did not affect ethanol-induced sleep time per se, and previous behavioral studies showing this route of administration and dose are behaviorally active but sub-sedative in mice (Bongers et al., 2004; Easton et al., 2004).
2.8. Effect of stress on desipramine potentiation of ethanol-induced sedation/hypnosis
Mice were placed into a transparent Plexiglas cylinder (20 cm diameter) filled halfway with water (24 ± 1 °C) for 10 min once daily for 14 consecutive days, as previously described (Boyce-Rustay et al., 2007; Izquierdo et al., 2006). Non-stressed controls were co-housed with the stressed mice. Twenty-four hours after the final stressor, mice were injected i.p. with 0, 7.5, 15, or 30 mg/kg DMI and 30 min later, injected i.p. with 4.0 g/kg ethanol and tested for sleep time. A higher dose of ethanol was used in this experiment because we have previously shown that this stress regime produces reliable effects on ethanol-induced sedation/hypnosis at 4 g/kg ethanol (Boyce-Rustay et al., 2007).
2.9. Drugs
Ethanol (200 proof) was prepared in 0.9% v/v saline to produce 20% v/v solutions. Citalopram hydrobromide, fluoxetine hydrochloride, desipramine hydro-chloride, and pentobarbital (all Sigma, St. Louis, MO) and atipamezole hydrochloride and dexmedetomidine hydrochloride (Orion, Espoo, Finland) were dissolved in 0.9% v/v saline and injected at 10 mL/kg body weight; 0.9% v/v saline served as the vehicle control for all drugs.
2.10. Statistical analysis
The effect of drug treatment on ethanol-induced sleep time and blood ethanol concentration was measured using analysis of variance (ANOVA) followed by Newman Keuls post hoc tests where appropriate. Two-way ANOVA was used to measure the effects of drug treatment × NE or ×5-HT depletion on ethanol-induced sleep time and brain monoamine levels. Two-way ANOVA was used to measure the effects of drug × time on core body temperature, with repeated measures for time and paired t-tests where appropriate. Data are presented as means ± standard error of the mean. The threshold for statistical significance was set at p < 0.05.
3. Results
3.1. Effects of desipramine, fluoxetine and citalopram on ethanol-induced sedation/hypnosis
Treatment with 30 mg/kg FLX, but not 30 mg/kg CIT, significantly increased ethanol-induced sleep time relative to vehicle-treated controls (F2,25 = 6.35, p < 0.01) (Fig. 1A).
Fig. 1.
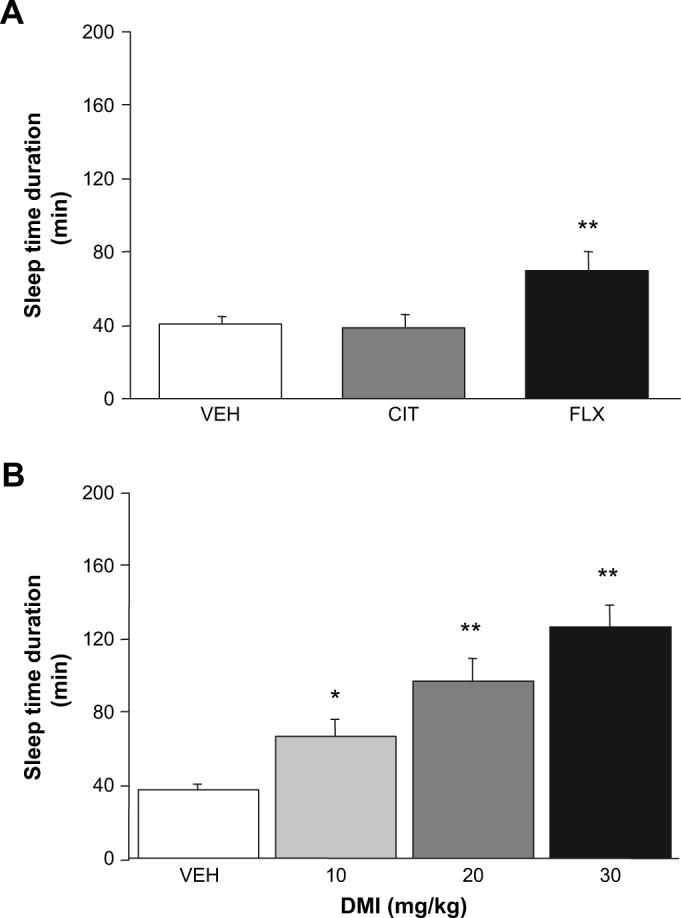
Effects of desipramine, fluoxetine and citalopram on the depressant effects of ethanol. (A) Treatment with a high dose (30 mg/kg) of fluoxetine (FLX) but not citalopram (CIT) increased the sleep time response to 3.0 g/kg ethanol (n = 9−10/dose). (B) Desipramine (DMI) dose-dependently increased the sleep time response to 3.0 g/kg ethanol (n = 11−22/dose). Data in Figs. 1-7 are means ± SEM. **p < 0.01, *p < 0.05 vs. vehicle (VEH).
DMI treatment dose-dependently increased ethanol-induced sleep time relative to vehicle-treated controls (F3,58 = 13.13, p < 0.01) (Fig. 1B). Blood ethanol concentration was significantly lower at awakening in the mice treated with 20 or 30 mg/kg DMI than vehicle-treated controls (F3,58 = 3.10, p < 0.05) (0 mg/kg = 329.0 ± 5.0 mg/dL, 10 mg/kg = 324.3 ± 5.6, 20 mg/kg 305.8 ± 8.7, 30 mg/kg = 307.2 ± 6.3).
3.2. Effects of desipramine on pentobarbital-induced sedation/hypnosis
Pre-treatment with DMI significantly increased pentobarbital-induced sleep time relative to vehicle pre-treated controls (F3,45 = 10.19, p < 0.01) in a dose-dependent manner (Fig. 2).
Fig. 2.
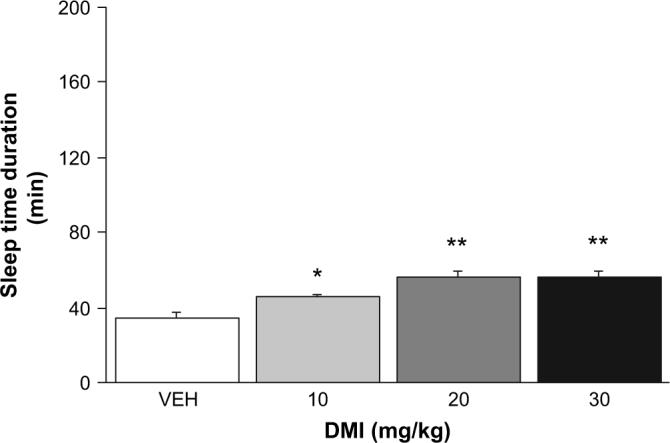
Effects of desipramine on the depressant effects of pentobarbital. Desipramine (DMI) dose-dependently potentiated the sedative/hypnotic effects of 30 mg/kg pentobarbital (n = 9−14/dose). **p < 0.01, *p < 0.05 vs. vehicle (VEH).
3.3. Effects of desipramine, fluoxetine and citalopram on ethanol-induced hypothermia
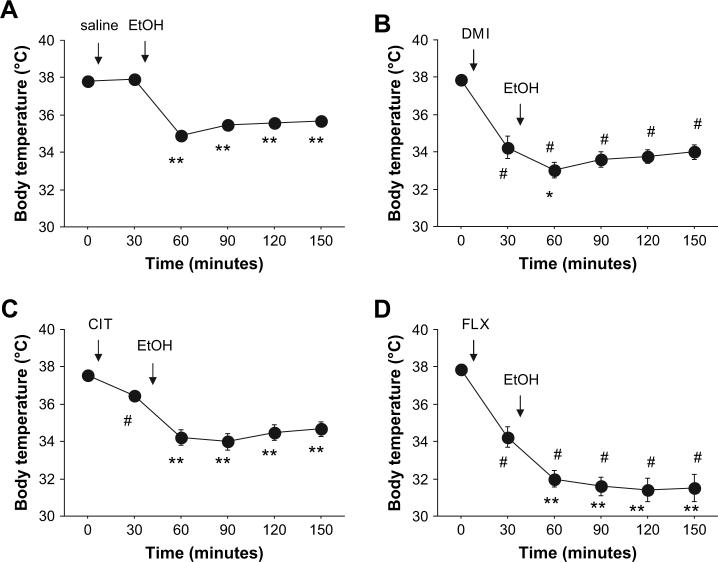
There was a significant interaction between drug treatment and time for core body temperature (F15,175 = 9.88, p < 0.01). Prior to ethanol injection (i.e., time point 30 min), CIT, FLX and DMI, but not saline, caused a significant decrease in temperature relative to baseline (i.e., time point 0 min). Following ethanol injection, there was a significant decrease in temperature across all time points in saline, CIT and FLX treated mice, as compared to time point 30 min. Temperatures in FLX + ethanol, but not in CIT + ethanol, treated mice were significantly lower at corresponding time points in saline + ethanol mice. Following ethanol injection, DMI treated mice showed a temperature decrease at time point 60 min, but returned to pre-ethanol levels at later time points. Temperatures in DMI + ethanol mice remained significantly lower than at corresponding time points in saline + ethanol mice (Fig. 3).
Fig. 3.
Effects of desipramine, fluoxetine and citalopram on the hypothermic effects of ethanol. (A) Hypothermic effects of 3.0 g/kg ethanol in saline-treated controls. (B) Desipramine (DMI) produced hypothermia per se, and transiently potentiated the hypothermic effects of ethanol. (C) Citalopram (CIT) produced hypothermia per se, but did not potentiate the hypothermic effects of ethanol. (D) Fluoxetine (FLX) produced hypothermia per se, and produced a sustained potentiation of the hypothermic effects of ethanol. n = 9−10/group. **p < 0.01, *p < 0.05 vs. time point 30/same drug group; #p < 0.05 vs. saline group at corresponding time point.
3.4. Effects of α2-adrenoreceptor agonist and antagonist on ethanol-induced sedation/hypnosis
Treatment with the highest dose of MED significantly increased ethanol-induced sleep time relative to vehicle-treated controls, while treatment with ATI had no significant effect at this dose range (F6,54 = 7.51, p < 0.01) (Fig. 4).
Fig. 4.
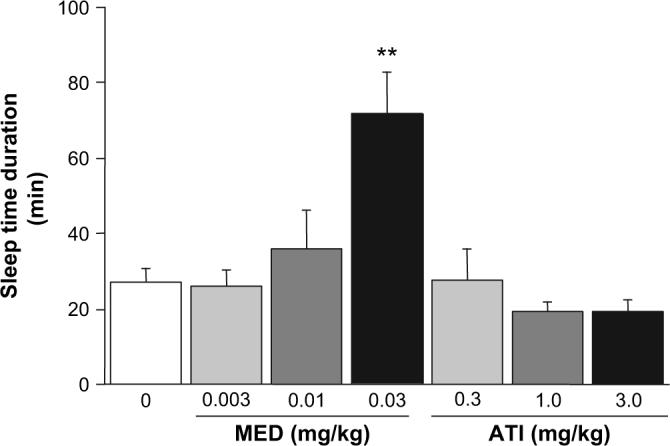
Effects of an α2-adrenoreceptor agonist and an α2-adrenoreceptor antagonist on the depressant effects of ethanol. The α2-adrenoreceptor agonist dexmedetomidine (MED) potentiated ethanol-induced sleep time, while the α2-adrenoreceptor atipamezole (ATI) produced a trend for an effect in the opposite direction (n = 8−9/group). **p < 0.01 vs. vehicle (VEH).
3.5. Effects of α2-adrenoreceptor agonist and antagonist on DMI-potentiation of ethanol-induced sedation/hypnosis
Treatment with 1.0 mg/kg ATI per se had no effect on sleep time, but blocked the potentiation of ethanol-induced sleep time by DMI (F3,30 = 5.63, p < 0.01) (Fig. 5A) or FLX (F3,31 = 4.29, p < 0.05) (Fig. 5B).
Fig. 5.
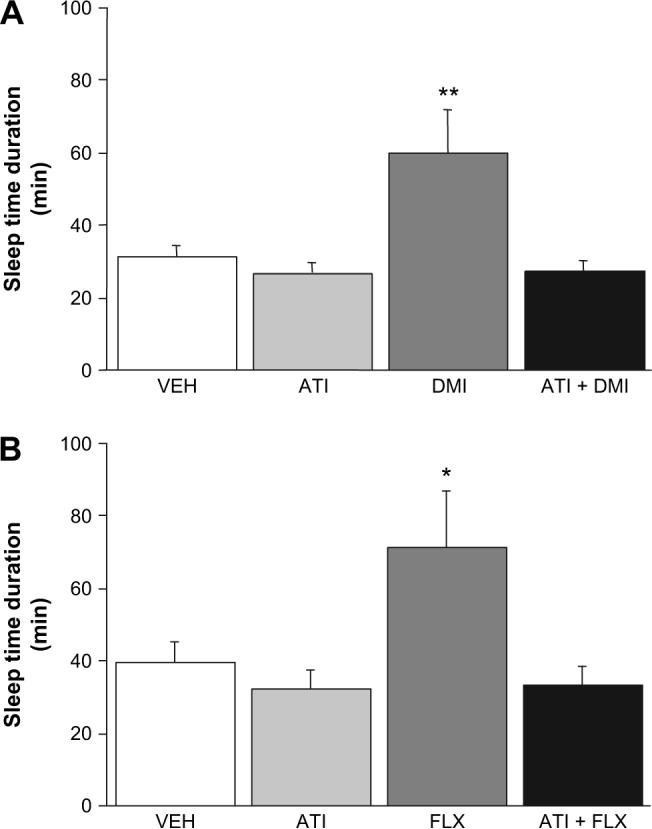
Effects of an α2-adrenoreceptor agonist and an α2-adrenoreceptor antagonist on the desipramine potentiation of ethanol's depressant effects. (A) Desipramine (DMI) increased the sleep time response to 3.0 g/kg ethanol in vehicle-treated mice but not in mice treated with 1 mg/kg of ATI (n = 7−9/group). (B) Fluoxetine (FLX) increased the sleep time response to 3.0 g/kg ethanol in vehicle-treated mice but not in mice treated with 1 mg/kg of ATI (n = 8−9/group). **p < 0.01, *p < 0.05 vs. vehicle (VEH).
3.6. Effects of NE and 5-HT depletion on DMI-potentiation of ethanol-induced sedation/hypnosis
Treatment with 20 mg/kg DMI significantly increased ethanol-induced sleep time relative to vehicle-treated controls regardless of DSP-4 treatment (effect of DMI: F1,44 = 17.56, p < 0.01; effect of DSP-4: ns; DSP-4 × DMI interaction, ns)(Fig. 6A). HPLC analysis confirmed that DSP-4 treatment significantly reduced levels of NE in hippocampus (F1,33 = 70.66, p < 0.01) and cortex (F1,32 24.21, p < 0.01) without affecting levels of 5-HT (Table 1).
Fig. 6.
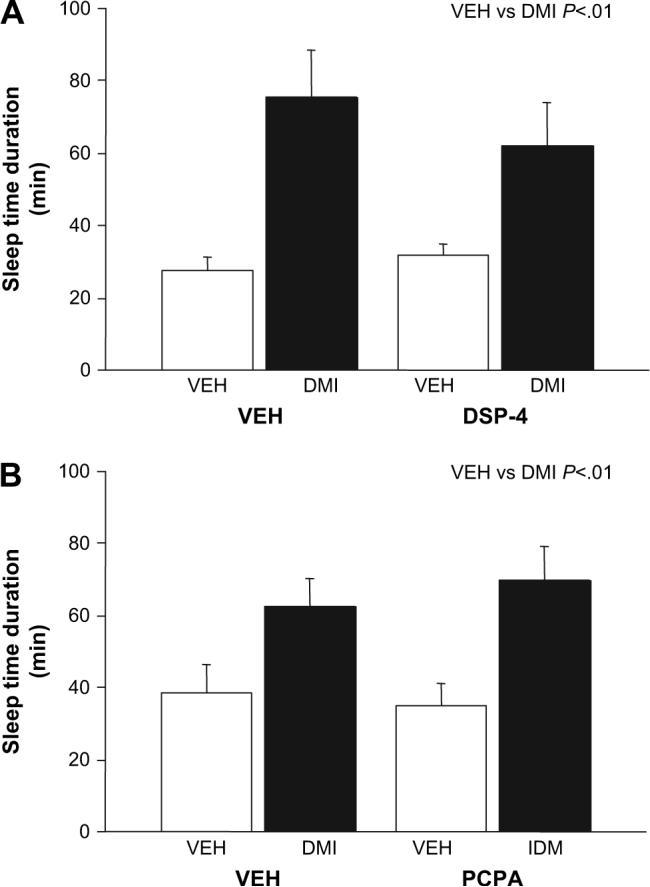
Effects of NE or 5-HT depletion on desipramine potentiation of ethanol's depressant effects. (A) Desipramine (DMI) increased the sleep time response to 3.0 g/kg ethanol in vehicle treated and mice treated with the NE depleting agent DSP-4 (n = 12/group). (B) DMI increased the sleep time response to 3.0 g/kg ethanol in vehicle treated and mice treated with the 5-HT depleting agent PCPA (n = 15−17/group). **p < 0.01, *p < 0.05 vs. vehicle (VEH).
Table 1.
Effects of DSP-4 on norepinephrine and 5-HT levels in cortex and hippocampus
| Vehicle | DSP-4 | Percentage change | |
|---|---|---|---|
| Norepinephrine | |||
| Cortex | 451.0 ± 73.6 | **62.6 ± 11.8 | −86 |
| Hippocampus | 582.5 ± 54.6 | **114.8 ± 16.4 | −80 |
| 5-HT | |||
| Cortex | 314.8 ± 38.9 | 318.6 ± 47.1 | +1 |
| Hippocampus | 811.1 ± 59.3 | 731.5 ± 36.9 | −10 |
Data are mean ± SEM ng/g tissue. n = 7−9/group. **p < 0.01 vs. vehicle.
Treatment with 20 mg/kg DMI significantly increased ethanol-induced sleep time relative to vehicle-treated controls irrespective of PCPA treatment (effect of DMI: F1,61 =13.27, p < 0.01; effect of PCPA: ns; PCPA × DMI interaction, ns) (Fig. 6B). HPLC analysis confirmed that PCPA treatment significantly reduced levels of 5-HT in hippocampus (F1,28 = 78.70, p < 0.01) and cortex (F1,30 = 12.53, p < 0.01) while NE levels were unaltered (Table 2).
Table 2.
Effects of PCPA on 5-HT and norepinephrine levels in cortex and hippocampus
| Vehicle | DSP-4 | Percentage change | |
|---|---|---|---|
| 5-HT | |||
| Cortex | 387.9 ± 52.56 | **183.8 ± 23.7 | −53 |
| Hippocampus | 639.8 ± 23.75 | **327.7 ± 25.8 | −49 |
| Norepinephrine | |||
| Cortex | 496.9 ± 22.3 | 505.9 ± 27.8 | +1 |
| Hippocampus | 586.7 ± 38.9 | 547.6 ± 22.9 | −7 |
Data are mean ± SEM ng/g tissue. n = 7−9/group.
p < 0.01 vs. vehicle.
3.7. Effects of H1 histamine receptor antagonist on desipramine potentiation of ethanol-induced sedation/hypnosis
Treatment with DMI dose-dependently increased ethanol-induced sleep time regardless of pyrilamine pre-treatment (effect of DMI: F1,24 = 16.36, p < 0.01; effect of pyrilamine: ns; pyrilamine DMI interaction, ns: vehicle/vehicle = 23.9 ± 3.2 min sleep, vehicle/DMI = 39.0 ± 3.5, pyrilamine/vehicle = 27.0 ± 2.6, pyrilamine/DMI = 37.0 ± 3.1).
3.8. Effect of stress on DMI-potentiation of ethanol-induced sedation/hypnosis
Treatment with DMI dose-dependently increased ethanol-induced sleep time in non-stressed controls, but did not alter sleep time in stressed mice (stress × DMI interaction; F3,73 = 4.14, p < 0.01) (Fig. 7).
Fig. 7.
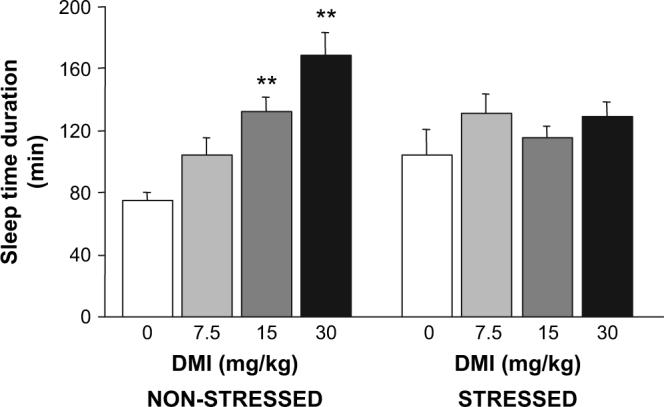
Effects of stress on desipramine potentiation of ethanol's depressant effects. Desipramine (DMI) increased the sleep time response to 4.0 g/kg ethanol in non-stressed controls but not in mice subjected to repeated swim stress. n = 9−12/dose/stress condition. **p < 0.01, *p < 0.05 vs. vehicle (VEH).
4. Discussion
The major findings of the present study were that the NET blocker desipramine strongly potentiated the depressant effects of ethanol, and these effects were reversed by antagonism of α2-adrenoreceptors and by exposure to repeated stress.
A recent study found that desipramine or another NET blocker, nisoxetine, increased the depressant effects of acute ethanol injection, as assayed by the duration of ethanol-induced sleep time in inbred long sleep (ILS) and inbred short sleep (ISS) mice (Haughey et al., 2005) (see also Cott and Ogren, 1980). Present data replicate these findings in the C57BL/6J mouse strain: pre-treatment with DMI markedly potentiated sleep time in a dose-dependent manner. By contrast, treatment with a relatively high dose (30 mg/kg) of the SERT-selective blocker citalopram had no effect on ethanol's depressant effects, a finding consistent with our previous findings across a wider dose range (Daws et al., 2006). On the other hand, the same dose of another SERT blocker, fluoxetine, significantly prolonged ethanol-induced sleep time. Dissociations between the effects of fluoxetine and citalopram on for example depression-related behavior and stress-induced serotonin release have been reported and attributed to the noradrenergic actions of fluoxetine (Cryan et al., 2004). In this context, given the prior observation that the ethanol-potentiating effect of fluoxetine was not produced by low, SERT-selective, doses of fluoxetine and remained extant in SERT knockout mice even at high doses (Daws et al., 2006), the effect is parsimoniously explained by effects of the drug's known affinity for NET at high doses (Bymaster et al., 2002; Frazer, 1997; Hyttel, 1994; Sanchez and Hyttel, 1999). Taken together, these data are consistent with an ethanol-potentiating effect following NET, but not SERT, blockade.
The ethanol-potentiating effects of desipramine generalized to another sedative/hypnotic, the prototypical barbiturate pentobarbital. The actions of desipramine did not, however, robustly alter another measure of ethanol's acute effects, hypothermia, even though this response was measured after the same ethanol dose and over the same post-treatment period as sleep time. Treatment with 30 mg/kg desipramine, fluoxetine or citalopram all produced a hypothermic response per se. Following ethanol treatment there was a further decrease in core body temperature in all groups. However, while the combination of fluoxetine and ethanol produced a sustained decrease in temperature relative to ethanol alone, desipramine produced only a modest transient additive effect, and citalopram had no additive effect at all. The apparent dissociation between desipramine's effects on sleep time and hypothermia suggests that increased hypothermia is not driving the potentiation of ethanol's sedative/hypnotic effects. It could also potentially provide an insight into the neural basis of the drug's effect on ethanol responses. Locus coeruleus neurons are thought to subserve noradrenergic modulation of ethanol's depressant effects (Aston-Jones et al., 1982; Corrodi et al., 1966; Gursey and Olson, 1960; Pohorecky and Brick, 1977; Verbanck et al., 1990), while the hypothermic effects of ethanol are likely mediated by the hypothalamus, a region receiving predominant input from the lateral tegmentum rather than locus coeruleus (Grzanna and Fritschy, 1991). In this context, the pattern of desipramine × ethanol interactions could reflect an effect of desipramine primarily on locus coeruleus noradrenergic neurons. However, specific lesions of the lateral tegmentum would be needed to directly address this possibility. Brain lesion studies would also address the possibility that some of the observed effects of desipramine and fluoxetine are mediated via effects on vascular tissue rather than in brain.
To confirm the role of norepinephrine in desipramine's ethanol-potentiating effects, we depleted brain tissue levels of norepinephrine and, for comparison, 5-HT. DSP-4 and PCPA produced significant and neurotransmitter-specific reductions in forebrain levels of norepinephrine and 5-HT, respectively, that were comparable to previous studies in mice (Dailly et al., 2006; Dursun and Handley, 1993; Fornai et al., 1996). Neither treatment affected the sleep time responses to ethanol per se, which is consonant with earlier work (Spuhler et al., 1987; Sutoo and Sano, 1984). Surprisingly, however, norepinephrine depletion failed to attenuate the magnitude of desipramine's effects on this measure (whether the desipramine effect also remained sensitive to α2-adrenoreceptor mediation was not determined, but this would be of interest given the discussion below). 5-HT depletion was also without effect. While this negative effect is puzzling at first glance, there are a number of possible explanations. While DSP-4 depleted hippo-campal and cortical norepinephrine to less than 20% of controls levels, it is possible that residual norepinephrine could have maintained desipramine's effects. A seemingly more likely explanation is that compensatory changes in the norepinephrine system mitigated the consequences of the gross loss in neurotransmitter substrate. Of particular note in this context, previous studies have shown that DSP-4-induced loss of norepinephrine tissue content is associated with a compensatory increase in extracellular levels of norepinephrine, which are further augmented by desipramine treatment (Hughes and Stanford, 1998). Furthermore, DSP-4 treatment causes downregulation of presynaptic α2-adrenoceptor receptors and upregulation of postsynaptic α2-adrenoceptor receptors (Dooley et al., 1983a,b; Kask et al., 1997). The net effect of these changes could be to maintain the ability of NET blockade via desipramine to exert effects on the norepinephrine system. A similar scenario is posited to explain the inability of DSP-4 to prevent other behavioral effects of desipramine, such as the drug's antidepressant-related effects (Cryan et al., 2002; Danysz et al., 1986; Esposito et al., 1987; Kostowski et al., 1984; O'Leary et al., 2007; Stanford, 1995).
Previous work has clearly established a role for α2-adrenoreceptors, and specifically the a2A subtype, in mediating norepinephrine's depressant actions (Aantaa and Scheinin, 1993; Kable et al., 2000; Lakhlani et al., 1997; Millan et al., 1994). Providing support for the contribution of α2-adrenoceptors to desipramine potentiation of ethanol's depressant effects, the selective α2-adrenoceptor antagonist atipamezole blocked these effects at a dose of atipamezole that was without significant effect per se. Moreover, atipamezole blocked the ethanol-potentiating effects of high dose fluoxetine. This would be consistent with these effects of fluoxetine being mediated via the norepinephrine rather than 5-HT system; although direct interactions between 5-HT and α2-adrenoceptors cannot be discounted (Haddjeri et al., 2004). Present data also showed that, consistent with previous studies (Idanpaan-Heikkila et al., 1995; Lister et al., 1989; Millan et al., 2000; Seppala et al., 1994), selective stimulation of α2-adrenoceptors via treatment with the agonist dexmedetomidine, potentiated ethanol's depressant effects in a manner that mimicked the profile of desipramine.
Taken together these data suggest that stimulation of α2-adrenoceptors following NET-mediated uptake blockade of norepinephrine is an important molecular step underlying the effects of desipramine on ethanol-induced sleep. α2-adrenoreceptors function as postsynaptic receptors and presynaptic autoreceptors, and present data cannot dissociate their respective roles. However, a simple working model would be that activation of presynaptic α2-adrenoreceptors leading to a decrease locus coeruleus firing would exacerbate the central depressant effects of ethanol. It should be noted in this context that while some studies have suggested that ethanol might act as a NET blocker (Lin et al., 1997, 1993) (but see Haughey et al., 2005; Sun et al., 1977), the present data do not directly speak to the issue of ethanol's potential actions at NET, but rather the interaction between NET inactivating compounds and ethanol. Another important caveat is that the depressant effects of ethanol itself are likely the result of actions on multiple sites that includes norepinephrine but also other systems, notably g-amino-butyric-acid (GABA) (Boehm et al., 2006). Moreover, desipramine and high dose fluoxetine have actions at sites other than NET that could contribute to the potentiating of ethanol-induced sedation, including activation of histamine H1 receptors (Savard et al., 1982; Taylor and Richelson, 1980; Wong et al., 1983). Furthermore, one known downstream effect of systemic atipamezole is to decrease hypothalamic histamine release (Laitinen et al., 1995). Therefore, although we found that silent doses of an α2-adrenoceptor agonist are sufficient to prevent desipramine's effects and saw no evidence of interaction between desipramine, ethanol and the H1 antagonist pyrilamine, we cannot fully exclude the possible contribution of histamine to the effects we observed.
Notwithstanding these mechanistic issues, the observation that desipramine strongly accentuates ethanol's depressant effects raises the issue of whether alterations in endogenous NET function would also affect this response. The norepinephrine system is potently activated by exposure to stress in rodents and humans (Aston-Jones and Cohen, 2005; Bremner et al., 1996; Stanford, 1995; Valentino and Van Bockstaele, 2008), and stress-related disorders such as anxiety and depression are frequently comorbid with alcoholism (Schildkraut, 1965; Stinson et al., 2005). Miner et al. (2006) recently found that repeated (14 days) cold stress caused a significant increase in plasma membrane-bound NET expression in the rat forebrain. Present data show that exposing mice to repeated (14 days) forced swim stress abolished the ability of desipramine to potentiate ethanol-induced sleep time. It is tempting to speculate that this effect results from an increase in the availability of plasmalemmal NET, as seen in Miner et al.'s study, but this remains to be tested directly. Indeed, other molecular changes following stress should also be examined. Clearly in this regard, it will be interesting to examine possible changes in α2-adrenoreceptor expression/function following stress given prior evidence that these receptors are sensitive to stress (Flugge et al., 2003; Pavcovich et al., 1990; Sallinen et al., 1999) and current findings demonstrating their modulation of desipramine's effects on ethanol. Another interesting avenue for future studies will be to test for genetic modulation of stress-induced changes in NET and α2-adrenoreceptor function, for example via comparison of different strains of mice known to differ in stress sensitivity (Boyce-Rustay et al., 2008; Jacobson and Cryan, 2007; Millstein and Holmes, 2007). Aside from these remaining questions, current data provide strong evidence that desipramine ethanol interactions on this measure can be dynamically regulated by stress. This extends earlier work suggesting that genetic variation in NET binding, mRNA levels and synaptosomal NE reuptake are also associated with differences in sensitivity to desipramine's ethanol-potentiating effects (Bennett and Johnson, 1998; Haughey et al., 2005; Markel et al., 1997). Collectively these findings provide growing support for a role for NET and α2-adrenoreceptors in modulating the depressant effects of ethanol.
In summary, the present study provides further evidence that the NET blocker desipramine potentiates the depressant effects of ethanol. Similar effects were produced by a high, non-selective dose of fluoxetine, but not by the selective SERT blocker citalopram. Desipramine also potentiated the depressant effects of the barbiturate pentobarbital but only weakly augmented the hypothermic effects of ethanol. Demonstrating a mechanistic role for α2-adrenoceptors, a ‘silent’ dose of the α2-adrenoceptor antagonist atipamezole blocked the ethanol-potentiating effects of desipramine while the α2-adrenoceptor agonist dexmedetomidine mimicked desipramine's effects. By contrast, depletion of neither forebrain norepinephrine nor 5-HT diminished desipramine's effects. Finally, desipramine's effects on the ethanol response were ablated by exposure to repeated stress. Elucidating the basis of desipramine - × ethanol interaction and the role of NET in modulating ethanol's depressant effects could ultimately have implications for understanding the pathophysiology and treatment of alcohol abuse and alcoholism.
Acknowledgements
Research supported by the Intramural Research Program of the National Institute of Alcohol Abuse and Alcoholism (Z01-AA000411).
References
- Aantaa R, Scheinin M. Alpha 2-adrenergic agents in anaesthesia. Acta Anaesthesiol. Scand. 1993;37:433–448. doi: 10.1111/j.1399-6576.1993.tb03743.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J. Neurosci. 1981;1:876–886. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Foote SL, Bloom FE. Low doses of ethanol disrupt sensory responses of brain noradrenergic neurones. Nature. 1982;296:857–860. doi: 10.1038/296857a0. [DOI] [PubMed] [Google Scholar]
- Bealer SL. Histamine releases norepinephrine in the paraventricular nucleus/anterior hypothalamus of the conscious rat. J. Pharmacol. Exp. Ther. 1993;264:734–738. [PubMed] [Google Scholar]
- Bennett B, Johnson TE. Development of congenics for hypnotic sensitivity to ethanol by QTL-marker-assisted counter selection. Mamm. Genome. 1998;9:969–974. doi: 10.1007/s003359900908. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Jung S, Alva H, Wallace D, Rosahl T, Whiting PJ, Harris RA. Deletion of the alpha1 or beta2 subunit of GABAA receptors reduces actions of alcohol and other drugs. J. Pharmacol. Exp. Ther. 2003;304:30–36. doi: 10.1124/jpet.102.042960. [DOI] [PubMed] [Google Scholar]
- Blum K, Merritt JH, Wallace JE, Owen R, Hahn JW, Geller I. Effects of catecholamine synthesis inhibition on ethanol narcosis in mice. Curr. Ther. Res. Clin. Exp. 1972;14:324–329. [PubMed] [Google Scholar]
- Boehm SL, 2nd, Ponomarev I, Blednov YA, Harris RA. From gene to behavior and back again: new perspectives on GABAA receptor subunit selectivity of alcohol actions. Adv. Pharmacol. 2006;54:171–203. doi: 10.1016/s1054-3589(06)54008-6. [DOI] [PubMed] [Google Scholar]
- Bongers G, Leurs R, Robertson J, Raber J. Role of H3-receptor-mediated signaling in anxiety and cognition in wild-type and Apoe–/– mice. Neuropsychopharmacology. 2004;29:441–449. doi: 10.1038/sj.npp.1300352. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol. Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl.) 2006;187:455–466. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav. Brain Res. 2008;186:133–137. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety. I. Preclinical studies. Synapse. 1996;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, Wong DT, Perry KW. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl.) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Corrodi H, Fuxe K, Hokfelt T. The effect of ethanol on the activity of central catecholamine neurones in rat brain. J. Pharm. Pharmacol. 1966;18:821–823. doi: 10.1111/j.2042-7158.1966.tb07817.x. [DOI] [PubMed] [Google Scholar]
- Cott JM, Ogren SO. Antidepressant drugs and ethanol: behavioral and pharmacokinetic interactions in mice. J. Neural Transm. 1980;48:223–240. doi: 10.1007/BF01250658. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, Page ME, Dalvi A, Thomas SA, Lucki I. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur. J. Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Dailly E, Chenu F, Petit-Demouliere B, Bourin M. Specificity and efficacy of noradrenaline, serotonin depletion in discrete brain areas of Swiss mice by neurotoxins. J. Neurosci. Methods. 2006;150:111–115. doi: 10.1016/j.jneumeth.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Danysz W, Kostowski W, Kozak W, Hauptmann M. On the role of noradrenergic neurotransmission in the action of desipramine and amitriptyline in animal models of depression. Pol. J. Pharmacol. Pharm. 1986;38:285–298. [PubMed] [Google Scholar]
- Daws LC, Montanez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J. Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley DJ, Bittiger H, Hauser KL, Bischoff SF, Waldmeier PC. Alteration of central alpha 2- and beta-adrenergic receptors in the rat after DSP-4, a selective noradrenergic neurotoxin. Neuroscience. 1983a;9:889–898. doi: 10.1016/0306-4522(83)90277-4. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Mogilnicka E, Delini-Stula A, Waechter F, Truog A, Wood J. Functional supersensitivity to adrenergic agonists in the rat after DSP-4, a selective noradrenergic neurotoxin. Psychopharmacology (Berl.) 1983b;81:1–5. doi: 10.1007/BF00439263. [DOI] [PubMed] [Google Scholar]
- Dursun SM, Handley SL. The effects of alpha 2-adrenoceptor antagonists on the inhibition of 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI)-induced head shakes by 5-HT1A receptor agonists in the mouse. Br. J. Pharmacol. 1993;109:1046–1052. doi: 10.1111/j.1476-5381.1993.tb13727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A, Norton J, Goodwillie A, Pfaff DW. Sex differences in mouse behavior following pyrilamine treatment: role of histamine 1 receptors in arousal. Pharmacol. Biochem. Behav. 2004;79:563–572. doi: 10.1016/j.pbb.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Erwin VG, Cornell K. Effects of 6-hydroxydopamine on brain catecholamines and on acute actions of ethanol in LS/Ibg and SS/Ibg mice. Alcohol. Clin. Exp. Res. 1986;10:285–289. doi: 10.1111/j.1530-0277.1986.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Esposito E, Ossowska G, Samanin R. Further evidence that noradrenaline is not involved in the anti-immobility activity of chronic desipramine in the rat. Eur. J. Pharmacol. 1987;136:429–432. doi: 10.1016/0014-2999(87)90319-0. [DOI] [PubMed] [Google Scholar]
- Flugge G, van Kampen M, Meyer H, Fuchs E. Alpha2A and alpha2C-adrenoceptor regulation in the brain: alpha2A changes persist after chronic stress. Eur. J. Neurosci. 2003;17:917–928. doi: 10.1046/j.1460-9568.2003.02510.x. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Bassi L, Torracca MT, Alessandri MG, Scalori V, Corsini GU. Region- and neurotransmitter-dependent species and strain differences in DSP-4-induced monoamine depletion in rodents. Neurodegeneration. 1996;5:241–249. doi: 10.1006/neur.1996.0032. [DOI] [PubMed] [Google Scholar]
- Fratta W, Biggio G, Mercurio G, Di Vittorio P, Tagliamonte A, Gessa GL. Letter: the effect of d- and l-p-chlorophenylalanine on the metabolism of 5-hydroxytryptamine in brain. J. Pharm. Pharmacol. 1973;25:908–909. doi: 10.1111/j.2042-7158.1973.tb09972.x. [DOI] [PubMed] [Google Scholar]
- Frazer A. Pharmacology of antidepressants. J. Clin. Psychopharmacol. 1997;17(Suppl 1):2S–18S. doi: 10.1097/00004714-199704001-00002. [DOI] [PubMed] [Google Scholar]
- French TA, Masserano JM, Weiner N. Further studies on the neurochemical mechanisms mediating differences in ethanol sensitivity in LS and SS mice. Alcohol. Clin. Exp. Res. 1988;12:215–223. doi: 10.1111/j.1530-0277.1988.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neuroscience. 1989;30:181–197. doi: 10.1016/0306-4522(89)90364-3. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Selective effects of DSP-4 on locus coeruleus axons: are there pharmacologically different types of noradrenergic axons in the central nervous system? Prog. Brain Res. 1991;88:257–268. doi: 10.1016/s0079-6123(08)63815-7. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Fritschy JM. Efferent projections of different subpopulations of central noradrenaline neurons. Prog. Brain Res. 1991;88:89–101. doi: 10.1016/s0079-6123(08)63801-7. [DOI] [PubMed] [Google Scholar]
- Gursey D, Olson RE. Depression of serotonin and norepinephrine levels in brain stem of rabbit by ethanol. Proc. Soc. Exp. Biol. Med. 1960;104:280–281. doi: 10.3181/00379727-104-25807. [DOI] [PubMed] [Google Scholar]
- Haapalinna A, Viitamaa T, MacDonald E, Savola JM, Tuomisto L, Virtanen R, Heinonen E. Evaluation of the effects of a specific alpha 2-adrenoceptor antagonist, atipamezole, on alpha 1- and alpha 2-adrenoceptor subtype binding, brain neurochemistry and behaviour in comparison with yohimbine. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:570–582. doi: 10.1007/pl00005092. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Lavoie N, Blier P. Electrophysiological evidence for the tonic activation of 5-HT(1A) autoreceptors in the rat dorsal raphe nucleus. Neuropsychopharmacology. 2004;29:1800–1806. doi: 10.1038/sj.npp.1300489. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Kaiser AL, Johnson TE, Bennett B, Sikela JM, Zahniser NR. Norepinephrine transporter: a candidate gene for initial ethanol sensitivity in inbred long-sleep and short-sleep mice. Alcohol. Clin. Exp. Res. 2005;29:1759–1768. doi: 10.1097/01.alc.0000183009.57805.a6. [DOI] [PubMed] [Google Scholar]
- Hefner K, Holmes A. An investigation of the behavioral actions of ethanol across adolescence in mice. Psychopharmacology (Berl.) 2007 doi: 10.1007/s00213-006-0646-2. [DOI] [PubMed] [Google Scholar]
- Howerton TC, Collins AC. Ethanol-induced inhibition of norepinephrine release from brain slices obtained from LS and SS mice. Alcohol. 1984;1:47–53. doi: 10.1016/0741-8329(84)90036-3. [DOI] [PubMed] [Google Scholar]
- Howerton TC, Marks MJ, Collins AC. Norepinephrine, gamma-aminobutyric acid, and choline reuptake kinetics and the effects of ethanol in long-sleep and short-sleep mice. Subst. Alcohol Actions Misuse. 1982;3:89–99. [PubMed] [Google Scholar]
- Hughes ZA, Stanford SC. A partial noradrenergic lesion induced by DSP-4 increases extracellular noradrenaline concentration in rat frontal cortex: a microdialysis study in vivo. Psychopharmacology (Berl.) 1998;136:299–303. doi: 10.1007/s002130050569. [DOI] [PubMed] [Google Scholar]
- Hwang BH, Wang GM, Wong DT, Lumeng L, Li TK. Norepinephrine uptake sites in the locus coeruleus of rat lines selectively bred for high and low alcohol preference: a quantitative autoradiographic binding study using [3H]-tomoxetine. Alcohol. Clin. Exp. Res. 2000;24:588–594. [PubMed] [Google Scholar]
- Hyttel J. Pharmacological characterization of selective serotonin reuptake inhibitors (SSRIs). Int. Clin. Psychopharmacol. 1994;9(Suppl 1):19–26. doi: 10.1097/00004850-199403001-00004. [DOI] [PubMed] [Google Scholar]
- Idanpaan-Heikkila JJ, Bjorn M, Seppala T. The effects of ethanol in combination with the alpha 2-adrenoceptor agonist dexmedetomidine and the alpha 2-adrenoceptor antagonist atipamezole on brain monoamine metabolites and motor performance of mice. Eur. J. Pharmacol. 1995;292:191–199. doi: 10.1016/0926-6917(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson LH, Cryan JF. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav. Genet. 2007;37:171–213. doi: 10.1007/s10519-006-9106-3. [DOI] [PubMed] [Google Scholar]
- Jonsson G, Hallman H, Ponzio F, Ross S. DSP4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) – a useful denervation tool for central and peripheral noradrenaline neurons. Eur. J. Pharmacol. 1981;72:173–188. doi: 10.1016/0014-2999(81)90272-7. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. In vivo gene modification elucidates subtype-specific functions of alpha(2)-adrenergic receptors. J. Pharmacol. Exp. Ther. 2000;293:1–7. [PubMed] [Google Scholar]
- Kask A, Harro J, Tuomaine P, Rago L, Mannisto PT. Overflow of noradrenaline and dopamine in frontal cortex after [N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine] (DSP-4) treatment: in vivo microdialysis study in anaesthetized rats. Naunyn Schmiedebergs Arch. Pharmacol. 1997;355:267–272. doi: 10.1007/pl00004942. [DOI] [PubMed] [Google Scholar]
- Kostowski W, Danysz W, Nowakowska E. Studies on brain noradrenergic neurons in animal model for antidepressive activity. Psychopharmacol. Bull. 1984;20:320–322. [PubMed] [Google Scholar]
- Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol. Biochem. Behav. 1996;53:585–591. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- Laitinen KS, Tuomisto L, MacDonald E. Effects of a selective alpha 2-adrenoceptor antagonist, atipamezole, on hypothalamic histamine and noradrenaline release in vivo. Eur. J. Pharmacol. 1995;285:255–260. doi: 10.1016/0014-2999(95)00410-m. [DOI] [PubMed] [Google Scholar]
- Lakhlani PP, MacMillan LB, Guo TZ, McCool BA, Lovinger DM, Maze M, Limbird LE. Substitution of a mutant alpha2a-adrenergic receptor via “hit and run” gene targeting reveals the role of this subtype in sedative, analgesic, and anesthetic-sparing responses in vivo. Proc. Natl. Acad. Sci. U.S.A. 1997;94:9950–9955. doi: 10.1073/pnas.94.18.9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Bickford PC, Palmer MR, Cline EJ, Gerhardt GA. Effects of ethanol and nomifensine on NE clearance in the cerebellum of young and aged Fischer 344 rats. Brain Res. 1997;756:287–292. doi: 10.1016/s0006-8993(97)00229-1. [DOI] [PubMed] [Google Scholar]
- Lin AM, Bickford PC, Palmer MR, Gerhardt GA. Ethanol inhibits the uptake of exogenous norepinephrine from the extracellular space of the rat cerebellum. Neurosci. Lett. 1993;164:71–75. doi: 10.1016/0304-3940(93)90860-n. [DOI] [PubMed] [Google Scholar]
- Lister RG, Durcan MJ, Nutt DJ, Linnoila M. Attenuation of ethanol intoxication by alpha-2 adrenoceptor antagonists. Life Sci. 1989;44:111–119. doi: 10.1016/0024-3205(89)90528-6. [DOI] [PubMed] [Google Scholar]
- Magnuson DS, Staines WA, Marshall KC. Electrophysiological changes accompanying DSP-4 lesions of rat locus coeruleus neurons. Brain Res. 1993;628:317–320. doi: 10.1016/0006-8993(93)90972-p. [DOI] [PubMed] [Google Scholar]
- Markel PD, Bennett B, Beeson M, Gordon L, Johnson TE. Confirmation of quantitative trait loci for ethanol sensitivity in long-sleep and short-sleep mice. Genome Res. 1997;7:92–99. doi: 10.1101/gr.7.2.92. [DOI] [PubMed] [Google Scholar]
- Masserano JM, Weiner N. Investigations into the neurochemical mechanisms mediating differences in ethanol sensitivity in two lines of mice. J. Pharmacol. Exp. Ther. 1982;221:404–409. [PubMed] [Google Scholar]
- Millan MJ, Bervoets K, Rivet JM, Widdowson P, Renouard A, Le Marouille-Girardon S, Gobert A. Multiple alpha-2 adrenergic receptor subtypes. II. Evidence for a role of rat R alpha-2A adrenergic receptors in the control of nociception, motor behavior and hippocampal synthesis of noradrenaline. J. Pharmacol. Exp. Ther. 1994;270:958–972. [PubMed] [Google Scholar]
- Millan MJ, Lejeune F, Gobert A, Brocco M, Auclair A, Bosc C, Rivet JM, Lacoste JM, Cordi A, Dekeyne A. S18616, a highly potent spiroimidazoline agonist at alpha(2)-adrenoceptors. II. Influence on monoaminergic transmission, motor function, and anxiety in comparison with dexmedetomidine and clonidine. J. Pharmacol. Exp. Ther. 2000;295:1206–1222. [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci. Biobehav. Rev. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Miner LH, Jedema HP, Moore FW, Blakely RD, Grace AA, Sesack SR. Chronic stress increases the plasmalemmal distribution of the norepinephrine transporter and the coexpression of tyrosine hydroxylase in norepinephrine axons in the prefrontal cortex. J. Neurosci. 2006;26:1571–1578. doi: 10.1523/JNEUROSCI.4450-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman-Tancredi A, Nicolas JP, Audinot V, Gavaudan S, Verriele L, Touzard M, Chaput C, Richard N, Millan MJ. Actions of alpha2 adrenoceptor ligands at alpha2A and 5-HT1A receptors: the antagonist, atipamezole, and the agonist, dexmedetomidine, are highly selective for alpha2A adrenoceptors. Naunyn Schmiedebergs Arch. Pharmacol. 1998;358:197–206. doi: 10.1007/pl00005243. [DOI] [PubMed] [Google Scholar]
- Norcross M, Mathur P, Enoch AJ, Karlsson RM, Brigman J, Cameron HA, Harvey-White J, Holmes A. Fluoxetine treatment during adolescence does not cause lasting deficits in fear-, anxiety- or stress-related behaviors in mice. Psychopharmacology (Berl.) doi: 10.1007/s00213-008-1215-7. in press, doi:10.1007/s00213-008-1215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary OF, Bechtholt AJ, Crowley JJ, Valentino RJ, Lucki I. The role of noradrenergic tone in the dorsal raphe nucleus of the mouse in the acute behavioral effects of antidepressant drugs. Eur. Neuropsychopharmacol. 2007;17:215–226. doi: 10.1016/j.euroneuro.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Sharpe AL, Burkhart-Kasch S, McKinnon CS, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor overexpression decreases ethanol drinking and increases sensitivity to the sedative effects of ethanol. Psychopharmacology (Berl.) 2004;176:386–397. doi: 10.1007/s00213-004-1896-5. [DOI] [PubMed] [Google Scholar]
- Pavcovich LA, Cancela LM, Volosin M, Molina VA, Ramirez OA. Chronic stress-induced changes in locus coeruleus neuronal activity. Brain Res. Bull. 1990;24:293–296. doi: 10.1016/0361-9230(90)90219-p. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. Interaction of ethanol and stress: research with experimental animals – an update. Alcohol Alcohol. 1990;25:263–276. doi: 10.1093/oxfordjournals.alcalc.a045000. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Brick J. Activity of neurons in the locus coeruleus of the rat: inhibition by ethanol. Brain Res. 1977;131:174–179. doi: 10.1016/0006-8993(77)90039-7. [DOI] [PubMed] [Google Scholar]
- Powell SB, Palomo J, Carasso BS, Bakshi VP, Geyer MA. Yohimbine disrupts prepulse inhibition in rats via action at 5-HT1A receptors, not alpha2-adrenoceptors. Psychopharmacology (Berl.) 2005;180:491–500. doi: 10.1007/s00213-005-2193-7. [DOI] [PubMed] [Google Scholar]
- Ritzmann RF, Tabakoff B. Dissociation of alcohol tolerance and dependence. Nature. 1976;263:418–420. doi: 10.1038/263418a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Cortical noradrenaline, attention and arousal. Psychol. Med. 1984;14:13–21. doi: 10.1017/s0033291700003032. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Longu G, Mercuro G, Hmaidan Y, Gessa GL. Biphasic effect of ethanol on noradrenaline release in the frontal cortex of awake rats. Alcohol Alcohol. 1992;27:477–480. [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, MacDonald E, Viitamaa T, Lahdesmaki J, Rybnikova E, Pelto-Huikko M, Kobilka BK, Scheinin M. Genetic alteration of the alpha2-adrenoceptor subtype c in mice affects the development of behavioral despair and stress-induced increases in plasma corticosterone levels. Mol. Psychiatry. 1999;4:443–452. doi: 10.1038/sj.mp.4000543. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell. Mol. Neurobiol. 1999;19:467–489. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard P, Merand Y, Dupont A. 3H-mepyramine binding in various regions of rat brain following chronic treatment with different classes of antidepressant drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 1982;6:449–454. doi: 10.1016/s0278-5846(82)80126-7. [DOI] [PubMed] [Google Scholar]
- Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- Seppala T, Idanpaan-Heikkila JJ, Stromberg C, Mattila MJ. Ethanol antagonism by atipamezole on motor performance in mice. Life Sci. 1994;55:245–251. doi: 10.1016/0024-3205(94)00886-8. [DOI] [PubMed] [Google Scholar]
- Shefner SA, Tabakoff B. Basal firing rate of rat locus coeruleus neurons affects sensitivity to ethanol. Alcohol. 1985;2:239–243. doi: 10.1016/0741-8329(85)90052-7. [DOI] [PubMed] [Google Scholar]
- Spuhler K, Gerhardt G, Palmer MR. CNS monoamine levels and the effect of DSP4 on ethanol sensitivity in LS and SS mice. Alcohol. 1987;4:419–424. doi: 10.1016/0741-8329(87)90078-4. [DOI] [PubMed] [Google Scholar]
- Stanford SC. Central noradrenergic neurones and stress. Pharmacol. Ther. 1995;68:242–297. doi: 10.1016/0163-7258(95)02010-1. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Swann AC, Silverman PB. Development of ethanol tolerance not altered by 6-OHDA lesions of dorsal bundle. Pharmacol. Biochem. Behav. 1989;33:729–731. doi: 10.1016/0091-3057(89)90417-6. [DOI] [PubMed] [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sun AY, Seaman RN, Middleton CC. Effects of acute and chronic alcohol administration on brain membrane transport systems. Adv. Exp. Med. Biol. 1977;85A:123–138. doi: 10.1007/978-1-4899-5181-6_9. [DOI] [PubMed] [Google Scholar]
- Sutoo D, Sano K. Modulating effects of biogenic amines on calcium and ethanol-induced sleeping time. Alcohol. 1984;1:141–144. doi: 10.1016/0741-8329(84)90070-3. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF. The effects of 6-hydroxydopamine on tolerance to and dependence on ethanol. J. Pharmacol. Exp. Ther. 1977;203:319–331. [PubMed] [Google Scholar]
- Tasaka K, Chung YH, Sawada K, Mio M. Excitatory effect of histamine on the arousal system and its inhibition by H1 blockers. Brain Res. Bull. 1989;22:271–275. doi: 10.1016/0361-9230(89)90053-1. [DOI] [PubMed] [Google Scholar]
- Taylor JE, Richelson E. High affinity binding of tricyclic antidepressants to histamine H1-receptors: fact and artifact. Eur. J. Pharmacol. 1980;67:41–46. doi: 10.1016/0014-2999(80)90006-0. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur. J. Pharmacol. 2008 doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanck P, Seutin V, Dresse A, Scuvee J, Massotte L, Giesbers I, Kornreich C. Electrophysiological effects of ethanol on monoaminergic neurons: an in vivo and in vitro study. Alcohol. Clin. Exp. Res. 1990;14:728–735. doi: 10.1111/j.1530-0277.1990.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Rust NC, Miller NS, Palmiter RD. Ethanol-associated behaviors of mice lacking norepinephrine. J. Neurosci. 2000;20:3157–3164. doi: 10.1523/JNEUROSCI.20-09-03157.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP, Reid LR, Threlkeld PG. Fluoxetine and two other serotonin uptake inhibitors without affinity for neuronal receptors. Biochem. Pharmacol. 1983;32:1287–1293. doi: 10.1016/0006-2952(83)90284-8. [DOI] [PubMed] [Google Scholar]