Abstract
Menopause is characterized by depletion of ovarian follicles, a reduction of ovarian hormones to castrate levels and elevated levels of serum gonadotropins. Rather than degenerating, the reproductive neuroendocrine axis in postmenopausal women is intact and responds robustly to the removal of ovarian hormones. Studies in both humans and non-human primates provide evidence that the gonadotropin hypersecretion in postmenopausal women is secondary to increased gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus. In addition, menopause is accompanied by hypertrophy of neurons in the infundibular (arcuate) nucleus expressing KiSS-1, neurokinin B (NKB), substance P, dynorphin and estrogen receptor α (ERα) mRNA. Ovariectomy in experimental animals induces nearly identical findings, providing evidence that these changes are a compensatory response to ovarian failure. The anatomical site of the hypertrophied neurons, as well as the extensive data implicating kisspeptin, NKB and dynorphin in the regulation of GnRH secretion, provide compelling evidence that these neurons are part of the neural network responsible for the increased levels of serum gonadotropins in postmenopausal women. We propose that neurons expressing KiSS-1, NKB, substance P, dynorphin and ERα mRNA in the infundibular nucleus play an important role in sex-steroid feedback on gonadotropin secretion in the human.
Keywords: steroid feedback, GnRH, estrogen, progesterone, aging, reproduction, pituitary, ovary
1. Introduction
Menopause marks the cessation in reproductive cycles of middle-aged women. It is heralded by depletion of ovarian follicles leading to the loss of ovarian hormones with repercussions throughout the body. The removal of sex-steroid negative feedback results in a marked increase in serum luteinizing hormone (LH) and follicle stimulating hormone (FSH). This open-loop condition is accompanied by increased GnRH gene expression and cellular hypertrophy of a subpopulation of neurons within the human infundibular nucleus, the homologue of the arcuate nucleus in other species [80,81]. Although postmenopausal neuronal hypertrophy was first described in 1966 [96], a major breakthrough in our understanding of this phenomenon occurred in 2007, when KiSS-1 mRNA was identified within the hypertrophied neurons [86]. To place this discovery in perspective, this article will review aging of the reproductive axis in women and the marked changes in hypothalamic morphology and gene expression in the postmenopausal human hypothalamus. As it will become apparent, the studies of kisspeptin and neurokinin B gene expression in the human hypothalamus provide considerable insight into reproductive neuroendocrine regulation in postmenopausal women.
2. Ovarian aging and the menopausal transition
Ovarian failure is the critical determinant of menopause in women. At birth, there are approximately 500,000 to 1,000,000 primordial ovarian follicles. Recent stereological studies have shown that degeneration of non-growing ovarian follicles continually accelerates from the time of birth to menopause [43]. Although it has been argued that the loss of follicles accelerates after the age of 37 [25,82], this conclusion has not been supported by mathematical modeling studies [60]. Regardless of the rate of decline, the ovary is virtually depleted of follicles after the menopause [6,60,82]. Thus, the postmenopausal phase of life is dominated by the hormonal milieu of ovarian failure.
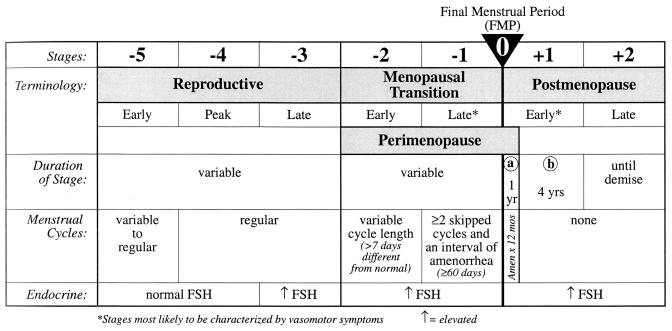
Guidelines for the classification of stages of reproductive life are shown in Figure 1 [101]. The reproductive stage is followed by the menopausal transition, which marks the onset of irregular cycle lengths [101]. However, before the onset of irregular cycles, fertility begins to decline and ovarian hormone levels change. The first hormone alteration is an elevation in FSH in the early follicular phase, accompanied by declining levels of inhibin B [50,51,97,107]. Inhibin B, secreted by granulosa cells in small antral follicles, is a useful marker of ovarian reserve [85,103]. Because inhibin B is the dominant form of inhibin suppressing FSH in the early follicular phase [85], the early rise in FSH secretion is likely due to decreased secretion of inhibin B. Anti-Mullerian hormone is another marker of ovarian function that decreases in the late reproductive stage [38]. Thus, the decline in inhibin B and anti-Mullerian hormone are signs of ovarian follicle depletion preceding the menopausal transition. It has been proposed that hypothalamic signals could trigger the transition [111], but this hypothesis is controversial because much of it is based on a rat model that exhibits significant ovarian function after the loss of reproductive capacity [67]. While altered frequency of gonadotropin pulses can have deleterious effects upon ovarian follicle development [75], there is currently conflicting evidence on whether this mechanism could contribute to the loss of ovarian follicles in the human [39].
Figure 1.
The STRAW (Stages of Reproductive Aging Workshop) staging system. The menopause is defined as the time of the final menstrual period. The onset of variable cycle lengths characterizes the menopausal transition. Note that the earliest change is increased FSH secretion before cycles become irregular. This increase in FSH secretion is inversely correlated with decreased levels of inhibin B.
From Soules, et al. [101], reproduced with permission from Elsevier.
During the early menopausal transition, when cycles are irregular, many women exhibit elevated levels of FSH throughout the cycle and lower levels of inhibin A, inhibin B, and progesterone [37]. Serum estrogen levels of the early menopausal transition are either preserved or increased. A rise in follicular phase FSH may lead to accelerated follicular development with shortening of the length of the menstrual cycle [49]. The late menopausal transition is marked by periods of amenorrhea, frequent anovulatory cycles, and substantial variability in ovarian hormone levels and cycle lengths [37,59]. In a subgroup of perimenopausal women, anovulatory cycles are characterized by normal estrogen and an LH surge but inadequate formation of corpora lutea [106]. In others, a rise in follicular phase estrogen is seen, but this is not accompanied by an LH surge [106]. These findings and others [104] have been interpreted as indicative of insensitivity to estrogen positive feedback, but carefully controlled studies to test this hypothesis are not available [40]. In any case, the marked fluctuation of ovarian hormones in the late menopausal transition gives rise to many of the clinical symptoms of the perimenopausal period. Erratic levels of serum estrogen, rather than the absolute level of estrogen, is a major factor in the occurrence of hot flashes in the perimenopausal phase [77].
3. The reproductive neuroendocrine axis in postmenopausal women
By the time of the postmenopausal period, the degeneration of ovarian follicles is complete and circulating estrogen and progesterone are reduced to castrate levels [4,9,66,105]. The profound deficiency in ovarian hormones results in elevated levels of serum gonadotropins due to the removal of steroid negative feedback and loss of the restraining action of inhibin on FSH secretion. In addition, ovarian failure results in a shift in the composition of the gonadotropins to acidic isoforms, retarding the clearance of LH from the peripheral circulation [94,108]. Due to the long duration of the postmenopausal phase, there may also be alterations in biological rhythms, energy homeostasis and the secretion of adrenal and thyroid hormones and growth hormone [58,100]. As chronological age advances after the menopause, there are also changes in the reproductive neuroendocrine axis. Between 50 and 80 years of age, mean levels of serum LH and FSH decline and LH pulse frequency decreases [88,91]. Decreased GnRH pulse frequency has also been documented using pulsatile gonadotropin free α-subunit pulses as a marker [41]. These studies show that after the menopausal transition, there is aging of the hypothalamic-pituitary axis independent from the loss in gonadal function.
Despite the continued aging of the central nervous system, there is compelling evidence that many aspects of reproductive neuroendocrine function remain intact after menopause. The hypothalamic/pituitary axis of postmenopausal women is capable of responding to steroid positive feedback signals with increased LH secretion [69,71]. In addition, studies using indirect pharmacological methods provide evidence that GnRH secretion is increased in postmenopausal women compared to premenopausal women [39]. Importantly, the ability of estrogen feedback to decrease GnRH secretion and gonadotropin secretion is not diminished by age [30,31]. Similarly, administration of progesterone will still suppress serum gonadotropin levels, GnRH secretion and free α-subunit pulse frequency in older postmenopausal women pretreated with estrogen [30]. Because the hypothalamus has been shown to be the major site of steroid negative feedback in the human [8,31], these studies demonstrate intact hypothalamic function in the postmenopausal period. Interestingly, the quantity of GnRH secretion continues to increase with age after the menopause [31]. Although it is not understood why GnRH secretion would increase in the postmenopausal period, this finding provides clear evidence that GnRH neurons in older women remain capable of releasing significant quantities of GnRH. Overall, these studies indicate that removal of steroid negative feedback in postmenopausal women is linked to increased GnRH secretion from the hypothalamus.
Autopsy studies of premenopausal and postmenopausal women provide additional evidence that the reproductive neuroendocrine axis in postmenopausal women responds to the removal of ovarian steroids. The content of the GnRH decapeptide is decreased in the hypothalamus of postmenopausal women [72]. A similar change is observed in young oophorectomized women [72], suggesting that the decrease in hypothalamic GnRH content in postmenopausal women is a consequence of ovarian failure and may represent decreased storage due to increased release of decapeptide into the portal circulation [110]. Moreover, GnRH mRNA is increased in the hypothalamus of postmenopausal women, as would be expected with removal of steroid negative feedback [80]. The elevation of GnRH gene expression occurs within a subpopulation of neurons scattered in the ventral preoptic region, retrochiasmatic area and the infundibular nucleus but not within the dorsal preoptic area or septal region [80]. Combined with the studies cited in the preceding paragraph, these data reinforce the concept that removal of steroid negative feedback leads to increased GnRH synthesis and secretion, leading to the gonadotropin hypersecretion characteristic of the postmenopausal state.
Experiments using non-human primates support the hypothesis that loss of steroid negative feedback in postmenopausal women leads to increased GnRH gene expression and secretion. Removal of the ovaries in young rhesus monkeys results in increased GnRH secretion into the portal capillary system [10]. Ovariectomy of young cynomolgus monkeys also results in increased GnRH gene transcripts and these changes are similar in distribution and magnitude to the changes in GnRH gene expression in postmenopausal women [90]. Conversely, in ovariectomized monkeys, estrogen replacement decreases GnRH release into the stalk-median eminence [11,64] and GnRH gene expression in the ventral hypothalamus [22,54]. Moreover, estrogen markedly suppresses the bursts of multiunit activity within the primate medial basal hypothalamus that are correlated with pulses of LH in peripheral plasma (the GnRH pulse generator) [47]. These data provide evidence that estrogen negative feedback occurs at the level of the GnRH neurons in the primate, although they do not address whether these effects on GnRH neurons are direct or indirect. They also indicate that increased GnRH gene expression in ovariectomized primates is linked with increased GnRH secretion from the hypothalamus. Taken together, these studies provide compelling evidence that the rise in hypothalamic GnRH gene expression and gonadotropin hypersecretion in postmenopausal women is secondary to ovarian failure, with withdrawal of estrogen being an important factor [80].
Similar to humans, menopause in non-human primates is accompanied by ovarian failure and gonadotropin hypersecretion [112]. However, menopause occurs very late in the lifespan of the monkey compared to the mid-life menopausal transition of humans [5]. In a recent study, GnRH secretion was compared in young and aged rhesus monkeys using push-pull perfusion [34]. The older monkeys exhibited low estrogen levels characteristic of ovarian follicle depletion and would be considered postmenopausal or within the late menopausal transition by the STRAW (Stages of Reproductive Aging Workshop) classification [101]. Remarkably, the amount of pulsatile GnRH secretion was dramatically increased in the aged monkeys, while GnRH pulse frequency was not significantly different between groups [34]. These findings correlate well with the increase in GnRH gene expression and secretion observed in postmenopausal women [31,80] and demonstrate remarkable preservation of GnRH neuronal function in the non-human primate, even in very advanced age.
4. Changes in morphology and NKB gene expression in the infundibular nucleus of postmenopausal women
More than four decades ago, Sheehan and Kovacs described pronounced differences in hypothalamic neuronal morphology between pre- and postmenopausal women [96]. The neurons were larger in postmenopausal women, in a subregion of the infundibular (arcuate) nucleus which they named the subventricular nucleus [68,96]. The enlarged neurons exhibited other signs of hypertrophy, including increased nuclear size, larger nucleoli and prominent Nissl substance. There was no evidence of increased storage material, chromatolysis, swelling or any other pathological changes that explained the change in neuron size. The hypertrophied neurons were identified in women over the age of 50 and in women with a history of post-partum hypopituitarism, but were inconspicuous in men of any age [96]. Because the neuronal hypertrophy was strongly correlated with uterine atrophy in patients with post-partum hypopituitarism, Sheehan proposed that the hypertrophy of neurons in postmenopausal women was related to loss of ovarian estrogen secretion [95].
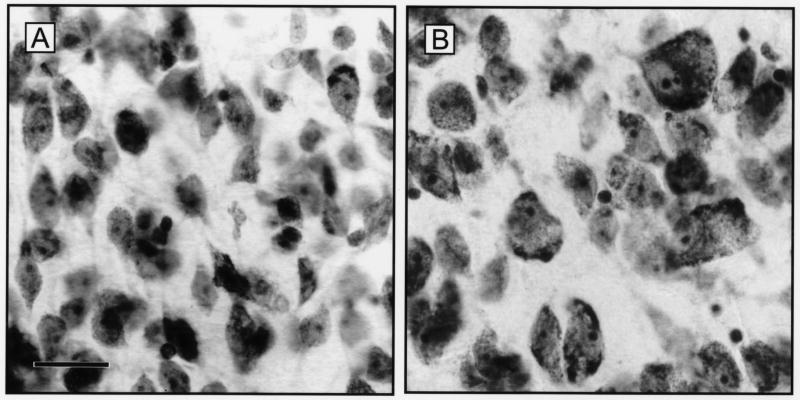
Subsequent analysis using computer microscopy showed a 30 to 40% increase in the size of neurons in the infundibular nucleus of postmenopausal women (Fig. 2, [2,79]). These studies also demonstrated that the neuronal hypertrophy occurred in a subpopulation of neurons within the infundibular nucleus [2,79]. Stereological studies revealed no neuronal cell loss in the infundibular nucleus of older women [2]. Thus, the neuronal hypertrophy is not a compensatory response to adjacent neuronal cell death.
Figure 2.
Representative photomicrographs of cresyl violet-stained sections of the infundibular nucleus of young, premenopausal (A) and older, postmenopausal (B) women. Note the considerably enlarged neurons in the older subject with increased size of nuclei and nucleoli as well as increased Nissl substance. Scale bar = 25 microns in A (applies to A,B). From Abel and Rance [2], reproduced with permission from Wiley-Liss Inc.
The development of in situ hybridization allowed characterization of mRNA expression in the hypertrophied neurons of postmenopausal women. The hypertrophied neurons express ERα mRNA but do not express GnRH [79]. The increase in GnRH gene expression in postmenopausal women occurs in a separate subpopulation of neurons scattered diffusely in the ventral hypothalamus and these GnRH neurons do not exhibit changes in cell size [80]. Hybridization of hypothalamic sections with a variety of cDNA probes revealed that the majority of hypertrophied neurons express neurokinin B (NKB) and substance P (SP) gene transcripts [81]. In addition to the increase in cell size, there are increased amounts of NKB and SP mRNA per cell and a striking increase in the number of cells expressing tachykinin gene transcripts in postmenopausal women. Ovariectomy of young, cynomolgus monkeys produces NKB neuronal hypertrophy and increased gene expression that is nearly identical to that seen in postmenopausal women [90]. Conversely, the expression of NKB mRNA in the infundibular nucleus of young ovariectomized cynomolgus monkeys is markedly reduced by estrogen replacement therapy [3]. These studies strongly support the hypothesis that the hypertrophy and increased NKB gene expression in the infundibular nucleus of older women is secondary to ovarian failure.
Reciprocal changes in neuropeptide Y (NPY) and proopiomelanocortin (POMC) gene expression occurs within separate subgroups of neurons in the hypothalamus of older women [1,23]. Specifically, the number of neurons expressing POMC gene transcripts decreases in the infundibular nucleus of postmenopausal women [1] whereas the gene expression of NPY neurons increases in both the infundibular nucleus and retrochiasmatic region [24]. However, unlike the NKB and ERα mRNA expressing neurons in the infundibular nucleus, NPY and POMC neurons do not exhibit changes in cell size. Furthermore, the changes in NPY and POMC gene expression in postmenopausal women are not mimicked by ovariectomy of young cynomolgus monkeys [23,90]. Thus, not all of the changes in gene expression observed within the hypothalamus of older women can be explained by ovarian failure.
5. Evidence in animal models that NKB neurons in the infundibular/arcuate nucleus play a role in the sex-steroid feedback on gonadotropin secretion
In postmenopausal women and ovariectomized monkeys, the hypertrophy and increased gene expression of NKB/ERα neurons occurs in association with removal of ovarian steroids. These changes are accompanied by increased hypothalamic GnRH gene expression and elevated levels of serum gonadotropins consistent with removal of steroid negative feedback (see sections 3 and 4). These findings suggest that NKB neurons in the human infundibular nucleus play a role in the hypothalamic circuitry regulating steroid negative feedback [79,81]. Multiple lines of evidence in experimental animals provide support for this hypothesis. Similar to humans, virtually all the NKB neurons in the arcuate nucleus of sheep and rats colocalize ERα [7,36] and estrogen replacement suppresses NKB gene expression in rat, mouse, sheep and monkeys, indicating that this circuit is highly conserved [3,15,17,73]. ERα is essential for estrogen negative feedback [19,45] and for the suppressive effects of estrogen on NKB gene expression [17]. Arcuate NKB neurons are sexually dimorphic [12,36] and NKB gene expression varies with the rat estrous cycle [78]. Finally, LH secretion is modulated by central injections of senktide, an agonist for the NK3 receptor (the preferential receptor for NKB). Initial studies showed a negative effect of senktide injection on LH secretion in ovariectomized rats with very low levels of exogenous estrogen [89]. However, in the ewe, central injection of senktide dramatically stimulates LH secretion (more than 15 fold) in the follicular phase, but not in the luteal phase [61]. Thus, the outcome of NK3 receptor activation on LH secretion depends on the hormonal milieu. Taken together, these data provide strong support for a role of NKB in the sex-steroid feedback on gonadotropin secretion.
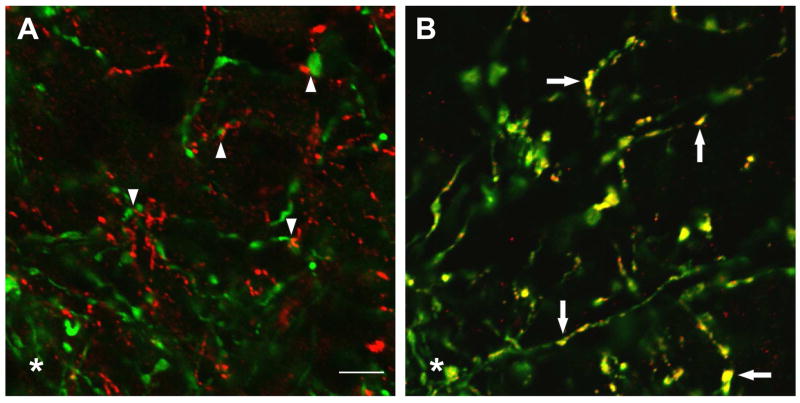
Morphological studies in the rat suggest that arcuate NKB neurons could influence LH secretion via projections to GnRH axons in the median eminence. Tract-tracing studies show that arcuate NKB neurons project to the median eminence as well as multiple hypothalamic sites [55]. Because arcuate NKB neurons fail to take up retrograde tracer after systemic injections, these projections do not link to the portal capillary system [56]. Within the median eminence, NKB and GnRH axons are closely apposed [36,56] and NK3 receptors are identified on GnRH axons (Fig. 3)[56]. Ultrastructural studies show that NKB varicosities are in direct contact with GnRH axons without classical synapses [12]. These data suggest that NKB may modulate GnRH secretion through non-synaptic transmission, a common mechanism of peptide signaling [62]. The convergence of GnRH axons and terminals in the median eminence represents a final coordinating site for synchronization of pulsatile GnRH secretion [65]. NKB neurons in the arcuate nucleus could provide a sex-steroid responsive input to the GnRH neuronal network via NK3 receptors in the median eminence (Fig. 4).
Figure 3.
Confocal microscopy of the rat median eminence. The images were captured at a single focal plane of approximately 0.80 μm in thickness. A: Color-combined image of GnRH (green) and proNKB (red)- immunofluorescence showing dense intermingling and multiple foci of close apposition (arrowheads). B: In contrast, combined images of GnRH (green) and NK3R (red)-immunofluorescence show punctate colocalization of NK3R on GnRH fibers (yellow, arrows). The asterisks in A and B mark the edge of the lateral palisade zone. These studies provide morphological evidence that NKB modulates GnRH secretion at the level of the rat median eminence. Scale bar = 5μm in A (applies to A, B). From Krajewski et al., [56] reproduced with permission Wiley-Liss Inc.
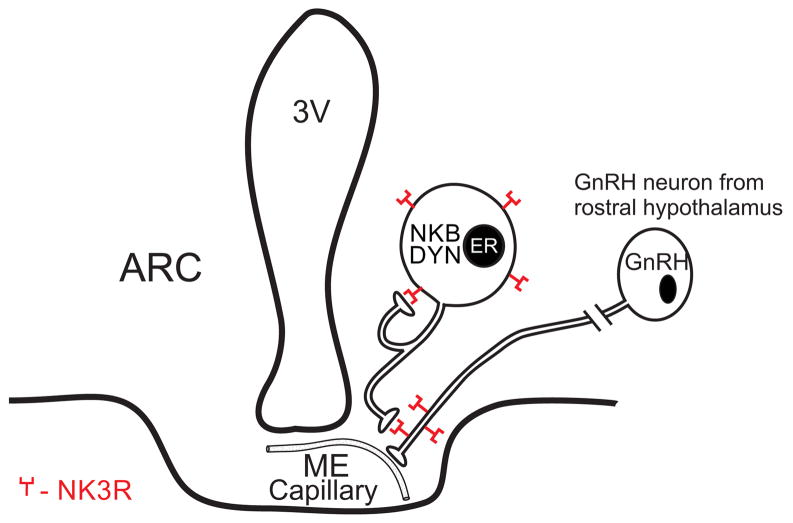
Figure 4.
Schematic diagram of the relationship between NKB (neurokinin B), Dynorphin (DYN), ER (estrogen receptor α) and GnRH (gonadotropin releasing-hormone) in the arcuate nucleus and median eminence of the rat. Although the presence of NK3 receptors is shown as an autofeedback loop, it is not known if these connections represent recurrent collaterals or synapses between NKB/dynorphin neurons. This diagram is based on immunocytochemical and tract-tracing studies from several sources [7,55,56].
NKB is extensively colocalized with dynorphin within arcuate neurons of the ewe and rat [7,12,26]. Because dynorphin modulates progesterone’s effects on pulsatile GnRH secretion [32], the colocalization of NKB and dynorphin provides additional evidence that arcuate NKB neurons participate in the reproductive axis. Immunocytochemical studies reveal close apposition of dual-labeled NKB/dynorphin terminals on NKB/dynorphin neurons in the arcuate nucleus of the rat and ewe [7,26]. In the ewe, ultrastructural examination reveals synaptic contacts at the site of closely apposing dynorphin-immunoreactive terminals and dynorphin-immunoreactive somata [27]. Because the majority of dynorphin neurons in the rat arcuate nucleus express NK3 receptor-immunoreactivity [7], a putative synapse between dynorphin/NKB fibers and dynorphin/NKB cell bodies could be mediated through the NK3 receptor (Fig. 4). It is not known if these inputs represent recurrent innervation or synapses between different dynorphin/NKB neurons within the arcuate nucleus. Although speculative, these connections could provide a mechanism to synchronize neuronal activity among dynorphin/NKB neurons within the arcuate nucleus and thus regulate pulsatile secretion of GnRH [7,26]
6. Kisspeptin neurons in the human hypothalamus and changes in KiSS-1 gene expression in postmenopausal women
Numerous studies have recently documented the importance of kisspeptin, the endogenous ligand of the G protein-coupled receptor 54 (GPR54), in the regulation of reproduction and the initiation of puberty [16,74,76,83,93]. Inactivating mutations of GPR54 in the human results in a failure of pubertal development with low levels of circulating gonadotropins and low serum sex hormones [16,93]. Moreover, a GPR54-activating mutation has been shown to be associated with central precocious puberty [102]. GnRH neurons in experimental animals express GPR54 mRNA [42,46] and are closely apposed by kisspeptin-immunoreactive fibers [13,48]. Exogenous administration of kisspeptin excites GnRH neurons, stimulates GnRH secretion and raises levels of LH and FSH in peripheral plasma [42,63,76,92]. The stimulatory action of kisspeptin on the reproductive axis is conserved among a wide variety of mammalian species, including humans [18,20,92].
Studies in experimental animals reveal many similarities between kisspeptin and NKB neurons in the arcuate nucleus. Like NKB neurons [81], arcuate kisspeptin neurons have been proposed to play a role in estrogen negative feedback [35]. Arcuate kisspeptin neurons express ERα [29,99] and kisspeptin (KiSS-1) gene expression is increased in the arcuate nucleus after ovariectomy [48,78,84,90,98,99]. Similarly, both KiSS-1 and NKB gene expression in the arcuate nucleus is suppressed by estrogen replacement [3,15,48,84,98,99] and ERα is required for this effect [17,99]. Based on these findings, it seemed likely that kisspeptin and NKB would be colocalized in arcuate neurons. If this hypothesis is correct, the hypertrophied neurons in the infundibular nucleus of postmenopausal women would express KiSS-1 mRNA, in addition to NKB and ERα mRNA.
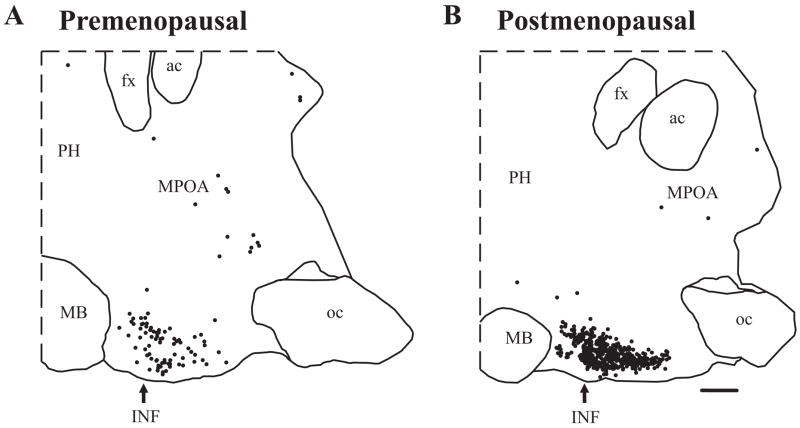
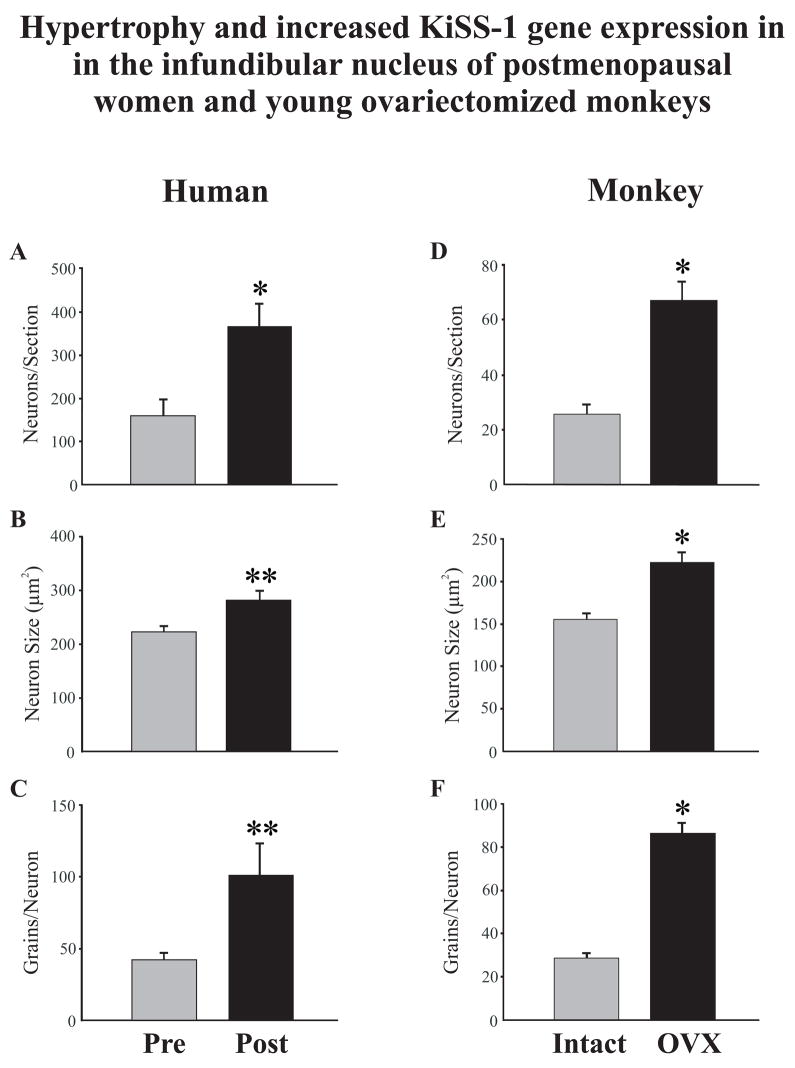
Studies were initiated in our laboratory to map the location of KiSS-1 mRNA expressing neurons in serial sections throughout the medial hypothalamus of pre- and postmenopausal women (Fig. 5). These studies showed a preferential distribution of KiSS-1 mRNA-containing neurons in the infundibular nucleus with only a few scattered KiSS-1 mRNA cell bodies in the medial preoptic area [86]. Significantly, the hypertrophied neurons in the infundibular nucleus of postmenopausal women were strongly labeled by the KiSS-1 probe, with a distribution and morphology identical to the hypertrophied NKB and ERα-containing neurons described earlier (see Section 4). Quantitative analyses revealed that the mean cross-sectional area of neurons expressing KiSS-1 mRNA increased in the infundibular nucleus of postmenopausal women, accompanied by an increase in the number of autoradiographic grains per neuron (Fig. 6). In addition, there was a marked increase in the number of neurons expressing KiSS-1 mRNA in the infundibular nucleus of postmenopausal women. Nearly identical changes in cell size and KiSS-1 gene expression occurred in young cynomolgus monkeys in response to ovariectomy (Fig. 6). Conversely, estrogen replacement of young ovariectomized cynomolgus monkeys reduced the number of KiSS-1 neurons in the infundibular nucleus to virtually undetectable levels [86]. These data provide strong evidence that the hypertrophy and increased gene expression of KISS-1 neurons in postmenopausal women are secondary to the loss of ovarian estrogen.
Figure 5.
Computer-assisted maps showing the distribution of neurons expressing KiSS-1 mRNA in representative parasagittal sections from a premenopausal (A) and a postmenopausal (B) woman. Each filled circle represents one labeled neuron. Neurons expressing KiSS-1 mRNA were predominantly located in the infundibular nucleus of both groups. A marked increase in the number of neurons expressing KiSS-1 mRNA was observed in the infundibular nucleus of postmenopausal women. The arrow indicates the location of the infundibular nucleus. Abbreviations: ac, anterior commissure; fx, fornix; INF, infundibular nucleus; MB, mammillary body; MPOA, medial preoptic area; oc, optic chiasm; PH, posterior hypothalamus. Scale Bar = 2 mm.
From Rometo et al. [86], reproduced with permission from The Endocrine Society.
Figure 6.
Changes in neuronal morphology and KiSS-1 gene expression in the infundibular nucleus of premenopausal and postmenopausal women (A,B,C) or the infundibular nucleus of young, intact and ovariectomized cynomolgus monkeys (D, E, F). Figure 6A shows the mean number of neurons expressing KiSS-1 mRNA in sagittal human sections and 6D shows the mean number of neurons in unilateral coronal sections in the monkey. 6B and E show the mean profile area (μm2) of KiSS-1 neurons and C and F show the mean number of autoradiographic grains for each labeled neuron. Postmenopausal women exhibited increased number, size and gene expression of KiSS-1 neurons that was similar to that seen in young, ovariectomized cynomolgus monkeys. Values are expressed as mean ± SEM. * Significantly different from premenopausal (A) or intact (D, E, F), p< 0.001. ** Significantly different from premenopausal (B, C), p< 0.05.
From Rometo et al. [86], reproduced with permission from The Endocrine Society.
The changes in KiSS-1 neuronal morphology and gene expression in the human and monkey infundibular nucleus were virtually identical to those observed in NKB neurons [81,90]. Nearly 75% of the hypertrophied neurons express KiSS-1 mRNA, similar to the percentage of the hypertrophied neurons previously shown to express NKB, SP or ERα mRNA [81] providing indirect evidence that KiSS-1, NKB, SP and ERα mRNAs are colocalized within the human infundibular nucleus. Dynorphin mRNA has also been identified within the hypertrophied neurons, but the number of neurons expressing dynorphin mRNA is decreased in postmenopausal women [87]. These findings are in agreement with the colocalization of kisspeptin, NKB and dynorphin in neurons demonstrated within the arcuate nucleus of the ewe [33], and NKB, dynorphin and ERα colocalization in the arcuate nucleus of the rat and ewe [7,26]. Definitive proof of KiSS-1, NKB, SP, dynorphin and ERα colocalization in the human infundibular nucleus awaits multiple labeling studies. However, the identification of each of these mRNAs in the hypertrophied neurons provides strong evidence that this circuit is preserved among mammalian species. A future challenge will be to understand the mechanism of the differential effects of sex-steroids on neuropeptide gene expression within the human infundibular nucleus and the contribution of each of these neuropeptides to reproductive regulation.
Studies of GPR54 mutations document the essential nature of kisspeptin/GPR54 signaling in reproductive control mechanisms [16,93]. Therefore, the relatively restricted distribution of neurons expressing KiSS-1 mRNA in the infundibular (arcuate) nucleus of the human underscores the importance of this region in the regulation of the reproductive axis. They also agree with clinical studies showing a hypothalamic site of estrogen negative feedback in the human [8,31,44]. These findings are consistent with classic studies in rhesus monkeys implicating the medial basal hypothalamus as the reproductive control center. For example, surgical isolation of the arcuate nucleus, median eminence, and portions of the ventromedial nuclei and premammillary areas from the rest of the brain does not interfere with estrogen negative or positive feedback [57]. Moreover, destruction of the arcuate region in rhesus monkeys abolishes pulsatile gonadotropin release [53]. Finally, electrodes placed in, or adjacent to, the arcuate nucleus will detect electrical activity synchronized with pulsatile release of LH (the GnRH pulse generator)[109]. The number of multiunit volleys increases after ovariectomy over a time course consistent with cellular remodeling and hypertrophy [70]. Conversely, the volleys are inhibited by estrogen replacement in ovariectomized monkeys [47], reminiscent of the dramatic inhibition of NKB and KiSS-1 gene expression by estrogen [86,90]. These data raise the intriguing possibility that the numerous KiSS-1/NKB neurons with the infundibular nucleus could contribute to the multiunit activity known as the GnRH pulse generator.
7: Summary and Conclusions
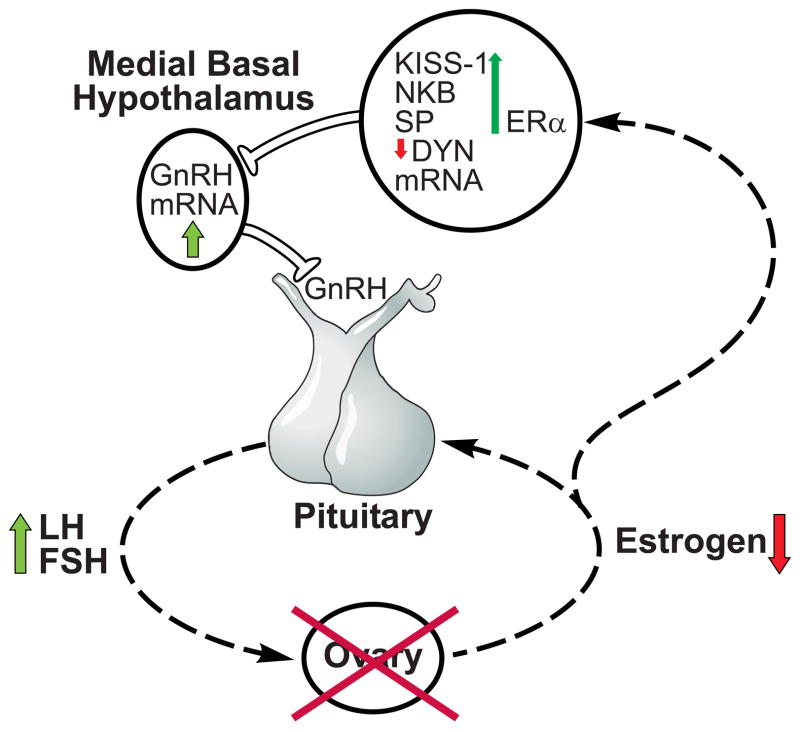
The hormonal milieu of postmenopausal women is characterized by the depletion of ovarian follicles, loss of ovarian steroid secretion and secondary gonadotropin hypersecretion from the anterior pituitary gland. Rather than showing signs of degeneration, the reproductive neuroendocrine axis in postmenopausal women responds robustly to the removal of ovarian hormones. Throughout the postmenopausal period, administration of exogenous sex steroids is still effective in reducing GnRH and LH secretion. Moreover, postmenopausal women exhibit increased hypothalamic GnRH gene expression and indirect evidence suggests that this is linked to increased GnRH secretion. These findings, combined with studies of non-human primates, provide strong evidence that the hypersecretion of gonadotropins in postmenopausal women is secondary to the increased synthesis and secretion of GnRH. In the infundibular nucleus of postmenopausal women, hypertrophy occurs in a subpopulation of neurons expressing KiSS-1, NKB, SP, dynorphin and ERα mRNA. Postmenopausal neuronal hypertrophy is accompanied by increased KiSS-1, NKB and substance P gene transcripts and decreased expression of dynorphin mRNA. Ovariectomy of young experimental animals induces nearly identical findings [28,86,90], providing evidence that the changes in GnRH, KiSS-1, NKB and dynorphin gene expression in the infundibular nucleus of postmenopausal women reflects a compensatory response to ovarian failure. Conversely, estrogen replacement reduces GnRH, KiSS-1 and NKB gene expression in ovariectomized cynomolgus monkeys [3,54,86]. Because GnRH and KiSS-1/NKB neurons in postmenopausal women exhibit changes similar to those in young monkeys after ovariectomy, these studies support the concept that reproductive hypothalamic function is preserved after menopause. The anatomical site of the hypertrophied neurons, the colocalization of ERα, as well as the extensive data implicating NKB, kisspeptin, and dynorphin in the regulation of GnRH secretion provide compelling evidence that the hypertrophied neurons are part of the neural network responsible for the increased levels of serum gonadotropins in postmenopausal women (Fig. 7).
Figure 7.
Schematic diagram of neuroendocrine regulation of LH secretion in postmenopausal women. The hallmark of menopause is ovarian aging with follicle depletion resulting in castrate levels of ovarian hormones. Removal of estrogen leads to hypertrophy of a subpopulation of infundibular neurons expressing KiSS-1, NKB, substance P, dynorphin and ERα mRNA in the human infundibular nucleus. Within these neurons, there is increased expression KiSS-1, NKB and substance P gene transcripts and the decreased gene expression of dynorphin mRNA. We hypothesize that stimulatory effects of kisspeptins and NKB, combined with a reduction in the inhibitory effects of dynorphin, ultimately results in increased GnRH gene expression. Increased GnRH secretion leads to the gonadotropin hypersecretion characteristic of the postmenopausal period. These studies provide evidence that neurons expressing KiSS-1, NKB, substance P, dynorphin and ERα mRNA play a role in the regulation of steroid negative feedback in the human. See text for justification of this model.
We hypothesize that the stimulatory effects of kisspeptin and NKB, combined with a reduction in the inhibitory effects of dynorphin, ultimately results in increased GnRH gene expression and secretion in postmenopausal women. While it is well recognized that GnRH neurons are influenced by multiple converging inputs, studies of GPR54 mutations in humans [16,93] and transgenic mice emphasize the essential nature of this circuitry in the regulation of reproduction. ERα is a critical component because estrogen negative feedback on GnRH gene expression and secretion will not occur in ERα knockout mice [14,19]. Similarly, ERα is essential for the suppressive effects of estrogen on KiSS-1 and NKB gene expression in the arcuate nucleus [17,99]. GPR54 receptor signaling is required for initiation of puberty [93], basal secretion of gonadotropins [93], the postovariectomy rise in LH [21] and the stimulatory effects of kisspeptin on GnRH neurons [63]. Further studies will be necessary to determine if NKB or dynorphin signaling are also critical factors in the reproductive axis. Another challenge will be to characterize putative connections between the hypertrophied neurons and GnRH neurons in the human hypothalamus. Because there is a wealth of information showing the critical role of kisspeptin signaling in reproduction, the identification of alterations in KiSS-1 gene expression in the infundibular of postmenopausal women sheds considerable light on our understanding of human reproductive neuroendocrine regulation. These studies provide strong evidence that a subpopulation of neurons in the infundibular nucleus coexpressing kisspeptin, NKB, SP, dynorphin and ERα mediates estrogen negative feedback on GnRH secretion in the human.
Acknowledgments
This research was supported by a grant from the NIH (AG-09214). The author gratefully acknowledges the comments and support from Dr. Nathaniel McMullen, Adonna Rometo, Penny Dacks and Sally Krajewski.
Footnotes
The author has nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Abel TW, Rance NE. Proopiomelanocortin gene expression is decreased in the infundibular nucleus of postmenopausal women. Mol Brain Res. 1999;69:202–208. doi: 10.1016/s0169-328x(99)00111-4. [DOI] [PubMed] [Google Scholar]
- 2.Abel TW, Rance NE. Stereologic study of the hypothalamic infundibular nucleus in young and older women. J Comp Neurol. 2000;424:679–688. doi: 10.1002/1096-9861(20000904)424:4<679::aid-cne9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 3.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab. 1999;84:2111–2118. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 4.Baird DT, Guevara A. Concentration of unconjugated estrone and estradiol in peripheral plasma in nonpregnant women throughout the menstrual cycle, castrate and postmenopausal women and in men. J Clin Endocrinol Metab. 1969;29:149–156. doi: 10.1210/jcem-29-2-149. [DOI] [PubMed] [Google Scholar]
- 5.Bellino FL, Wise PM. Nonhuman primate models of menopause workshop. Biol Reprod. 2003;68:10–18. doi: 10.1095/biolreprod.102.005215. [DOI] [PubMed] [Google Scholar]
- 6.Block E. Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat. 1952;14:108–123. doi: 10.1159/000140595. [DOI] [PubMed] [Google Scholar]
- 7.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 8.Cemeroglu AP, Kletter GB, Guo W, Brown MB, Kelch RP, Marshall JC, Padmanabhan V, Foster CM. In pubertal girls, naloxone fails to reverse the suppression of luteinizing hormone secretion by estradiol. J Clin Endocrinol Metab. 1998;83:3501–3506. doi: 10.1210/jcem.83.10.5207. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarti S, Collins WP, Forecast JD, Newton JR, Oram DH, Studd JWW. Hormonal profiles after the menopause. Br Med J. 1976;2:784–786. doi: 10.1136/bmj.2.6039.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5:41–50. doi: 10.1111/j.1365-2826.1993.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 11.Chongthammakun S, Terasawa E. Negative feedback effects of estrogen on luteinizing hormone- releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology. 1993;132:735–743. doi: 10.1210/endo.132.2.8425492. [DOI] [PubMed] [Google Scholar]
- 12.Ciofi P, Leroy D, Tramu G. Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience. 2006;141:1731–1745. doi: 10.1016/j.neuroscience.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 13.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone (GnRH) neurons. Endocrinology. 2006;147:5817–5825. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 15.Danzer SC, Price RO, McMullen NT, Rance NE. Sex steroid modulation of neurokinin B gene expression in the arcuate nucleus of adult male rats. Mol Brain Res. 1999;66:200–204. doi: 10.1016/s0169-328x(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 16.de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;145:736–742. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- 18.Dhillo WS, Chaudhri OB, Thompson EL, Murphy KG, Patterson M, Ramachandran R, Nijher GK, Amber V, Kokkinos A, Donaldson M, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J Clin Endocrinol Metab. 2007;92:3958–3966. doi: 10.1210/jc.2007-1116. [DOI] [PubMed] [Google Scholar]
- 19.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 20.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 21.Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. doi: 10.1523/JNEUROSCI.2748-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Majdoubi M, Sahu A, Plant TM. Effect of estrogen on hypothalamic transforming growth factor alpha and gonadotropin-releasing hormone gene expression in the female rhesus monkey. Neuroendocrinology. 1998;67:228–235. doi: 10.1159/000054318. [DOI] [PubMed] [Google Scholar]
- 23.Escobar CM, Krajewski SJ, Sandoval-Guzmán T, Voytko ML, Rance NE. Neuropeptide Y gene expression is increased in the hypothalamus of older women. J Clin Endocrinol Metab. 2004;89:2338–2343. doi: 10.1210/jc.2003-031899. [DOI] [PubMed] [Google Scholar]
- 24.Escobar CM, Krajewski SJ, Sandoval-Guzmán T, Voytko ML, Rance NE. Neuropeptide Y gene expression is increased in the infundibular nucleus of postmenopausal women. J Clin Endocrinol Metab. 2004;89:2338–2343. doi: 10.1210/jc.2003-031899. [DOI] [PubMed] [Google Scholar]
- 25.Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Human Reproduction. 1992;7:1342–1346. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 26.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 27.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 28.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynormphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- 29.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–230. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 30.Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- 31.Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab. 2002;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- 32.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- 33.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 34.Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 35.Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254–255:91–96. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 36.Goubillon M-L, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 37.Hale GE, Burger HG. Perimenopausal reproductive endocrinology. Endocrinol Metab Clin North Am. 2005;34:907–922. doi: 10.1016/j.ecl.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Hale GE, Zhao X, Hughes CL, Burger HG, Robertson DM, Fraser IS. Endocrine features of menstrual cycles in middle and late reproductive age and the menopausal transition classified according to the Staging of Reproductive Aging Workshop (STRAW) staging system. J Clin Endocrinol Metab. 2007;92:3060–3067. doi: 10.1210/jc.2007-0066. [DOI] [PubMed] [Google Scholar]
- 39.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin North Am. 2004;33:637–659. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–351. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 41.Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab. 2000;85:1794–1800. doi: 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- 42.Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen KR, Knowlton NS, Thyer AC, Charleston JS, Soules MR, Klein NA. A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause. Hum Reprod. 2008;23:699–708. doi: 10.1093/humrep/dem408. [DOI] [PubMed] [Google Scholar]
- 44.Hayes FJ, Seminara SB, DeCruz S, Boepple PA, Crowley WF., Jr Aromatase inhibition in the human male reveals a hypothalamic site of estrogen feedback. J Clin Endocrinol Metab. 2000;85:3027–3035. doi: 10.1210/jcem.85.9.6795. [DOI] [PubMed] [Google Scholar]
- 45.Hewitt SC, Korach KS. Estrogen receptors: structure, mechanisms and function. Rev Endocr Metab Disord. 2002;3:193–200. doi: 10.1023/a:1020068224909. [DOI] [PubMed] [Google Scholar]
- 46.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 47.Kesner JS, Wilson RC, Kaufman J-M, Hotchkiss J, Chen Y, Yamamoto H, Pardo RR, Knobil E. Unexpected responses of the hypothalamic gonadotropin-releasing hormone “pulse generator” to physiological estradiol inputs in the absence of the ovary. Proc Natl Acad Sci USA. 1987;84:8745–8749. doi: 10.1073/pnas.84.23.8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K-I. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 49.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81:1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 50.Klein NA, Houmard BS, Hansen KR, Woodruff TK, Sluss PM, Bremner WJ, Soules MR. Age-related analysis of inhibin A, inhibin B, and activin A relative to the intercycle monotropic follicle-stimulating hormone rise in normal ovulatory women. J Clin Endocrinol Metab. 2004;89:2977–2981. doi: 10.1210/jc.2003-031515. [DOI] [PubMed] [Google Scholar]
- 51.Klein NA, Illingworth PJ, Groome NP, McNeilly AS, Battaglia DE, Soules MR. Decreased inhibin B secretion is associated with the monotropic FSH rise in older, ovulatory women: a study of serum and follicular fluid levels of dimeric inhibin A and B in spontaneous menstrual cycles. J Clin Endocrinol Metab. 1996;81:2742–2745. doi: 10.1210/jcem.81.7.8675606. [DOI] [PubMed] [Google Scholar]
- 52.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- 53.Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G. Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science. 1980;207:1371–1373. doi: 10.1126/science.6766566. [DOI] [PubMed] [Google Scholar]
- 54.Krajewski SJ, Abel TW, Voytko ML, Rance NE. Ovarian steroids differentially modulate the gene expression of gonadotropin-releasing hormone neuronal subtypes in the ovariectomized cynomolgus monkey. J Clin Endocrinol Metab. 2003;88:655–662. doi: 10.1210/jc.2002-020887. [DOI] [PubMed] [Google Scholar]
- 55.Krajewski SJ, Anderson MJ, Burke MC, McMullen NT, Rance NE. Arcuate neurokinin B neurons project to the median eminence as well as multiple hypothalamic sites: An anterograde tract-tracing study using biotinylated dextran amine. Soc Neurosci Abstr. 2005;(No 7607) [Google Scholar]
- 56.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphological evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 57.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I Gonadotropin secretion. Endocrinology. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- 58.Lamberts SWJ, van den Beld AW, van der Lely A-J. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 59.Landgren B-M, Collins A, Csemiczky G, Burger HG, Baksheev L, Robertson DM. Menopause transition: Annual changes in serum hormonal patterns over the menstrual cycle in women during a nine-year period prior to menopause. J Clin Endocrinol Metab. 2004;89:2763–2769. doi: 10.1210/jc.2003-030824. [DOI] [PubMed] [Google Scholar]
- 60.Leidy LE, Godfrey LR, Sutherland MR. Is follicular atresia biphasic? Fertil Steril. 1998;70:851–859. doi: 10.1016/s0015-0282(98)00316-1. [DOI] [PubMed] [Google Scholar]
- 61.McManus CJ, Valent M, Connors JM, Lehman MN, Goodman R. A neurokinin-B agonist stimulates LH secretion in follicular, but not luteal, phase ewes. Soc Neurosci Abstr. 2005;(No 7608) [Google Scholar]
- 62.Merighi A. Costorage and coexistence of neuropeptides in the mammalian CNS. Prog Neurobiol. 2002;66:161–190. doi: 10.1016/s0301-0082(01)00031-4. [DOI] [PubMed] [Google Scholar]
- 63.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A, Aparicio SAJR. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuno M, Terasawa E. Search for neural substrates mediating inhibitory effects of oestrogen on pulsatile luteinising hormone-releasing hormone release in vivo in ovariectomized female rhesus monkeys (Macaca mulatta) J Neuroendocrinol. 2005;17:238–245. doi: 10.1111/j.1365-2826.2005.01295.x. [DOI] [PubMed] [Google Scholar]
- 65.Moenter SM, DeFazio RA, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- 66.Monroe SE, Jaffe RB, Midgley AR., Jr Regulation of human gonadotropins. XIII Changes in serum gonadotropins in menstruating women in response to oophorectomy. J Clin Endocrinol Metab. 1972;34:420–422. doi: 10.1210/jcem-34-2-420. [DOI] [PubMed] [Google Scholar]
- 67.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: How basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nauta WJH, Haymaker W. In: The Hypothalamus. Haymaker W, Anderson E, Nauta WJH, editors. Springfield, Ill: Charles C. Thomas; 1969. pp. 136–209. [Google Scholar]
- 69.Nishi T, Yagi S, Nakano R. Feedback of estrogen in postmenopausal women. Acta Obstet Gynecol Scand. 1987;66:309–313. doi: 10.3109/00016348709103642. [DOI] [PubMed] [Google Scholar]
- 70.O’Byrne KT, Chen MD, Nishihara M, Williams CL, Thalabard JC, Hotchkiss J, Knobil E. Ovarian control of gonadotropin hormone-releasing hormone pulse generator activity in the rhesus monkey: duration of the associated hypothalamic signal. Neuroendocrinology. 1993;57:588–592. doi: 10.1159/000126411. [DOI] [PubMed] [Google Scholar]
- 71.Odell WD, Swerdloff RS. Progestogen-induced luteinizing and follicle-stimulating hormone surge in postmenopausal women: a simulated ovulatory peak. Proc Natl Acad Sci USA. 1968;61:529–536. doi: 10.1073/pnas.61.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Parker CR, Jr, Porter JC. Luteinizing hormone-releasing hormone and thyrotropin-releasing hormone in the hypothalamus of women: Effects of age and reproductive status. J Clin Endocrinol Metab. 1984;58:488–491. doi: 10.1210/jcem-58-3-488. [DOI] [PubMed] [Google Scholar]
- 73.Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol. 2003;15:749–753. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- 74.Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol. 2006;155:S11–S16. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- 75.Pohl CR, Richardson DW, Hutchison JS, Germak JA, Knobil E. Hypophysiotropic signal frequency and the functioning of the pituitary-ovarian system in the rhesus monkey. Endocrinology. 1983;112:2076–2080. doi: 10.1210/endo-112-6-2076. [DOI] [PubMed] [Google Scholar]
- 76.Popa SM, Clifton DK, Steiner RA. The Role of Kisspeptins and GPR54 in the Neuroendocrine Regulation of Reproduction. Annu Rev Physiol. 2008;70:213–238. doi: 10.1146/annurev.physiol.70.113006.100540. [DOI] [PubMed] [Google Scholar]
- 77.Prior JC. Perimenopause: The complex endocrinology of the menopausal transition. Endocr Rev. 1998;19:397–428. doi: 10.1210/edrv.19.4.0341. [DOI] [PubMed] [Google Scholar]
- 78.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 79.Rance NE, McMullen NT, Smialek JE, Price DL, Young WS., III Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- 80.Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab. 1996;81:3540–3546. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- 81.Rance NE, Young WS., III Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 82.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: Evidence for acclelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 83.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 84.Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 85.Roberts VJ, Barth S, El-Roeiy A, Yen SSC. Expression of inhibin/activin subunits and follistatin messenger ribonucleic acids and proteins in ovarian follicles and the corpus luteum during the human menstrual cycle. J Clin Endocrinol Metab. 1993;77:1402–1410. doi: 10.1210/jcem.77.5.8077341. [DOI] [PubMed] [Google Scholar]
- 86.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 87.Rometo AM, Rance NE. Changes in morphology and gene expression of dynrophin neurons in the infundibular nucleus of postmenopausal women. Soc Neurosci Abstr. 2007;(No 1941) [Google Scholar]
- 88.Rossmanith WG, Scherbaum WA, Lauritzen C. Gonadotropin secretion during aging in postmenopausal women. Neuroendocrinology. 1991;54:211–218. doi: 10.1159/000125878. [DOI] [PubMed] [Google Scholar]
- 89.Sandoval-Guzmán T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 90.Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 91.Santoro N, Banwell T, Tortoriello D, Lieman H, Adel T, Skurnick J. Effects of aging and gonadal failure on the hypothalamic-pituitary axis in women. Am J Obstet Gynecol. 1998;178:732–741. doi: 10.1016/s0002-9378(98)70483-1. [DOI] [PubMed] [Google Scholar]
- 92.Seminara SB. Metastin and its G protein-coupled receptor, GPR54: Critical pathway modulating GnRH secretion. Front Neuroendocrinol. 2005;26:131–138. doi: 10.1016/j.yfrne.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 93.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 94.Sharpless JL, Supko JG, Martin KA, Hall JE. Disappearance of endogenous luteinizing hormone is prolonged in postmenopausal women. J Clin Endocrinol Metab. 1999;84:688–694. doi: 10.1210/jcem.84.2.5433. [DOI] [PubMed] [Google Scholar]
- 95.Sheehan HL. Variations in the subventricular nucleus. J Path Bact. 1967;94:409–416. doi: 10.1002/path.1700940222. [DOI] [PubMed] [Google Scholar]
- 96.Sheehan HL, Kovács K. The subventricular nucleus of the human hypothalamus. Brain. 1966;89:589–614. doi: 10.1093/brain/89.3.589. [DOI] [PubMed] [Google Scholar]
- 97.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. The Journal of Clinical Investigation. 1975;55:699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic adic expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 99.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 100.Smith RG, Betancourt L, Sun Y. Molecular endocrinology and physiology of the aging central nervous system. Endocr Rev. 2005;26:203–250. doi: 10.1210/er.2002-0017. [DOI] [PubMed] [Google Scholar]
- 101.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of reproductive aging workshop (STRAW) Fertil Steril. 2001;76:874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 102.Teles MG, Bianco SD, Brito VN, Trarbach EB, Kuohung W, Xu S, Seminara SB, Mendonca BB, Kaiser UB, Latronico AC. A GPR54-activating mutation in a patient with central precocious puberty. N Engl J Med. 2008;358:709–715. doi: 10.1056/NEJMoa073443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tinkanen H, Bläuer M, Laippala P, Tuohimaa P, Kujansuu E. Correlation between serum inhibin B and other indicators of the ovarian function. Eur J Obstet Gynecol Reprod Biol. 2001;94:109–113. doi: 10.1016/s0301-2115(00)00319-5. [DOI] [PubMed] [Google Scholar]
- 104.Van Look PFA, Hunter WM, Fraser IS, Baird DT. Impaired estrogen-induced luteinizing hormone release in young women with anovulatory dysfunctional uterine bleeding. J Clin Endocrinol Metab. 1978;46:816–823. doi: 10.1210/jcem-46-5-816. [DOI] [PubMed] [Google Scholar]
- 105.Wallach EE, Root AW, Garcia C-R. Serum gonadotropin responses to estrogen and progestogen in recently castrated human females. J Clin Endocrinol Metab. 1970;31:376–381. doi: 10.1210/jcem-31-4-376. [DOI] [PubMed] [Google Scholar]
- 106.Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- 107.Welt CK, McNicholl DJ, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–111. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 108.Wide L, Bakos O. More basic forms of both human follicle-stimulating hormone and luteinizing hormone in serum at midcycle compared with the follicular or luteal phase. J Clin Endocrinol Metab. 1993;76:885–889. doi: 10.1210/jcem.76.4.8473400. [DOI] [PubMed] [Google Scholar]
- 109.Wilson RC, Kesner JS, Kaufman J-M, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39:256–260. doi: 10.1159/000123988. [DOI] [PubMed] [Google Scholar]
- 110.Wise PM, Rance N, Selmanoff M, Barraclough CA. Changes in radioimmunoassayable luteinizing hormone-releaseing hormone in discrete brain areas of the rat at various times on proestrus, diestrous day 1, and after phenobarbital administration. Endocrinology. 1981;108:2179–2185. doi: 10.1210/endo-108-6-2179. [DOI] [PubMed] [Google Scholar]
- 111.Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Böttner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- 112.Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab. 2002;87:5160–5167. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]