Abstract
We have utilized soluble HIV Gag-specific T-cell receptor (TCR) D3 with low affinity and TCR-like antibody 25-D1.16 recognizing its natural peptide-MHC (pMHC) ligand with high affinity to determine how affinity and off-rate of the receptor-pMHC interactions affect the sensitivity of pMHC detection on the cell surface. We found that with soluble TCR cognate pMHCs can be detected only at relatively high cell surface densities when the TCR was oligomerized using either Strepavidin or quantum dots (QD) scaffolds. While the higher affinity probe led to a greater sensitivity of pMHC detection, monomers and oligomers of the probe showed essentially the same detection limit, which is restricted by the sensitivity of standard flow cytometry technique. We have also shown that imaging of QD/TCR specifically bound to cognate pMHC on the cell surface yielded a very bright fluorescent signal that can enhance the sensitivity of viral peptide detection on infected cells.
Keywords: soluble TCR oligomers, detection of cognate pMHC on the cell surface, quantum dot/TCR conjugates
INTRODUCTION
Effective presentation of virus peptide epitopes that leads to the development of strong and long-lasting virus-specific cytotoxic T lymphocytes (CTL) is necessary and essential to contain the virus. To be effectively presented, viral peptides should be naturally processed in infected cells and displayed at adequate density for a sufficient period of time on the cell surface. This is especially important in the acute phase of infection, since eliciting an earlier strong CTL response may have an impact on anti-virus immunity later in the infection. Therefore, it is important to evaluate how the density and lifetime of cognate peptide-MHC (pMHC) on infected cells can influence peptide immunogenicity. For this purpose, specific reagents that recognize virus peptide epitopes bound to MHC protein on the infected cells are required.
One way to develop a pMHC-specific reagent is to make use of a soluble TCR recognizing the pMHC complex of interest. However, the intrinsic affinity of the TCR-pMHC interaction is usually low (micromolar range) [1; 2] and precludes the detection of cognate pMHC on the cell surface. It has been shown that oligomerization of TCR molecules allows soluble TCR oligomers to interact with several cognate cell surface pMHCs resulting in much more stable interactions [3; 4; 5; 6], similar to the binding of pMHC oligomers to TCR on T cells [7; 8]. In addition, the pMHC detection can be facilitated by increasing intrinsic affinity of a TCR-pMHC interaction using various strategies for in vitro TCR affinity maturation [9; 10; 11; 12]. Moreover, the development of effective approaches for selection of TCR-like antibodies would further advanced the ability to follow presentation of cognate pMHC [13; 14; 15; 16; 17; 18].
Here we compare 2 different strategies to increase the sensitivity of detection of cognate pMHCs. We have found that oligomerization of soluble TCR is effective only at a sufficient pMHC density on the cell surface. At a lower density, the average separating distance between cognate pMHCs becomes larger than the distance between binding sites of the TCR oligomer making the oligomerization inefficient. Fluorescent signal amplification technique enhanced the quality of the analysis but hardly improved the limit of detection. We have also exploited a TCR-like antibody with a significantly higher affinity for the pMHC ligand. While this higher affinity probe exhibited a greater sensitivity of pMHC detection, the monomeric and oligomeric probes showed essentially the same detection limit. At the same time, the oligomer exhibited the same sensitivity in the detection of ligands with higher (strong agonist) and lower (weak agonist) affinity. Thus, the sensitivity of detection appears to be restricted not only by the average separating distance between cell surface cognate pMHC, but also by limitations of standard flow cytometry technique. Finally, we have resorted to quantum dots (QD) conjugated with soluble TCR molecules, i.e. QD/TCR conjugates, and show that imaging of QD/TCRs specifically bound to cognate pMHCs on the cell surface can further advance the sensitivity of detection of peptide epitopes on the cell surface. The latter is an essential parameter for vaccine development and design of new immunotherapeutic interventions.
MATERIAL AND METHODS
Cells and reagents
The EBV-transformed B cell line JY (HLA-A2, B7, Cw7) was grown in supplemented RPMI 1640 medium containing 10% fetal calf serum as described [19]. The mouse thymoma EL4 (H-2b) was kindly provided by Herman Eisen and was grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% inactivated FCS [20].
Streptavidin labeled with Phycoerythrin (PE) was purchased from Invitrogen and Streptavidin conjugated with Horse Radish Peroxidase (HRP) was supplied by Sigma.
HIV Gag-derived peptide SLYNTVATL (SL9) [21], influenza matrix protein peptide GILGFVFTL (GL9) [22; 23], peptide from ovalbumin SIINFEKL (pOV8) [24], vesicular stomatitis virus RGYVYQGL (VSV) [25], as well as pOV8-R4, a variant of pOV8 peptide [26] were synthesized by Research Genetics.
Soluble peptide-MHC complexes
Soluble HLA-A2 was expressed in Drosophila S2 cells and was purified from culture supernatant as previously describe [27; 28]. “Empty” soluble HLA-A2 molecules in PBS were loaded overnight with the peptide of interest at saturating concentration (10-4 M), subjected to gel-filtration on Superdex HR-200 (Pharmacia), and stored in phosphate buffered saline (PBS) with protease inhibitors (1 mM EDTA, 10 μM pepstatin and leupeptin, and 100 μM PMSF) and excess of the peptide (10-4 M) at 4°C.
TCR expression, purification and biotinylation
We have utilized genes encoding TCR α and β from CTL clone D3 specific for the immunodominant HIV-derived peptide SL9; these genes have been previously cloned and used to produce a soluble analog of D3 TCR [27; 29]. We introduced the sequence (GLNDIFEAQKIEWHE), which is receptive to a single biotin molecule, at the C-terminal end of the D3 TCR α-chain upstream of His6 tag. The C-terminus of the TCR β-chain was not modified and was terminated shortly after the interchain disulfide bridge. The modified genes were cloned into a commercially available Drosophila expression vector pMT/V5-His under metallothionine promoter (Invitrogen) and expressed in S2 cells. Soluble TCR was purified essentially as described [29] and was biotinylated using Biotin transferase (BirA) according to the manufacturer’s protocol (Avidity).
TCR tetramer assembly and characterization
To assemble the tetramer we have used Streptavidin labeled with either PE or covalently modified with HRP. The Streptavidin was added drop-wise sequentially (10 portions) to the biotinylated D3 TCR (1.7 mg/ml) in 5 min intervals. The final molar ratio of Streptavidin:D3 TCR in the reaction mixture was 1:4. The formation of oligomeric TCR was confirmed by SDS PAGE.
Expression, purification and biotinilation of 25-D.1.16 antibody Fab fragments and assembly of the Fab tetrameric complex
Genes encoding the heavy and light chains of 25-D1.16 were previously derived [26] from the hybridoma that was kindly provided by Ronald Germain [14]. The light chain (L) gene and the N-terminal half of the heavy chain (Fd) gene were cloned into commercially available pMT/V5-His expression vector (Invitrogen). A biotinylation site was introduced upstream of the His6 at the C-termini of the Fd fragment to be used for detection and purification purposes. Plasmids containing L chain, Fd fragments, and the neomycin–resistance gene were co-transfected into Drosophila S2 cells. Protein expression was controlled by methallothionein promoter [27; 29]. The yield of recombinant Fab was 4 mg per 1L. The identity of purified proteins was confirmed be SDS-PAGE and the specificity was demonstrated in binding experiments with soluble SIINFEKL-Kb (pOV8-Kb) using Biacore 3000 as previously described [26] (data not shown). The detailed procedure for the expression, purification, and characterization of the recombinant 25-D1.16 Fab fragment will be described elsewhere (Mareeva et al., in preparation). 25-D1.16 Fab tetramer was assembled and characterized as described above.
Preparation of DHLA-capped QD
Single-crystal core-shell CdSe/ZnS TOPO (trioctylphosphine oxide)-capped QDs with emission wavelength 520 were purchased from Evident Technologies. To produce water-soluble aggregate-free QDs, we utilized a previously described method [30]. Briefly, 20 mg of the QDs in 2 ml of toluene were precipitated drop wise with 4 ml of methanol under argon stream. After centrifugation (2300 g, 10 min) QDs were dissolved in 200 μl of chloroform and precipitation procedure was repeated. 200 μg of DHLA (Sigma) was added to the QDs and the vial with QDs was stirred and heated at 69°C in an oil bath under argon. After 6 hours incubation, the vial was removed from the bath, and QDs were diluted in 1 ml of absolute dimethylformamide. 600 mg of potassium tert-butoxide was dissolved in 1 ml dimethylformamide and was added dropwise to the QD solution with intensive stirring. After centrifugation, the supernatant was removed and 8 ml of water was added. Soluble QDs were separated from insoluble material by centrifugation and the solution was filtered through a 0.22 μm membrane. QDs were dialyzed against water followed by 10 mM sodium tetraborate buffer, pH 9.3 using Amicon centrifugal filter devices (30,000 MW cut off). Concentration of the final QD(520) solution was measured by absorption at 495 nm. This procedure has proved to yield 2 ml of DHLA-covered aggregate-free QDs(520) with a concentration of 10 μM.
Lipid encapsulated QDs with Ni-NTA functional group
QDs coated with Ni-NTA modified lipids were provided by Evident Technologies. QDs with emission at ~620nm were coated with PEGylated phospholipids and purified following the procedure described by Dubertret et al. [31]. Approximately 25% of PEGylated lipids were covalently functionalized with metal affinity Ni-NTA groups such that these groups were connected with lipid molecules through the PEG spacer.
Assembly of QD/D3 TCR conjugate
QD/D3 TCR conjugate formation was driven by self-assembly between His6-terminated D3 TCR protein and QDs at the desired QD-to-TCR molar ratios in 10 mM sodium tetraborate buffer, 25 mM NaCl, pH 8.0 for 15 minutes at 22-25°C. We have shown that this produces stable solutions of QD/D3 TCR conjugates with the defined protein-to-QD ratio using both DHLA-capped and lipid encapsulated QD.
ELISA assay
The specificity of D3/tetramer, assembled with Streptavidin conjugated with HRP, was examined in ELISA assay. Flat-bottom 96-well plates were covered overnight (4°C) with soluble peptide-HLA-A2 complexes at 10 μg/ml in 50 mM PBS, pH 7.4. The unbound material was removed and the plate was blocked with 1% BSA/PBS for 2 hour at room temperature. D3/tetramer was then added at various concentrations and the plates were incubated 2 hours at room temperature (22-24°C). The plates were washed free of unreacted D3/tetramer, and the amount of bound tetramer was quantified by measuring OD at 490 nm in the presence of o-phenylendiamine. Indicated concentrations refer to the concentration of D3 in the preparation of the tetramer.
Flow cytometry
2×105 JY cells were loaded with SL9 or control peptide GL9 at various concentrations for 1 hour at 37°C. The cell aliquots were incubated with indicated concentrations of PE-conjugated TCR tetramer in 50 μl 1% BSA/PBS during 45 min at room temperature. Cells were washed 3 times with chilled 1% BSA/PBS, fixed and were analyzed on an MCL/XL automated analytical cytometer (Beckman Coulter Inc.).
In some experiment, JY cells loaded with indicated peptide were stained with a QD/D3 TCR conjugates at various concentrations for 30-45 min at room temperatures, washed free of unreacted QD conjugates, and analyzed by flow cytometry as above.
EL4 cells were pulsed with pOV8 or pOV8-R4 or VSV peptides at various concentrations for 1 hour at 37°C. The cells were incubated for 30 min at room temperature (22-24°C) with either 25-D1.16 Fab monomer or 25-D1.16 Fab tetramer at a 40 nM concentration of Fab in both preparations. The cells were washed free of unreacted proteins, and PE-labeled Streptavidin (4 μg/ml) was added to the cells treated with biotinylated Fab monomer. All samples were further incubated for 30 min at 4°C. The cells were washed with chilled 1% BSA/PBS and were stored on ice prior to FACS analysis.
Amplification of TCR tetramer fluorescence signal
2×105 JY cells were loaded with peptides as mentioned above and then the cells were incubated with TCR tetramer at various concentrations, in which Streptavidin was conjugated with HRP. The cells were washed three times with 5 ml 0.5% BSA/PBS and one time with PBS. After the last centrifugation, the cells were quickly suspended in 50 μl of tyramine biotin mixture from the Enzymatic Amplification Staining Kit (Flow-Amp System Ltd.) and the amplification reaction was developed at room temperature during 15 min. The cells were washed with stop buffer (PBS with 1% sodium azide) follow by 1% BSA/ PBS with 0.05% sodium azide. After washing the cells were stained with Streptavidin-PE conjugate at a concentration 10 μg/ml for 30 min at 4°C. The cells were washed, fixed and analyzed by flow cytometry on an MCL/XL automated analytical cytometer (Beckman Coulter Inc.).
Live cell fluorescent microscopy
JY cells were loaded with cognate SL9 or irrelevant GL9 peptides at 10-4 M and were labeled with soluble TCR (200 μg/ml) conjugated with either Streptavidin-PE or DHLA-capped QD(520) for 30 min at room temperature. After washing the cells were fixed and analyzed by fluorescence microscopy. Images were collected with a Quantix charged- coupled device camera with a Kodak 1401E chip attached to a Zeiss Axiovert 200 inverted microscope using a Plan-Apo 63X 1.40 NA objective and MetaMorph software (Universal Imaging Inc.)
RESULTS
Soluble D3 TCR tetramer assembly and characterization
Since the tetramer can productively engage only 3 arms at the same time in binding to cognate pMHC on the cell surface [32], preparations containing trimers and tetramers are optimal. To achieve maximal saturation of Streptavidin with biotinylated TCR an excess of biotinylated D3 over available biotin binding sites was maintained during tetramer assembly. SDS-PAGE established the efficiency of the TCR biotinylation and tetrameric complex formation. Figure 1 shows that all biotinylated D3 was bound to Streptavidin. 4 molecular species, namely, Streptavidin bound to 1, 2, 3, and 4 D3 molecules, were present in the mixture. The trimer (≈240 kD) was dominant. Lower order oligomers are often observed in tetramer preparations and the relative amount may vary between different experiments, but the amount was always significantly lower than that of the trimer and the tetramer.
Figure 1. Assembly of D3/TCR tetramer.

SDS PAGE at non-reducing condition shows that all biotinylated D3 were bound to Streptavidin after D3/tetramer assembly. Although trimer (Strepavidin + 3 molecules of D3) dominates the tetramer preparation, other species (monomer, dimer, and tetramer) were present as well (Lane 1); small amount of aggregates was also evident (upper band in Lane 1). Lane 2: Streptavidin; lane 3: D3 after freezing and storage at -70°C (aggregates and a small amount of degradation products are visible); lane 4: freshly isolated D3 TCR.
D3 TCR tetramer specifically binds SL9-HLA-A2 immobilized on a plastic surface
To determine the ability of D3/tetramer to recognize its natural ligand, SL9-HLA-A2 complex, we immobilized the SL9-HLA-A2 protein or an irrelevant GL9-HLA-A2 complex on 96-well plates and determined the amount of specifically bound D3/tetramer by ELISA assay. For this purpose Streptavidin conjugated with HRP was utilized. D3/tetramer specifically binds to SL9-HLA-A2, but not to GL9-HLA-A2 complex in dose dependent manner (Fig. 2).
Fig. 2. D3/tetramer specifically bound to immobilized SL9-HLA-A2 but not GL9-HLA-A2 complex.
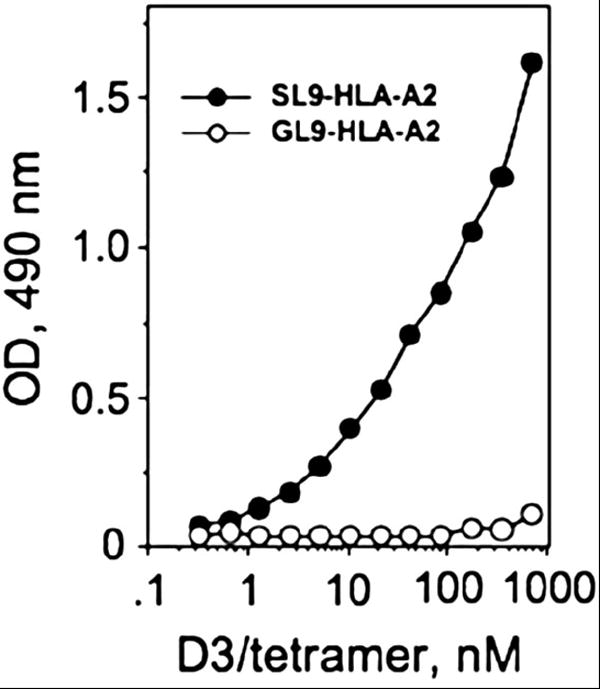
Flat-bottom 96-well plate was covered overnight (4°C) by soluble peptide-HLA-A2 complex and the plates were blocked with 1% BSA/PBS. D3/tetramer was then added at various concentrations and the amount of bound tetramer was quantified by measuring OD at 490 nm. Indicated concentrations refer to the concentration of D3 in the preparation of the tetramer.
Tetrameric D3 TCR specifically stains HLA-A2+ target cells bearing SL9 peptide epitopes
We next examined the ability of D3/tetramer to specifically stain live target cells bearing cognate SL9-HLA-A2 complexes. Figure 3 demonstrates that D3/tetramer/PE specifically bound to HLA-A2+ JY cells sensitized with SL9-HLA-A2. In control experiments, the staining of JY cells sensitized with irrelevant Flu-derived peptide GL9 was indistinguishable from the negative control, i.e., self fluorescence of JY cells. Addition of β2m to JY cells during peptide loading led to enhanced the staining intensity of the staining presumably due to increased stability of heavy chains-β2m MHC class I heterodimers. When JY cells were pulsed with SL9 peptide for 1 hour and then washed free of unbound peptide, the staining with D3 tetramer decreased (data not shown). Because free peptide was not present in the extracellular medium, the epitope density on target cells was solely determined by the half-life time of the SL9-HLA-A2 complex. This suggests that SL9-HLA-A2 complexes on JY cells have a relatively short lifetime resulting in a rapid disappearance of SL9-HLA-A2 complexes from the cell surface. To lessen the peptide dissociation from cell surface HLA-A2, free peptide was kept in the buffer during staining procedure in all other experiments.
Fig. 3. D3/tetramer specifically stains HLA-A2+ JY cells sensitized with HIV gag-derived peptide SLYNTVATL (SL9) but not with GILGFVFTL (GL9) peptide from influenza virus.
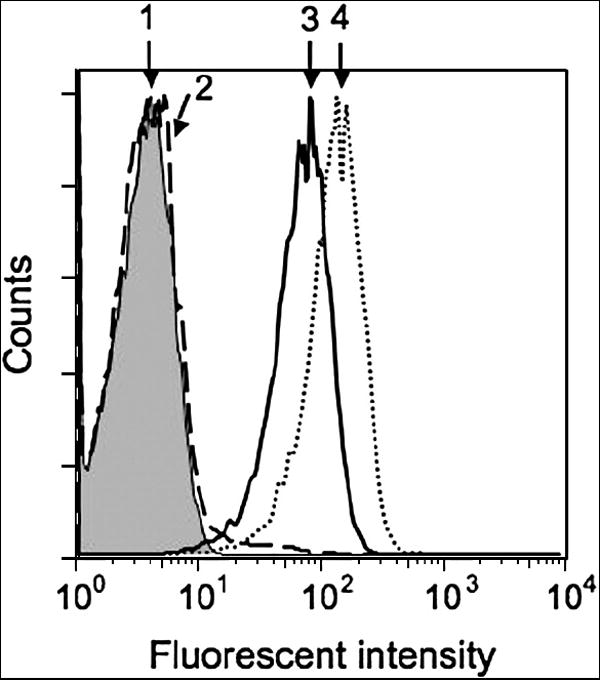
The cells (106/ml in R10) were incubated with respected peptides at 100 μM 60 minutes at 37°C. To some samples soluble β2m was added at 100 μg/ml. The culture supernatant was removed by centrifugation, and the cells were stained with 5 μM D3 TCR tetramer: (1), intact cells; (2), cells sensitized with irrelevant GL9 peptide; (3) cells sensitized with cognate SL9 peptide; (4) cells sensitized with cognate SL9 peptide in the presence of β2m. The presence of soluble β2m facilitated staining of target cells bearing cognate pMHC complexes by soluble fluorescence-labeled TCR/tetramer. Staining of the cells sensitized with Flu-derived peptide GL9 was indistinguishable from the negative control.
To understand how the intensity of the staining depends on the amount of the specific pMHC complexes on target cell surface, cell surface HLA-A2 were loaded at various peptide concentrations. At peptide concentrations below 1 μM the staining was not evident (data not shown), while CTL can still respond well to target cells sensitized at these peptide concentrations [28].
Enzymatic amplification increases the fluorescence intensity of TCR tetramer bound to cognate pMHC on the cell surface
To further increase the sensitivity of epitope detection we resorted to a signal amplification technique developed by Kaplan and colleagues [33]. It has been estimated that less than a hundred molecules per cell could be detected by this method. For this purpose, D3 TCR tetramer, in which Streptavidin is covalently bound to Peroxidase, was allowed to bind to the cells bearing SL9-HLA-A2 complexes. Biotin tyramine, a biotinylated substrate for peroxidase, was then added to the cells. The activated substrate forms covalent bonds with protein molecules in close vicinity of the peroxidase, introducing multiple biotin tags, which can serve as ligands for fluorochrome-labeled Streptavidin molecules. Figures 4A and B demonstrate that the mean fluorescence intensity of JY cells sensitized with SL9 peptide at 10-4 M and then tagged with D3/Strepavidin/HRP followed by enzymatic amplification was increased by approximately 100-fold as compare to the staining with D3/Streptavidin/PE. However, staining of JY cells sensitized at 10-7 M of SL9 with D3/tetramer followed by enzymatic amplification was still not enough to detect SL9-HLA-A2 complexes on JY cells (Fig. 4C). Although enzymatic amplification resulted in an increase in the staining intensity, the sensitivity of pMHC detection has not been enhanced. These data suggest that at peptide concentrations lower than 10-7 M the D3/tetramer molecules were mostly bound to the cell surface pMHC by one binding site, presumably due to a low epitope density and large average distance between pMHCs. Alternatively, instability of the SL9-HLA-A2 could lead to rapid disappearance of detectable pMHC complexes from the cell surface during the staining procedure, and, even with the assumption that remaining pMHCs are clustered to allow multivalent tetramer binding, the number of cell surface bound tetramers is too low to be detected by Flow Cytometry.
Fig. 4. Enzymatic amplification of fluorescent signal results in profound increase of the staining intensity, while the sensitivity of the epitope detection was similar to the standard staining procedure.
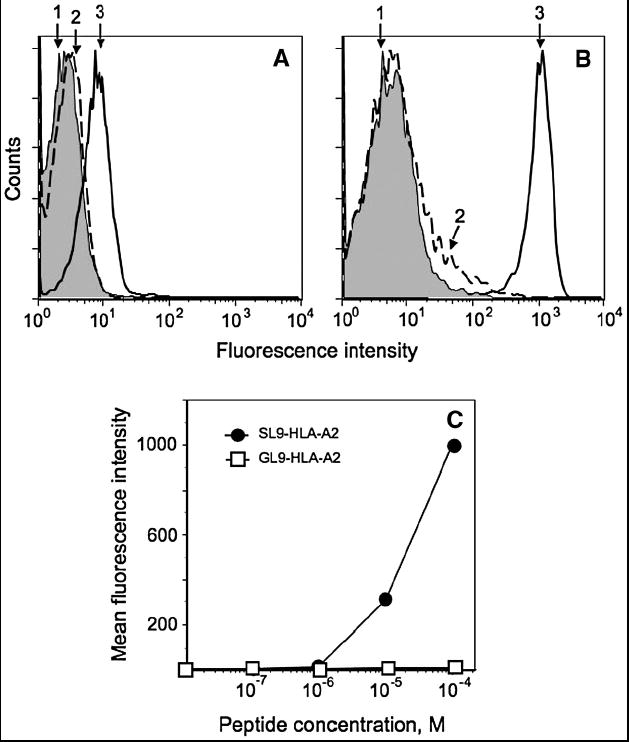
A. Intact JY cells (1) and JY cells bearing either cognate SL9-HLA-A2 (3) or irrelevant GL9-HLA-A2 (2) complexes stained with 0.2 μM of PE-labeled D3/tetramer. B. The same cells stained with HRP-labeled D3/tetramer at TCR concentration 0.2 μM followed by tyramine-based signal amplification technique (see Material and Methods). C. JY cells were sensitized with indicated concentration of the SL9 or GL9 peptides and were stained with D3/tetramer using the amplification technique.
QD/D3 TCR conjugate shows the same sensitivity as D3 TCR/tetramer despite higher level of TCR oligomerization
To increase the level of TCR oligomerization, we used QD as a scaffold to assemble QD/TCR conjugates that have a higher valency than the TCR tetramer. Depending on the QD size, each nanoparticle can bind from 10 to 100 TCR molecules, all interacting with the QDs via C-terminal His6-tag and, therefore, having the same orientation relative to the QD surface. QD (520) with an average diameter ~5nm carried 10-12 TCR molecules per QD. QDs (620) encapsulated into lipid micelles had a larger diameter and, consequently, higher binding capacity, i.e., up to 100 TCR molecules per QD. As shown in Figure 5A and B, both types of the QDs can specifically bind to cognate cell surface pMHCs. However, the peptide titration curve indicates that a greater extent of TCR oligomerization did not significantly improve the sensitivity of the specific pMHC detection (Fig. 5C). This suggests that further increase of TCR oligomerization is not sufficient to compensate for a low intrinsic affinity of the TCR-pMHC interaction. The results imply that a pMHC-specific reagent with higher affinity and/or longer TCR-pMHC lifetime has to be utilized to detect cognate cell surface pMHC at low density.
Fig. 5. Multivalent QD/D3 TCR conjugate shows similar specificity and sensitivity as the D3 TCR/tetramer.
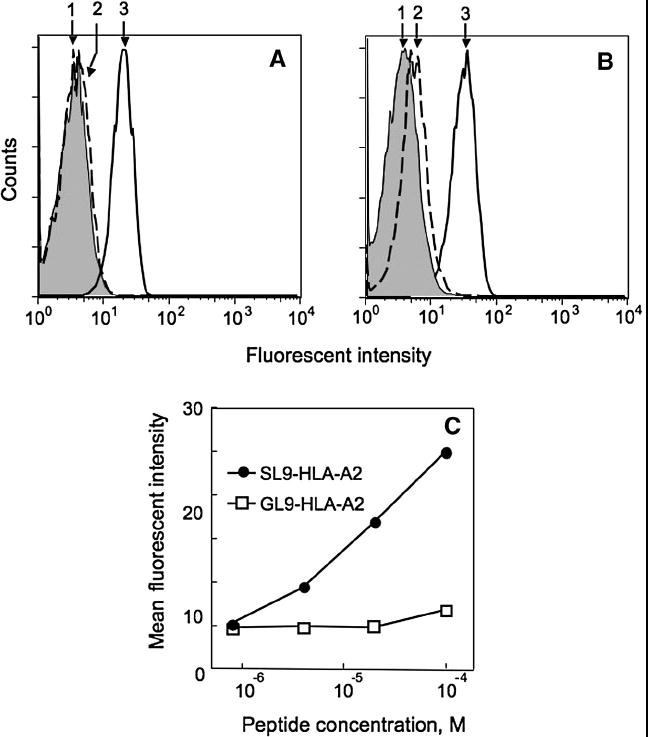
QD/TCR conjugates specifically stain HLA-A2+ JY cells sensitized with cognate SL9 (3). Staining of cells sensitized with control GL9 peptide (2) was compared with unlabeled cells (1). A. JY cells bearing with SL9-HLA-A2 (3) or GL9-HLA-A2 (2) complexes stained with QD(520)/TCR conjugate (0.5 μM of QDs) accommodating 10 TCR molecules per dot. B. The same cells stained with QD(620)/TCR conjugate (20 nM of QDs) accommodating 100 TCR molecules per dot. C. JY cells were sensitized with indicated concentration of the SL9 or GL9 peptide and were stained with QD(620)/TCR conjugate at QDs concentration 20 nM.
Monovalent Fab fragments of a TCR-like antibody with high affinity for cognate pMHC permits detection of cell surface pMHCs with greater sensitivity
To determine how the intrinsic affinity of interactions and the lifetime of the specific TCR-pMHC complex influence the sensitivity of detection of cognate pMHC on the surface of live cells, we utilized recombinant Fab fragments from 25-D1.16 TCR-like antibodies specific for pOV8 peptide in association with H-2Kb. We compared the staining of EL4 cells sensitized with either strong agonist (pOV8) or weak agonist (pOV8-R4) peptides with similar affinity for Kb protein by monomeric and tetrameric 25-D1.16 Fab. Intrinsic affinity of 25-D1.16 for pOV8-Kb complex measured in a cell free system is about 10-times higher than that for pOV8-R4-Kb variant [26]. While both monomeric and tetrameric 25-D1.16 Fab unambiguously stained EL4 cells sensitized with either pOV8 or pOV8-R4, the staining intensity with the tetrameric probe was substantially larger than with the monomeric one (Fig. 6A and B). This suggests that lowering the intrinsic affinity of the probe for cognate pMHC within a certain range affects the sensitivity of staining with the monomeric probe to a larger extent than that for the tetrameric probe. Consistent with this, 25-D1.16/tetramer staining of EL4 cells sensitized with pOV8 and pOV8-R4 was similar over all range of peptide concentrations tested (Fig. 6C) yielding approximately the same limit of detection (10-9 M peptide concentration). This is despite more than a 10-fold difference in intrinsic affinity of the Fab probe for strong and weak agonist ligands, as well as about a 5-fold difference in half-life time (t1/2) of these ligands with 25-D1.16 Fab [26]. These data suggest that an increase in receptor-pMHC lifetime beyond a certain threshold would unlikely enhance the sensitivity of pMHC detection with oligomeric TCR or TCR-like antibodies by standard flow cytometry.
Fig. 6. The sensitivity of pOV8-Kb detection with monomeric and tetrameric 25-D1.16 Fab are very similar, while the staining for pOV8-R4-Kb with the tetramer is more sensitive than with monomeric reagent.
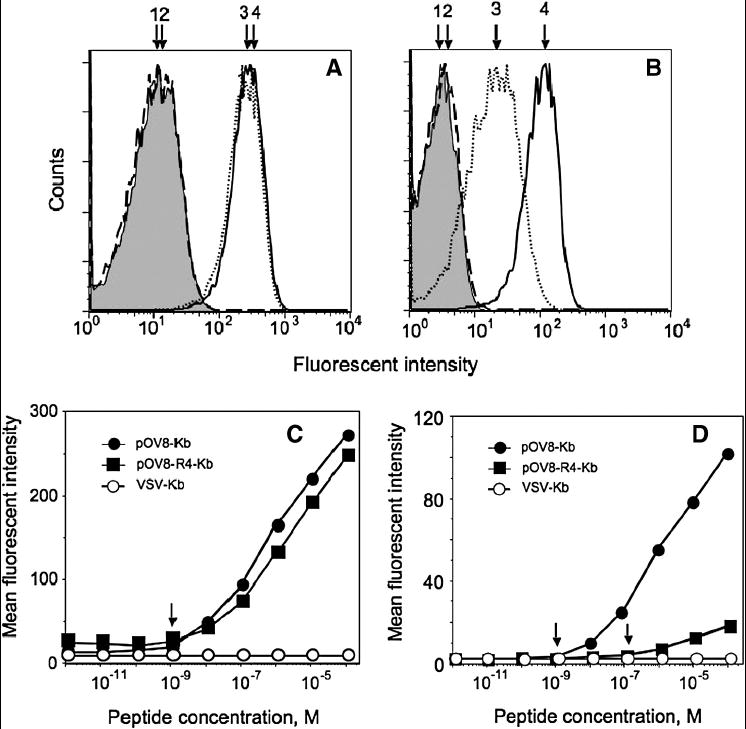
25-D1.16 Fab tetramer (A) and 25-D1.16 Fab monomer (B) specifically stain EL-4 cells pulsed with 10-4 M pOV8 (4, solid line) and pOV8-R4 (3, dotted line). Staining of EL4 cells with irrelevant peptide VSV (2, dashed line) was not different from self fluorescence of intact cells (1, shown in grey). The intensity of the staining with the tetramer was somewhat higher than with the monomeric Fab; slight difference in the background staining was evident.
EL-4 cells were pulsed with either strong agonist pOV8 or weak agonist pOV8-R4 or a null peptide VSV at various concentrations and were stained with 25-D1.16 Fab tetramer (C) or 25-D1.16 Fab monomer (D) at 40 nM concentration of the Fab.
In contrast, we observed a striking difference in the staining of pOV8- and pOV8-R4-sensitized EL4 cells with monomeric 25-D1.16 Fab fragments: the limit of detection of pOV8-Kb complexes was two orders of magnitude lower than that of pOV8-R4-Kb complexes (Fig. 6D). Importantly, the sensitivity of the staining of EL4 cells bearing pOV8-Kb complexes with monomeric 25-D1.116 Fab fragment was comparable to the sensitivity obtained with tetrameric Fab (Fig. 6C and D) suggesting that oligomerization of a high affinity receptor does not enhance the sensitivity of the detection.
Target cells presenting viral peptides that are specifically stained with fluorescent-labeled TCR/tetramer or QD/TCR conjugate can be detected with fluorescence microscopy
We utilized fluorescent-labeled D3 TCR tetramers or QD/D3 TCR conjugates to detect SL9-HLA-A2 complexes on the surface of JY cells by imaging the cells after staining with these reagents. JY cells carrying non-cognate GL9-HLA-A2 proteins were used as a negative control. As shown on Fig. 7, both reagents clearly stained cells loaded with SL9 peptide, while staining of intact cells or cells sensitized with Flu-derived peptide GL9 was practically not evident. The TCR tetramer fluorescence pattern was less intense and more diffuse compared to that observed with QD/TCR conjugates even though flow cytometric analysis showed a higher fluorescence intensity staining with the TCR/tetramer/PE than with QD/TCR (see Fig. 3, 5 and 7). The discrepancy can be explained by difference in fluorescence properties of PE and QDs fluorochromes. The PE is rapidly bleached during the imaging as opposed to QDs with exceptional photostability. These exceptional properties of QDs permit to track single molecules on the cell surface [34].
Fig. 7. QD/TCR conjugate is a better reagent for pMHC epitope detection by fluorescent microscopy as compared to D3 TCR/tetramer.
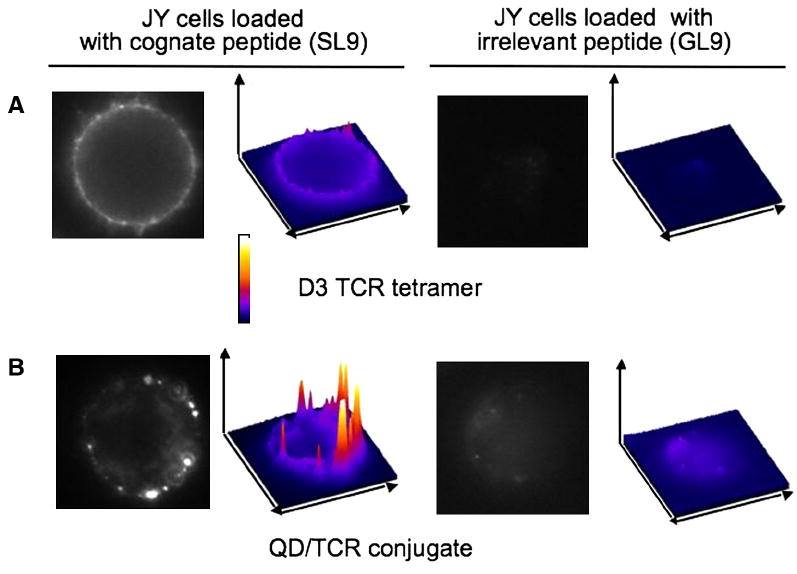
HLA-A2+ JY cells sensitized with SL9-HLA-A2 at peptide concentration 10-4 M were stained with D3/tetramer/PE (A) and QD/D3 TCR (B). Staining of the cells sensitized with control peptide GL9 was not evident. The staining pattern with D3 tetramer was less intense and more diffuse as compared to the QD/D3 TCR staining. Surface plots of the staining were built with ImageJ software.
DISCUSSION
The significance of quantitative analysis of the epitope density on antigen-presenting and target cells is evident from countless experiments showing strong impact of the number of cell surface cognate pMHC on the manifestation and magnitude of T cell responses. In particular, the level of viral presentation can significantly influence anti-virus T cell responses. It will be especially important to compare the presentation of distinct viral epitopes that differ in their ability to elicit CTL responses in the acute phase of viral infection. This will allow the development of a framework for the selection of peptide epitopes, which are expected to be strongly immunogenic, an important requirement for the design of effective immunotherapeutic interventions.
The number of the viral and tumor specific pMHC complexes on a target cell surface is influenced by many factors and can vary significantly. Calculations and direct measurements show that even few cognate pMHC on the cell surface may active T cells and induce a functional response [35; 36; 37]. Traditional reagents and methods cannot measure such low number of cell surface molecules, and new techniques ought to be developed.
In this study we attempted to understand the limitations of various approaches that we have applied to detect cognate pMHC proteins on the cell surface. These strategies include: (i) TCR oligomerization, (ii) increasing the intrinsic affinity of receptor-pMHC interactions, (iii) amplification of fluorescence signal intensity, and (iv) detection of pMHC with fluorescence microscopy.
D3 TCR has an intrinsic affinity for its cognate ligand of 2×105 L/M with a half-life time of 4.6 sec (kdiss = 0.15 s-1) [27], which makes the D3 TCR monomer useless for the detection of SL9-HLA-A2 epitopes on the cell surface. D3 TCR assembly into tetramers let us to detect SL9-HLA-A2 complexes on JY target cells. However, we have found that the sensitivity of cognate pMHC detection with the TCR tetramer is limited. The intensity of target cell staining with the tetramer rapidly declined as the density of SL9-HLA-A2 complexes was titrated down: positive tetramer staining was not evident at epitope densities that are sufficient to induce a strong cytolytic response of D3 CTL against JY target cells. The low stability of the SL9-HLA-A2 complex is one reason. Indeed, when JY cells were sensitized at a saturating SL9 concentration (10-5 M) and then washed free of unreacted peptide, incubation for only 30 min at room temperature (22-24°C) and about 10 minutes at 37°C resulted in complete loss of the ability to detect cell surface SL9-HLA-A2 complexes by D3 TCR tetramer (data not shown). Dissociation of the peptide from the MHC molecule led to a rapid decrease in the epitope density and, most likely, an increase of separating distances between cognate pMHCs on the cell surface; this precludes the TCR tetramer from engaging all available binding sites resulting in a decrease in the binding avidity. Because of the tetrahedral geometry of the Streptavidin molecule, only 3 binding sites of the tetramer may be engaged [32] by the pMHC on the cell surface. Thus, the inability to engage 2 or 3 binding sites at the same time practically abolishes the tetramer potency. In addition, reduction of the number of epitopes may also result in a decrease in the total amount of bound fluorescent tetramer below the detection limit of flow cytometry.
With this in mind, we have applied two different approaches to increase the sensitivity of SL9-HLA-A2 detection, namely, the enzymatic amplification technique and a probe with higher valency. However, the higher order TCR oligomer, QD/TCR conjugate, cannot significantly enhance the sensitivity of detection (Fig. 5C). Although QD/TCR conjugates have a higher valency (up to 100 TCR molecules per nanoparticle), it still does not improve the binding of QD/TCR to allow the detection of a relatively small number of cognate ligands on the cell surface. While the valency of QD/TCR conjugates is significantly larger than the valency of TCR/tetramer, the maximal distance between TCR binding sites on QD is about 24 nm (based on the diameter of lipid encapsulated QDs [31] and dimensions of the TCR molecule [38]), which is very similar to the distance between TCR binding sites within TCR/tetramer, i.e. 20 nm. Therefore, when separating distances between cognate pMHC are greater than 24 nm, the valency increase does not translate into higher sensitivity. Perhaps, the QD/TCR conjugates could be more useful in studying the distribution of cognate pMHCs on target cells that have been delivered to the cell surface via a physiological pathway. It is thought that the delivery of pMHC to the cell surface enables clustering of MHC molecules loaded with the same peptide [39; 40] and, thus, facilitates their detection with oligomeric TCR.
An enzymatic amplification technique has been developed to enhance the detection limit of flow cytometric analysis [33]. It makes use of the formation of reactive biotin-tyramine intermediates by peroxidase conjugated primary antibodies. The short-lived intermediate can form covalent linkages to phenolic residues in close vicinity to the primary reaction site, which can subsequently be detected by Streptavidin-fluorochrome conjugates. We have adapted this tyramine-based signal amplification (TSA) technique to enhance the fluorescent signal of stained target cells displaying cognate pMHCs. Although this approach gave raise to 10-100-fold enhancement in fluorescent signal as compared to the standard tetramer staining method, the sensitivity of epitope detection did not improve (Fig. 4). This suggests that pMHC lifetime and intrinsic affinity of the TCR-pMHC interactions are major factors limiting the sensitivity of SL9 epitope detection. Therefore, TCR with enhanced affinity or high-affinity TCR-like antibodies with the same specificity appear to be good candidates for sensing of cogante pMHC on the cell surface.
To understand how increases in the lifetime of pMHC complexes and affinity of the pMHC-probe interaction can improve the detection sensitivity, we utilized recombinant Fab fragments from 25-D1.16 TCR-like antibodies specific for pOV8 and pOVA8-R4 peptides in association with H-2Kb. Both pOVA8 and pOVA-R4 peptides form very stable complexes with H-2Kb with an affinity constant 1.2×108 L/M [41], while the pMHC complexes have similar affinities for 25-D1.16 antibodies (3.9×106 L/M and 3.2×105 L/M, respectively) [26]. We have shown that a 10-fold difference in affinity with a 5-fold difference in the lifetime between complexes of 25-D1.16 Fab with either pOV8-Kb or pOV8-R4-Kb complexes resulted in 2 orders of magnitude difference in the staining sensitivity by monomeric reagent (Fig. 6). However, 25-D1.16 Fab tetramer stains EL-4 cells loaded with strong agonist pOV8 or weak agonist pOV8-R4 equally well showing the same detection limit. Moreover, 25-D1.16 tetramer and monomer displayed a similar sensitivity of detection of pOV8-Kb on EL4 cells (see Figure 6). These data indicate that even at the peptide concentration 10-9 M multivalent engagement is prevalent, and the epitope detection limit is rather restricted by the sensitivity of flow cytometric analysis, i.e., 200-500 molecules of PE per cell (BD Bioscience). Thus, further raising the intrinsic affinity and lessening the off-rate of the pMHC-specific probe without improving detection techniques is insufficient.
Flow cytometry detection limit can be improved by increasing the molar ratio of fluorochrome to probe. This principle was successfully utilized in a new amplification technique based on conjugation of antibody to fluorescein-filled liposomes containing a large number of fluorochrome molecules [42]. It has been shown that depending on the size of the liposomes the sensitivity can be substantially increased without altering the background. This technique allows detection of 50-100 molecules of IFN-γ and IL-10 on the surface of a cell, which are undetectable by other available methods. The development of more uniform and stable nanoparticles with strong fluorescent signal will likely make the suggested approach even more efficient.
While flow cytometry analysis measures integral fluorescence intensity from each individual cell, fluorescence microscopy permits analysis of fluorescence intensity of individual clusters or even single molecules on the cell surface. Therefore, the sensitivity of pMHC detection at low densities has been improved up to 10 times with single-molecule imaging [36; 43]. To further modify the approach, we have suggested using QDs as a fluorochrome. The QDs have several unique features that make them useful for the detection of cells with low epitope density (Table 1). First, QDs can be easily conjugated with His6-tagged protein through affinity interaction with either Zn2+ atom of the QD shell or Ni-NTA group of lipid encapsulated QDs. Second, QDs are characterized by a large separation between the excitation and emission wavelengths that makes it possible to collect fluorescence over a wide range of emission spectra improving the sensitivity. Third, QDs have exceptional photostability compared with typical fluorophores (hours vs minutes), and due to the inorganic nature QDs are resistant to metabolic degradation making them suitable for sensitive in vitro and in vivo imaging. Compared with D3 TCR/tetramer, QD/D3 TCR conjugate showed a very bright, contrast, and specific staining of the peptide-loaded cells with low background (Fig. 7). This difference becomes even more striking as analysis of the same cells by flow cytometry shows a higher fluorescence intensity with D3 TCR/tetramer than with QD/D3 TCR conjugate (see Fig. 3, 5A, and 7). These data suggest new potential opportunities to use QD conjugates for the imaging of virus-infected and tumor cells presenting perhaps only several dozen of cognate pMHC per cell.
Table 1.
| Quantum Dot properties | Influence on the method of detection | |||
|---|---|---|---|---|
| Flow cytometry | Microscopy | |||
| Sensitivity | Other factors | Sensitivity | Other factors | |
| Photostability | No | No | Significantly increases | Allows to detect a single QD |
| Serve as a scaffold | Increases | Permits detection of cells with low affinity receptors | Increases | Permits imaging of low affinity receptor on the cell surface |
| Tendency to aggregate | Decreases | Increases background, requires freshly prepared QDs of high quality | Decreases | Increases background, requires freshly prepared QDs of high quality |
| Narrow emission bandwidths | No | Allows multicolor detection | No | Allows multicolor detection |
| Wide excitation range | Varied depending on excitation wavelength | Allows usage of a single excitation source for multicolor detection | Varied depending on excitation wavelength | Allows usage of a single excitation source for multicolor detection |
Acknowledgments
This work was supported by NIH research grants AI058755 and AI052812 to Y.S. T.M. was supported in part by research grant CA56036-08 to the Kimmel Cancer Center. N.A. was supported in part by NRSA Training Programs in AIDS Research 5-T32-AI07523. We thank Angel Porgador and Ronald Germain for providing hybridoma 25-D.1.16.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisen HN, Sykulev Y, Tsomides TJ. The antigen-specific T-cell receptor and its reactions with peptide-MHC complexes. In: Haber E, editor. Antigen-Binding Molecules Antibodies and T-cell Receptors. Academic Press; San Diego: 1997. pp. 1–56. [Google Scholar]
- 2.Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y. Ligand recognition by alpha beta T cell receptors. Annual Review of Immunology. 1998;16:523–44. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- 3.Sykulev Y, Anikeeva N, Kalams SA. Soluble TCR/tetramer as a tool to study the distribution of immunodominant HIV Gag peptide epitope on the surface of target cells. Experimental Biology Meeting; New Orlean, LA. 2002. [Google Scholar]
- 4.Subbramanian RA, Moriya C, Martin KL, Peyerl FW, Hasegawa A, Naoi A, Chhay H, Autissier P, Gorgone DA, Lifton MA, Kuus-Reichel K, Schmitz JE, Letvin NL, Kuroda MJ. Engineered T-cell receptor tetramers bind MHC-peptide complexes with high affinity. Nat Biotechnol. 2004;22:1429–34. doi: 10.1038/nbt1024. [DOI] [PubMed] [Google Scholar]
- 5.Laugel B, Boulter JM, Lissin N, Vuidepot A, Li Y, Gostick E, Crotty LE, Douek DC, Hemelaar J, Price DA, Jakobsen BK, Sewell AK. Design of soluble recombinant T cell receptors for antigen targeting and T cell inhibition. J Biol Chem. 2005;280:1882–92. doi: 10.1074/jbc.M409427200. [DOI] [PubMed] [Google Scholar]
- 6.Zhu X, Belmont HJ, Price-Schiavi S, Liu B, Lee HI, Fernandez M, Wong RL, Builes J, Rhode PR, Wong HC. Visualization of p53(264-272)/HLA-A*0201 complexes naturally presented on tumor cell surface by a multimeric soluble single-chain T cell receptor. J Immunol. 2006;176:3223–32. doi: 10.4049/jimmunol.176.5.3223. [DOI] [PubMed] [Google Scholar]
- 7.Altman J, Moss P, Goulder P, Barouch D, McHeyzer-Williams M, Bell J, McMichael A, Davis M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 8.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–82. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 9.Holler PD, Holman PO, Shusta EV, O’Herrin S, Wittrup KD, Kranz DM. In vitro evolution of a T cell receptor with high affinity for peptide/MHC. Proc Natl Acad Sci U S A. 2000;97:5387–92. doi: 10.1073/pnas.080078297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shusta EV, Holler PD, Kieke MC, Kranz DM, Wittrup KD. Directed evolution of a stable scaffold for T-cell receptor engineering. Nat Biotechnol. 2000;18:754–9. doi: 10.1038/77325. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Moysey R, Molloy PE, Vuidepot AL, Mahon T, Baston E, Dunn S, Liddy N, Jacob J, Jakobsen BK, Boulter JM. Directed evolution of human T-cell receptors with picomolar affinities by phage display. Nat Biotechnol. 2005;23:349–54. doi: 10.1038/nbt1070. [DOI] [PubMed] [Google Scholar]
- 12.Dunn SM, Rizkallah PJ, Baston E, Mahon T, Cameron B, Moysey R, Gao F, Sami M, Boulter J, Li Y, Jakobsen BK. Directed evolution of human T cell receptor CDR2 residues by phage display dramatically enhances affinity for cognate peptide-MHC without increasing apparent cross-reactivity. Protein Sci. 2006;15:710–21. doi: 10.1110/ps.051936406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc Natl Acad Sci U S A. 1996;93:1820–4. doi: 10.1073/pnas.93.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–26. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- 15.Polakova K, Plaksin D, Chung DH, Belyakov IM, Berzofsky JA, Margulies DH. Antibodies directed against the MHC-I molecule H-2Dd complexed with an antigenic peptide: similarities to a T cell receptor with the same specificity. J Immunol. 2000;165:5703–12. doi: 10.4049/jimmunol.165.10.5703. [DOI] [PubMed] [Google Scholar]
- 16.Chames P, Willemsen RA, Rojas G, Dieckmann D, Rem L, Schuler G, Bolhuis RL, Hoogenboom HR. TCR-like human antibodies expressed on human CTLs mediate antibody affinity-dependent cytolytic activity. J Immunol. 2002;169:1110–8. doi: 10.4049/jimmunol.169.2.1110. [DOI] [PubMed] [Google Scholar]
- 17.Wittman VP, Woodburn D, Nguyen T, Neethling FA, Wright S, Weidanz JA. Antibody targeting to a class I MHC-peptide epitope promotes tumor cell death. J Immunol. 2006;177:4187–95. doi: 10.4049/jimmunol.177.6.4187. [DOI] [PubMed] [Google Scholar]
- 18.Biddison WE, Turner RV, Gagnon SJ, Lev A, Cohen CJ, Reiter Y. Tax and M1 peptide/HLA-A2-specific Fabs and T cell receptors recognize nonidentical structural features on peptide/HLA-A2 complexes. J Immunol. 2003;171:3064–74. doi: 10.4049/jimmunol.171.6.3064. [DOI] [PubMed] [Google Scholar]
- 19.Lebedeva T, Anikeeva N, Kalams SA, Walker BD, Gaidarov I, Keen JH, Sykulev Y. Major histocompatibility complex class I-intercellular adhesion molecule-1 association on the surface of target cells: implications for antigen presentation to cytotoxic T lymphocytes. Immunology. 2004;113:460–71. doi: 10.1111/j.1365-2567.2004.01985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walden PR, Eisen HN. Cognate peptides induce self-destruction of CD8+ cytolytic T lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9015–9. doi: 10.1073/pnas.87.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brander C, Hartman K, Trocha A, Jones N, Johnson R, Korber B, Wentworth P, Buchbinder S, Wolinsky S, Walker B, Kalams S. Lack of strong immune selection pressure by the immunodominant, HLA-A*0201-restricted cytotoxic T lymphocyte response in chronic human immunodeficiency virus-1 infection. J Clin Invest. 1998;101:2559–2566. doi: 10.1172/JCI2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotch F, Rothbard J, Howland K, Townsend A, McMichael A. Cytotoxic T lymphocytes recognize a fragment of influenza virus matrix protein in association with HLA-A2. Nature. 1987;326:881–2. doi: 10.1038/326881a0. [DOI] [PubMed] [Google Scholar]
- 23.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carbone FR, Sterry SJ, Butler J, Rodda S, Moore MW. T cell receptor a-chain pairing determines the specificity of residue 262 within the Kb-restricted, ovalbumin 257-264 determinent. Int Immunol. 1992;4:861–867. doi: 10.1093/intimm/4.8.861. [DOI] [PubMed] [Google Scholar]
- 25.van Bleek GM, Nathenson SG. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990;348:213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- 26.Mareeva T, Lebedeva T, Anikeeva N, Manser T, Sykulev Y. Antibody specific for the peptide-MHC complex: is it TCR-like? J Biol Chem. 2004;279:44243–49. doi: 10.1074/jbc.M407021200. [DOI] [PubMed] [Google Scholar]
- 27.Anikeeva N, Lebedeva T, Krogsgaard M, Tetin SY, Martinez-Hackert E, Kalams SA, Davis MM, Sykulev Y. Distinct molecular mechanisms account for the specificity of two different T-cell receptors. Biochemistry. 2003a;42:4709–4716. doi: 10.1021/bi026864+. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Hackert E, Anikeeva N, Kalams SA, Walker BD, Hendrickson WA, Sykulev Y. Structural basis for degenerate recognition of natural HIV peptide variants by cytotoxic lymphocytes. J Biol Chem. 2006;281:20205–12. doi: 10.1074/jbc.M601934200. [DOI] [PubMed] [Google Scholar]
- 29.Anikeeva N, Lebedeva T, Sumaroka M, Kalams SA, Sykulev Y. Soluble HIV-specific T-cell receptor: expression, purification and analysis of the specificity. J Immunol Meth. 2003b;277:75–86. doi: 10.1016/s0022-1759(03)00179-0. [DOI] [PubMed] [Google Scholar]
- 30.Mattoussi H, Mauro JM, Goldman ER, Anderson GP, Sundar VC, Mikulec FV, Bawendi MG. J Am Chem Soc. 2000;122:12142–12150. [Google Scholar]
- 31.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–62. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 32.Boniface J, Rabinowitz J, Wulfing C, Hampl J, Reich Z, Altman J, Kantor R, Beeson C, McConnell H, Davis M. Initiation of signal transduction through the T cell receptor requires the multivalent engagement of peptide/MHC ligands. Immunity. 1998;9:459–466. doi: 10.1016/s1074-7613(00)80629-9. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan D, Smith D. Enzymatic amplification staining for flow cytometric analysis of cell surface molecules. Cytometry. 2000;40:81–5. doi: 10.1002/(sici)1097-0320(20000501)40:1<81::aid-cyto11>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 34.Dahan M, Levi S, Luccardini C, Rostaing P, Riveau B, Triller A. Diffusion dynamics of glycine receptors revealed by single-quantum dot tracking. Science. 2003;302:442–5. doi: 10.1126/science.1088525. [DOI] [PubMed] [Google Scholar]
- 35.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4:565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 36.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 37.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, Wong HC, Chakraborty AK, von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–91. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–66. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 39.Spiliotis ET, Osorio M, Zuniga MC, Edidin M. Selective export of MHC class I molecules from the ER after their dissociation from TAP. Immunity. 2000;13:841–851. doi: 10.1016/s1074-7613(00)00081-9. [DOI] [PubMed] [Google Scholar]
- 40.Neefjes JJ, Schumacher TN, Ploegh HL. Assembly and intracellular transport of major histocompatibility complex molecules. Curr Opin Cell Biol. 1991;3:601–9. doi: 10.1016/0955-0674(91)90029-x. [DOI] [PubMed] [Google Scholar]
- 41.Sykulev Y, Brunmark A, Jackson M, Cohen RJ, Peterson PA, Eisen HN. Kinetics and affinity of reactions between an antigen-specific T-cell receptor and peptide-MHC complexes. Immunity. 1994a;1:15–22. doi: 10.1016/1074-7613(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 42.Scheffold A, Assenmacher M, Reiners-Schramm L, Lauster R, Radbruch A. High-sensitivity immunofluorescence for detection of the pro- and anti-inflammatory cytokines gamma interferon and interleukin-10 on the surface of cytokine-secreting cells. Nat Med. 2000;6:107–10. doi: 10.1038/71441. [DOI] [PubMed] [Google Scholar]
- 43.Purbhoo MA, Sutton DH, Brewer JE, Mullings RE, Hill ME, Mahon TM, Karbach J, Jager E, Cameron BJ, Lissin N, Vyas P, Chen JL, Cerundolo V, Jakobsen BK. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J Immunol. 2006;176:7308–16. doi: 10.4049/jimmunol.176.12.7308. [DOI] [PubMed] [Google Scholar]


