Abstract
Spontaneous atrophy of basal forebrain cholinergic neurons occurs with aging in the non-human primate brain. Short-term reversal of this atrophy has been reported following ex vivo Nerve Growth Factor (NGF) gene delivery, but long-term effects of in vivo NGF gene delivery in the aged primate brain have not to date been examined. We tested the hypothesis that long-term lentiviral NGF intraparenchymal gene delivery would reverse age-related cholinergic decline, without induction of adverse effects previously observed following sustained intracerebroventricular growth factor protein exposure. Three aged rhesus monkeys underwent intraparenchymal lentiviral NGF gene delivery to the cholinergic basal forebrain. One year later, cholinergic neuronal numbers were quantified stereologically and compared to findings in four control, non-treated aged monkeys and four young adult monkeys. Safety was assessed on several variables related to growth factor exposure. We now report that lentiviral gene delivery of NGF to the aged primate basal forebrain sustains gene expression for at least one year, and significantly restores cholinergic neuronal markers to levels of young monkeys. Aging resulted in a significant 17% reduction (p<0.05) in the number of neurons labeled for the cholinergic marker p75 among basal forebrain neurons. Lentiviral NGF gene delivery induced significant (p<0.05) and nearly complete recovery of p75-labeled neuronal numbers in aged subjects to levels observed in young monkeys. Similarly, the size of cholinergic neurons in aged monkeys was significantly reduced by 16% compared to young subjects (p<0.05), and lentiviral NGF delivery to aged subjects induced complete recovery of neuronal size. Intraparenchmyal NGF gene delivery over a one-year period did not result in systemic leakage of NGF, activation of inflammatory markers in the brain, pain, weight loss, Schwann cell migration, or formation of anti-NGF antibodies. These findings indicate that extended trophic support to neurons in the non-human primate brain reverses age-related neuronal atrophy. These findings also support the safety and feasibility of lentiviral NGF gene transfer for potential testing in human clinical trials to protect degenerating cholinergic neurons in Alzheimer’s disease.
Keywords: Alzheimer’s disease, cholinergic, chronic, gene therapy, neurotrophic factor, neurotrophin, p75, rhesus
INTRODUCTION
A complete description of mechanisms underlying cognitive decline with aging in the non-human primate brain remains elusive. Age-related decrements in white matter volume, loss of gray matter in some structures, and reductions in dendritic arborization/spines have been detected in non-human primates (Cupp and Uemura, 1980; Peters et al., 1998; Smith, 2004; Smith et al., 1999; Wisco et al., 2007). However, cell death as a function of aging either does not occur or is mild in extent in most brain regions examined to date, including the entorhinal cortex and hippocampus (Gazzaley et al., 1997; Merrill et al., 2000; Peters et al., 1996). These findings suggest that functional decline associated with aging across species does not primarily result from cell loss, but from other mechanisms including age-related decrements in gene expression and resultant cell signaling and biochemistry. Indeed, decrements in ERK/Map Kinase activation, Arc expression and functional neuronal transport include a set of atrophic cellular events that have been associated with functional decline in the nervous system with aging (De Lacalle et al., 1996; Niewiadomska et al., 2005; Small, 2004; Williams et al., 2006).
The general preservation of cell number in the nervous system as a function of aging raises the possibility that targeted interventions could ameliorate age-related atrophic changes. In rodent studies, aging is clearly associated with reductions in functional markers of basal forebrain cholinergic neurons (Chen and Gage, 1995; Fischer et al., 1987). These neurons project to extensive regions of the hippocampus and neocortex, modulate cortical neuronal excitability, and are required for some forms of cortical plasticity and learning (Mesulam et al., 1983a; Mesulam et al., 1983b). Notably, age-related atrophy of basal forebrain cholinergic neurons can be reversed by nerve growth factor (NGF) delivery to the brain, resulting in reversal of age-related cognitive decline (Chen and Gage, 1995; Fischer et al., 1987; Markowska et al., 1994). Age-related atrophy of basal forebrain cholinergic neurons has also been reported in the rhesus monkey brain (Smith et al., 1999; Stroessner-Johnson et al., 1992), and short-term (1–3 month) reversal of this decline was previously reported using techniques of either NGF protein infusion into the ventricular system (Koliatsos et al., 1991; Tuszynski et al., 1991; Tuszynski et al., 1990) or ex vivo gene delivery in which cells genetically modified to secrete NGF were grafted into the brain (Conner et al., 2001; Emerich et al., 1994; Smith et al., 1999; Tuszynski et al., 1996). However, whether age-related degenerative events in the cholinergic systems can be reversed by extended growth factor administration has not to date been tested. Addressing long-term consequences of growth factor gene delivery in the nervous system is of particular importance, as several clinical trials using this approach in human neurodegenerative disorders are in progress, including adeno-associated virus (AAV) NGF gene delivery in Alzheimer’s disease (Arvanitakis, 2007; Tuszynski et al., 2005) and Neurturin gene delivery in Parkinson’s disease (Marks et al., 2008).
The present study tested the hypothesis that lentiviral delivery of NGF to the basal forebrain of the aged rhesus monkey would reverse age-related cholinergic degenerative changes over extended time periods. We further hypothesized that previously reported adverse effects of growth factor administration, including pain, weight loss and Schwann cell hyperplasia (Emmett et al., 1996; Eriksdotter Jonhagen et al., 1998; Williams, 1991; Winkler et al., 1997), would be avoided by restricting gene delivery to cholinergic basal forebrain regions.
While several clinical trials of adeno-associated virus (AAV) vector delivery to the brain are in progress currently, lentiviral vector delivery to the CNS has been less thoroughly explored. Yet lentiviral vectors have potential benefits in a therapeutic context. First, lentiviral vectors more rapidly induce transgene expression than single-stranded AAV vectors in vitro and in vivo (Blesch and Tuszynski, 2007; Blesch et al., 2000; Naldini et al., 1996; Zufferey et al., 1998). Second, some AAV vector serotypes may undergo remote transport from their sites of injection into the nervous system (Hollis et al., 2008; Kaspar et al., 2002; Mandel and Burger, 2004), whereas lentiviral vectors are not known to exhibit remote transport properties; this could be an advantage in limiting therapeutic gene expression to a defined brain region. Third, relatively little is known regarding the long-term safety of viral vectors in the human CNS, and it is prudent to continue to explore the efficacy and safety profile of several viral gene delivery candidates, including lentiviral vectors.
METHODS
Experimental Subjects
11 monkeys served as experimental subjects. Three groups were examined: 4 aged un-operated control monkeys (mean age 23.9 ± 1.8 years; all males), 3 aged monkeys that received intraparenchymal injections of lentiviral vectors expressing human NGF (mean age 22.8 ± 0.7 years; 1 male and 2 females), and 4 adult, non-aged monkeys. The latter non-aged group consisted of 2 young adult monkeys that received injections of lentiviral vectors expressing the control reporter gene Green Fluorescent Protein, GFP (ages 4.5 and 8.8 years; 1 male and 1 female) and 2 un-operated monkeys (ages 8.7 and 15.3 years; both males). We did not include an aged group of subjects injected with lentiviral vectors expressing GFP because aged primates are a limited resource, and the enrollment of additional aged subjects assigned to this control group would potentially add little information for two reasons. First, previous studies from our group indicate that injection of a large volume (25 microliters) of control, GFP-expressing fibroblasts into the brains of aged monkeys do not result in detectable alterations of cholinergic cell number or basal forebrain anatomy, do not activate immune responses, do not induce NGF upregulation, and do not elicit detectable adverse effects in the brain. Second, previous studies in the non-human primate brain (Kordower et al., 2000) and findings of the current study (below) did not reveal a difference in cell parameters or inflammation when comparing GFP-injected and control, unoperated non-aged brains. For these reasons, and because primates in general and aged primates in particular are a limited resource, we did not include a group of aged, Lenti-GFP-injected controls. All subjects were from the California National Primate Center. Prior to the present study, animals were maintained under similar social conditions and were not involved in behavioral studies, surgical procedures or pharmacological experiments. All animal care procedures adhered to AAALAC and institutional guidelines.
Lentiviral Vector Production
For NGF delivery to aged subjects, lentiviral vectors genetically modified to secrete human NGF were prepared as previously described (Blesch et al., 2005). Briefly, a self-inactivating lentiviral vector (Zufferey et al., 1998) derived from pRRL (Follenzi et al., 2000) was used for virus production. For the constitutive expression of GFP and NGF, the vector p156sinRRLpptCAG-GFP-PRE containing (Pfeifer et al., 2002) the eGFP cDNA was digested with NheI and BamHI. The NheI site was filled and a HpaI/BamHI fragment containing the human NGF cDNA including a Kozak consensus sequence was cloned in. The GFP cDNA was removed by BamHI/BsrGI digestion and the vector was religated. Expression of GFP and NGF genes, respectively, was driven by the CMV/β-actin promoter (CAG) (Niwa et al., 1991).
For lentivirus production a third generation lentivirus packaging system was utilized as previously described (Blesch et al., 1999; Dull et al., 1998). Viral supernatants were concentrated by ultracentrifugation. Titers were determined by measurement of p24 levels in serial dilutions of vector stock by ELISA (Perkin Elmer). Lentivirus was diluted to a concentration of 100 μg/ml for injection. For GFP expressing virus, infectious units (I.U.) were also determined by infection of 293 cells with serial dilutions of the vector stock and correlated with p24 titers. Virus titers were 100 μg/ml (p24) for the GFP expressing virus and 100 μg/ml (p24) for the NGF expressing virus. Vector was stored at −80°C in Eppendorf tubes until the day of surgery.
Surgery
Monkeys underwent pre-operative MRI scans to visualize basal forebrain targets. After generating stereotaxic coordinates from MRI scans, each monkey received intraparenchymal injections of lentiviral-NGF or control lentiviral-GFP vectors. Monkeys were preanesthetized with 25 mg/kg ketamine IM and anesthetized with isofluorane. Vector was injected into each of 3 sites spaced over the rostral-caudal extent of the intermediate component of the basal forebrain cholinergic region (corresponding to the Ch4 region in the classification of Mesulam (Mesulam et al., 1983b)) bilaterally (6 injections total per animal). Injections were targeted to a position slightly dorsal to but within 500 μm of cell clusters constituting the cholinergic basal forebrain nucleus. 10 μl vector volumes were injected into each site with a 26-gauge Hamilton syringe at a rate of 1 μl/min. Postoperatively, all subjects were observed closely for signs of discomfort or toxicity and received the analgesic buprenorphine. Subjects were sacrificed one year after gene delivery. For perfusion, subjects were sedated with 25 mg/kg IM ketamine and were then deeply anesthetized with sodium pentobarbital (30 mg/kg IP). All reflex responses to cutaneous stimulation were verifiably absent before perfusion procedures were begun. Subjects were perfused with saline, followed by 4% paraformaldehyde or 2% paraformaldehyde/0.2% parabenzoquinone solution. Brains were stereotaxically blocked in the coronal plane.
Histology and Stereology
Serial coronal sections through the brain were cut on a freezing microtome set at 40 μm. Every twelfth section was processed for p75 immunoreactivity (monoclonal hybridoma cell line generated by M. Bothwell, dilution 1:100) as previously described (Smith et al., 1999). p75 has been reported to exhibit 95% co-association with cholinergic neurons of the basal forebrain, and labels no other neuronal types in this brain region (Smith et al., 1999). Stereological procedures were used to quantify the number of cholinergic neurons in the intermediate division of the basal forebrain cholinergic system (Ch4i) using p75-immunolabeled sections. The following anatomical boundaries were used to demarcate Ch4i: 1) the rostral boundary of Ch4i is marked by the appearance of the ansa peduncularis as it begins to penetrate Ch4, beginning dorsomedially and traversing diagonally through Ch4i (dorsomedial to ventrolateral). 2) The caudal boundary of Ch4i is marked by the completion of the passage of the ansa peduncularis through Ch4 at its ventrolateral extent, and the subsequent grouping of cholinergic neurons laterally into a single cluster, the posterior division (Ch4p). 3) The dorsal boundary of Ch4i is formed by the globus pallidus. 4) The ventral boundary of Ch4i at its rostral pole is formed by the Ch3 cell group. The cells of Ch3 are smaller than those of Ch4, hypochromic on p75 labeling, and are fusiform in shape with their long axis oriented along the ventral surface of the brain. The ventral boundary at the caudal aspect of Ch4i is formed by the ventral surface of the brain. There were no significant differences among animal groups in the mean number of tissue sections containing the Ch4i region (7.7 ± 0.6) used for stereological quantification (ANOVA, p = 0.34).
Stereological counts were performed on one in twelve sections through the entire extent of Ch4i, as previously described (Smith et al., 1999). The stereology computer programs controlled movement from one counting frame to the next by moving a Ludl motorized stage mounted on an Olympus BX60 microscope. Ch4i neurons were counted using a 40X high numerical aperture (1.00) oil objective. Unbiased stereological analysis was performed using StereoInvestigator software (Microbrightfield) with a 60×60 μm counting frame and a 240×240 μm sampling grid. Immunolabeled cells were included in the count if: a) they were p75-positive; b) the cell nucleus was within the counting frame (or touching the inclusion boundary) but did not touch the exclusion boundary; and c) the cell body was best in focus within the inclusion volume. Thus, unbiased estimates of p75-labeled neurons in Ch4i per subject were generated and compared among groups. Immunolabeling was noted to be uniform through the z-axis of sections from young and aged brains, allowing accurate comparisons between subjects.
Quantification of neuronal area
Stereological methods were used to determine differences in p75-immunolabeled neuronal area between groups. Cells were included in the area quantification if they met the morphological criteria described above. The mean cross-sectional area for Ch4i neurons was calculated for each animal, and means were averaged to derive values for each group.
Quantification of NGF transduction
Quantification of the NGF-transduced region was performed on a 1:12 series of sections that were immunolabeled with an NGF antibody (described below). Using Stereoinvestigator software, regions of transduction were outlined as determined by the presence of NGF immunolabeling within neurons, glia, and the surrounding extracellular space. Volume of transduction was generated as a function of area of transduction per section and converted to a volume estimate by stereological software based on inter-section distances.
Immunocytochemistry
Immunolabeling for CD3, CD8, and CD45 were performed to investigate the presence of activated microglia and several classes of leukocytes including T cells, monocytes, and granulocytes at the site of NGF or GFP lentivirus infusion. CD3, CD8, and CD45 (BD Pharmingen) were used at 1:500 dilution and processed with avidin/biotin amplification, ABC kit (Vector Laboratories), and with DAB reaction. Immunolabeling for NGF was performed with an NGF antibody (1:3000) raised in rabbit, as previously described (Conner and Varon, 1992).
NGF ELISA and serum NGF antibody assay
NGF levels in the cerebrospinal fluid were assayed one year after gene delivery. Cerebrospinal fluid was obtained by C1 puncture and stored at −80°C until assayed in a two-site ELISA specific for NGF (Conner and Varon, 1996). This ELISA is sensitive to 1 picogram NGF per mg. The potential formation of anti-NGF antibodies was assessed by immunoprecipitation of serum, using a known anti-NGF antibody as a positive control (Conner and Varon, 1996)
Statistics
Multiple group comparisons were made by analysis of variance (ANOVA) with post-hoc analysis using Fisher’s least square difference. Biological significance was established at the 95% confidence level. Data are presented as mean ± standard error of the mean, except for stereology data that is customarily presented as ± standard deviation.
RESULTS
Persistence and Pattern of In Vivo Gene Expression
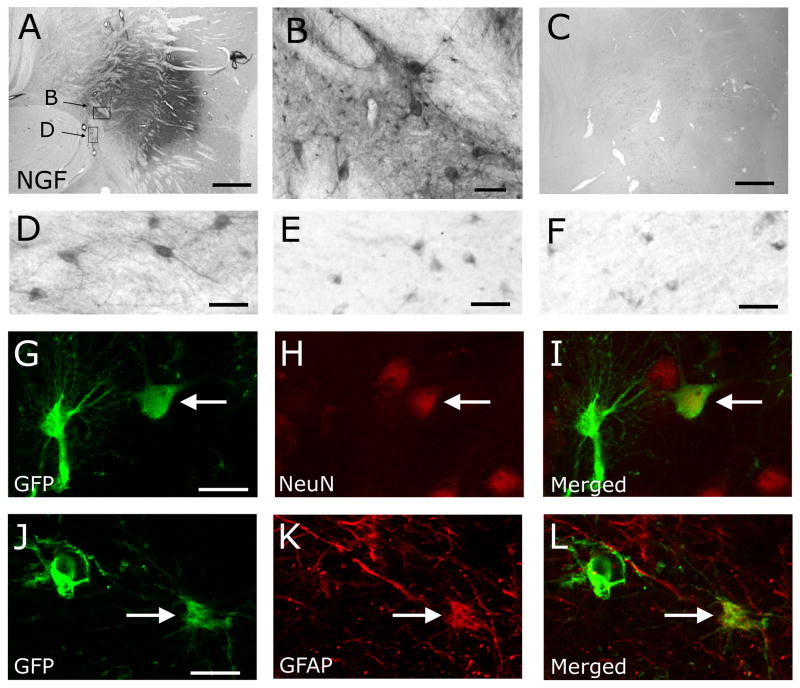
Lentiviral gene expression was readily detectable in the primate brain for at least one year after therapeutic gene delivery, indicated by GFP expression in young subjects and NGF immunolabeling in aged monkeys (Fig. 1). NGF lentiviral injections were targeted adjacent to the nucleus basalis, and resulted in enhanced NGF labeling within both neuronal perikarya and in the extracellular matrix compared to young control and aged subjects (Fig. 1A–F). A mean parenchymal volume of 16.0 ± 2.1 mm3 was transduced per injection site, based on quantification of spread of NGF immunolabeling from the injection site along x, y, z axes in aged, Lenti-NGF treated subjects. The mean distance of NGF spread laterally from the site of transduction per subject (in coronal sections) was 2.83 ± 1.83 mm, and most injections were located within 1 mm of p75-labeled basalis neurons. Enhanced levels of NGF immunolabeling within cortical regions were not detected in aged subjects, suggesting a lack of distant NGF transport to locations remote from the injection sites. Lentiviral vectors infected both host neurons and glia within the region of the nucleus basalis (Fig. 1), with a nearly equal proportion of infected cell types based on double immunoableing for GFP and neuron- or glia-specific markers: 49.2 ± 0.8% of GFP-labeled cells co-expressed neuronal markers, and 50.8 ± 0.8% of GFP-labeled cells co-localized with GFAP.
Figure 1. NGF and GFP immunolabeling in the gene delivery site.
(A) NGF immunoreactivity in the basal forebrain region containing cholinergic neurons, in an aged recipient of Lenti-NGF (see also Fig. 2; scale bar 1000 μm). (B) At higher magnification, dense NGF labeling is present both intra- and extra-cellularly after Lenti-NGF injection (scale bar 50 μm) (C) In contrast, extracellular NGF immunolabeling is not detectable in a young control monkey that underwent injection of Lenti-GFP; thus, needle passage or control vector delivery did not detectably enhance NGF expression (injection site shown; scale bar 1000 μm). (D) At the level of individual neurons, NGF immunolabeling is relatively dense after Lenti-NGF injection in aged monkeys. Scale bar 70 μm in D–F. (E) In contrast, intraneuronal NGF labeling is substantially lighter in young, lenti-NGF-injected control and (F) aged control subjects. Lentiviral vectors infect both (G–I) neurons and (J–L) glia, indicated by double labeling for the neuron-specific marker NeuN and GFP, or the glial-specific marker GFAP and GFP, respectively (scale bar 20 μm). Arrows indicate double-labeled cells.
Normal Aging Results in a Decline in Primate Cholinergic Neuronal Number and Size
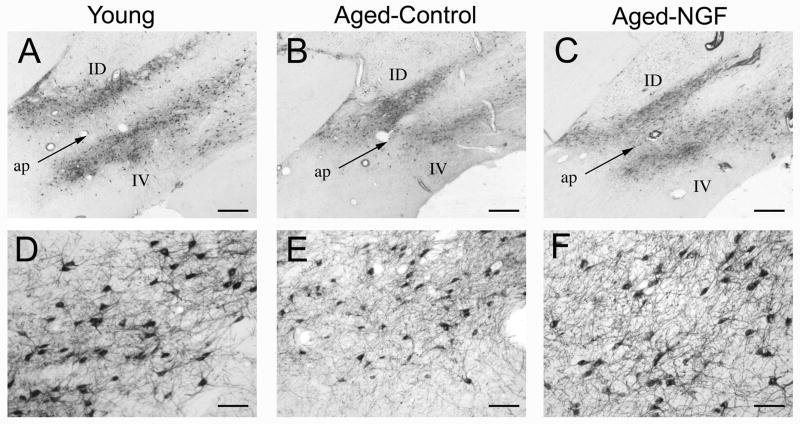
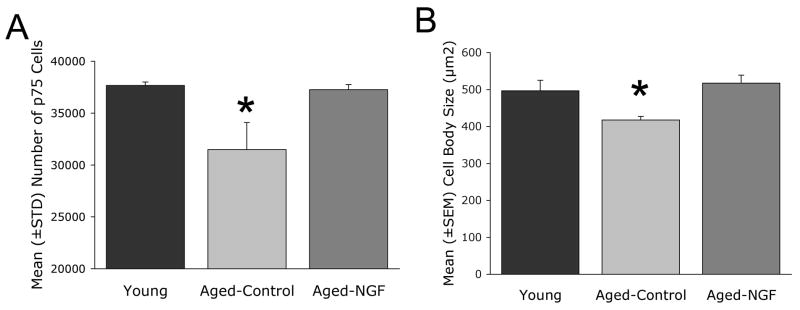
Aging resulted in a significant decline in both the number and size of neurons immunolabeled for p75 in the intermediate region of the cholinergic basal forebrain (Ch4i; Figs. 2, 3), consistent with previous reports (Smith et al., 1999). The number of p75-labeled neurons was significantly reduced in 4 aged control monkeys by 17% compared to young monkeys (Young: 37,656 ± 345 (SD) vs. Age Control: 31,482 ± 2,606; p<0.05). The size of remaining p75-labeled neurons also significantly declined by 16% in control, aged monkeys (Young: 496.5 ± 28.7 μm2 vs. Aged: 417.6 ± 9.9 μm2; p<0.05). Thus, aging resulted in a significant reduction in the number and size of basal forebrain cholinergic neurons labeled for a specific marker of basal forebrain cholinergic neurons, the p75 low-affinity neurotrophin receptor (p75NTR). We previously reported that these reductions represent a decline of functional markers in cholinergic neurons, but not cell death, based on p75NTR and Nissl-stained cell counts in aged monkeys (Smith et al., 1999).
Figure 2. Cholinergic neuronal labeling in the Nucleus Basalis.
Representative images of p75 immunolabeling (a specific marker for cholinergic neurons of the basal forebrain) in the intermediate dorsal (ID) and ventral (IV) components of the cholinergic basal forebrain (Ch4i) in: (A) young control monkeys, (B) aged control monkeys, and (C) aged, lenti-NGF treated monkeys. ap: ansa peduncularis; scale bar 500 μm. Higher magnification suggests reductions in neuronal number and size in (E) aged control subjects compared to (D) young monkeys, and (F) possible reversal with lenti-NGF treatment in aged subjects (scale bar, 100 μm).
Figure 3. Quantification of cholinergic neuronal number and size.
(A) Aged monkeys exhibit a reduction in the number of p75-labeled neurons in Ch4i compared to young monkeys; NGF gene delivery reverses this decline (ANOVA, p<0.05; post-hoc Fisher’s, *p<0.05; aged control vs. young and aged control vs. NGF-Aged groups). Data presented here as mean ± standard deviation, as is customary for stereological data. (B) Aged monkeys also exhibit shrinkage in mean neuronal size compared to young monkeys; lenti-NGF delivery reverses cell atrophy (ANOVA, p<0.05; post-hoc Fisher’s, *p<0.05; aged control vs. young and aged control vs. NGF-Aged groups). Cell size data are presented as mean ± standard error.
Reversibility of age-related cholinergic neuronal atrophy by NGF gene delivery
Age-related declines in p75-labeled neuronal number and size were reversed in recipients of Lenti-NGF injections (Figs. 2, 3). Mean p75-immunolabeled neuronal numbers in aged recipients of NGF-secreting cells were restored to within 1.1% of values in non-aged subjects (37,259 ± 498 neurons), an amount that did not differ significantly from young subjects and was significantly greater than aged control subjects (p<0.05) on post-hoc analysis. Similarly, neuronal size in aged recipients of lenti-NGF vectors recovered compared to aged controls (p<0.05), and exceeded the mean size of cholinergic neurons in young animals by 4% (517.4 ± 21.6 μm2).
Safety
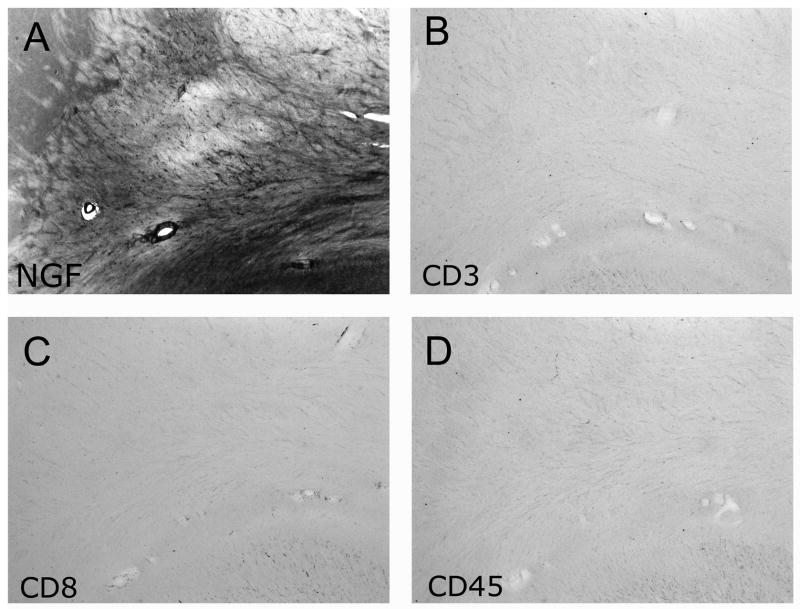
Regions of gene delivery evidenced minimal or no activation of inflammatory markers after one year in the primate brain. Immunolabeling for CD45, CD3, and CD8 detects activated microglia and several classes of leukocytes including T cells, monocytes, and granulocytes. NGF treated-aged monkeys did not exhibit an elevation of CD3, CD8 or CD45 labeling in regions of lenti-NGF injections (Fig 4). There was also no evidence of tumor formation in any cell type near or remote from the gene delivery site. Adverse effects of non-targeted NGF delivery were also not observed: 1) subjects did not lose weight over the post-delivery period (mean pre-treatment weight in lenti-NGF subjects, 13.0 ± 2.1 kg; weight at one year, 12.8 ± 2.8 kg); 2) Schwann cell infiltrations were not observed in the brainstem or spinal cord (not shown); and 3) sprouting of sympathetic axons was not detected around the cerebral vasculature in the subarachnoid space (not shown).
Figure 4. Inflammatory markers are not induced at sites of lenti-NGF gene delivery.
(A) NGF-immunolabeling reveals robust in vivo gene expression in the same regions that CD3, CD8 and CD45 are examined in subsequent panels B–D, in serial sections. (B) CD3, (C) CD8, and (D) CD45 immunolabeling are not activated in the non-human primate brain one year after lenti-NGF gene delivery. Labeling of peripheral leukocytes was performed as a positive control for each label (not shown).
Cerebrospinal fluid was assayed for NGF levels by ELISA one year after lenti-NGF gene delivery. In no case was NGF detectable, using an assay sensitive to concentrations of NGF of 1 pg/ml. Further, serum was assayed for potential formation of anti-NGF antibodies using an ELISA that could readily detect NGF binding antibodies present in solution at a concentration of < 0.5 ng/ml. No detectable NGF antibodies were found in serum samples taken either before or after NGF lentiviral treatment.
DISCUSSION
Findings of this study indicate that lentiviral-mediated in vivo NGF gene delivery sustains growth factor production for at least one year in the aged non-human primate brain, and that this sustained delivery reverses age-related neuronal atrophy. Trophic effects on neurons are reflected in both the number of neurons expressing the specific basal forebrain cholinergic marker, p75 (Kordower et al., 1994), and the size of cholinergic neurons. Sustained lentiviral NGF delivery was safe, without evidence of significant immune response, and without NGF spread beyond the targeted brain region.
Previous studies have utilized ex vivo techniques to deliver NGF to the aged primate brain (Emerich et al., 1994; Kordower et al., 1994; Smith et al., 1999). With natural aging in primates, there is a significant reduction in the size and number of cholinergic markers, but without frank cell loss by Nissl stain (Smith et al., 1999), indicating that cholinergic neurons undergo atrophy but not death as a function of aging. Previous studies also reveal that declines in p75-labeled neurons parallel declines in choline acetyltransferase (ChAT)-labeled neurons in aged monkeys (Smith et al., 1999), and that ameliorative effects of NGF administration are reflected on quantification of both p75 and ChAT neuron number. Analysis of the aged control group in the present study confirms previous observations of age-related cholinergic atrophy by p75 labeling. Ex vivo NGF gene therapy has been reported to reverse atrophic effects of aging on cholinergic markers three months after treatment in primates (Smith et al., 1999). We now report neuroprotection using lentiviral in vivo NGF gene delivery, and persistence of these effects over prolonged time frames of one year in the non-human primate brain. Thus, atrophic effects of aging can be reversed for extended time periods by sustained growth factor delivery to the primate brain.
Lentiviral NGF delivery to the primate basal forebrain sustained gene expression over one year, evidenced by persistent GFP and NGF immunolabeling. Further, neither local nor remote toxicity was observed. Specifically, there was no evidence of local neuronal loss, immune response or tumor formation. Adverse effects of NGF distribution throughout the neuraxis did not occur: subjects did not lose weight (Eriksdotter Jonhagen et al., 1998; Williams, 1991), did not exhibit evidence of pain (Eriksdotter Jonhagen et al., 1998) (as reflected by general appetite and activity in the home cage), and showed no Schwann cell hyperplasia in the subarachnoid space (Winkler et al., 1997). Supporting this safety profile, NGF spread into the spinal fluid was not detectable at the conclusion of the study. Further, serum anti-NGF antibodies were not detected. Thus, prolonged NGF gene delivery appears to restrict delivery of NGF to the targeted brain region without induction of detectable adverse effects and without a detectable build up of NGF levels over time.
Ex vivo and now in vivo NGF gene delivery (using AAV vectors) have undergone clinical testing in the most common human neurodegenerative disorder, Alzheimer’s disease (Tuszynski et al., 2005). The prolonged restorative effects and safety of in vivo NGF gene delivery in this study support the rationale underlying these clinical trials. Ongoing controlled trials in humans will ultimately determine whether NGF specifically will slow neuronal loss and improve cognitive performance in Alzheimer’s disease. Yet a point of paramount importance in testing the potential value of growth factors for any neurodegenerative condition is the availability of a delivery method that can both achieve effective concentrations of growth factors in regions of degenerating neurons, while restricting availability of those growth factors only to degenerating neurons to avoid adverse effects that invariably result from spread beyond the targeted region. Lentiviral gene delivery exhibits the potential to provide such a method. We now present evidence that lentiviral NGF gene delivery in the primate brain, like AAV-NGF gene delivery in the rodent brain (Bishop et al., 2008), elicits trophic effects in the adult CNS, sustains in vivo gene expression for extended time periods, and results in no detectable adverse effects. Lentiviral vector delivery of NGF does not result in detectable remote expression of NGF in other brain regions; the restricted expression of NGF to sole sites of vector injection could prove to be a useful property in future clinical application. Thus, AAV and lentiviral vector systems both represent potentially useful vehicles for clinical gene delivery in the adult CNS, meriting continued development.
Acknowledgments
We thank R. Torres, H. Zhang, and M. Culbertson for technical support. Supported by the NIH (AG10435), the Veterans Administration, the Alzheimer’s Association (TTL-03-5814), State of California Department of Health Services (04-35530), and the Shiley Family Foundation.
Abbreviations
- CAG
CMV/β-actin promoter
- Ch4i
intermediate division of cholinergic nucleus 4 (nucleus basalis)
- Ch4p
posterior division of cholinergic nucleus 4 (nucleus basalis)
- CMV
cytomegalovirus
- GFP
green fluorescent protein
- NBM
nucleus basalis of Meynert
- NGF
Nerve growth factor
Footnotes
Conflict of interest statement: MT is a consultant to Ceregene, Inc.; AR and MG are employees of Ceregene, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arvanitakis Z, Tuszynski MH, Bakay R, Arends D, Potkin S, Bartus R, Bennett D. Interim data from a phase 1 clinical trial of AAV-NGF (CERE-110) gene delivery in Alzheimers disease. Abstr Amer Acad Neurol. 2007:05.071. [Google Scholar]
- Bishop KM, Hofer EK, Mehta A, Ramirez A, Sun L, Tuszynski M, Bartus RT. Therapeutic potential of CERE-110 (AAV2-NGF): targeted, stable, and sustained NGF delivery and trophic activity on rodent basal forebrain cholinergic neurons. Exp Neurol. 2008;211:574–584. doi: 10.1016/j.expneurol.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Pfeiffer A, Conner JM, Britton W, Verma I, Tuszynski MH. Regulated lentiviral NGF genetransfer controls rescue of medial septal cholinergic neurons. Molec Ther. 2005;11:916–925. doi: 10.1016/j.ymthe.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J Neurosci. 2007;27:10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesch A, Uy HS, Diergardt N, Tuszynski MH. Neurite outgrowth can be modulated in vitro using a tetracycline-repressible gene therapy vector expressing human nerve growth factor. J Neurosci Res. 2000;59:402–409. doi: 10.1002/(SICI)1097-4547(20000201)59:3<402::AID-JNR14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Blesch A, Uy HS, Grill RJ, Cheng JG, Patterson PH, Tuszynski MH. Leukemia Inhibitory Factor augments neurotrophin expression and corticospinal axon growth after adult CNS injury. J Neurosci. 1999;19:3556–3566. doi: 10.1523/JNEUROSCI.19-09-03556.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KS, Gage FH. Somatic gene transfer of NGF to the aged brain: Behavioral and morphological amelioration. J Neurosci. 1995;15:2819–2825. doi: 10.1523/JNEUROSCI.15-04-02819.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Darracq MA, Roberts J, Tuszynski MH. Non-tropic actions of neurotrophins: Subcortical NGF gene delivery reverses age-related degeneration of primate cortical cholinergic innervation. Proc Nat Acad Sci. 2001;98:1941–1946. doi: 10.1073/pnas.98.4.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Varon S. Distribution of nerve growth factor-like immunoreactive neurons in the adult rat brain following colchicine treatment. J Comp Neurol. 1992;326:347–362. doi: 10.1002/cne.903260304. [DOI] [PubMed] [Google Scholar]
- Conner JM, Varon S. Characterization of antibodies to nerve growth factor: assay-dependent variability in the cross-reactivity with other neurotrophins. J Neurosci Methods. 1996;65:93–99. doi: 10.1016/0165-0270(95)00151-4. [DOI] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol. 1980;69:143–163. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- De Lacalle S, Cooper JD, Svendsen CN, Dunnett SB, Sofroniew MV. Reduced retrograde labelling with fluorescent tracer accompanies neuronal atrophy of basal forebrain cholinergic neurons in aged rats. Neuroscience. 1996;75:19–27. doi: 10.1016/0306-4522(96)00239-4. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DW, Winn S, Harper J, Hammang JP, Baetge EE, Kordower JH. Implants of polymer-encapsulated human NGF-secreting cells in the nonhuman primate: Rescue and sprouting of degenerating cholinergic basal forebrain neurons. J Comp Neurol. 1994;349:148–164. doi: 10.1002/cne.903490110. [DOI] [PubMed] [Google Scholar]
- Emmett CJ, Stewart GR, Johnson RM, Aswani SP, Chan RL, Jakeman LB. Distribution of radioiodinated recombinant human nerve growth factor in primate brain following intracerebroventricular infusion. Exp Neurol. 1996;140:151–160. doi: 10.1006/exnr.1996.0125. [DOI] [PubMed] [Google Scholar]
- Eriksdotter Jonhagen M, Nordberg A, Amberla K, Backman L, Ebendal T, Meyerson B, Olson L, Seiger, Shigeta M, Theodorsson E, Viitanen M, Winblad B, Wahlund LO. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 1998;9:246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- Fischer W, Wictorin K, Bjorklund A, Williams LR, Varon S, Gage FH. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature. 1987;329:65–68. doi: 10.1038/329065a0. [DOI] [PubMed] [Google Scholar]
- Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging. 1997;18:549–553. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Hollis ER, 2nd, Kadoya K, Hirsch M, Samulski RJ, Tuszynski MH. Efficient retrograde neuronal transduction utilizing self-complementary AAV1. Mol Ther. 2008;16:296–301. doi: 10.1038/sj.mt.6300367. [DOI] [PubMed] [Google Scholar]
- Kaspar BK, Vissel B, Bengoechea T, Crone S, Randolph-Moore L, Muller R, Brandon EP, Schaffer D, Verma IM, Lee KF, Heinemann SF, Gage FH. Adeno-associated virus effectively mediates conditional gene modification in the brain. Proc Natl Acad Sci U S A. 2002;99:2320–2325. doi: 10.1073/pnas.042678699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliatsos VE, Clatterbuck RE, Nauta HJ, Knusel B, Burton LE, Hefti FF, Mobley WC, Price DL. Human nerve growth factor prevents degeneration of basal forebrain cholinergic neurons in primates. Ann Neurol. 1991;30:831–840. doi: 10.1002/ana.410300613. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Déglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Winn SR, Liu YT, Mufson EJ, Sladek JR, Jr, Hammang JP, Baetge EE, Emerich DF. The aged monkey basal forebrain: rescue and sprouting of axotomized basal forebrain neurons after grafts of encapsulated cells secreting human nerve growth factor. Proc Natl Acad Sci U S A. 1994;91:10898–10902. doi: 10.1073/pnas.91.23.10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges. Curr Opin Mol Ther. 2004;6:482–490. [PubMed] [Google Scholar]
- Markowska AL, Koliatsos VE, Breckler SJ, Price DL, Olton DS. Human nerve growth factor improves spatial memory in aged but not in young rats. J Neurosci. 1994;14:4815–4824. doi: 10.1523/JNEUROSCI.14-08-04815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, Taylor R, Cahn-Weiner DA, Stoessl AJ, Olanow CW, Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III and V/VI of aged primates. J Comp Neurol. 2000;422:396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BJ, Level AI. Central cholinergic pathways in the rat: An overview based on an alternative nomenclature (Ch1–Ch6) Neuroscience. 1983a;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983b;214:170–197. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Nat Acad Sci. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiadomska G, Baksalerska-Pazera M, Riedel G. Altered cellular distribution of phospho-tau proteins coincides with impaired retrograde axonal transport in neurons of aged rats. Ann N Y Acad Sci. 2005;1048:287–295. doi: 10.1196/annals.1342.026. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Peters A, Rosene DL, Moss MB, Kemper TL, Abraham CR, Tigges J, Albert MS. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J Neuropathol Exp Neurol. 1996;55:861–874. doi: 10.1097/00005072-199608000-00001. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Pfeifer A, Ikawa M, Dayn Y, Verma IM. Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos. Proc Natl Acad Sci U S A. 2002;99:2140–2145. doi: 10.1073/pnas.251682798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differntially vulnerable to aging. Proc Nat Acad Sci. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24:4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Roberts J, Gage FH, Tuszynski MH. Age-associated neuronal atrophy occurs in the primate brain and is reversible by growth factor gene therapy. Proc Nat Acad Sci. 1999;96:10893–10898. doi: 10.1073/pnas.96.19.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroessner-Johnson HM, Rapp PR, Amaral DG. Cholinergic cell loss and hypertrophy in the medial septal nucleus of the behaviorally characterized aged rhesus monkey. J Neurosci. 1992;12:1936–1944. doi: 10.1523/JNEUROSCI.12-05-01936.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Roberts J, Senut MC, U HS, Gage FH. Gene therapy in the adult primate brain: intraparenchymal grafts of cells genetically modified to produce nerve growth factor prevent cholinergic neuronal degeneration. Gene Therapy. 1996;3:305–314. [PubMed] [Google Scholar]
- Tuszynski MH, Thal L, Pay M, Salmon DP, U HS, Bakay R, Patel P, Blesch A, Vahlsing HL, Ho G, Tong G, Potkin SG, Fallon J, Hansen L, Mufson EJ, Kordower JH, Gall C, Conner J. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11:551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- Tuszynski MHUH-S, Gage FH. Recombinant human nerve growth factor infusions prevent cholinergic neuronal degeneration in the adult primate brain. Ann Neurol. 1991;30:625–636. doi: 10.1002/ana.410300502. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, U HS, Amaral DG, Gage FH. Nerve growth factor infusion in primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990;10:3604–3614. doi: 10.1523/JNEUROSCI.10-11-03604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berl) 2006;188:605–618. doi: 10.1007/s00213-006-0477-1. [DOI] [PubMed] [Google Scholar]
- Williams LR. Hypophagia is induced by intracerebroventricular administration of nerve growth factor. Exp Neurol. 1991;113:31–37. doi: 10.1016/0014-4886(91)90143-z. [DOI] [PubMed] [Google Scholar]
- Winkler J, Ramirez GA, Kuhn HG, Peterson DA, Day-Lollini PA, Stewart GR, Tuszynski MH, Gage FH, Thal LJ. Reversible Schwann cell hyperplasia and sprouting of sensory and sympathetic neurites after intraventricular administration of nerve growth factor. Ann Neurol. 1997;41:82–93. doi: 10.1002/ana.410410114. [DOI] [PubMed] [Google Scholar]
- Wisco JJ, Killiany RJ, Guttmann CR, Warfield SK, Moss MB, Rosene DL. An MRI study of age-related white and gray matter volume changes in the rhesus monkey. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]