Abstract
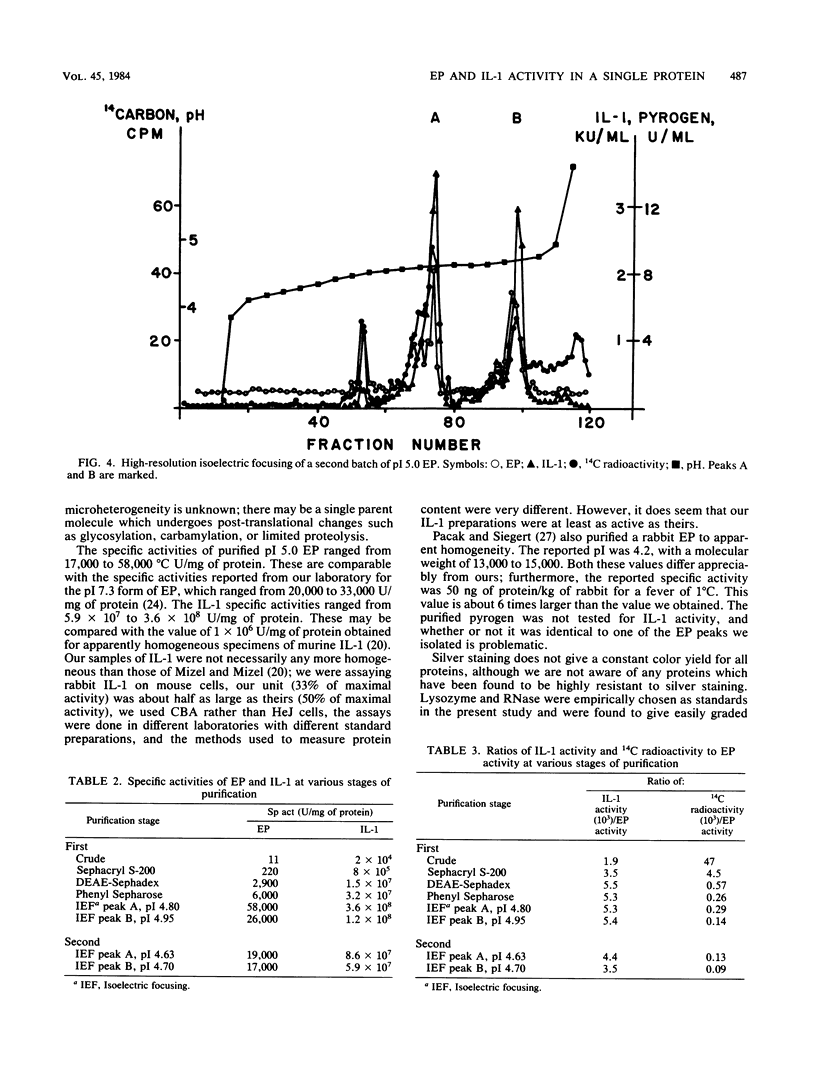
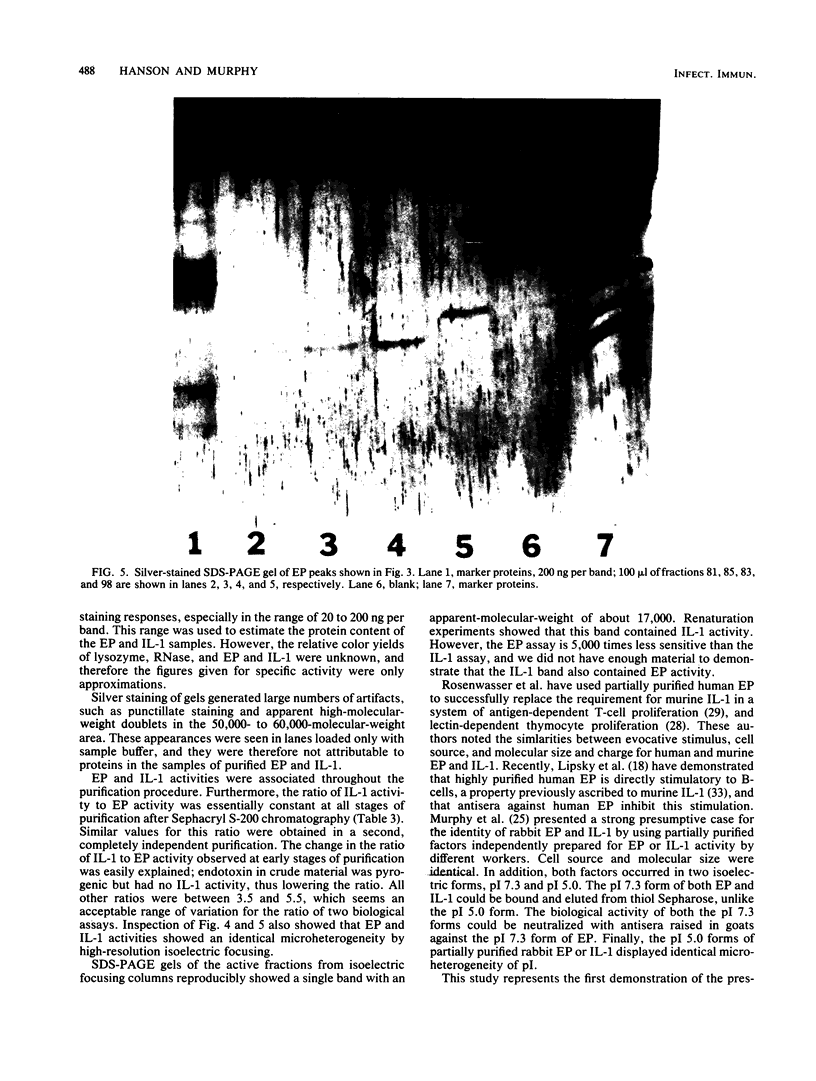
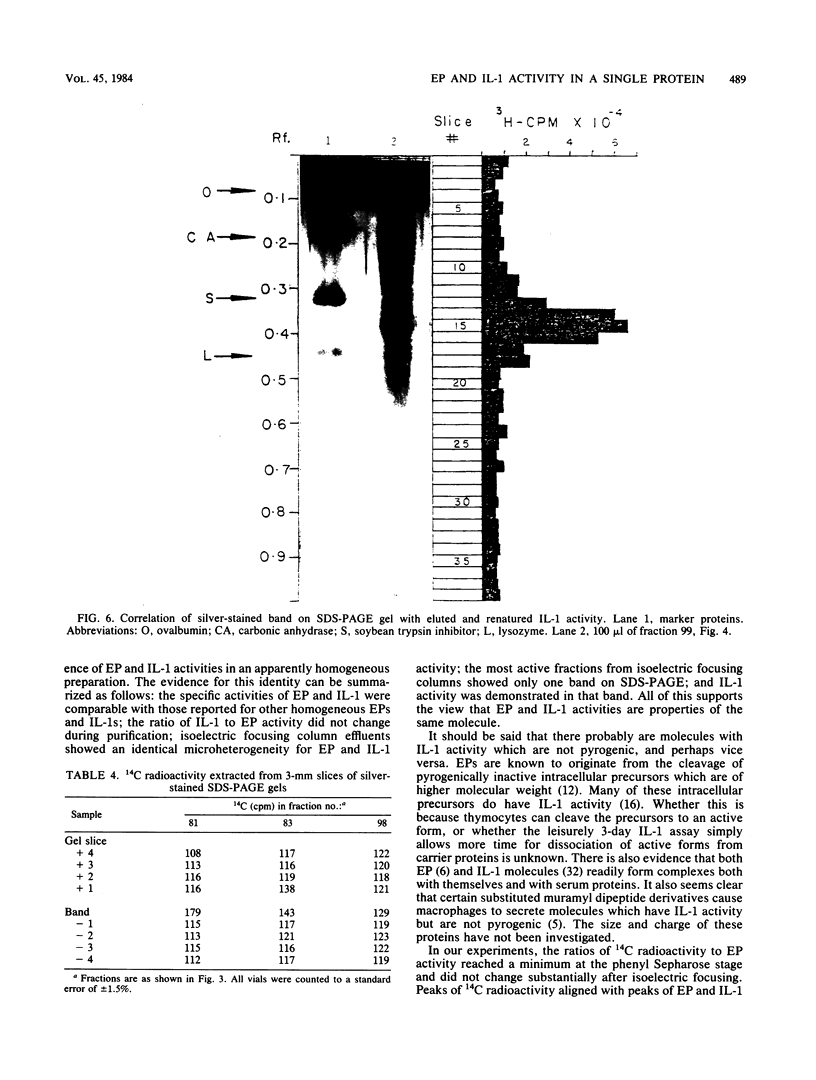
Rabbit mononuclear cells from oil-induced peritoneal exudates were purified by centrifugation on Percoll gradients, suspended in tissue culture medium, and stimulated with opsonized Staphylococcus epidermidis. The supernatants from these macrophages caused fever when injected intravenously into rabbits (endogenous pyrogen [EP] activity). The EP activity was contained in two protein fractions, with pIs of 7.3 and ca. 5.0. The same fractions caused mouse thymocytes to incorporate tritiated thymidine when incubated in vitro with small quantities of phytohemagglutinin (interleukin 1 [IL-1] activity). The pI 5.0 form of EP was purified to apparent homogeneity by sequential use of ammonium sulfate precipitation, gel filtration, ion-exchange chromatography, hydrophobic chromatography, and high-resolution isoelectric focusing. EP and IL-1 activities were not separable by any of these procedures. Active fractions from isoelectric focusing were analyzed by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. Only one band was visible as judged by a silver staining method, and IL-1 activity could be recovered by renaturing eluates from the same region of sodium dodecyl sulfate gels run in parallel. An estimate of specific activity was made by comparing the intensity of stained bands of EP with the intensity of bands containing known quantities of lysozyme or RNase. By this criterion, the specific activity of purified pI 5 EP was between 17,000 and 58,000 degrees C U/mg of protein, and the specific activity in terms of IL-1 was between 59 million and 360 million U per mg of protein. These observations suggest that both EP and IL-1 activities can be expressed by a single molecular species. The implications of this coincidence are discussed. It was also shown that highly purified pI 5 EP obtained from macrophages stimulated in the presence of 14C-labeled amino acids contained significant 14C radioactivity. This suggests that the pI 5.0 EP, like the pI 7.3 form, was synthesized de novo from amino acid precursors.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cebula T. A., Hanson D. F., Moore D. M., Murphy P. A. Synthesis of four endogenous pyrogens by rabbit macrophages. J Lab Clin Med. 1979 Jul;94(1):95–105. [PubMed] [Google Scholar]
- Damais C., Riveau G., Parant M., Gerota J., Chedid L. Production of lymphocyte activating factor in the absence of endogenous pyrogen by rabbit or human leukocytes stimulated by a muramyl dipeptide derivative. Int J Immunopharmacol. 1982;4(5):451–462. doi: 10.1016/0192-0561(82)90020-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Renfer L., Wolff S. M. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar J. J., Benjamin W. R., Hilfiker M. L., Howard M., Farrar W. L., Fuller-Farrar J. The biochemistry, biology, and role of interleukin 2 in the induction of cytotoxic T cell and antibody-forming B cell responses. Immunol Rev. 1982;63:129–166. doi: 10.1111/j.1600-065x.1982.tb00414.x. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Mizel S. B., Farrar J. J. Participation of lymphocyte activating factor (Interleukin 1) in the induction of cytotoxic T cell responses. J Immunol. 1980 Mar;124(3):1371–1377. [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hahn H. H., Cheuk S. F., Elfenbein S., Wood W. B., Jr Studies on the pathogenesis of fever. XIX. Localization of pyrogen in granulocytes. J Exp Med. 1970 Apr 1;131(4):701–709. doi: 10.1084/jem.131.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson D. F., Murphy P. A., Windle B. E. Failure of rabbit neutrophils to secrete endogenous pyrogen when stimulated with staphylococci. J Exp Med. 1980 Jun 1;151(6):1360–1371. doi: 10.1084/jem.151.6.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman W. J., Farrar J. J., Fuller-Bonar J. Evidence for the identification of lymphocyte activating factor as the adherent cell-derived mediator responsible for enhanced antibody synthesis by nude mouse spleen cells. Cell Immunol. 1978 Jan;35(1):92–98. doi: 10.1016/0008-8749(78)90129-6. [DOI] [PubMed] [Google Scholar]
- Lachman L. B., Metzgar R. S. Characterization of high and low molecular weight lymphocyte-activating factor (interleukin I) from P388D and J774.1 mouse macrophage cell lines. J Reticuloendothel Soc. 1980 Jun;27(6):621–629. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipsky P. E., Thompson P. A., Rosenwasser L. J., Dinarello C. A. The role of interleukin 1 in human B cell activation: inhibition of B cell proliferation and the generation of immunoglobulin-secreting cells by an antibody against human leukocytic pyrogen. J Immunol. 1983 Jun;130(6):2708–2714. [PubMed] [Google Scholar]
- Mizel S. B., Ben-Zvi A. Studies on the role of lymphocyte-activating factor (Interleukin 1) in antigen-induced lymph node lymphocyte proliferation. Cell Immunol. 1980 Sep 1;54(2):382–389. doi: 10.1016/0008-8749(80)90218-x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Mizel D. Purification to apparent homogeneity of murine interleukin 1. J Immunol. 1981 Mar;126(3):834–837. [PubMed] [Google Scholar]
- Moore D. M., Murphy P. A., Chesney P. J., Wood W. B., Jr Synthesis of endogenous pyrogen by rabbit leukocytes. J Exp Med. 1973 May 1;137(5):1263–1274. doi: 10.1084/jem.137.5.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Murphy P. A., Cebula T. A., Levin J., Windle B. E. Rabbit macrophages secrete two biochemically and immunologically distinct endogenous pyrogens. Infect Immun. 1981 Oct;34(1):177–183. doi: 10.1128/iai.34.1.177-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy P. A., Chesney P. J., Wood W. B., Jr Further purification of rabbit leukocyte pyrogen. J Lab Clin Med. 1974 Feb;83(2):310–322. [PubMed] [Google Scholar]
- Murphy P. A., Simon P. L., Willoughby W. F. Endogenous pyrogens made by rabbit peritoneal exudate cells are identical with lymphocyte-activating factors made by rabbit alveolar macrophages. J Immunol. 1980 May;124(5):2498–2501. [PubMed] [Google Scholar]
- Pacák F., Siegert R. Chemical characterization of a rabbit leukocytic pyrogen. Eur J Biochem. 1982 Oct;127(2):375–380. doi: 10.1111/j.1432-1033.1982.tb06882.x. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation. Cell Immunol. 1981 Sep 1;63(1):134–142. doi: 10.1016/0008-8749(81)90034-4. [DOI] [PubMed] [Google Scholar]
- Rosenwasser L. J., Dinarello C. A., Rosenthal A. S. Adherent cell function in murine T-lymphocyte antigen recognition. IV. Enhancement of murine T-cell antigen recognition by human leukocytic pyrogen. J Exp Med. 1979 Sep 19;150(3):709–714. doi: 10.1084/jem.150.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P. L., Willoughby W. F. The role of subcellular factors in pulmonary immune function: physicochemical characterization of two distinct species of lymphocyte-activating factor produced by rabbit alveolar macrophages. J Immunol. 1981 Apr;126(4):1534–1541. [PubMed] [Google Scholar]
- Smith K. A., Lachman L. B., Oppenheim J. J., Favata M. F. The functional relationship of the interleukins. J Exp Med. 1980 Jun 1;151(6):1551–1556. doi: 10.1084/jem.151.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa A., Oppenheim J. J., Mizel S. B. Characterization of lymphocyte-activating factor (LAF) produced by human mononuclear cells: biochemical relationship of high and low molecular weight forms of LAF. J Immunol. 1979 May;122(5):2112–2118. [PubMed] [Google Scholar]