Abstract
Bone morphogenetic protein 2 (BMP2) was originally found by its osteoinductive ability, and recent genetic analyses have revealed that it plays critical roles during early embryogenesis, cardiogenesis, decidualization as well as skeletogenesis. During a course of evaluation of the conditional allele for Bmp2, we found that the presence of a neo cassette, a selection marker needed for gene targeting events in embryonic stem cells, in the 3’ untranslated region of exon 3 of Bmp2, reduced the expression levels of Bmp2 both in embryonic and maternal tissues. Some of the embryos that were genotyped as transheterozygous for the floxed allele with the neo cassette over the conventional null allele (fn/−) showed a lethal phenotype including defects in cephalic neural tube closure and ventral abdominal wall closure. Embryos exhibiting these abnormalities were increased when genotypes of the pregnant females were different; when expression levels of Bmp2 in maternal tissues were lower, a larger proportion of fn/− embryos exhibit these abnormalities. These results suggest that the expression levels of Bmp2 together in both in embryonic and maternal tissues influence the normal neural tube closure and body wall closure with different thresholds.
Keywords: BMP2, hypomorphic, neural tube closure, decidualization, omphalocele
Introduction
TGF-β superfamily members are important for embryogenesis, cell growth and differentiation including sexual differentiation [de Caestecker, 2004]. BMPs are a subfamily of TGF-β superfamily that were originally identified due to their osteoinductive ability but played various roles during development [Kishigami and Mishina, 2005]. BMP2 is one of the first members to be identified and its conventional gene disruption results in early embryonic lethality soon after gastrulation [Zhang and Bradley, 1996]. Using this mutant null (−) allele in heterozygotes (+/−), it is revealed that BMP2 is a critical factor for the migration but not induction of neural crest cells [Correia et al., 2007; Kanzler et al., 2000].
To overcome the embryonic lethality, floxed mouse lines for Bmp2 have recently become available, and BMP2 function in the later stages of development including cardiogenesis and skeletogenesis have begun to emerge [Ma et al., 2005; Tsuji et al., 2006]. Recently, it is reported that a uterine-specific disruption of Bmp2 leads to failure of decidualization causing abortion of embryos [Lee et al., 2007]. We independently developed a conditional allele of Bmp2 (manuscript in preparation). Besides two loxP sites for conditional removal of exon3, we inserted a neo resistant cassette driven by a polII promoter flanked by FRT sites into the 3’-untranslated region (UTR) of Bmp2 as a selection marker for gene targeting events (manuscript in preparation and fig. 1).
Figure 1. Schematic representation of different alleles of Bmp2 locus.

A loxP site (red triangle) was inserted in intron 2 and a polII-neo cassette flanked by 2 FRT sites (red square) followed by the other loxP site and human alkaline phosphatase gene (blue line) was inserted into the 3’ untranslated region of exon 3 (white box). Coding regions in exon 2 and 3 were highlighted as light blue. Gene targeting in ES cells resulted in the floxed-neo (fn) allele. The neo cassette was removed by breeding with an FLPe mouse line to generate the floxed allele (fx). Structure of the conventional null allele [Zhang and Bradley, 1996] was also shown. Examples of PCR genotyping were shown in the lower panel. M; 100 bp marker ladder.
It is known that the presence of neo selection cassette in the targeted locus sometimes compromises gene function, generating a hypomorphic allele [Meyers et al., 1998; Olson et al., 1996]. During a course of evaluation of the gene activity of the floxed allele for Bmp2, with or without the neo cassette, we found that the embryos genotyped as floxed with the neo cassette over the conventional null allele (fn/−) were at a disadvantage for survival, and this phenomenon depended on the genotype of the mother. These results suggest that the appropriate levels of BMP2 in embryos are critical for their normal development and the levels of Bmp2 from mothers also influence the penetrance of these abnormalities when combined with lower BMP2 levels in the embryo.
Materials and Methods
Mouse strains
Detailed description for generation of a floxed allele of Bmp2 will be provided elsewhere (manuscript in preparation). In brief, a polII-neo cassette flanked by FRT sites followed by one loxP site, a splicing acceptor and human alkaline phosphatase expression cassette was introduced into the 3’-UTR of exon 3 of Bmp2 via homologous recombination in embryonic stem (ES) cells. The resulting allele was designated as floxed-neo (fn) allele (fig. 1). After germline transmission of the targeted allele, heterozygous mice for the fn allele were bred with FLPe mice [Farley et al., 2000] to remove the neo selection cassette. This allele was designated floxed (fx) allele (fig. 1). Mice carrying a conventional null allele were obtained from Allan Bradley [Zhang and Bradley, 1996]. These mouse lines were maintained in mixture of 129SvEv and C57BL6/J background. All animal experiments were conducted according to the U.S. Public Health Service policy on the humane care and use of animals. All animal procedures used in this study were approved by the National Institute of Environmental Health Sciences Institutional Animal Care and Use Committee.
Genotyping
Genotype of the embryos and pups was determined by PCR analyses of genomic DNA extracted from amnion for embryos or from ear punch for adults, with the following set of primers A:5’-AGCATGAACCCTCATGTGTTGG-3’, B:5’-GTGACATTABBCTGCTGTAGCA-3’, and C:5’-GAGACTAGTGAGACGTGCTACT-3’. Positions of the primers and examples of the genotyping results are shown in figure 1. Sizes of the PCR products are; 322 bp for the wild type, 357 bp for the null (−) allele, 422 bp for fn and fx alleles. The fx allele was differentiated from the fn allele with primers amplifying between exon 3 and human alkaline phosphatase; 5’-AGGGTTTCAGGTCAGTTTCCG-3’ and 5’-GATGATGAGGTTCTTGGGCGG-3’ (450 bp for fx allele, 2 kb for fn allele).
Quantitative PCR
For embryonic samples, pregnant mice were sacrificed at E9.5 and extra-embryonic and embryonic tissues were dissected. The extra-embryonic tissues were used for subsequent genotyping and the embryos were surgically divided into anterior and posterior regions. For maternal samples, pregnant mice were scarified at E6.5, and uteri and deciduas were removed. RNA was isolated from these tissues using TRIzol (Invitrogen) and cDNA was synthesized using the SuperScript First-Strand Synthesis System (Invitrogen) and random hexamer primers. Real time PCR measurements of individual cDNAs were performed with the ABI Prism 7700 sequence detection system. Gene-specific primers for Bmp2 (Mm01340178_m1) were purchased as the pre designed TaqMan gene expression assays gene-specific probe and primer mixture (PE Applied Biosystems). The TaqMan rodent glyceraldehyde-3-phosphate dehydrogenase (Gapdh) control reagent (PE Applied Biosystems) was used as an internal control. All measurements were performed in triplicate. Values were normalized to Gapdh using the 2-DDCt method and expressed as means ± SD. Statistical differences were determined by a Student’s t test. Statistical significances are relative to the corresponding wild type control animals.
Results
Floxed-neo (fn) allele for Bmp2 is disadvantageous for survival
To address whether presence of the neo cassette in the Bmp2 locus compromises its function, first we set up the breeding between heterozygous mutant mice for the floxed-neo (fn) allele and heterozygous mutant mice for conventional null allele to generate pups transheterozygous for fn and null allele for Bmp2 (fn/−). In this case, 4 different genotypes of pups with the same ratio were expected. However, individuals whose genotype was fn/−were underrepresented (table 1). Proportions of the fn/− pups were more severely underrepresented when mothers were heterozygous for null (+/−) versus heterozygous for floxed-neo (fn/+) (table 1). Body weight of the surviving pups of the genotype fn/− at 38 days after birth was significantly lower than that of the wild type littermates (fig. 2). Many of the fn/− individuals had kinked tails (fig. 3A, B) and this phenotype was evident during embryogenesis (fig. 3C-E). When fathers homozygous for the floxed allele without the neo cassette (fx/fx) were bred with heterozygous null females (+/−); we found the expected ratio of genotypes of pups (table 1). These findings prompted us to hypothesize that the fn allele, but not the fx allele, was functionally hypomorphic, and more importantly, a reduction of BMP2 in the mother as in lower levels of maternal BMP2 has an influence during the pregnancy when combined with the reduced levels of BMP2 in the embryo. Various types of breeding and analysis were set up to test this idea.
Table 1.
Under representation of fn/− pups
| Number of pups for each genotype (%) | |||||
|---|---|---|---|---|---|
| father | mother | +/+ | +/− | fn/+ | fn/− |
| +/− | fn/+ | 22 (25) | 20 (23) | 35 (41) | 10 (11) |
| fn/+ | +/− | 23 (28) | 36 (43) | 20 (24) | 4 (5) |
| +/− | fn/fn | N/A | N/A | 59 (65) | 32 (35) |
| fn/fn | +/− | N/A | N/A | 100 (81) | 23 (19) |
| fx/fx | +/− | N/A | N/A | 26 (53) | 23 (47) |
Figure 2. Reduction of body weight in fn mice.
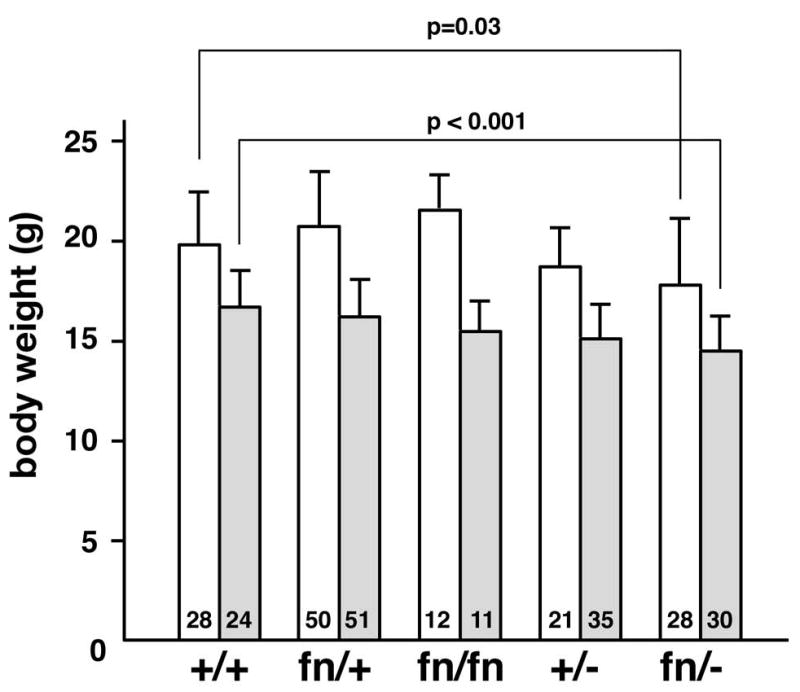
Body weight was measured at 38 days after birth. Numbers at the bottom of each bar represent the total number of mice that were measured. Open bar; male, shaded bar; female. Student t-test was done for statistical analyses.
Figure 3. Kinky tail phenotype.
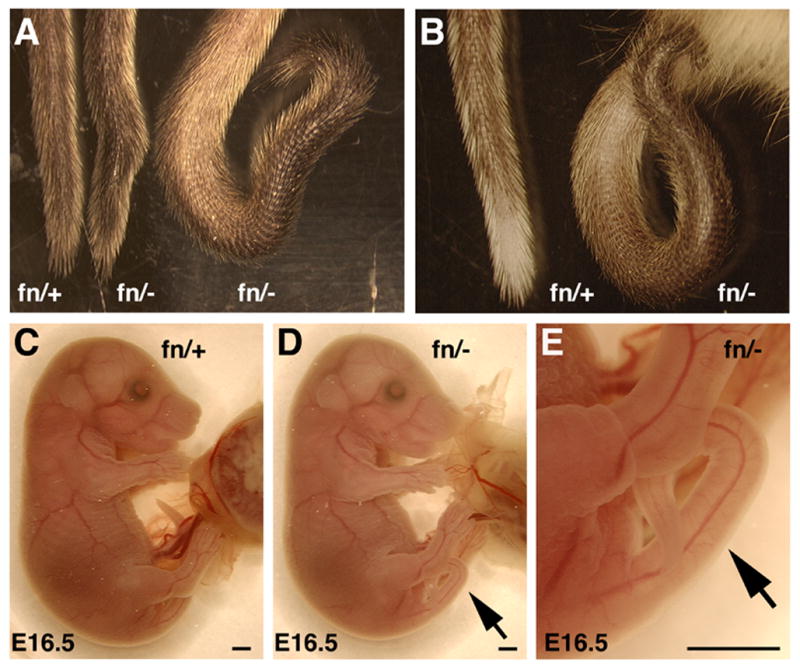
Three week old fn/− mice showed kinky tail with a varying degree (A, B). Right two in panel A and right one in panel B are fn/−. Tails from control littermates were shown on the left. The kinky tail phenotype is developed during embryogenesis (arrows). E16.5 embryos that developed the phenotype in a fn/+ mother were shown (C, control, D-E, fn/−). Scale bar = 1 mm (C-E).
If the fn allele is hypomorphic, then the mice with the genotype of fn over the conventional null (fn/−) should exhibit more severe phenotype than the conventional heterozygous mutants (+/−). The mating schedule shown in table 2 was set up to determine whether distortion of the genotype ratio would become more prominent, if mothers’ genotypes were fn/−. As shown in table 2, the proportion of fn/− pups at weaning stage was lower than expected (33%) in both the +/− mothers and fn/− mothers (14% and 20%, respectively). However, ratio of fn/− pups in the litters from fn/− mothers was not drastically reduced compared with that from +/− mothers. For the case of intercross between fn/− mice, 47% of fn/− individuals reached weaning stage instead of the expected 67% (the expected ratio of fn/fn:fn/−: −/− at weaning stage is 1:2:0). In the case of an intercross of fx/− mice, expected ratio of fx/− individuals were obtained at the weaning stage (table 2), supporting the idea that the neo cassette was having an influence. Interestingly, intervals of conception to delivery for fn/− females were slightly longer than those of +/− females (27.5 ±6.4 day, n=11 and 22.8 ±1.6 day, n=21, respectively), while litter size was dramatically reduced (3.9±2.2, n=9 and 6.2±1.9, n=14, respectively, p<0.01). An average litter size for wild type females over the past several years in our colony with similar genetic background is 7.3. These results reinforce the idea that the fn allele, not the fx allele, is hypomorphic, and suggest that reduced BMP2 function in fn/− mothers compromise survival of embryos regardless of the genotype of embryos.
Table 2.
Under representation of fn/− pups
| Number of pups for each genotype (%) | ||||||
|---|---|---|---|---|---|---|
| father | mother | +/− | −/− | fn/+ | fn/− or fx/− | fn/fn or fx/fx |
| fn/− | +/− | 14 (41) | 0 (0) | 15 (45) | 5 (14) | N/A |
| +/− | fn/− | 21 (36) | 0 (0) | 25 (44) | 12 (20) | N/A |
| fn/− | fn/− | N/A | 0 (0) | N/A | 25 (47) | 28 (53) |
| fx/− | fx/− | N/A | 0 (0) | N/A | 42 (63) | 24 (36) |
Floxed-neo (fn) allele for Bmp2 causes closure defects in cephalic neural tube and ventral abdominal body wall
To identify the cause(s) of lethality, we set up timed matings in several combinations of the genotypes for parents. As previously reported, homozygous null mice were morphologically distinguished as early as embryonic day 8.5 (E8.5) [Zhang and Bradley, 1996], with their defects in embryonic turning and neural tube closure at E9.5 (fig. 4A). All of the fn/− embryos turned by E9.5, but some of them showed closure defects of the cephalic neural tube (fig. 4B, C) and develop exencephaly during mid-gestation (fig. 4D-F). Frequency of the closure defects for the neural tube found in fn/− embryos strongly depended on the genotype of the mothers (table 3). For example, if the mother was fn/fn, +/−, or fn/−, the frequency of neural tube defects in fn/− embryos was 25%, but if the mother was fn/+, the frequency was 13% in the fn/− embryos (table 3). Two of the fn/fn embryos out of 27 collected from fn/− mothers showed similar types of closure defects (fig. 4G), while none of the fn/fn embryos collected from fn/+ and fn/fn mothers showed the defects. These facts again demonstrate that a reduction of BMP2 in the mother affects the development of the neural tube closure defects of fn/fn embryos.
Figure 4. Neural tube closure defects.

At E9.5, homozygous null mutant embryos (−/−) were distinct by their defects in turning and neural tube closure along the body axis (A). Some of the fn/− embryos showed closure defects in the cephalic neural tube at E9.5 (B, white arrow). The closure defects persist during embryogenesis, but limited in the cephalic region and resulted in exencephaly as shown in (C) E10.0, (D) E11.5, (E-F) E16.5. Some of the fn/fn embryos developed in fn/− mothers developed similar closure defects as shown in (G), E15.5. Genotype of mothers is +/− (A), fn/+ (B), +/− (C), fn/− (D), +/− (E, F) and fn/− (G). Scale bar = 1 mm.
Table 3.
Abnormalities found in fn/− embryos
| Number of abnormal embryos (%) | ||||||
|---|---|---|---|---|---|---|
| mother | embryo | total | NTD | hernia | kinked | dying |
| fn/+ | fn/− | 8 | 1 (13) | 0 (0) | 1 (13) | 1 (13) |
| fn/fn | fn/− | 31 | 7 (23) | 1 (3) | 1 (3) | 0 (0) |
| +/− | fn/− | 44 | 11 (25) | 6 (14) | 6 (14) | 1 (2) |
| fn/− | fn/− | 17 | 4 (24) | 1 (6) | 3 (18) | 2 (12) |
NTD, neural tube closure defects.
Some of the fn/− embryos also developed a body wall hernia in the ventral abdominal region (fig. 5A-D). In many cases, this abnormality coincided with the defects in neural tube closure, but not always (fig. 5F). As shown in table 3, frequency of this phenotype also varied among the genotype of mothers with the highest frequency in fn/− embryos when the mother was heterozygote for conventional null allele (+/−). Although the numbers and frequency are small for this analysis, they clearly suggest a maternal influence on development of this abdominal abnormality.
Figure 5. Body wall closure defects found in the ventral abdominal region of fn/− embryos.
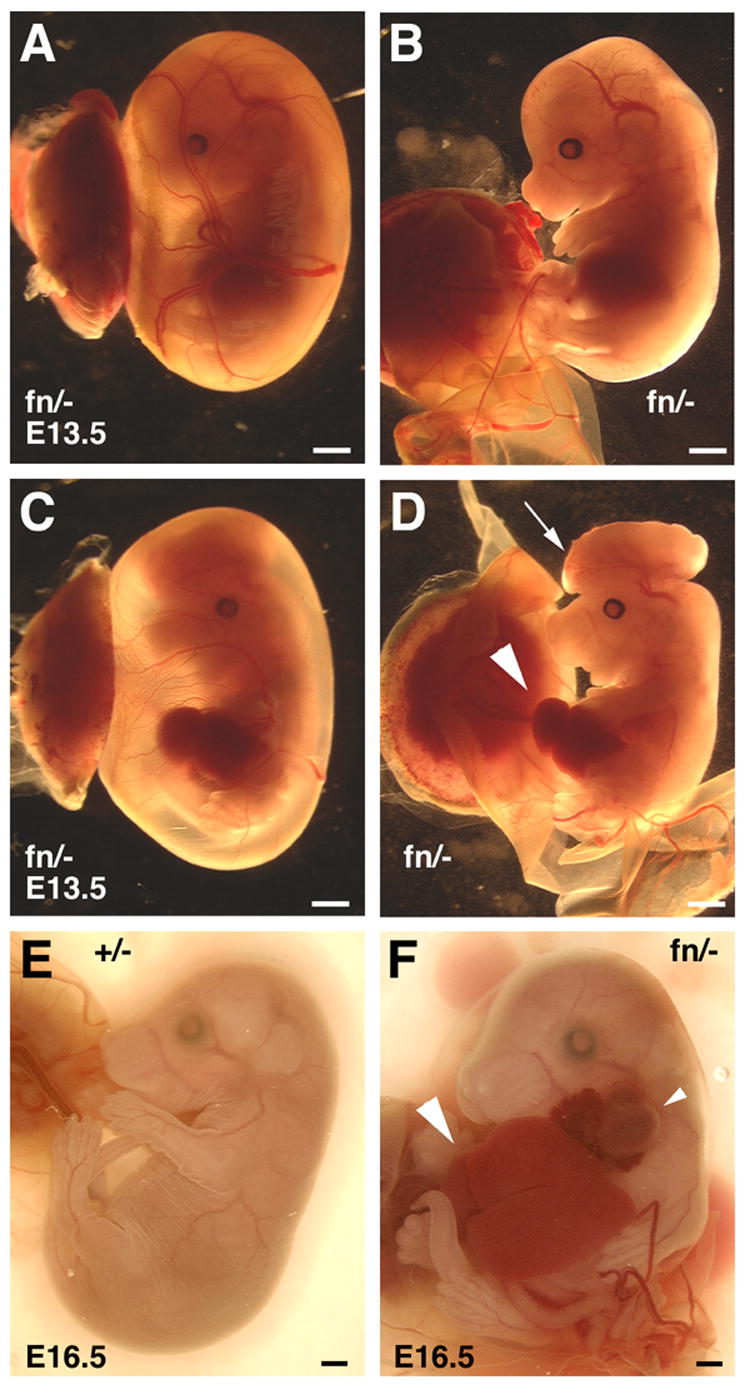
Two fn/− embryos at E13.5 from the same litter. One looked normal (A, B) and the other showed body wall closure defects (hernia, white arrowhead) along with exencephaly (white arrow) (C, D). Gross morphology before (A, C) and after (B, D) removal of the yolk sac were shown. Some of the fn/− embryos showed only the body wall closure defect (white arrowhead), but not exencephaly (E, F), E15.5. Genotypes of mothers for these embryos are +/−. Scale bar = 1 mm.
Expression levels of the floxed neo allele is lower
The direct hypothesis is that the amount of BMP2 transcript generated form the fn allele is lower due to the presence of the neo cassette in the 3’-UTR. To address this issue, we extracted RNA from embryos at E9.5 and uteri at E6.5 for quantification of BMP2 transcript levels. RNA was also extracted from decidual tissues at E6.5 since Bmp2 is known to be highly expressed in the decidua around this stage and believed to play critical roles for the establishment of implantation and subsequent embryogenesis [Li et al., 2007; Paria et al., 2001; Ying and Zhao, 2000]. As expected, expression levels of Bmp2 in homozygous embryos for the fn allele were dramatically reduced both in the anterior and posterior halves compared to those of wild type (Fig. 6A-B, 6% and 7% respectively). Expression levels of Bmp2 in fn/fn embryos were much lower than that of heterozygous null (fig. 6A, B, 17% for the anterior half and 10% for the posterior half relative to the wild type). Expression levels of Bmp2 in fn/− embryos were slightly lower than that of fn/fn embryos (fig. 6A, B, 5% for the anterior half and 4% for the posterior half). Results from both uterus and decidua showed the same expression profile as embryos (fig. 6C, D). Expression levels of Bmp2 in decidua and uterus in fn/+ were 84% and 81%, respectively, while in heterozygous mice for the conventional null allele were 36% and 28%, respectively. These results suggest that the different degree of under representation of fn/− pups between fn/+ mothers and +/− mothers (11% and 5%, respectively) is due to the difference in expression of Bmp2 in the maternal tissues adjacent to the embryos. However, expression levels of Bmp2 in the maternal tissues in fn/fn individuals were similar to or lower when compared to those of +/− individuals (38% vs. 36% in decidua and 14% vs. 28% in uterus), suggesting that there could be more complex reasons to explain the difference shown in table 1 for the expected number of pups with the various genotypes. As expected from the breeding results, expression levels of Bmp2 in fx/fx mothers were comparable to those in the wild type (Fig. 6C, D, 95% in decidua and 87% in uterus) and significantly higher than those in fn/fn mothers reinforcing the idea that presence of the neo cassette, not loxP sites or the human alkaline phosphatase cassette in the 3’-UTR makes the Bmp2 allele hypomorphic.
Figure 6. Lowered expression of Bmp2 from the fn allele.
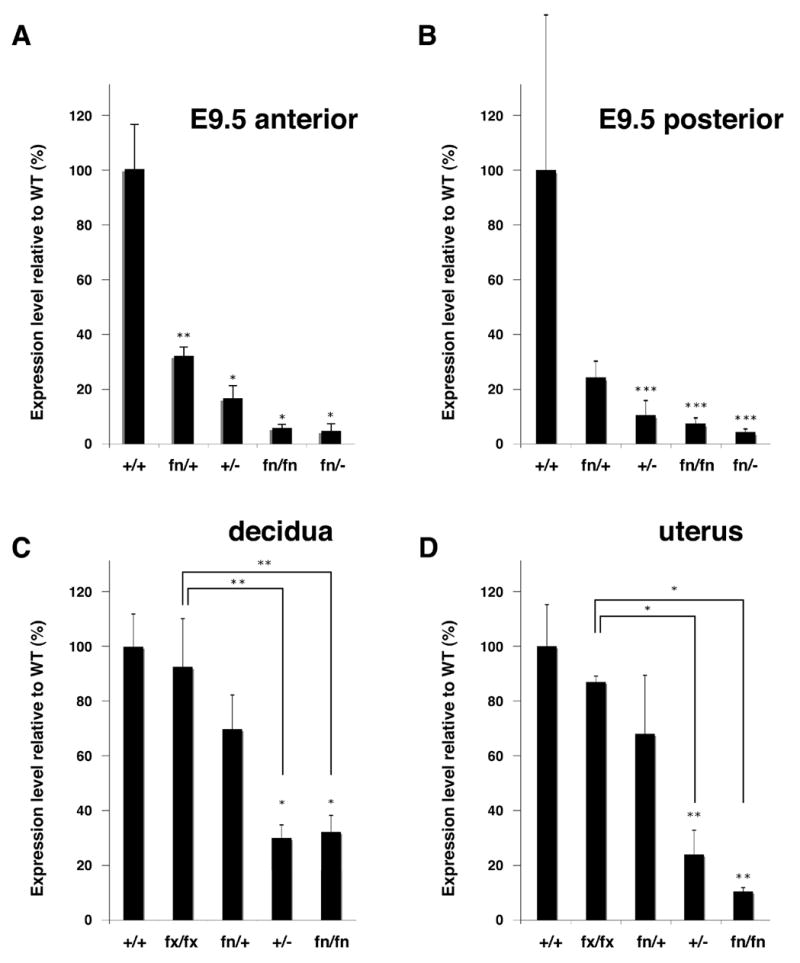
Expression levels of Bmp2 are measured by quantitative RT-PCR and their relative levels against that of the wild type are shown. Average of at least 3 independent samples are shown with standard deviation. (A) E9.5 embryos, anterior half. (B) E9.5 embryos, posterior half. (C) Decidual tissues at E6.5. (D) Uterus at E6.5. Genotypes are shown at the bottom of each bar. Student’s t-test was done for statistical analyses. *, p<0.025; **, p<0.05; ***, p<0.1.
Discussion
Here we showed that the presence of the neo cassette in the 3’-UTR of Bmp2 lowered the expression level of the BMP2 gene, and caused abnormalities in adults, including lower body weight and a kinked tail. Lowered expression of Bmp2 also caused an embryonic phenotype, including neural tube closure defects and body wall closure defects, leading to lethality. Interestingly, genotypes of mothers influenced the frequency of these abnormalities found in embryos. When there was lower levels of maternal BMP2 expression in the uterus and/or decidua, higher frequency of embryonic defects where observed in embryos with already reduced BMP2 levels. These abnormalities were rescued after removal of the neo cassette from the locus that lead to the resumed levels of normal Bmp2 expression levels.
All of the homozygous with the embryos for conventional null allele (−/−) show abnormalities including defects in the turning, defects in neural tube closure, and failure to develop limb bud (Fig. 4) [Zhang and Bradley, 1996]. Recently, we found some portion of the heterozygous embryos for the conventional null allele (+/−) also developed neural tube closure defects in cephalic regions depending on, in part, strain background (TC and YM, manuscript submitted). These abnormalities found in +/− embryos were resembled with those shown in this study (fig. 4). In both cases, closure defects in the trunk region were never observed suggesting that the cephalic region of the neural tube is much more sensitive to the level of BMP2 than that of the trunk region. It is proposed that morphological changes of the neural plate to form paired dorsolateral hinge points (DLHPs) are necessary for neural tube closure at the trunk level [Copp et al., 2003]. It is recently reported that antagonisms of BMP2 stimulate neural tube closure in the trunk region trough formation of DLHPs [Ybot-Gonzalez et al., 2007]. However, in the cephalic region, the formation of DLHPs is not required for neural tube closure [Copp, 2005; Ybot-Gonzalez et al., 2002]. Together with our results in this study, these reports support the idea that BMP2 plays an important role in neural tube closure, presumably with different mechanisms or thresholds of BMP2 required between cephalic and trunk regions. Interestingly, frequencies of the neural tube defects in the cephalic region of fn/− embryos were dramatically increased when the genotypes of the mothers are fn/fn, +/−, or fn/− compared to fn/+ (table 3), suggesting that maternal factors significantly contribute to the frequency of the defects. It is still an outstanding question whether maternal BMP2 is directly involved in the closure process of embryos. It is possible that downstream target genes of BMP2 signaling, either in maternal tissues or embryos, are associated in this closure process.
We also found that some of the fn/− embryos developed closure defects in ventral body wall resulting in abdominal hernia (fig. 5). This phenotype resembles a human condition known as omphalocele [Brewer and Williams, 2004; Wilson and Johnson, 2004]. We recently reported that mesoderm-derived tissue-specific disruption of Bmpr1a, a receptor for BMPs including BMP2, results in omphalocele-like defect [Sun et al., 2007]. It is also reported that double homozygous mutant embryos for Msx1 and Msx2, known downstream target genes of BMP signaling, develop similar defects [Ogi et al., 2005]. These results suggest that lowered expression of Bmp2 would decrease the expression levels of its downstream targets, including Msx1 and Msx2, and cause a failure of proper closure of the ventral abdominal wall. We have never observed the body wall closure defects in over one thousand of Bmp2 +/− embryos dissected over 10 years (unpublished results), suggesting that proper development of ventral abdominal body wall requires less BMP2 than that of cephalic neural tube closure.
Bmp2 is highly expressed in decidual tissues as well as the lumen of the uterus soon after implantation [Li et al., 2007; Paria et al., 2001; Ying and Zhao, 2000]. Recent studies using a conditional gene disruption technology have revealed that BMP2 from the maternal side plays a role during implantation and subsequent embryogenesis. A uterus epithelium-specific disruption of Bmp2 results in failure of decidua formation and implantation [Lee et al., 2007]. Smaller litter size from fn/− females could be explained by this hypothesis, as the levels of BMP2 in uterus and/or decidua of fn/− females are too low, and thereby cannot fully support the development of embryos regardless of their genotype. Indeed, we observed an increase in the numbers of resorption sites in fn/− mother when dissected at early to mid-gestation (data not shown). It is notable that among the surviving embryos in fn/− mothers, some of the fn/fn embryos develop neural tube closure defects providing more supporting evidence that expression levels of Bmp2 in mothers have an impact for development of abnormalities in the those embryos.
The remaining question is how the presence of the neo cassette is negatively influencing the expression level of Bmp2. It is believed that the 3’-UTR play a role in degradation of mRNA, providing a binding site for enzymes for RNA degradation [Blackshear, 2002; Jacobson and Peltz, 1996]. There are accumulating evidences that alteration of the 3’-UTR changes steady state levels of transcripts. Therefore, it is reasonable to speculate that presence of the neo cassette in the 3’-UTR would stimulate degradation of the Bmp2 transcript. Alternatively, but not exclusively, presence of the strong polII promoter may be influencing regulation, transcription or splicing of the BMP2 gene. It is very intriguing that after the removal of the neo cassette from the Bmp2 locus (fx allele), expression levels of Bmp2 become comparable to the wild type (fig. 6C, D), despite the presence of the human alkaline phosphatase cassette at the 3’-UTR of Bmp2. This implies that not only the location but also the sequence of the neo cassette or the polII promoter is altering the regulation and the levels of Bmp2 expression. Recent SNP analyses in humans have revealed that there are 6 SNPs located in 3’-UTR of BMP2 (http://www.genecards.org/cgi-bin/carddisp.pl?gene=BMP2&search=bmp2). At this moment, no correlation of these SNPs to peculiar phenotypes is reported. However, one of the SNP, rs15705, results in disruption of a putative posttranscriptional regulatory motif [Fritz et al., 2006]. Together with the results found in this study, these data suggest that the 3’-UTR of the Bmp2 locus controls the amount of the BMP2 transcript and alteration of this 3’-UTR function may result in embryonic abnormalities.
Acknowledgments
We gratefully thank Drs. Hongbing Zhang and Allan Bradley for Bmp2 conventional mice. We thank Ms. Leigh E. Davis, Ijeoma Nwosu, Gloria MacDonald, and Kelly McCann for their technical support, Ms. Tonya Miller for her excellent service of maintenance for the mouse colonies. This work was supported by the Intramural Research Program of the NIEHS/NIH to Y.M. (ES071003-10).
References
- Blackshear PJ. Tristetraprolin and other CCCH tandem zinc-finger proteins in the regulation of mRNA turnover. Biochem Soc Trans. 2002;30:945–952. doi: 10.1042/bst0300945. [DOI] [PubMed] [Google Scholar]
- Brewer S, Williams T. Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays. 2004;26:1307–1321. doi: 10.1002/bies.20137. [DOI] [PubMed] [Google Scholar]
- Copp AJ. Neurulation in the cranial region--normal and abnormal. J Anat. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–793. doi: 10.1038/nrg1181. [DOI] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;236:2493–2501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Farley FW, Soriano P, Steffen LS, Dymecki SM. Widespread recombinase expression using FLPeR (flipper) mice. Genesis. 2000;28:106–110. [PubMed] [Google Scholar]
- Fritz DT, Jiang S, Xu J, Rogers MB. A polymorphism in a conserved posttranscriptional regulatory motif alters bone morphogenetic protein 2 (BMP2) RNA:protein interactions. Mol Endocrinol. 2006;20:1574–1586. doi: 10.1210/me.2005-0469. [DOI] [PubMed] [Google Scholar]
- Jacobson A, Peltz SW. Interrelationships of the pathways of mRNA decay and translation in eukaryotic cells. Annu Rev Biochem. 1996;65:693–739. doi: 10.1146/annurev.bi.65.070196.003401. [DOI] [PubMed] [Google Scholar]
- Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478. doi: 10.1128/MCB.00342-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007;282:31725–31732. doi: 10.1074/jbc.M704723200. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- Ogi H, Suzuki K, Ogino Y, Kamimura M, Miyado M, Ying X, Zhang Z, Shinohara M, Chen Y, Yamada G. Ventral abdominal wall dysmorphogenesis of Msx1/Msx2 double-mutant mice. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:424–430. doi: 10.1002/ar.a.20180. [DOI] [PubMed] [Google Scholar]
- Olson EN, Arnold HH, Rigby PW, Wold BJ. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci U S A. 2001;98:1047–1052. doi: 10.1073/pnas.98.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Liu YH, Chen H, Nguyen MP, Mishina Y, Upperman JS, Ford HR, Shi W. Deficient Alk3-mediated BMP signaling causes prenatal omphalocele-like defect. Biochem Biophys Res Commun. 2007;360:238–243. doi: 10.1016/j.bbrc.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- Wilson RD, Johnson MP. Congenital abdominal wall defects: an update. Fetal Diagn Ther. 2004;19:385–398. doi: 10.1159/000078990. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad1, during mouse decidualization. Biol Reprod. 2000;63:1781–1786. doi: 10.1095/biolreprod63.6.1781. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]


