Abstract
BACKGROUND
Block of ultra-rapid delayed rectified potassium current (IKur), present in atria, but not in ventricles, is thought to be a promising approach for atrial-specific therapy of atrial fibrillation (AF). However, it has been shown that IKur block may abbreviate atrial repolarization and that loss-of-function mutations in KCNA5, which encodes Kv 1.5 channels responsible for IKur, is associated with familial AF.
OBJECTIVE
Our objective in this study was to use low concentrations of 4-aminopyridine (4-AP, 10–50 µM), known to selectively block IKur, to assess the pro-and antiarrhythmic effects of IKur block in “healthy” and “remodeled” atria.
METHODS
Isolated canine coronary-perfused right atrial preparations were used. Acetylcholine or ischemia/reperfusion was utilized to acutely “remodel” the atria. Transmembrane action potentials and a pseudo-ECG were simultaneously recorded.
RESULTS
Normal (“healthy”) atria typically displayed action potentials (AP) with a prominent plateau, whereas remodeled atria displayed triangular-shaped APs (“remodeled”). In “healthy” atria, in which AF could not be induced with programmed stimulation, 4-AP abbreviated action potential measured at 90% repolarization (APD90) and effective refractory period (ERP), permitting the induction of AF in 4/12 preparations (33%). In “remodeled” atria, 4-AP produced little (50 µM) to no (10–25 µM) prolongation of APD90 or ERP and was either ineffective or poorly effective in terminating AF or preventing its induction.
CONCLUSIONS
Our findings suggest that block of IKur can provide the substrate for development of AF in “healthy” canine atria, presumably via abbreviation of APD and ERP.
Keywords: atrial fibrillation, antiarrhythmic agents, action potential
INTRODUCTION
Ultra-rapid delayed rectified potassium current (IKur), carried by Kv 1.5 channels encoded by the KCNA5 gene, is present in atria, but not in the ventricles.1–3 Block of IKur is thought to be a promising approach for atrial-specific therapy of atrial fibrillation (AF). However, a recent report has associated KCNA5 loss-of-function mutations with familial AF.4 It has been shown also that reduction of IKur abbreviates action potential duration (APD90) in non-remodeled (“healthy”) atrial cells, but prolongs APD90 in remodeled atrial cells (displaying a triangular-shaped action potential).5, 6 Knowing that abbreviation of atrial repolarization promotes AF, we hypothesized that IKur block could promote AF in “healthy” atria. We used low concentrations of 4-AP (10–50 µM), shown to be specific for IKur block,2, 3 to assess the pro-and antiarrhythmic effects of IKur block in “healthy” vs. “remodeled” canine isolated coronary-perfused atria.
METHODS
Experiments were performed using isolated arterially-perfused canine right atrial preparations. The methods used for isolation and perfusion of these preparations have been described in previous publications.5, 7 Briefly, right atria were dissected from hearts removed from anesthetized (sodium pentobarbital) adult mongrel dogs (20–25 kg). Unfolded atria with a rim of the right ventricle was cannulated and perfused through the ostium of the right coronary artery. Unperfused tissue was removed with a razor blade or scissors. The cut ventricular and atrial branches were ligated using silk thread. After these procedures (performed in cold cardioplegic solution, 4–8°C), the preparations were transferred to a temperature-controlled bath and arterially-perfused with Tyrode’s solution by use of a roller pump. The composition of the Tyrode’s solution was (in mM): NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, and D-glucose 5.5, buffered with 95% O2 and 5% CO2 (37±0.5 °C, pH=7.35)
Transmembrane action potential (AP) recordings were obtained using floating glass microelectrodes (2.7 M KCl, 10–25 MΩ DC resistance). A pseudo-electrocardiogram (ECG) was recorded using two electrodes consisting of Ag/AgCl half cells placed in the Tyrode’s solution bathing the preparation, 1.0 to 1.2 cm from opposite ends of the atrial preparations. Effective refractory period (ERP) was measured by delivering premature stimuli of twice diastolic threshold intensity after every 10th basic beat at a pacing CL of 500 ms.
“Healthy” and “Remodeled” atria
Arterially-perfused atrial preparations not subjected to any interventions were referred to as “Healthy” atria. Acute “remodeling” of atrial coronary perfused preparations was achieved with two experimental approaches known to abbreviate atrial APD and promote AF. In the first, acetylcholine (ACh, 0.5 µM) was used to abbreviate APD and ERP and mimic a “remodeled” atrium.7, 8 The second approach involved a recently developed AF model in which isoproterenol (ISO, 0.1–0.2 µM) was added to the coronary perfusate after the atrial preparations are exposed to ischemia and reperfusion.9, 10 Ischemia and reperfusion were produced by stopping (for 30–40 min) and reinitiating coronary flow with continuous superfusion flow. On the 15–30th minutes after the start of reperfusion, ISO was added to the coronary perfusate, which reproducibly generated non-sustained AF in the ischemia/reperfusion-damaged atria.9, 10 Note that β-adrenergic stimulation only mildly promotes AF in “healthy” atria.9, 11
Experimental Protocols
The equilibration period for the preparations was 30–40 min. The concentration of 4-aminopyridine (4-AP, 10–50 µM in “healthy” atria and 10–200 µM in “remodeled” atria) was increased in a step-wise manner (in ≥ 20 min after the start of each concentration). APs were recorded from the endocardial crista terminalis (CT) and pectinate muscle (PM). Arrhythmogenicity was tested using programmed electrical stimulation (PES) protocols at a CL of 500 ms (a single premature beat with a twice-threshold stimulus intensity) at three locations (two in PM and one in CT) under baseline conditions and in the presence of each concentration of 4-AP. In some experiments involving ACh, persistent AF was induced by a rapid pacing protocol (CL = 100–50 ms for 3–5 seconds).
Drugs
4-AP, ACh, and ISO (all SIGMA, MO) were dissolved in distilled water and prepared fresh as a stock of 1–100 mM before each experiment.
Statistics
Statistical analysis was performed using paired or unpaired t test and one way repeated measures or multiple comparison analysis of variance (ANOVA) followed by Bonferroni’s test, as appropriate. All data are expressed as mean ± SD.
RESULTS
In “healthy” coronary-perfused right atrial preparations, block of IKur with low concentrations of 4-AP significantly elevated the plateau level and prolonged the early repolarization phase (APD20 from 5±3 to 42±19 ms, p<0.001; in CT; 25 µM; n=10), but abbreviated APD90 and ERP (Fig. 1). The magnitude of phase 1 of the action potential was reduced from 30±6 mV in control to 22±5, 17±4, and 8±3 mV in the presence of 10, 25, 50 µM of 4-AP, respectively (all p<0.05 vs. control; CT, n=12 for each; CL = 500 ms; Fig. 1). Plateau voltage (dome) was significantly elevated from −4±4 mV in control to +3±5, +10±3, and +15±6 mV after 10, 25, and 50 µM of 4-AP, respectively (all p<0.05 vs. control; CT, n=12 for each; CL = 500 ms).
Figure 1.
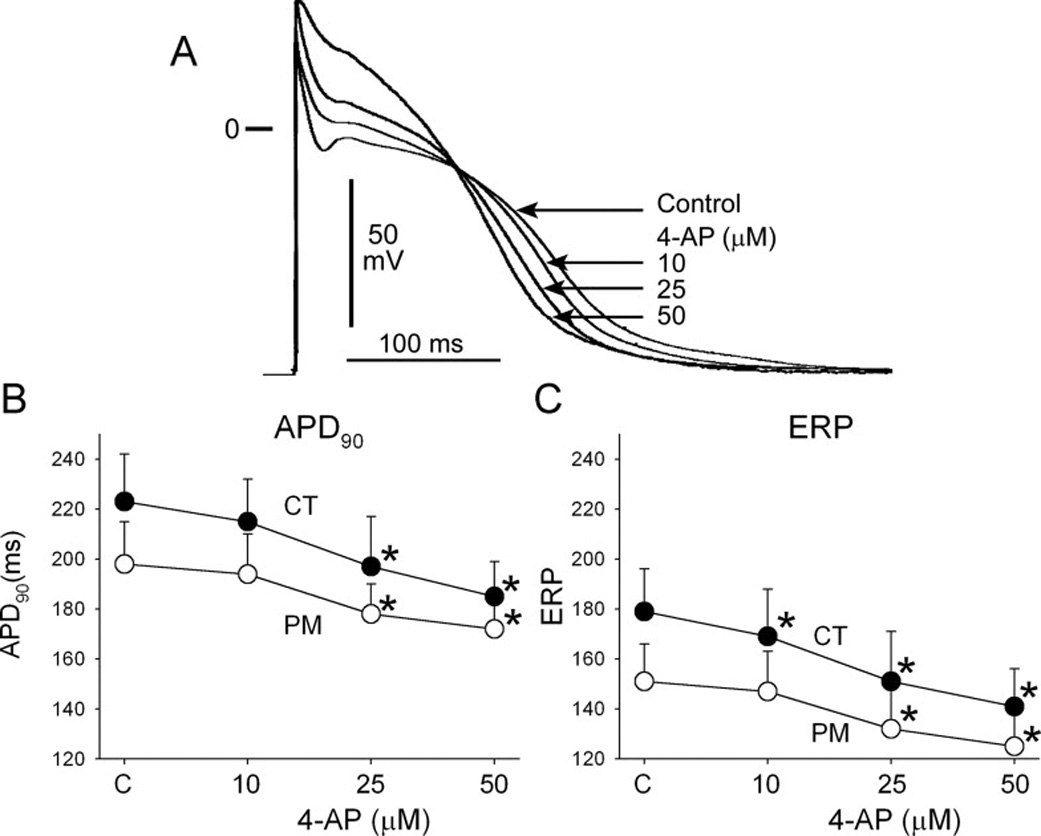
Inhibition of IKur using low concentrations of 4-aminopyridne (4-AP) abbreviates APD90 and ERP in “healthy” atrial preparations. Shown are superimposed action potentials recorded from the crista terminalis (CT) under control conditions (C) and after the addition of 4-AP (A). Graphs plot summary data of APD90 (B) and ERP (C) recorded from CT and pectinate muscle (PM) sites as a function of 4-AP concentration. CL = 500 ms. n=7–12. * − p<0.05 vs. respective control. Note that atrial ERP corresponds to the level of APD75.
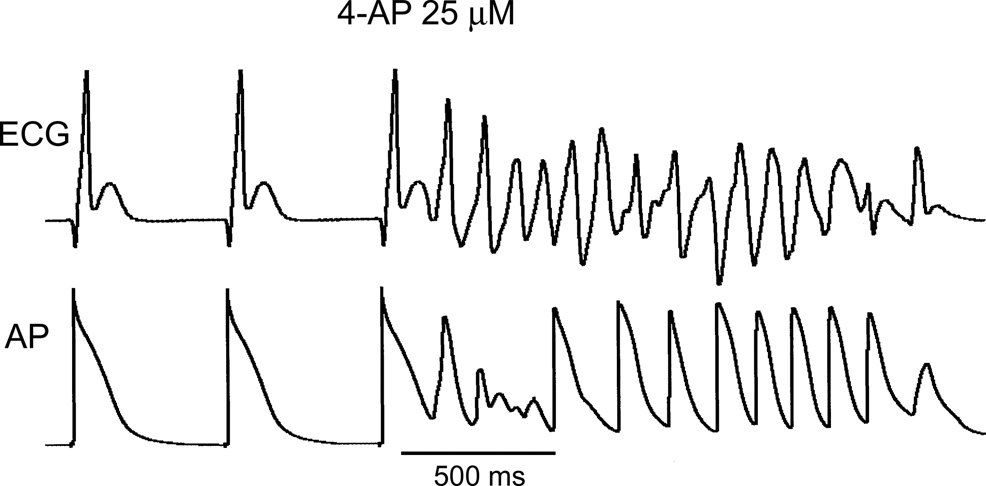
Under baseline conditions, 2 out of 14 “healthy” atria developed short AF (<1 sec) in response to PES. These two preparations were removed from further study. In the presence of low concentrations of 4-AP (10–50 µM), AF could be induced in up to 33% of “healthy” atrial preparations, which did not develop AF in the absence of 4-AP (Fig. 2 and Table I). All episodes of AF were non-sustained (≤3 sec). Spontaneous arrhythmias were not observed.
Figure 2.
Non-sustained AF induced by a single premature beat (S1–S2 = 115 ms) in the presence of 25 µM 4-AP in a “healthy” atrial preparation.
Table I.
Incidence of PES-induced non-sustained AF in “healthy” atrial preparations in the presence of low concentration of 4-AP.
| Control | 4-AP 10 µM | 4-AP 25 µM | 4-AP 50 µM |
|---|---|---|---|
| 0/12 (0%)* | 1/12 (8%) | 3/12 (25%) | 4/12 (33%) |
PES induced non-sustained arrhythmias (≤ 1 sec) in 2 out of 14 atrial preparations under control conditions. These two preparations were excluded from further study.
In contrast to “healthy” atria, block of IKur using 10 and 25 µM 4-AP produced little to no change in APD90 or ERP in “remodeled” atria (Fig. 3). Higher concentrations of 4-AP (50 and 100 µM) prolonged APD90 and ERP in the “remodeled” atrial preparations (Fig. 4).
Figure 3.
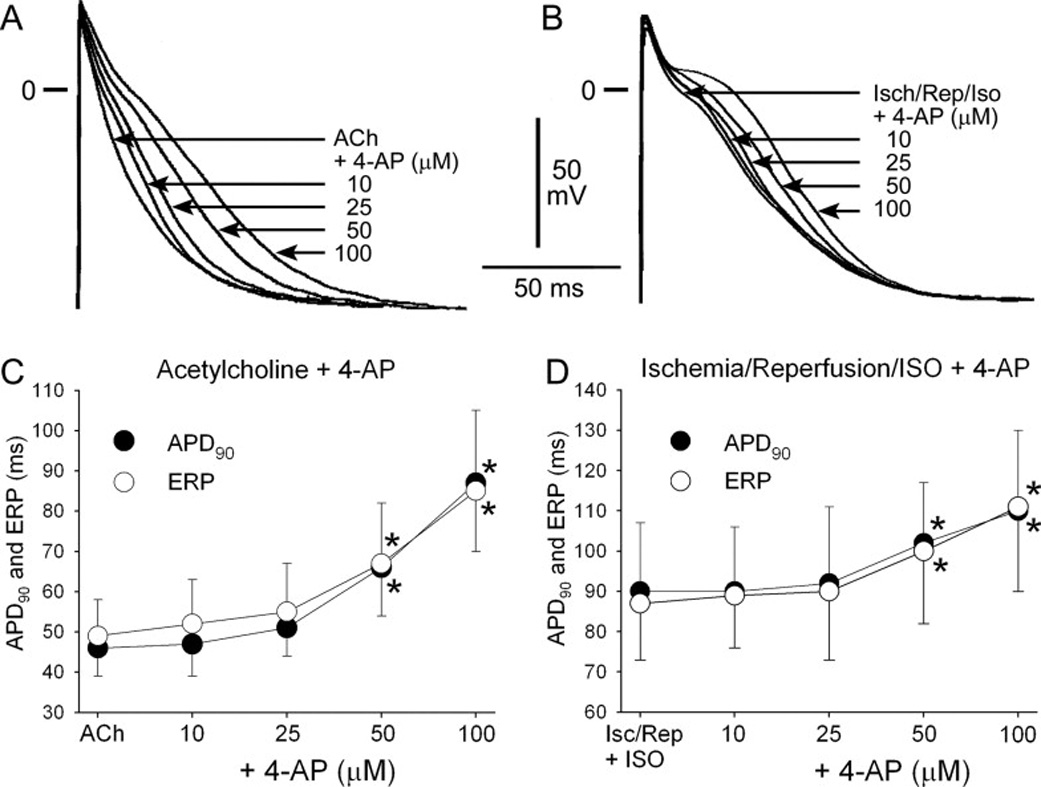
Effects of 4-AP on APD90 and ERP in “acutely remodeled” atrial preparations. Shown are superimposed action potentials obtained from atria pre-treated with acetylcholine (ACh, A) and from atria exposed to ischemia and reperfusion + isoproterenol (Isch/Rep/Iso, B) before and after addition of 4-AP. C–D: Summary APD90 and ERP data from pectinate muscle. n=7–12. CL = 500 ms. * − p<0.05 vs. respective control.
Figure 4.
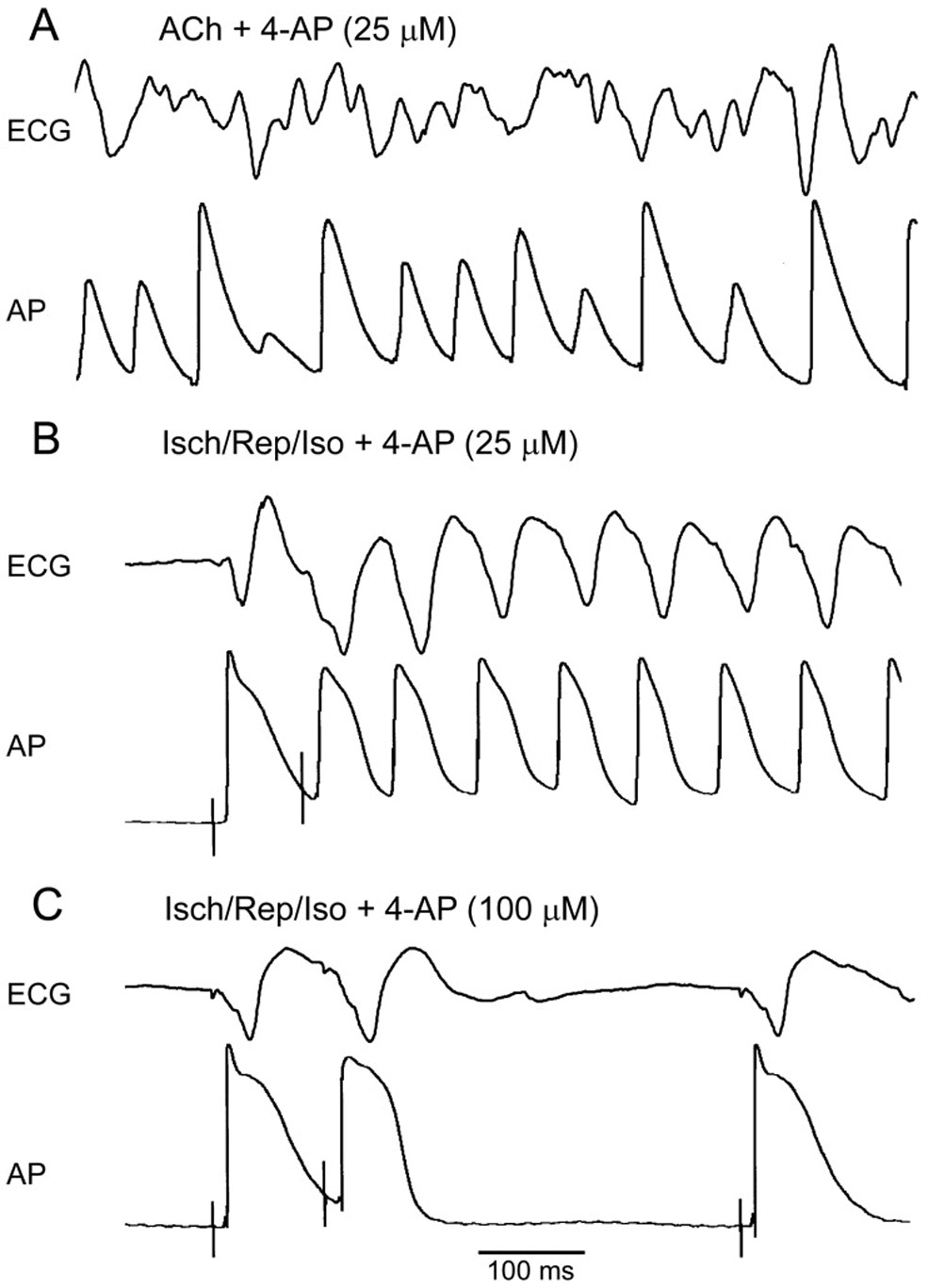
Concentrations of 4-AP that selectively block IKur fail to prevent or terminate atrial fibrillation in “remodeled” atria. Shown are simultaneously recorded ECG and AP traces. A: 25 µM 4-AP fails to terminate persistent AF induced in the presence of acetylcholine (ACh, 0.5 µM). B: 25 µM 4-AP fails to prevent the induction of non-sustained AF by a single premature beat (S1–S2 = 85 ms, which is ERP) in ischemia/reperfusion+isoproterenol AF model (Isch/Rep/Iso). C: An increase of 4-AP concentration to 100 µM prolongs ERP and prevents the initiation of the arrhythmia by premature stimulation (S1–S2 = 110 ms, which is ERP).
4-AP at concentrations of 10–25 µM was not able to prevent or terminate AF and at a concentration of 50 µM exerted only mild antiarrhythmic actions in both experimental AF models used in our study (Table II and III). Higher concentrations of 4-AP (100–200 µM) were more effective in preventing AF initiation and termination of persistent AF, but still showed limited antiarrhythmic efficacy in our experimental AF models (Table II and III).
Table II.
Incidence of acetylcholine (ACh) -mediated AF and the ability of 4-AP to prevent the initiation of AF or terminate persistent AF in atrial preparations.
| Ach (0.5 µM) | ACh + 4-AP (10–25 µM) | ACh + 4-AP (50 µM) | ACh + 4-AP (100–200 µM) | |
|---|---|---|---|---|
| Incidence of AF initiation | 100% (10/10) | 100% (4/4) | 75% (3/4) | 50% (3/6) |
| Termination of persistent AF | 0% (0/10) | 0% (0/4) | 0% (0/5) | 33% (2/6) |
Experiments to prevent AF and terminate AF were performed in different preparations. Incidence of AF initiation: 4-AP was added to ACh-containing Tyrode’s solution and AF induction was attempted using PES. Termination of persistent AF: Persistent AF was induced in the exposure to Ach and 4-AP was added 5–7 min after the initiation of AF.
Table III.
Incidence of PES-induced non-sustained AF with and without 4-AP in ischemia/reperfusion + isoproterenol model of AF in atrial preparations.
| Ischemia/Reperfusion + isoproterenol | + 4-AP (10–25 µM) | + 4-AP (50 µM) | + 4-AP (100–200 µM) |
|---|---|---|---|
| 100% (5/5) | 100% (5/5) | 80% (4/5) | 40% (2/5) |
DISCUSSION
The main findings of our study are that block of IKur with low concentrations of 4-AP abbreviates APD90 and ERP and promotes AF in “healthy” canine coronary-perfused atria.
Ion channel specificity of 4-AP
Within the range of concentrations used in the present study to block IKur (10–50 µM), 4-AP is thought to exert little or no inhibition of Ito and not to affect other currents.2, 3 When measured in isolated atrial myocytes, 4-AP blocks IKur and Ito with an IC50 of 5 and 471 µM in dogs (potency ratio = 94),2 and 8 and 1000 µM in human (potency ratio = 125).3 Apparently, all IKur blockers inhibit Ito. The Ito/IKur blocking potency ratios for AVE0118, AVE1231, DPO-1, AZD7009, RSD1235, and NIP-141 are 3.1, 1.6, 8.0, 0.9, 2.3, and 3.1, respectively (data in most cases obtained from heterologous expression systems such as CHO or HEK cells).12–17 Apart from Ito, most of these IKur blockers also inhibit IK(ACh), and/or IKr, and/or INa with affinities comparable to that of IKur block. Thus, 4-AP at low concentrations is a more selective IKur blocker than the above-listed agents.
In mathematical models of canine or human “healthy” atrial action potential, 80–90% percent block of IKur results in about 20–25% reduction of phase 1 magnitude.6, 18, 19 The large reduction of the phase 1magnitude induced by 50 µM of 4-AP (by 71%; see Fig. 1) suggests that in the coronary-perfused canine atria 50 µM 4-AP may also block a substantial amount of Ito. A relatively selective IKur inhibition appears to be achieved with ≤25 µM of 4-AP (which causes a 43% reduction in phase 1magnitude). It is noteworthy that the pro-arrhythmic potency of 25 and 50 µM 4-AP was comparable (Table I).
Atrial-selective approaches for management of AF
A number of antiarrhythmic agents have been shown to be effective in terminating and/or preventing AF in the clinic. Most of these agents have as a primary action the ability to reduce INa (e.g., propafenone and flecainide) or IKr (e.g., dofetilide) or to block multiple ion channels (e.g., amiodarone). An important limitation of these antiarrhythmic agents is their potential ventricular pro-arrhythmic actions and/or organ toxicity at therapeutically effective doses.20, 21
As a consequence, much of the focus in recent years has been on development of atrial-selective drugs that take advantage of the presence of IKur channels in atrial but not in ventricular cells.1–3 A number of studies have demonstrated that agents capable of blocking IKur (AVE0118, AVE1231, S9947, S20951, ISQ1, DPO-1, RSD1235; AZD7009; NIP141, NIP-142) selectively prolongs atrial ERP both in electrically-remodeled and non-remodeled atria in vivo and in vitro.13, 22–24 17, 25–27
It is well appreciated that the effect of IKur inhibition on APD70–90 is dependent on the baseline AP morphology.6 IKur block has been reported to prolong APD70–90 in atrial cells displaying a triangular AP morphology (typical for remodeled atria) and abbreviate APD70–90 in atrial cells having a plateau AP morphology (typical for non-remodeled atria)6 (as observed in the current study as well). Such APD90 abbreviation is explained by elevation of AP plateau voltage (see fig. 1), which leads to augmentation of IKr and IKs.5, 6 IKur block-induced ERP prolongation in electrically-remodeled atria is consistent with APD70–90 prolongation. On the other hand, IKur block-induced ERP prolongation in non-remodeled atria is not readily explained by APD70–90 abbreviation,5, 6 and may be attributable to concomitant block of sodium channel current. INa blockers are known for their ability to prolong ERP without prolonging APD. This effect of INa is due to the development of post-repolarization refractoriness (PRR), secondary to the depression of excitability.10, 28 Atrial-selective ERP prolongation attributable to sodium channel blockade, has been reported secondary to development of PRR preferentially in atria.10, 29
Interestingly, atrial selective agents AZD7009 and RSD1235 (or vernakalant) have been shown to potently block INa, along with IKur, Ito, and IKr.16, 25, 26, 30 The atrial selectivity of these agents to prolong ERP is largely attributed to their ability to block IKur. However, AZD7009 also depresses sodium channel-dependent parameters (conduction velocity and diastolic threshold of excitation) in an atrial selective manner.26 AZD7009 prolongs atrial ERP much more than APD90, leading to capture failure; due to development of PRR.30 These observations suggest that atrial-selective or predominant sodium channel block may contribute prominently to the atrial-selective ERP prolongation of AZD7009. ISQ-1 may also block INa, since this agent slows down conduction velocity in atria in vivo.31 Note that with the exception of AZD7009,26 comparison of the effects of IKur blockers on INa or sodium channel-dependent parameters in atria and ventricles has not been conducted.
There is an apparent contradiction between our data and those of others6, 32 showing that 4-AP abbreviates atrial APD90 in healthy atrial preparations in vitro with data reported by Nattel et al33 demonstrating that 4-AP at a plasma concentration range of 25–50 µM significantly increases ERP in healthy canine atria in vivo. This discrepancy may be due to the fact that Nattel et al33 recorded ERP at relatively rapid pacing rates (300–400 ms CLs), at which AP shape tends to become triangular. Under these conditions, 4-AP may prolong late repolarization and thus ERP. The in vivo vs. in vitro differences may be due to the effect of 4-AP on autonomic tone in vivo.34
Inhibition of IKur promotes non-sustained AF in healthy atria
Most experimental models of AF, as well as clinical cases of AF, are associated with an abbreviation of atrial repolarization.7, 8, 35 It is therefore no surprise that abbreviation of APD90 and ERP by 4-AP in “healthy” atria promoted AF in our study. The extent of APD and ERP abbreviation induced by 4-AP is much smaller that that induced by ACh or electrical remodeling. This may account for the relatively low incidence of AF and its brief duration in our experiments. Our findings relative to the ability of IKur inhibition to promote AF are consistent with a recent report demonstrating an association of KCNA5 loss-of-function mutations with familial AF.4
Can IKur block alone suppress AF?
At concentrations that terminate AF, IKur blockers relatively potently inhibit Ito and/or IKACh (e.g., AVE0118 or NIP-142)22, 27 and/or INa (e.g., AZD7009 and vernakalant).16, 26, 36 Our experimental findings suggest that “pure” IKur block may be incapable of effectively suppressing AF. Note the IC50 of 4-AP block of atrial Ito is one third of that of ventricular Ito.3, 33 If it is the case with the other IKur/Ito blockers, then Ito block may importantly contribute to the atrial-selectivity of IKur blockers.
A possible reason for the failure of “pure” IKur block to terminate AF in our experimental models may be the fact that IKur density is reduced at rapid activation rates,37, 38 so that at rates encountered during AF IKur may already be greatly reduced without much left to block.
It is commonly believed that anti-AF actions of potassium channel blockers are mediated through the prolongation of APD90/ERP. A recent mathematical modeling study suggests that IKur and/or Ito blockade may terminate AF by prolonging APD at the level of the plateau, but not the terminal phase of the action potential, leading to random tip meander and wavebreak, resulting in rotor termination.39
Study limitations
Our experiments were performed in isolated atria coronary-perfused with Tyrode’s solution. The presence of autonomic influences and other factors present in vivo may modulate the effect of IKur inhibition, resulting in outcomes different from those observed in the present study. IKur is known to be modulated by β-and α-adrenergic sympathetic activity.40 IKur blockers, including 4-AP, are known to increase atrial contractility (i.e., intracellular calcium activity), apparently secondary to elevation of plateau voltages and augmentation of ICa and reverse mode of sodium-calcium exchange.41 It is not known whether these influences secondary to IKur block, or possibly 4-AP directly, contribute to the arrhythmogenesis observed. If due to IKur block, this effect should be relevant to all IKur blockers.
Our results obtained in “acutely remodeled” atrial preparations, lacking a number of principal electrical (ICa,L, Ito, IK1, etc) and structural changes observed in chronically remodeled atria,35 should be interpreted with due caution. In that there are important inter-atrial electrophysiological differences, the data obtained from the right atrium in the present study may not be directly applicable to the left atrium.
CONCLUSIONS
Our findings suggest that inhibition of IKur can create the substrate for development of AF in “healthy” canine atria, via an abbreviation of ERP. These observations advance our understanding of why KCNA5 loss-of-function mutations are associated with the development of AF.4
ACKNOWLEDGMENTS
We gratefully acknowledge the expert technical assistance of Judy Hefferon, Robert Goodrow, and Kathy Sullivan.
Supported by grant HL47678 from NHLBI (CA) and grants from Eighth Manhattan Masonic District and the Masons of NYS and Florida.
ABBREVIATIONS LIST
- AF
atrial fibrillation
- AP
transmembrane action potential
- APD
action potential duration
- ERP
effective refractory period
- IKur
ultra-rapid delayed rectified potassium current
- Ito
transient outward potassium current
- PES
programmed electrical stimulation
- PRR
post-repolarization refractoriness
- RMP
resting membrane potential
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Wang ZG, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes: Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73:1061–1076. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 2.Yue L, Feng J, Li GR, et al. Characterization of an ultrarapid delayed rectifier potassium channel involved in canine atrial repolarization. J Physiol. 1996;496(Pt 3):647–662. doi: 10.1113/jphysiol.1996.sp021716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amos GJ, Wettwer E, Metzger F, et al. Differences between outward currents of human atrial and subepicardial ventricular myocytes. J Physiol. 1996;491(Pt 1):31–50. doi: 10.1113/jphysiol.1996.sp021194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson TM, Alekseev AE, Liu XK, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 5.Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially-perfused atrium: effect of IKr and Ito/IKur block. Am J Physiol. 2004;286:H2393–H2400. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- 6.Wettwer E, Hala O, Christ T, et al. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 7.Burashnikov A, Antzelevitch C. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation. 2003;107:2355–2360. doi: 10.1161/01.CIR.0000065578.00869.7C. [DOI] [PubMed] [Google Scholar]
- 8.Zipes DP, Mihalick MJ, Robbins GT. Effects of selective vagal and stellate ganglion stimulation of atrial refractoriness. Cardiovasc Res. 1974;8:647–655. doi: 10.1093/cvr/8.5.647. [DOI] [PubMed] [Google Scholar]
- 9.Burashnikov A, Antzelevitch C. Beta-adrenergic stimulation is highly arrhythmogenic following ischemia/reperfusion injury in the isolated canine right atrium. Heart Rhythm. 2005;2:S179. (Abstract) [Google Scholar]
- 10.Burashnikov A, Di Diego JM, Zygmunt AC, et al. Atrium-selective sodium channel block as a strategy for suppression of atrial fibrillation: differences in sodium channel inactivation between atria and ventricles and the role of ranolazine. Circulation. 2007;116:1449–1457. doi: 10.1161/CIRCULATIONAHA.107.704890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 12.Gogelein H, Brendel J, Steinmeyer K, et al. Effects of the atrial antiarrhythmic drug AVE0118 on cardiac ion channels. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:183–192. doi: 10.1007/s00210-004-0957-y. [DOI] [PubMed] [Google Scholar]
- 13.Wirth KJ, Brendel J, Steinmeyer K, et al. In vitro and in vivo effects of the atrial selective antiarrhythmic compound AVE1231. J Cardiovasc Pharmacol. 2007;49:197–206. doi: 10.1097/FJC.0b013e318032002f. [DOI] [PubMed] [Google Scholar]
- 14.Lagrutta A, Wang J, Fermini B, et al. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 2006;317:1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- 15.Persson F, Carlsson L, Duker G, et al. Blocking characteristics of hERG, hNav1.5, and hKvLQT1/hminK after administration of the novel anti-arrhythmic compound AZD7009. J Cardiovasc Electrophysiol. 2005;16:329–341. doi: 10.1046/j.1540-8167.2005.40427.x. [DOI] [PubMed] [Google Scholar]
- 16.Fedida D, Orth PM, Chen JY, et al. The mechanism of atrial antiarrhythmic action of RSD1235. J Cardiovasc Electrophysiol. 2005;16:1227–1238. doi: 10.1111/j.1540-8167.2005.50028.x. [DOI] [PubMed] [Google Scholar]
- 17.Seki A, Hagiwara N, Kasanuki H. Effects of NIP-141 on K currents in human atrial myocytes. J Cardiovasc Pharmacol. 2002;39:29–38. doi: 10.1097/00005344-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Courtemanche M, Ramirez RJ, Nattel S. Ionic targets for drug therapy and atrial fibrillation-induced electrical remodeling: insights from a mathematical model. Cardiovasc Res. 1999;42:477–489. doi: 10.1016/s0008-6363(99)00034-6. [DOI] [PubMed] [Google Scholar]
- 19.Ramirez RJ, Nattel S, Courtemanche M. Mathematical analysis of canine atrial action potentials: rate, regional factors, and electrical remodeling. Am J Physiol Heart Circ Physiol. 2000;279:H1767–H1785. doi: 10.1152/ajpheart.2000.279.4.H1767. [DOI] [PubMed] [Google Scholar]
- 20.CAST Investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 21.Antzelevitch C, Belardinelli L, Wu L, et al. Electrophysiologic properties and antiarrhythmic actions of a novel anti-anginal agent. J Cardiovasc Pharmacol Therapeut. 2004;9 Suppl 1:S65–S83. doi: 10.1177/107424840400900106. [DOI] [PubMed] [Google Scholar]
- 22.Blaauw Y, Gogelein H, Tieleman RG, et al. "Early" class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–1724. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 23.Knobloch K, Brendel J, Rosenstein B, et al. Atrial-selective antiarrhythmic actions of novel Ikur vs. Ikr, Iks, and IKAch class Ic drugs and beta blockers in pigs. Med Sci Monit. 2004;10:BR221–BR228. [PubMed] [Google Scholar]
- 24.Regan CP, Stump GL, Wallace AA, et al. In vivo cardiac electrophysiologic and antiarrhythmic effects of an isoquinoline IKur blocker, ISQ-1, in rat, dog, and nonhuman primate. J Cardiovasc Pharmacol. 2007;49:236–245. doi: 10.1097/FJC.0b013e3180325b2a. [DOI] [PubMed] [Google Scholar]
- 25.Dorian P, Pinter A, Mangat I, et al. The effect of vernakalant (RSD1235), an investigational antiarrhythmic agent, on atrial electrophysiology in humans. J Cardiovasc Pharmacol. 2007;50:35–40. doi: 10.1097/FJC.0b013e3180547553. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein RN, Khrestian C, Carlsson L, et al. Azd7009: a new antiarrhythmic drug with predominant effects on the atria effectively terminates and prevents reinduction of atrial fibrillation and flutter in the sterile pericarditis model. J Cardiovasc Electrophysiol. 2004;15:1444–1450. doi: 10.1046/j.1540-8167.2004.04354.x. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda T, Takeda K, Ito M, et al. Atria selective prolongation by NIP-142, an antiarrhythmic agent, of refractory period and action potential duration in guinea pig myocardium. J Pharmacol Sci. 2005;98:33–40. doi: 10.1254/jphs.fpj04045x. [DOI] [PubMed] [Google Scholar]
- 28.Campbell TJ. Kinetics of onset of rate-dependent effects of Class I antiarrhythmic drugs are important in determining their effects on refractoriness in guinea-pig ventricle, and provide a theoretical basis for their subclassification. Cardiovasc Res. 1983;17:344–352. doi: 10.1093/cvr/17.6.344. [DOI] [PubMed] [Google Scholar]
- 29.Burashnikov A, Antzelevitch C. Atrial-selective sodium channel blockers. Do they exist? J Cardiovasc Pharmacol. 2008 doi: 10.1097/FJC.0b013e31817618eb. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson L, Chartier D, Nattel S. Characterization of the in vivo and in vitro electrophysiological effects of the novel antiarrhythmic agent AZD7009 in atrial and ventricular tissue of the dog. J Cardiovasc Pharmacol. 2006;47:123–132. doi: 10.1097/01.fjc.0000196242.04384.c3. [DOI] [PubMed] [Google Scholar]
- 31.Regan CP, Kiss L, Stump GL, et al. Atrial antifibrillatory effects of structurally distinct IKur blockers 3-[(dimethylamino)methyl]-6-methoxy-2-methyl-4-phenylisoquinolin-1(2H)-one and 2-phenyl-1,1-dipyridin-3-yl-2-pyrrolidin-1-yl-ethanol in dogs with underlying heart failure. J Pharmacol Exp Ther. 2008;324:322–330. doi: 10.1124/jpet.107.127654. [DOI] [PubMed] [Google Scholar]
- 32.Shibata EF, Drury T, Refsum H, et al. Contribution of a transient outward current to repolarization in human atrium. Am J Physiol. 1989;257:H1773–H1781. doi: 10.1152/ajpheart.1989.257.6.H1773. [DOI] [PubMed] [Google Scholar]
- 33.Nattel S, Matthews C, De Blasio E, et al. Dose-dependence of 4-aminopyridine plasma concentrations and electrophysiological effects in dogs : potential relevance to ionic mechanisms in vivo. Circulation. 2000;101:1179–1184. doi: 10.1161/01.cir.101.10.1179. [DOI] [PubMed] [Google Scholar]
- 34.Bowman WC, Marshall RJ, Rodger IW, et al. Actions of 4-aminopyridine on the cardiovascular systems of anaesthetized cats and dogs. Br J Anaesth. 1981;53:555–565. doi: 10.1093/bja/53.6.555. [DOI] [PubMed] [Google Scholar]
- 35.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415:219–226. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 36.Lofberg L, Jacobson I, Carlssonq L. Electrophysiological and antiarrhythmic effects of the novel antiarrhythmic agent AZD7009: a comparison with azimilide and AVE0118 in the acutely dilated right atrium of the rabbit in vitro. Europace. 2006;8:549–557. doi: 10.1093/europace/eul061. [DOI] [PubMed] [Google Scholar]
- 37.Feng J, Xu D, Wang Z, et al. Ultrarapid delayed rectifier current inactivation in human atrial myocytes: properties and consequences. Am J Physiol. 1998;275:H1717–H1725. doi: 10.1152/ajpheart.1998.275.5.H1717. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich JR, Ocholla H, Ziemek D, et al. Characterization of human cardiac Kv1.5 inhibition by the novel atrial-selective antiarrhythmic compound AVE1231. J Cardiovasc Pharmacol. 2008;51:380–387. doi: 10.1097/FJC.0b013e3181669030. [DOI] [PubMed] [Google Scholar]
- 39.Pandit SV, Berenfeld O, Anumonwo JM, et al. Ionic determinants of functional reentry in a 2-D model of human atrial cells during simulated chronic atrial fibrillation. Biophys J. 2005;88:3806–3821. doi: 10.1529/biophysj.105.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue L, Feng J, Wang Z, et al. Adrenergic control of the ultrarapid delayed rectifier current in canine atrial myocytes. J Physiol. 1999;516(Pt 2):385–398. doi: 10.1111/j.1469-7793.1999.0385v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schotten U, de HS, Verheule S, et al. Blockade of atrial-specific K+-currents increases atrial but not ventricular contractility by enhancing reverse mode Na+/Ca2+-exchange. Cardiovasc Res. 2007;73:37–47. doi: 10.1016/j.cardiores.2006.11.024. [DOI] [PubMed] [Google Scholar]