Abstract
Gold nanorods (GNRs) were encapsulated and dispersed into organic solvents by tetrabenzylthiol resorcinarene (TBTR) and by a poly(dithiocarbamate) derived from tetra- N-methyl(aminomethyl)resorcinarene (TMAR-DTC), formed by the in situ condensation of TMAR with carbon disulfide. The latter proved to be highly effective at enabling the redispersion of GNRs in various organic solvents. GNRs encapsulated in TMAR-DTC exhibited a strong solvatochromic response, with a refractive index sensitivity of over 300 nm/RIU. The resorcinarene-encapsulated GNRs could withstand high temperatures for a short period of time, and could be used to nucleate the growth of magnetic nanoshells.
Keywords: calixarenes, nanoparticles, encapsulation, nanorods, solvatochromism
Introduction
Anisotropic gold nanoparticles are well suited as contrast agents for optical imaging of biological tissues. Colloidal gold has a long history of clinical use, but only recently have methods been developed to fashion gold nanoparticles into structures with tunable plasmon modes, ranging from visible to near-infrared (NIR) wavelengths. The NIR region between 750 and 1300 nm is particularly favorable for optical imaging, as shorter wavelengths are extinguished by hemoglobin or other endogenous pigments, and longer wavelengths are strongly attenuated by water.1
Several types of NIR-resonant gold nanoparticles are currently being investigated as contrast agents for various biomedical imaging modalities, including nanoshells,2,3 nanocages,4 and nanorods.5,6,7 Gold nanorods (GNRs) with aspect ratios of 3–4 (ca. 15 nm wide and 45–50 nm in length) are especially attractive for such applications, as they can support a higher absorption cross section at NIR frequencies per unit volume than most other structures,8 and have narrower linewidths due to reduced radiative damping effects.9 We and others have been engaged in the development of GNRs as multifunctional agents for imaging and photoactivated therapies.5–7 In addition to their strong NIR absorption, we have found that GNRs are capable of producing significant two-photon luminescence (TPL) when excited at plasmon resonance with ultrashort laser pulses.6,10 This is an unusual property for metal nanoparticles, which are better known for their efficiency to quench fluorescence. Nevertheless, the TPL signals from GNRs can be detected with single-particle sensitivity and are useful for real-time in vitro and in vivo imaging.
GNRs can be prepared on a bulk scale in aqueous micellar surfactant solutions using seeded growth conditions in the presence of AgNO3,11,12 but this approach is often accompanied by a gradual blueshift in their longitudinal plasmon resonance due to the reshaping of GNRs by a slow growth process. Fortunately, this “optical drift” is easily remedied by treating freshly prepared GNRs with sodium sulfide to arrest further growth, thereby facilitating their practical application as NIR contrast agents.13 Stringent control over surface chemistry is also important in the development of GNRs for targeted imaging and therapy. For this purpose, we have developed a one-step method of surface functionalization based on the in situ formation of dithiocarbamate (DTC) ligands, derived from the corresponding amines.6,14 The chemisorption of DTCs to gold surfaces is considerably more robust than that of alkanethiols, which are prone to desorption under physiological conditions by competing adsorbates or oxidation.15
The longitudinal plasmon resonance (LPR) gives rise to polarization-dependent optical absorption and scattering,6a,10,16 suggesting additional opportunities for applying GNRs as imaging agents or as optical switches. In particular, it may be possible to align GNRs with an external field by incorporating a magnetically responsive coating, to create a dynamic form of optical signaling. However, while there are numerous examples of core–shell Fe3O4@Au nanoparticles, i.e. iron oxide nanoparticles with gold shells, examples of magnetic materials grown on Au nanoparticles have appeared only recently. Yu et al. demonstrated that alkanethiol-coated Au nanoparticles can nucleate the deposition of Fe(CO)5 in hot organic solvents, which upon air oxidation produced binary Au/Fe3O4 nanoparticles instead of a complete Fe3O4 shell.17 This suggests a feasible mechanism for incorporating magnetic materials onto GNRs, given a reliable method for stabilization in organic solvents. Other recent examples include the formation of a thin Fe3O4 nanoshell on GNRs by the coprecipitation of Fe(II) and Fe(III) salts,18 and the quasi-epitaxial growth of Ni on Pt-tipped GNRs.19
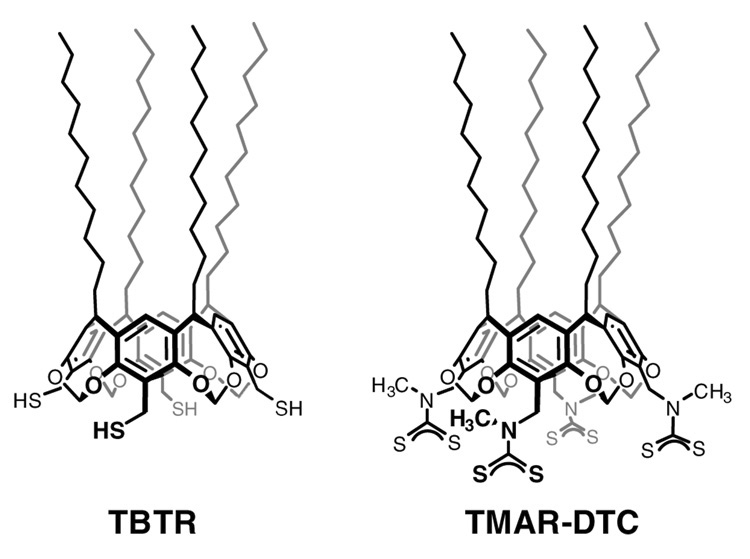
In this paper we use surfactants derived from C-undecylcalix[4]resorcinarene, namely tetrabenzylthiol resorcinarene (TBTR) and a poly(dithiocarbamate) derived from tetra-N-methyl( aminomethyl)resorcinarene (TMAR-DTC), for encapsulating GNRs and extracting them into organic solvents (see Figure 1). The resorcinarene-encapsulated GNRs were examined for their capacity to nucleate the growth of magnetic shells by the decomposition of Fe(III)(acac)3 or Fe(CO)5 at elevated temperatures. Resorcinarenes and other calixarene derivatives have previously been used to enhance the dispersion of colloidal metal particles in various organic solvents,20,21 as well as their self-assembly into well-defined nanostructures with novel collective properties.22 The resorcinarenes’ excellent dispersant properties are derived from their anisotropic presentation of several hydrocarbon chains: The tailgroups are spaced several angstroms apart on the macrocyclic framework and disfavor a close-packed organization, which translates into effective steric repulsion due to the loss of configurational entropy upon chain compression or interdigitation.20e The resorcinarene surfactant chains are complemented by a multivalent headgroup for robust chemisorption onto the nanoparticle surface.20f Although we have successfully used resorcinarenes to extract colloidal gold particles as large as 100 nm from aqueous solutions into organic solvents,20c we face additional challenges with GNRs from their stronger van der Waals interactions as well as competition from preexisting surfactant. We note that GNRs have recently been extracted from aqueous solutions into organic solvents by using a hydrophobic polystyrene coating, and deposited onto surfaces as rings by a dewetting mechanism.23 However, for our purposes we require a surfactant coating of minimal thickness, to enable subsequent nucleation of iron onto the GNR surface.
Figure 1.
Tetrabenzylthiol resorcinarene (TBTR) and multivalent dithiocarbamate derived from tetra-N-methyl(aminomethyl)resorcinarene (TMAR-DTC).
Results and Discussion
Extraction of resorcinarene-encapsulated Au nanorods
NIR-resonant GNRs were prepared in aqueous micellar solutions of cetyltrimethylammonium bromide (CTAB) (see Figure 2). TBTR was prepared as previously described20c and added to GNR dispersions as a 2 mM solution in THF. TMAR was prepared by a procedure similar to that described by Reinhoudt and coworkers,24 except that methylamine was substituted onto the corresponding tetra(bromomethyl)cavitand. The (poly)dithiocarbamate was generated in situ by combining a 2 mM solution of TMAR in THF with a dilute solution of CS2, to produce TMAR-DTC at a final concentration of 1.33 mM.
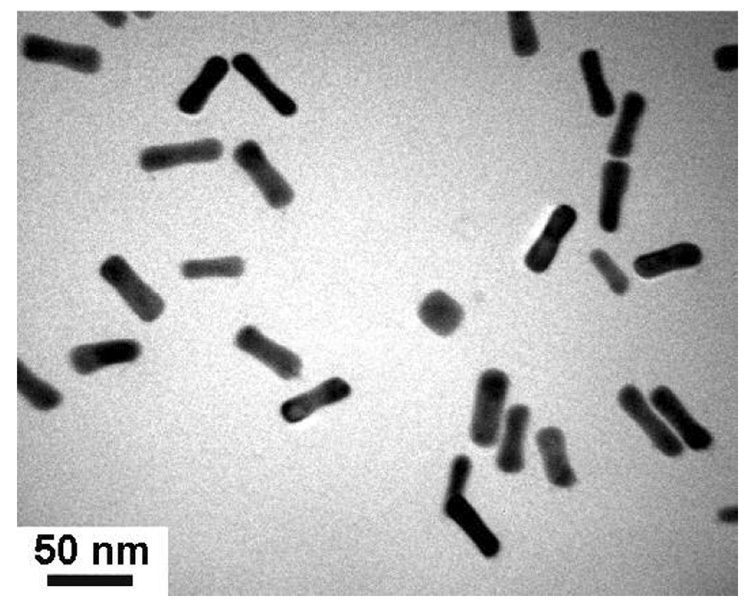
Figure 2.
TEM image (JEOL 2000FX, 200 keV) of GNRs prepared by seeded growth in aqueous micellar solutions.
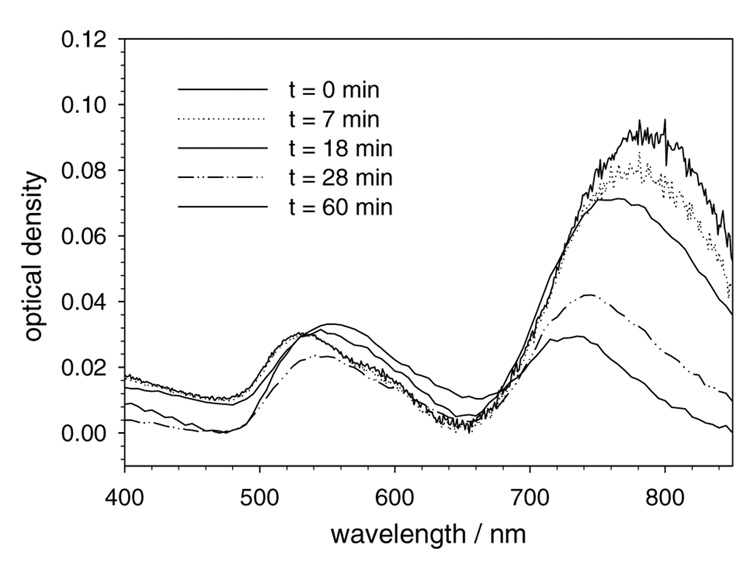
Aqueous solutions of GNRs were first treated with an equal volume of TBTR in THF, followed by the addition of a 10 µM solution of tetraoctylammonium bromide (TOAB) in toluene. The presence of TOAB has previously been used to mediate the direct extraction of colloidal gold nanoparticles with TBTR.20c,f However, in the case of GNRs, the dispersions were observed to be unstable in toluene, with aggregation in less than one hour (see Figure 3). Repeating the process with chloroform resulted in more stable dispersions, but these were also short-lived and substantial aggregation was observed by the following day.
Figure 3.
Absorbance spectra of GNRs extracted with TBTR into toluene, as a metric of dispersion stability over time.
Addition of TMAR-DTC had a rather different effect on the GNRs. Vigorous mixing resulted in a frothy suspension into which the resorcinarene-encapsulated GNRs were absorbed. This extraction procedure is analogous to a process known as flotation, which is often used in mining operations to recover precious metals from finely ground ore.25 However, in our case the GNRs are not dissolved into ionic species, but are instead partitioned into the foam or against the walls of the plastic culture tube. Once the GNRs are removed from the solution mixture, they can be recovered simply by decanting the remaining solution and redispersing the residue in CH2Cl2. It is worth noting that attempts to directly extract the TMAR-DTC-encapsulated GNRs into CH2Cl2 were unsuccessful; instead, most of the nanorods remained trapped at the biphasic interface and could not be recovered.
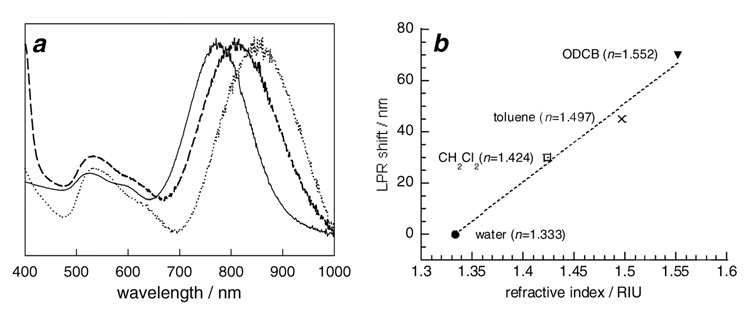
The CH2Cl2 suspensions of GNRs encapsulated by TMAR-DTC are stable at room temperature, and their optical densities remain largely unchanged after a small initial decrease (see Figure 4a). These GNRs were readily suspended in other higher-boiling organic solvents such as o-dichlorobenzene (ODCB) and dioctyl ether, simply by adding the desired solvent and removing CH2Cl2 by rotary evaporation. This facile solvent exchange allowed us to examine solvatochromic effects on their longitudinal plasmon resonance (see Figure 4b). Remarkably, the LPRs could be shifted toward longer wavelengths by as much as 70 nm from their initial resonance in aqueous solutions. Solvent-dependent shifts have previously been been noted for resorcinarene-encapsulated Au nanospheres20c as well as for polymer-stablilized nanoparticles,26 but the changes observed for GNRs are more pronounced. A linear fit of the LPR data in Figure 4b indicates a refractive index sensitivity of 306 nm/RIU. This is in accord with a study on the solvatochromism of Ag nanospheres, nanotriangles (prisms) and nanorods, in which the plasmon resonance of the latter was observed to be most sensitive to the solvent dielectric.27
Figure 4.
(a) Normalized absorbance spectra of GNRs stabilized by CTAB in water (—), and GNRs stabilized by TMAR-DTC in CH2Cl2 (---) and ODCB (‥‥). (b) Solvatochromatic shifts in longitudinal plasmon resonance (LPR) as a function of refractive index.
Gold nanorods with iron oxide nanoshells
To determine whether the resorcinarene-encapsulated GNRs could be used to nucleate the growth of iron at high temperatures, were first needed to confirm that they could withstand harsh thermal conditions, as GNRs have been reported to reshape into spheres when heated in the presence of CTAB.28 Extracted GNRs were dispersed in dioctyl ether and heated to reflux (286 °C) for 30 minutes with remarkably little change in their morphology, in contrast to earlier reports on GNR thermal stability. We attribute this stability to the robust nature of the TMAR-DTC coating which reduces the probability of sintering, and also to the absence of corrosive electrolytes such as those associated with CTAB. We note that the reshaping of GNRs into nanospheres occurred to a greater extent when heated in the presence of Fe(III)(acac)3 (see below).
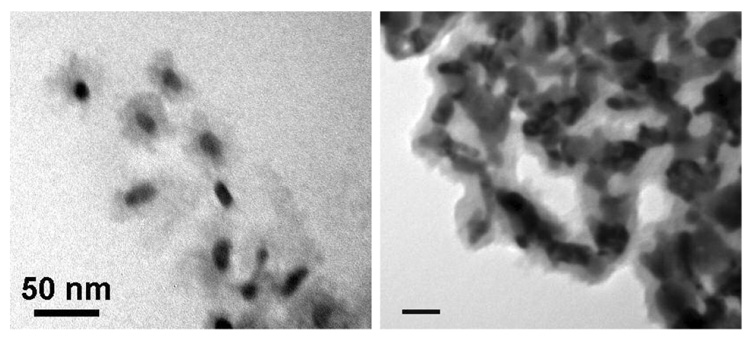
We first examined the seeded growth conditions reported by Sun and Zeng for the synthesis of Fe3O4 nanoparticles, based on the polyol reduction of Fe(III) salts.29 The TMAR-DTC-encapsulated GNRs were dispersed in a dioctyl ether solution of oleic acid, oleylamine, Fe(III)(acac)3, and tetraethylene glycol and heated to 250 °C for 1 hour, then cooled to room temperature and precipitated by the addition of ethanol to yield a dark brown solid. This residue contained some GNRs with amorphous iron oxide coatings (see Figure 5a), but more often resulted in the formation of free iron oxide nanoparticles as well as the partial reshaping of GNRs.
Figure 5.
(a) TEM image (JEOL 2000FX, 200 keV) of iron oxide-coated gold nanostructures, obtained from resorcinarene-encapsulated GNRs dispersed in hot dioctyl ether prior to thermal decomposition of Fe(acac)3 at 250 °C. (b) Iron oxide-coated gold nanostructures obtained from GNRs initially deposited onto a glass surface prior to thermal decomposition of Fe(acac)3. Scale bar = 50 nm.
We also examined the possibility of nucleating the thermal decomposition of Fe(III)(acac)3 on GNRs under heterogeneous conditions. GNRs were concentrated to dryness onto the surface of a round-bottomed flask, then immersed in the dioctyl ether solution described above and heated in a 250 °C sand bath. This condition was successful to produce iron oxide coatings (see Figure 5b), but many of the GNRs became misshapen and were difficult to release from the glass surface. We thus sought milder reaction conditions for functionalizing GNRs with magnetic materials.
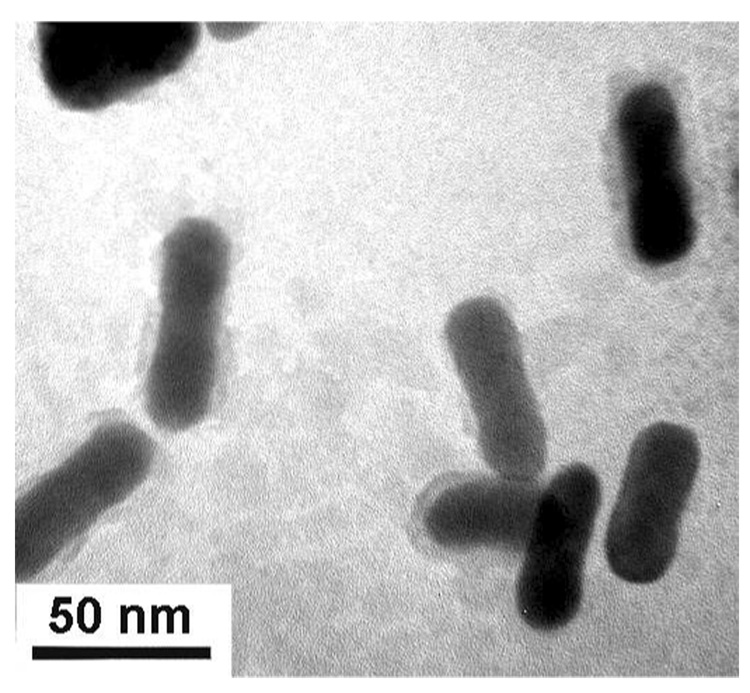
The formation of GNRs with iron nanoshells could be improved by replacing Fe(III)(acac)3 with Fe(CO)5 and by using o-dichlorobenzene (ODCB) as the solvent, which has a boiling point of 180 °C. The best results were obtained by preheating Fe(CO)5 with oleic acid at 120 °C, prior to introducing GNRs and oleylamine and heating to reflux. This condition is thought to produce an “Fe-oleate” complex, and has been used to synthesize uniform Fe3O4 nanoparticles with excellent control over the growth process.30 Subjecting resorcinarene-coated GNRs to these reaction conditions resulted in particles which responded to a strong magnetic field gradient. Transmission electron microscopy (TEM) analysis revealed that a significant fraction of GNRs were coated by a 4–6 nm layer of iron oxide (see Figure 6).
Figure 6.
TEM image (JEOL 2000FX, 200 keV) of GNRs with iron oxide shells (4–6 nm thickness), obtained from resorcinarene-encapsulated GNRs dispersed in hot ODCB prior to thermal decomposition of Fe(CO)5 under reflux conditions.
In summary, GNRs can be extracted and dispersed in organic solvents by the resorcinarene-based surfactant TMAR-DTC. These form more stable dispersions in organic solvents than GNRs coated by TBTR, due to the enhanced chemisorption properties of dithiocarbamates relative to that of thiols. The resorcinarene-encapsulated GNRs can be used as intermediates to form magnetic nanoshells and in the preparation of core−shell nanostructures. These can also be dispersed into hydrophobic polymers and liquid crystals for the preparation of multifunctional GNR-based composites, which will be described in due course.
Materials and Methods
Preparation of gold nanorods
GNRs were prepared in aqueous micellar solutions of cetyltrimethylammonium bromide (CTAB) using seeded growth conditions as previously described,11,12 followed by treatment with Na2S at the end of the rapid growth phase to arrest further changes in aspect ratio.13 The sulfide-treated GNRs used in this study were produced with lengths of 45–50 nm and a mean aspect ratio of 3.5, and longitudinal plasmon resonances between 765–800 nm. The GNRs were centrifuged and redispersed in deionized water twice (24000 g, 5 min per cycle) to remove most of the CTAB and residual metal salts, treated with a mixed-bed ion-exchange resin (Amberlite MB-3, Mallinckrodt) to further reduce the amount of CTAB, then diluted to an optical density in the range of 1.0–1.2. GNRs were characterized by TEM using a JEOL 2000-FX with an accelerating voltage of 200 keV, and by visible-NIR absorption spectroscopy with a Cary-50 spectrophotometer. Carbon-coated Cu TEM grids were purchased from Ted Pella, Inc. and used as supplied. High-purity water with measured resistivity above 18 MΩ cm was obtained using an ultrafiltration system (Milli-Q, Millipore) equipped with an additional 0.22-micron membrane filter.
Preparation of TMAR-DTC
Tetra-C-methylcavitand was synthesized according to known procedures,20c,24 and subjected to sodium bromite oxidation using the biphasic conditions reported by Iishi and coworkers.31 Tetra-C-methylcavitand (1.2 g, 0.79 mmol) was added to 7 mL of a 2.3 M NaBrO3 solution in EtOAc and stirred for 10 minutes at room temperature, then treated dropwise with 5 mL of a 3.2 M aqueous NaSO3 solution, producing a yellow-orange color. The reaction mixture was stirred for 5 hours and monitored by thin-layer chromatography to prevent overbromination of the product. The reaction mixture was then extracted with Et2O, washed with saturated Na2S2O3 and brine, dried over Na2SO4, and concentrated. Purification by silica gel chromatography yielded the desired tetra(bromomethyl)cavitand (0.96 g, 80% yield) as a viscous oil. This was dissolved in 10 mL of THF and treated with 100 mL of a 2 M solution of methylamine in THF. The reaction mixture was stirred at room temperature for 6 hours, then concentrated and redissolved in CH2Cl2. The product was washed with 1 M NaOH and water, then concentrated to dryness to yield TMAR as a yellow oil (1.2 g), which was used without further purification. 1H NMR (300 MHz, CDCl3): δ 7.06 (s, 4 H), 5.91 (d, 4 H, J = 6.9 Hz), 4.76 (t, 4 H, J = 6.1 Hz), 4.33 (d, 4 H, J = 6.9 Hz), 3.51 (s, 8 H), 2.20 (m, 8 H), 1.28 (m), 0.90 (t, 12 H, J = 6.3 Hz).
TMAR (26.6 mg, 0.02 mmol) was dissolved in 10 mL of freshly distilled THF and mixed with 5 mL of a 10 mM solution of CS2 in THF. The TMAR-DTC solution was allowed to sit at room temperature for 5 minutes prior to use.
Preparation of iron-coated nanorods by reduction of Fe(III)(acac)3
A CH2Cl2 suspension of GNRs stabilized by TMAR-DTC (20 mL, O.D. 0.6) was dried for 60 min over molecular sieves, then mixed with 10 mL of dioctyl ether and subjected to rotary evaporation to remove CH2Cl2. The GNR suspension (10 mL, O.D. 1.2) was charged with Fe(III)(acac)3, tetraethylene glycol, oleylamine, and oleic acid in a 1:5:3:3 ratio and heated to reflux under argon for 1 hour. The solution was cooled to room temperature and treated with 10 parts EtOH to induce precipitation of the nanoparticles. The residue was collected from the wall of the reaction flask and sonicated briefly in EtOH for redispersion, then deposited onto a carbon-coated Cu grid for TEM analysis.
Preparation of iron-coated nanorods by thermolysis of Fe(CO)5
GNRs stabilized by TMAR-DTC were dried and suspended in o-dichlorobenzene (10 mL, O.D. 1.2) using the procedure described above. Oleic acid (50 µL) was added to 2.5 mL of o-dichlorobenzene and heated to 120 °C, followed by an injection of Fe(CO)5 (7.5 µL) and further heating at 120 °C for 5 min. The suspension of resorcinarene-coated GNRs and oleylamine (25 µL) were added to the reaction mixture, then heated to reflux for 45 min in a 230 °C sand bath. The ratio of Au:Fe was estimated to be 1:2. The nanoparticle suspension was cooled to room temperature, diluted with isopropyl alcohol, then centrifuged at 24000 g for 20 minutes. The supernatant was discarded and the brown sediment were sonicated and redispersed in toluene, then collected by magnetic precipitation using a handheld NdFeB magnet and deposited onto a TEM grid.
Acknowledgments
The authors gratefully acknowledge financial support from the National Science Foundation (CHE-0243496) and the National Institutes of Health (EB-001777).
REFERENCES
- 1.Helmchen F, Denk W. Nature Methods. 2005;2:932. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 2.Loo C, Lowery A, West J, Halas N, Drezek R. Nano Lett. 2005;5:709. doi: 10.1021/nl050127s. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Xie X, Wang X, Ku G, Gill KL, O'Neal DP, Stoica G, Wang LV. Nano Lett. 2004;4:1689. [Google Scholar]
- 4.(a) Chen J, Saeki F, Wiley BJ, Cang H, Cobb MJ, Li Z-Y, Au L, Zhang H, Kimmey MB, Li X, Xia Y. Nano Lett. 2005;5:473. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]; (b) Chen J, Wang D, Xi J, Au L, Siekkinen AR, Warsen A, Li Z-YZH, Xia Y, Li X. Nano Lett. 2007;7:1318. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Huang X, El-Sayed IH, Qian W, El-Sayed MA. J. Am. Chem. Soc. 2006;128:2115. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]; (b) Takahashi H, Niidome T, Nariai A, Niidome Y, Yamada S. Chem. Lett. 2006;35:500. [Google Scholar]
- 6.(a) Wang H, Huff TB, Zweifel DA, He W, Low PS, Wei A, Cheng J-X. Proc. Natl. Acad. Sci. USA. 2005;102:15752. doi: 10.1073/pnas.0504892102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huff TB, Hansen MN, Zhao Y, Cheng J-X, Wei A. Langmuir. 2007;23:1596. doi: 10.1021/la062642r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huff TB, Tong L, Zhao Y, Hansen MN, Cheng J-X, Wei A. Nanomedicine. 2007;2:125. doi: 10.2217/17435889.2.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Tong L, Zhao Y, Huff TB, Hansen MN, Wei A, Cheng J-X. Adv. Mater. 2007;19 doi: 10.1002/adma.200701974. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Oldenburg AL, Hansen MN, Zweifel DA, Wei A, Boppart SA. Opt. Express. 2006;14:6724. doi: 10.1364/oe.14.006724. [DOI] [PubMed] [Google Scholar]; (b) Durr NJ, Larson T, Smith DK, Korgel BA, Sokolov K, Ben-Yakar A. Nano Lett. 2007;7:941. doi: 10.1021/nl062962v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Troutman TS, Barton JK, Romanowski M. Opt. Lett. 2007;32:1438. doi: 10.1364/ol.32.001438. [DOI] [PubMed] [Google Scholar]
- 8.Jain PK, Lee KS, El-Sayed IH, El-Sayed MA. J. Phys. Chem. B. 2006;110:7238. doi: 10.1021/jp057170o. [DOI] [PubMed] [Google Scholar]
- 9.Sönnichsen C, Franzl T, Wilk T, von Plessen G, Feldmann J, Wilson O, Mulvaney P. Phys. Rev. Lett. 2002;88:077402. doi: 10.1103/PhysRevLett.88.077402. [DOI] [PubMed] [Google Scholar]
- 10.Bouhelier A, Bachelot R, Lerondel G, Kostcheev S, Royer P, Wiederrecht GP. Phys. Rev. Lett. 2005;95:267405. doi: 10.1103/PhysRevLett.95.267405. [DOI] [PubMed] [Google Scholar]
- 11.(a) Jana NR, Gearheart L, Murphy CJ. J. Phys. Chem. B. 2001;105:4065. [Google Scholar]; (b) Sau TK, Murphy CJ. Langmuir. 2004;20:6414. doi: 10.1021/la049463z. [DOI] [PubMed] [Google Scholar]
- 12.Nikoobakht B, El-Sayed MA. Chem. Mater. 2003;15:1957. [Google Scholar]
- 13.Zweifel DA, Wei A. Chem. Mater. 2005;17:4256. doi: 10.1021/cm0506858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Pérez-Segarra W, Shi Q, Wei A. J. Am. Chem. Soc. 2005;127:7328. doi: 10.1021/ja050432f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Flynn NT, Tran TN, Cima MJ, Langer R. Langmuir. 2003;19:10909. [Google Scholar]; (b) Dasog M, Scott RWJ. Langmuir. 2007;23:3381. doi: 10.1021/la0627415. [DOI] [PubMed] [Google Scholar]
- 16.Van der Zande BMI, Pagès L, Hikmet RAM, van Blaaderen A. J. Phys. Chem. 1999;103:5761. [Google Scholar]
- 17.Yu H, Chen M, Rice PM, Wang SX, White RL, Sun S. Nano Lett. 2005;5:379. doi: 10.1021/nl047955q. [DOI] [PubMed] [Google Scholar]
- 18.Murphy CJ, Gole AM, Hunyadi SE, Orendorff CJ. Inorg. Chem. 2006;45:7544. doi: 10.1021/ic0519382. [DOI] [PubMed] [Google Scholar]
- 19.Grzelczak M, Rodríguez-González B, Pérez-Juste J, Liz-Marzán LM. Adv. Mater. 2007;19 in press. [Google Scholar]
- 20.(a) Stavens KB, Pusztay SV, Zou S, Andres RP, Wei A. Langmuir. 1999;15:8337. [Google Scholar]; (b) Balasubramanian R, Xu J, Kim B, Sadtler B, Wei A. J. Dispersion Sci. Tech. 2001;22:485. [Google Scholar]; (c) Balasubramanian R, Kim B, Tripp SL, Wang X, Lieberman M, Wei A. Langmuir. 2002;18:3676. [Google Scholar]; (d) Pusztay SV, Wei A, Stavens KB, Andres RP. Supramol. Chem. 2002;14:291. [Google Scholar]; (e) Wei A. ChemComm. 2006:1581. [Google Scholar]; (f) Balasubramanian R, Kwon Y-G, Wei A. J. Mater. Chem. 2007;17:105. doi: 10.1039/b614295h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Misra TK, Chen TS, Liu CY. J. Colloid Interface Sci. 2006;297:584. doi: 10.1016/j.jcis.2005.11.013. [DOI] [PubMed] [Google Scholar]; (b) Ben-Ishay ML, Gedanken A. Langmuir. 2007;23:5238. doi: 10.1021/la0633076. [DOI] [PubMed] [Google Scholar]
- 22.(a) Kim B, Tripp SL, Wei A. J. Am. Chem. Soc. 2001;123:7955. doi: 10.1021/ja0160344. [DOI] [PubMed] [Google Scholar]; (b) Wei A, Kim B, Pusztay SV, Tripp SL, Balasubramanian R. J. Inclusion Phenom. Macrocyclic Chem. 2001;41:83. [Google Scholar]; (c) Tripp SL, Pusztay SV, Ribbe AE, Wei A. J. Am. Chem. Soc. 2002;124:7914. doi: 10.1021/ja0263285. [DOI] [PubMed] [Google Scholar]; (d) Tripp SL, Dunin-Borkowski RE, Wei A. Angew. Chem. Int. Ed. 2003;42:5591. doi: 10.1002/anie.200352825. [DOI] [PubMed] [Google Scholar]; (e) Kim B, Carignano MA, Tripp SL, Wei A. Langmuir. 2004;20:9360. doi: 10.1021/la0488351. [DOI] [PubMed] [Google Scholar]; (f) Kim B, Balasubramanian R, Pérez-Segarra W, Wei A, Decker B, Mattay J. Supramol. Chem. 2005;17:173. [Google Scholar]
- 23.Khanal BP, Zubarev ER. Angew. Chem. Int. Ed. 2007;46:2195. doi: 10.1002/anie.200604889. [DOI] [PubMed] [Google Scholar]
- 24.Boerrigter H, Verboom W, Reinhoudt DN. J. Org. Chem. 1997;62:7148. doi: 10.1021/jo9703414. [DOI] [PubMed] [Google Scholar]
- 25.Woods R. J. Phys. Chem. 1971;75:354. [Google Scholar]
- 26.Underwood S, Mulvaney P. Langmuir. 1994;10:3427. [Google Scholar]
- 27.McFarland AD, van Duyne RP. Nano Lett. 2003;3:1057. [Google Scholar]
- 28.(a) Mohamed MB, Ismail KZ, Link S, El-Sayed MA. J. Phys. Chem. B. 1998;102:9370. [Google Scholar]; (b) Petrova H, Perez Juste J, Pastoriza-Santos I, Hartland GV, M L-ML, Mulvaney P. Phys. Chem. Chem. Phys. 2006;8:814. doi: 10.1039/b514644e. [DOI] [PubMed] [Google Scholar]
- 29.Sun SH, Zeng H. J. Am. Chem. Soc. 2002;124:8204. doi: 10.1021/ja026501x. [DOI] [PubMed] [Google Scholar]
- 30.Park J, Lee E, Hwang NM, Kang M, Kim SC, Hwang Y, Park J-G, Noh H-J, Kim J-Y, Park J-H, Hyeon T. Angew. Chem. Int. Ed. 2005;44:2873. doi: 10.1002/anie.200461665. [DOI] [PubMed] [Google Scholar]
- 31.Kikuchi D, Sakaguchi S, Ishii Y. J. Org. Chem. 1998;63:6023. doi: 10.1021/jo972263q. [DOI] [PubMed] [Google Scholar]